ABSTRACT
Introduction
Studies on economic evaluations of the 13-valent pneumococcal conjugate vaccine (PCV13) have been increasing over the last decade. No systematic reviews have synthesized the evidence of economic evaluations of the PCV13.
Areas covered
We systematically searched the literature which published on peer-reviewed journals from January 2010 to June 2022. The literature search was conducted in the following electronic databases: PubMed, Web of Science, Embase, the Cochrane Library, CNKI, Wanfang database, VIP database. We identified 1827 records from the database search. After excluding 511 duplicates, 1314 records were screened, of which 156 records were retained for the full-text reviews. A total of 44 studies were included in the review. Among the included studies, 33 studies were economic evaluations of PCV13 among children, and 11 studies were conducted among adults. The literature search initiated in April, 2022, and updated in June 2022.
Expert opinion
Vaccination with PCV13 was found to significantly reduce the mortality and morbidity of pneumococcal diseases and was cost-effective compared to no vaccine or several other pneumococcal vaccines (e.g. PCV10, PPV23). Future research is advised to expand economic evaluations of PCV13 combined with dynamic model to enhance methodologic rigor and prediction accuracy.
1. Introduction
Pneumococcal diseases (PDs) have become a serious public health problems around the world, and cause significant morbidity and mortality and a substantial burden for health care systems [Citation1–3]. The Streptococcus Pneumoniae (SPn) or pneumococcus is acknowledged as the main cause of PDs, such as bacterial pneumonia, meningitis, acute otitis media (AOM), and bacteremia in children and adults [Citation4]. It has been estimated that more than 300,000 children under 5 years old worldwide died every year from SPn infections [Citation5]. Given the increasing resistance to antibiotics treatments for SPn, there is an urgent need to use vaccines to control PDs [Citation6]. WHO recommends that all countries should incorporate pneumococcal conjugate vaccine (PCV) into their own National Immunization Programs (NIP), especially those countries with high childhood mortality [Citation7].
13-valent pneumococcal conjugate vaccine (PCV13) is one of the major PCVs that has been used since 2010 for prevent PDs among children and adults [Citation8]. In recent years, a number of studies have reported that the incidence of PDs, especially the invasive PDs in vaccinated populations decreased significantly with the use of the PCV13 [Citation9–12]. However, there are significant differences across countries in terms of the income levels and the epidemic intensity of PDs. Based on income levels categorized by the World Bank, the countries and territories were stratified into four
groups, which classified as high-income countries (HICs), upper-middle income countries (UMCs), lower-middle income countries (LMCs), and low-income countries (LICs), respectively [Citation13]. Health economic evaluation is a scientific method to evaluate cost-effectiveness or cost-benefits of health interventions, which can provide scientific evidence for health resource allocation and health care decision-making [Citation14]. In the last decade, studies on economic evaluations of PCV13 have been increasing worldwide. However, the evidence-based cost-effectiveness of PCV13 is unclear. Some studies have demonstrated that PCV13 is more cost-effective relative to no vaccine or other pneumococcal vaccines [Citation14,Citation15], while other studies have shown that the PCV13 vaccine was not cost-effective [Citation16–18]. There is a lack of evidence integration and systematic reviews of the economic evaluation studies for PCV13. No systematic reviews have summarized the cost-effectiveness results of PCV13 among populations of both children and adults in all countries, though a few systematic reviews examined cost-effectiveness of PCVs in children, or adults separately in some regions (e.g. in low- and middle-income countries) [Citation19–21]. Further, no previous systematic reviews have included both English and Chinese language studies. A comprehensive systematic reviews of cost-effectiveness PCV13 is important to provide evidence-based information as decision-makers need to know whether the PCV13 vaccination is cost-effective, and how the vaccination impacts the economic budget.
This systematic review aimed to synthesize the individual studies on the economic evaluation of PCV13.
2. Methods
2.1. Protocol and reporting
This systematic review registered with the International Prospective Register of Systematic Reviews (PROSPERO) (registration no. CRD42022327,960), available from https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=327960. The review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Citation22].
2.2. Eligibility criteria
Inclusion criteria: Studies were determined to be eligible if they met the following criteria: (1) economic evaluations that compared the PCV13 with other vaccines or no vaccination, including cost-effectiveness analysis, cost-utility analysis, cost-benefit analysis and cost-minimization analysis; (2) outcomes included quality-adjusted life-year (QALY), disability-adjusted life-year (DALY), and the study reported an incremental cost-effectiveness ratio (ICER), or incremental costs, incremental effectiveness; (3) the target populations included children and adults.
Exclusion criteria: (1) Studies describing vaccine effectiveness and immunogenicity; (2) no valid outcome data could be extracted from the studies; (3) studies on data duplication; (4) studies in multiple countries. (5) systematic review and meta-analysis studies, conference abstracts, articles and proceedings.
2.3. Information sources and search
We systematically searched the literature which published on peer-reviewed journals from January 2010 to June 2022. The literature search was conducted in the following electronic databases: PubMed, Web of Science, Embase, the Cochrane Library, CNKI, Wanfang database, VIP database. Search terms included ‘pneumococcal vaccines,’ ‘PCV13,’ ‘13-valent pneumococcal conjugate vaccine,’ ‘cost-benefit analysis,’ ‘cost-effectiveness analysis,’ ‘cost-utility analysis,’ ‘economic evaluation.’ We also screened the references in relevant reviews and meta-analyses to identify potential eligible studies. The search results from the databases were merged, and duplicates were removed. The literature search initiated in April, 2022, and updated in June 2022.
2.4. Selection of sources of evidence
According to inclusion and exclusion criteria, two authors independently conducted the study selection. The authors screened the titles and abstracts. If a decision could not be made based on the title and abstract, the full-text was retrieved for review to determine the eligibility. When there were disagreements on the eligibility of some studies between the two authors, the eligibility of the studies was resolved by discussion among all the authors.
2.5. Data charting process and data items
Subsequently, the full-text articles of all selected studies for the synthesis were retrieved for data extraction. Data were extracted by one author and checked by another author for accuracy and correction. We developed a customized data extraction sheet based on the WHO Guide for standardization of economic evaluations of immunization programs [Citation23]. Extracted information included basic information (i.e. author, year of publication, country and target population), description of the interventions (i.e. types of interventions and comparisons), details of the economic evaluations (i.e. model, currency, type and perspective, time horizon, reference year, discounting and sensitivity analysis) and study findings (i.e. incremental costs, incremental effects and incremental cost-effectiveness ratios).
2.6. Assessment of risk of bias
We used the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement to evaluate risk of bias of the included studies [Citation24]. The CHEERS statement is a report checklist with 24 items developed to assess methodological quality of economic evaluations. Each question was answered with ‘yes,’ ‘no,’ or ‘not clear.’ The question was assigned one score if a confirmative answer ‘yes’ was appropriate for the study, indicating that the study met the quality criteria. If the answer to the question was ‘no’ or ‘not clear,’ it was assigned a score of zero, indicating that the study did not entirely fulfill the quality criteria. The total score was computed as a sum of the score for each question. The quality of the selected studies was determined by the percentage of items that fulfilled the quality criteria. Studies were classified as ‘excellent,’ ‘good,’ ‘fair’ and ‘poor’ quality if they fulfilled 100% of the items, between >75% and <100% of the items, between >50% and ≤75% of the items, and ≤50% of the items respectively [Citation25,Citation26].
2.7. Synthesis of results
Narrative synthesis was used to summarize the findings of individual studies. The main findings of individual studies were presented using descriptive tables for qualitative comparisons.
3. Results
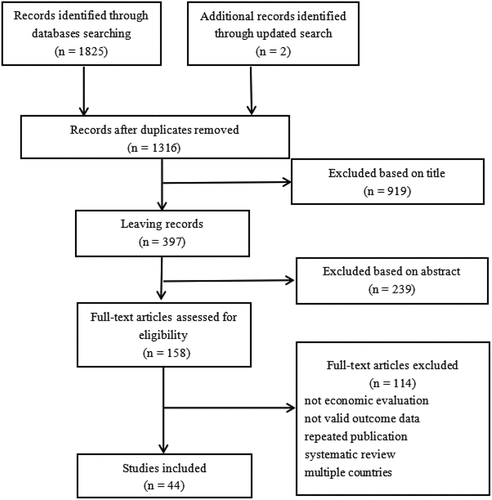
We identified 1827 records from the database search. After excluding 511 duplicates, 1314 records were screened, of which 156 records were retained for the full-text reviews. A total of 44 studies were included in the review. Study selection is shown in .
3.1. Characteristics of reviewed studies
The characteristics of included studies are presented in . The studies were published between 2010 and 2021, and were conducted among 31 countries. Two studies in Chinese language were PhD and Master degree students’ theses [Citation27,Citation28]. Among the included studies, 33 studies were economic evaluations of PCV13 among children [Citation14,Citation16–18,Citation27–55], and most of these studies included children less than 5 years old. These studies included 7 HICs [Citation29,Citation38,Citation41,Citation49–52], 19 UMCs [Citation14,Citation16–18,Citation27,Citation28,Citation30,Citation32–34,Citation37,Citation40,Citation42,Citation43,Citation45–47,Citation53,Citation54], 5 LMCs [Citation31,Citation35,Citation39,Citation44,Citation48] and 2 LICs [Citation36,Citation55]. Eleven studies were conducted among adults [Citation15,Citation56–65], where 10 studies selected adults aged 50 years older, and one study targeted adults > 18 years of age [Citation58]. Eleven studies were all HICs. All studies performed cost-effectiveness analysis. Twenty-five studies used a 3% discount rate for outcomes. All studies applied health economic models, and 13 studies applied a model time horizon of 5 years. Markov model (n = 22) and decision tree model were the most commonly used models in the included studies. Other models (i.e. TRIVAC model) were also used in some studies [Citation29,Citation34,Citation41–43,Citation54]. In terms of perspectives, 15 studies used the societal perspective, followed by the payer perspective (n = 8), healthcare perspective (n = 5), and government perspective (n = 4). Two studies did not report the perspective [Citation29,Citation59].
Table 1. Characteristics of the included studies.
Table 2. Characteristics of the included studies.
show characteristics of interventions and economic evaluations. In studies of children, 17 studies used three doses of PCV13 [Citation30,Citation31,Citation34,Citation36,Citation39,Citation41–46,Citation48,Citation50–53,Citation55], 15 studies used four doses of PCV13 [Citation16–18,Citation27–29,Citation32,Citation33,Citation35,Citation37,Citation38,Citation40,Citation47,Citation49,Citation54], and one study used three and four doses of PCV13 [Citation14]. In studies of adults, one dose of PCV13 was used in all studies. The vaccine coverage varied from country to country, and the coverage of PCV13 was assumed to be the same as diphtheria, tetanus toxoids, and pertussis vaccine coverage in some countries [Citation16,Citation18,Citation27–29,Citation35,Citation37,Citation38,Citation50,Citation55].
Table 3. Characteristics of interventions and economic evaluations.
Table 4. Characteristics of interventions and economic evaluations.
3.2. Sensitivity analysis
Sensitivity analysis was an important step to verify the reliability of economic evaluation conclusions. By controlling the changes of several major variables within a certain range, the influence of the changes of variables on the results was tested. Among the 44 included studies, 43 studies performed a sensitivity analysis. One way sensitivity analyses and probabilistic sensitivity analyses (PSA) were the most common. The results of the sensitivity analysis of most studies showed that the most sensitive parameter was the price of the vaccine, followed by the incidence of PDs and vaccine effectiveness ().
3.3. Cost-effectiveness results
Among the 44 included studies, incremental cost-effectiveness ratio (ICER) was reported in 36 studies, and incremental cost and incremental effect were reported in all studies. The specific ICER is shown in . Thirty-four studies used quality-adjusted life-years (QALYs), and 10 studies used disability-adjusted life-years (DALYs) [Citation34,Citation35,Citation39,Citation41–44,Citation48,Citation54,Citation55].
3.3.1. Children’s studies
3.3.1.1. HICs
Seven studies were identified [Citation29,Citation38,Citation41,Citation49–52], 5 studies concluded that the PCV13 vaccine was cost-effective [Citation29,Citation38,Citation49–51], 1 studies concluded that the PCV13 vaccine was unlikely to be cost-effective [Citation41] and only 1 studies concluded that it was not to be cost-effective [Citation52].
3.3.1.2. UMCs
Nineteen studies were identified [Citation14,Citation16–18,Citation27,Citation28,Citation30,Citation32–34,Citation37,Citation40,Citation42,Citation43,Citation45–47,Citation53,Citation54], 1 studies concluded that the PCV13 vaccine was highly cost-effective [Citation28], 2 studies concluded that it was more cost-effective [Citation45,Citation53], 11 studies concluded that it was cost-effective [Citation14,Citation30,Citation32–34,Citation37,Citation40,Citation42,Citation43,Citation46,Citation54] and 5 studies concluded that it was not to be cost-effective [Citation16–18,Citation27,Citation47].
3.3.1.3. LMCs
Five studies were identified [Citation31,Citation35,Citation39,Citation44,Citation48], 3 studies concluded that the PCV13 vaccine was highly cost-effective [Citation35,Citation39,Citation48] and 2 studies concluded that it was cost-effective [Citation31,Citation44].
3.3.1.4. LICs
Two studies were identified [Citation36,Citation55]. These 2 studies concluded that the PCV13 vaccine was cost-effective.
3.3.2. Adults’ studies
Eleven studies were identified [Citation15,Citation56–65], where 11 studies were all HICs. Of the included studies, 2 studies concluded that the PCV13 vaccine was highly cost-effective [Citation15,Citation64], 2 studies concluded that it was more cost-effective [Citation56,Citation62], and 6 studies concluded that it was cost-effective [Citation57–61,Citation65]. The remaining 1 study concluded that the PCV13 vaccine was unlikely to be cost-effective [Citation63].
3.4. Risk of bias assessment
The risk of bias assessment scores of the included studies were shown in . The studies had an average score of 21.5 (ranged 19–23), with quality scores ranging from 75% to 100%. All studies were evaluated as good quality. None of the studies met the excellent standard, and no studies were rated as fair or poor quality. The main quality problems included no characterizing heterogeneity, no measurement and valuation of preferences, no model assumptions or no source of funding.
Table 5. Risk of bias assessment among the studies reviewed.
4. Expert opinion
Our findings suggest the economically advantages profile of the PCV13 vaccination in children and adults. Vaccination with PCV13 was found to significantly reduce the mortality and morbidity of pneumococcal diseases. However, countries are reevaluating the impact and economic evaluations of PCV13 vaccination programs, because of the pressure of constrained health budgets [Citation66].
This study is the first to systematically summarize English and Chinese studies on the economic evaluation of PCV13. We found the evidence that PCV13 was cost-effective compared to no vaccine or several other pneumococcal vaccines (e.g. PCV10, PPV23). Among the 44 included studies, most studies on PCV13 concluded that PCV13 inoculation was cost-effective, and six studies reported that PCV13 inoculation was not cost-effective. All the six studies showing that PCV13 was not cost-effective targeted at children [Citation16–18,Citation27,Citation47,Citation52]. Only one study found that PCV13 inoculation was unlikely to be cost-effective among adults. These studies are all from HICs and UMCs. This may be related to three possible reasons. First of all, PCV13 is more often used in children, and there are fewer studies comparing PCV13 inoculation with other inoculation strategies in adults. Secondly, the adults selected in most studies are the elderly, who are susceptible to SPn due to the lack and decrease of circulating IgM memory B cells [Citation67]. A number of clinical trials have found that PCV13 has better immunogenicity than PPV23 in the elderly population, and can largely prevent SPn infection in the elderly population [Citation68,Citation69]. Thirdly, the introduction of vaccination programs in HICs and UMCs were not limited by financial barriers [Citation19].
4.1. Choice of study perspectives
WHO Guide for Standardization of Economic Evaluations of Immunization Programmes and the Consolidated Health Economic Evaluation Reporting Standards statement requires that a full economic evaluation study should clearly demonstrate the study perspective [Citation23]. Different countries, social systems and cultural backgrounds can lead to different study perspectives. Given the perspective impacts the costs and outcomes of the evaluation, it is important to specify the study perspective in order to determine the costs that should be considered in economic evaluations [Citation70]. The societal perspective is most broadly used in economic evaluation studies [Citation23]. The societal perspective refers to the total social costs that should be considered in health economic evaluations, including all costs incurred by society [Citation67]. In the present review, the societal perspective is the most frequently used perspective, followed by the payer perspective (n = 8) and health care perspective (n = 5). Previous research suggests that the societal perspective should be selected in order to gain a scientific result if there is no other specific purpose to be considered in the economic evaluations of PCV13 [Citation23,Citation70].
4.2. Choice of modeling methods and parameters
Cost-effectiveness analyses are useful for predicting potential costs and benefits, and can assist stakeholders in making informed decisions about the value of proposed interventions. The majority of the economic evaluation studies of PCV13 adopted static models, mainly Markov model and decision tree model. The static models have advantages of simplicity and ability of handling both costs and outcomes [Citation71]. One limitation of the cohort Markov model and decision tree model is that these models do not allow state transition probabilities to vary with the time in that state or previous states because it assumes that the probability of disease state is constant over time [Citation72]. In contrast, dynamic models account for interactions among entities, and allow modeling the change of a state to be dependent on the previous states (i.e. feedback) [Citation72,Citation73]. Previous studies have documented that static model in economic evaluations of PCV13 often underestimates the health benefit of PCV13, and further underestimates the cost-effectiveness of PCV13 in NIP. In recent years, an emerging number of studies have applied dynamic or hybrid models in economic evaluations [Citation74,Citation75]. The dynamic model and hybrid model had higher requirements for the model parameters, and are more complex than static models like cohort Markov models and decision tree models. However, the incidence and prevalence trend of PDs in the future can be more accurately predicted using a dynamic or hybrid model [Citation70]. Future research is needed to expand economic evaluations of PCV13 combined with dynamic model to enhance methodologic rigor and prediction accuracy.
Using high quality parameters in economic evaluations of vaccines is crucial in order to provide accurate simulations of the benefits of PCV13 inoculation. However, in some circumstances, ideal parameters for the effectiveness are difficult to be obtained, and researchers have to use proxy measures or parameters from other regions or countries. In this systematic review, we found that some studies used the epidemiological parameters selected from other regions or countries [Citation18,Citation34,Citation35,Citation43,Citation44,Citation52,Citation53,Citation76]. In order to better simulate the real-world PCV effectiveness in each country, and obtain more accurate and robust economic evaluation results, more epidemiological studies like surveillance of pneumococcal disease risk in general populations are required to evaluate the immune effect of PCV13.
We found that cost-effectiveness modeling methods on PCV13 varied with different countries. Studies in HICs tend to use more advanced modeling methods such as Markov model versus a simple decision tree model than studies in LMCs and LICs. Compared with HICs, studies in India, Egypt and other LMCs (i.e. Filipino, Iran) were more likely to use decision tree model in the economic evaluations of PCV13, characterizing as insufficient modeling methods (e.g. single decision tree model), and insufficient reported outcomes (e.g. no ICER report), or low parameters quality [Citation30,Citation31,Citation34,Citation44].
4.3. Reporting of the economic evaluations of PCV vaccines
The findings of this systematic review suggest that the reporting of economic evaluations of PCV13 needs to be further improved. We observed that major quality problems of some individual studies included a lack of clarification of the outcomes such as the immune effects of PCV13 and the valuation of preference for utility measure, a lack of description of model assumptions or source of funding. Future studies should illustrate clearly the measurement and valuation of preference for the vaccine effect, model assumptions, methods of improving the quality of model parameters, characteristics of heterogeneity, and the source of funding. To improve the quality of reporting, researchers should follow the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement [Citation77] in conducting and reporting economic evaluations of PCV.
4.4. Strengths and limitation of this systematic review
This study has several strengths. We conducted a comprehensive literature research for economic evaluation studies on PCV13 both children and adults populations. This study included economic evaluations of PCV13 reported in English and Chinese languages, and conducted in a variety of countries. In addition, reporting of the systematic review followed the PRISMA statement. A limitation of this study is that due to significant differences across countries in the economic levels and the epidemic intensity of PDs, studies have substantial heterogeneities with respect to the perspective, parameters used for the costs and effectiveness, modeling and time horizon, thus the comparability of the results of the included studies between countries is limited.
5. Conclusions
This systematic review suggests that the PCV13 vaccine may be cost-effective in children and adults from the perspective of health care providers, society and payers. However, the cost-effectiveness results may vary due to differences in health systems, the perspective, parameters for the effectiveness, modeling methods and cost-effectiveness thresholds within or across the countries. Future research is needed to evaluate the cost-effectiveness of PCV13 using dynamic transmission models to better capture and more accurately assess the effect of previous risk of PDs on future health state and vaccine costs.
Article highlights
Pneumococcal diseases (PDs) have become a serious public health problem around the world and the Streptococcus Pneumoniae (SPn) or pneumococcus is acknowledged as the main cause of PDs.
Pneumococcal vaccination is the most cost-effective measure to prevent SPn infection. However, the evidence-based cost-effectiveness of PCV13 is unclear.
The current body of situation is limited largely to a few systematic reviews of the cost-effectiveness of PCVs in children or adults separately in some regions, with no systematic reviews have summarized the cost-effectiveness results of PCV13 among populations of both children and adults in all countries.
We conducted a comprehensive literature research for economic evaluation studies on PCV13 both children and adults populations. This systematic review suggests that the PCV13 vaccine may be cost-effective in children and adults.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or mending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
CPW, ZWW and XYW conceived the study, performed the study selection, data extraction and analysis, and revised the draft manuscript. YZD, HWS, YFX, MY and JWC performed the literature search, study selection, data extraction and analysis. YZD and JJL wrote the original draft manuscript. XYW, YW, YYW and TZ reviewed and revised the draft manuscript. All authors wrote the manuscript, read and approved the submitted version.
Additional information
Funding
References
- Wahl B, O’Brien KL, Greenbaum A, et al. Burden of streptococcus pneumoniae and haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000-15. Lancet Glob Health. 2018 Jul;6(7):e744–e757.
- Song JY, Choi JY, Lee JS, et al. Clinical and economic burden of invasive pneumococcal disease in adults: a multicenter hospital-based study. BMC Infect Dis. 2013 May;4(13):202.
- Huang SS, Johnson KM, Ray GT, et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011 Apr 18;29(18):3398–3412.
- Shiri T, Khan K, Keaney K, et al. Pneumococcal disease: a systematic review of health utilities, resource use, costs, and economic evaluations of interventions. Value Health. 2019 Nov;22(11):1329–1344.
- Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in. 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis. 2018 Nov;18(11):1191–1210.
- Lewnard JA, Lo NC, Arinaminpathy N, et al. Childhood vaccines and antibiotic use in low- and middle-income countries. Nature. 2020 May;581(7806):94–99.
- Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper, February 2019. World Health Organization. https://apps.who.int/iris/bitstream/handle/10665/310968/WER9408.pdf.
- Wang Q, An J, Zhang M, et al. Pneumococcal disease immune prevention expert consensus. Chinese Journal Epidemiology. 2020;41(12):1945–1979.
- Waight PA, Andrews NJ, Ladhani SN, et al. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015 May;15(5):535–543.
- Picazo JJ, Ruiz-Contreras J, Casado-Flores J, et al. Impact of 13-valent pneumococcal conjugate vaccination on invasive pneumococcal disease in children under 15 years old in Madrid, Spain, 2007 to 2016: the HERACLES clinical surveillance study. Vaccine. 2019 Apr 10;37(16):2200–2207.
- Lepoutre A, Varon E, Georges S, et al. Impact of the pneumococcal conjugate vaccines on invasive pneumococcal disease in France, 2001-2012. Vaccine. 2015 Jan 3;33(2):359–366.
- Berman-Rosa M, O’Donnell S, Barker M, et al. Efficacy and effectiveness of the PCV-10 and PCV-13 vaccines against invasive pneumococcal disease. Pediatrics. 2020;145(4):2019–0377.
- Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020 Oct 17;396(10258):1223–1249.
- Li Y, Wang HQ, Furnback W, et al. The Cost-Effectiveness of 13-Valent Pneumococcal Conjugate Vaccine in Seven Chinese Cities. Vaccines (Basel). 2021;9(11):1368.
- Mangen MJJ, Rozenbaum MH, Huijts SM, et al. Cost-effectiveness of adult pneumococcal conjugate vaccination in the Netherlands. Eur Respir J. 2015 Nov;46(5):1407–1416.
- Bakir M, Turel O, Topachevskyi O. Cost-effectiveness of new pneumococcal conjugate vaccines in Turkey: a decision analytical model. BMC Health Serv Res. 2012;12:386.
- Mo X, Gai Tobe R, Liu X, et al. Cost-effectiveness and health benefits of pediatric 23-valent pneumococcal polysaccharide vaccine, 7-valent pneumococcal conjugate vaccine and forecasting 13-valent pneumococcal conjugate vaccine in China. Pediatr Infect Dis J. 2016 Nov;35(11):e353–e361.
- Zhou H, He JC, Wu B, et al. Cost-effectiveness analysis of routine 13-valent pneumococcal conjugate vaccinations in Chinese infants. Hum Vaccin Immunother. 2018;14(6):1444–1452.
- Saokaew S, Rayanakorn A, Wu DB, et al. Cost effectiveness of pneumococcal vaccination in children in low- and middle-income countries: a systematic Review. Pharmacoeconomics. 2016 Dec;34(12):1211–1225.
- Shao Y, Stoecker C. Cost-effectiveness of pneumococcal vaccines among adults over 50 years old in low- and middle-income countries: a systematic review. Expert Rev Vaccines. 2020 Dec;19(12):1141–1151.
- van de Vooren K, Duranti S, Curto A, et al. Cost effectiveness of the new pneumococcal vaccines: a systematic review of European studies. Pharmacoeconomics. 2014 Jan;32(1):29–45.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009 Jul;21(339):b2535.
- Walker DG, Hutubessy R, Beutels P. WHO Guide for standardisation of economic evaluations of immunization programmes. Vaccine. 2010 Mar 8;28(11):2356–2359.
- Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Value Health. 2013 Mar-Apr;16(2):e1–5.
- Hamberg-van Reenen HH, Proper KI. van den Berg M. Worksite mental health interventions: a systematic review of economic evaluations. Occup Environ Med. 2012 Nov;69(11):837–845.
- Mihalopoulos C, Chatterton ML. Economic evaluations of interventions designed to prevent mental disorders: a systematic review. Early Interv Psychiatry. 2015 Apr;9(2):85–92.
- Ning GJ. Study on the burden of childhood pneumonia and the economic evaluations of an immunization program in Baiyin City Chinese center for disease control and prevention; 2017. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CDFD&dbname=CDFDLAST2022&filename=1019196162.nh&uniplatform=NZKPT&v=jSPWW5xNOlBr7BMrZCQCRxbf7caTbVFpGCAttqp64A8Z8wj5VkMKxYP6ft5zyHNR]
- Lin L. Protective effect and health economic evaluations of 13-valent pneumococcal conjugate vaccine in children under 2 years old in Ningbo Ningbo University. 2020. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD202201&filename=1021532021.nh&uniplatform=NZKPT&v=7AdnDso9w1YD-_5PwOIz4my9tYS5eAzA-FKn0yvJridEshIQnh6qRuwE718GBQcf]
- Kim HY, Park SB, Kang ES, et al. Cost-effectiveness of a national immunization program with the 13-valent pneumococcal conjugate vaccine compared with the 10-valent pneumococcal conjugate vaccine in South Korea. Hum Vaccin Immunother. 2021 Mar;17(3):909–918.
- Perdrizet J, Santana CFS, Senna T, et al. Cost-effectiveness analysis of replacing the 10-valent pneumococcal conjugate vaccine (PCV10) with the 13-valent pneumococcal conjugate vaccine (PCV13) in Brazil infants. Hum Vaccin Immunother. 2021 Apr;17(4):1162–1172.
- Perdrizet J, Horn EK, Nua W, et al. Cost-effectiveness of the 13-Valent Pneumococcal Conjugate Vaccine (PCV13) versus lower-valent alternatives in Filipino infants. Infect Dis Ther. 2021 Dec;10(4):2625–2642.
- Wang CX, Su L, Mu QL, et al. Cost-effectiveness analysis of domestic 13-valent pneumococcal conjugate vaccine for children under 5 years of age in mainland China. Hum Vaccin Immunother. 2021 Jul;17(7):2241–2248.
- Dilokthornsakul P, Kengkla K, Saokaew S, et al. An updated cost-effectiveness analysis of pneumococcal conjugate vaccine among children in Thailand. Vaccine. 2019 Jul;37(32):4551–4560.
- Ezoji K, Yaghoubi M, Nojomi M, et al. Cost-effectiveness of introducing the pneumococcal conjugate vaccine for children under 5 years in the Islamic Republic of Iran. East Mediterr Health J. 2019 Oct;25(10):686–697.
- Krishnamoorthy Y, Eliyas SK, Nair NP, et al. Impact and cost effectiveness of pneumococcal conjugate vaccine in India. Vaccine. 2019 Jan;37(4):623–630.
- Dorji K, Phuntsho S, Pempa, et al. Towards the introduction of pneumococcal conjugate vaccines in Bhutan: a cost-utility analysis to determine the optimal policy option. Vaccine. 2018 Mar;36(13):1757–1765.
- Shen KL, Wasserman M, Liu DD, et al. Estimating the cost-effectiveness of an infant 13-valent pneumococcal conjugate vaccine national immunization program in China. PLoS One. 2018;13(7):201–245.
- Kuhlmann A. von der Schulenburg JMG. Modeling the cost-effectiveness of infant vaccination with pneumococcal conjugate vaccines in Germany. Eur J Health Econ. 2017 Apr;18(3):273–292.
- Sundaram N, Chen C, Yoong J, et al. Cost-effectiveness of 13-valent pneumococcal conjugate vaccination in Mongolia. Vaccine. 2017 Feb;35(7):1055–1063.
- Maurer KA, Chen HF, Wagner AL, et al. Cost-effectiveness analysis of pneumococcal vaccination for infants in China. Vaccine. 2016 Dec;34(50):6343–6349.
- Vucina VV, Filipovic SK, Koznjak N, et al. Cost-effectiveness of pneumococcal conjugate vaccination in Croatia. Vaccine. 2015;33:A209–A218.
- Mezones-Holguin E, Canelo-Aybar C, Clark AD, et al. Cost-effectiveness analysis of 10-and 13-valent pneumococcal conjugate vaccines in Peru. Vaccine. 2015;33:A154–A166.
- Kieninger MP, Caballero EG, Sosa AA, et al. Cost-effectiveness analysis of pneumococcal conjugate vaccine introduction in Paraguay. Vaccine. 2015;33:A143–A153.
- Sibak M, Moussa I, El-Tantawy N, et al. Cost-effectiveness analysis of the introduction of the pneumococcal conjugate vaccine (PCV-13) in the Egyptian national immunization program, 2013. Vaccine. 2015;33:A182–A191.
- Mezones-Holguin E, Bolanos-Diaz R, Fiestas V, et al. Cost-effectiveness analysis of pneumococcal conjugate vaccines in preventing pneumonia in Peruvian children. J Infect Developing Countries. 2014 Dec;8(12):1552–1562.
- Gomez JA, Tirado JC, Rojas AAN, et al. Cost-effectiveness and cost utility analysis of three pneumococcal conjugate vaccines in children of Peru. BMC public health. 2013;13:1025.
- Kulpeng W, Leelahavarong P, Rattanavipapong W, et al. Cost-utility analysis of 10-and 13-valent pneumococcal conjugate vaccines: protection at what price in the Thai context? Vaccine. 2013 Jun;31(26):2839–2847.
- Ayieko P, Griffiths UK, Ndiritu M, et al. Assessment of health benefits and cost-effectiveness of 10-valent and 13-valent pneumococcal conjugate vaccination in Kenyan children. PLoS One. 2013;8(6):e67324.
- Hoshi SL, Kondo M, Okubo I. Economic evaluation of vaccination programme of 13-valent pneumococcal conjugate vaccine to the birth cohort in Japan. Vaccine. 2013 Jun;31(25):2762–2771.
- Earnshaw SR, McDade CL, Zanotti G, et al. Cost-effectiveness of 2 + 1 dosing of 13-valent and 10-valent pneumococcal conjugate vaccines in Canada. Bmc Infectious Diseases. 2012;12:101.
- Díez-Domingo J, Ridao-López M, Gutiérrez-Gimeno MV, et al. Pharmacoeconomic assessment of implementing a universal PCV-13 vaccination programme in the Valencian public health system (Spain). Vaccine. 2011 Dec 6;29(52):9640–9648.
- Robberstad B, Frostad CR, Akselsen PE, et al. Economic evaluation of second generation pneumococcal conjugate vaccines in Norway. Vaccine. 2011 Nov;29(47):8564–8574.
- Mucino-Ortega E, Mould-Quevedo JF, Farkouh R, et al. Economic evaluation of a child immunization programme in Mexico based in 13-valent PCV. Value Health. 2011 Jul-Aug;14(5):S65–S70.
- Uruena A, Pippo T, Betelu MS, et al. Cost-effectiveness analysis of the 10-and 13-valent pneumococcal conjugate vaccines in Argentina. Vaccine. 2011 Jul;29(31):4963–4972.
- Kim SY, Lee G, Goldie SJ, et al. Economic evaluation of pneumococcal conjugate vaccination in The Gambia. Bmc Infectious Diseases. 2010;10:260.
- Igarashi A, Hirose E, Kobayashi Y, et al. Cost-effectiveness analysis for PCV13 in adults 60 years and over with underlying medical conditions which put them at an elevated risk of pneumococcal disease in Japan. Expert Rev Vaccines. 2021 Sep;20(9):1153–1165.
- Gouveia M, Jesus G, Ines M, et al. Cost-effectiveness of the 13-valent pneumococcal conjugate vaccine in adults in Portugal versus “no vaccination” and versus vaccination with the 23-valent pneumococcal polysaccharide vaccine. Hum Vaccin Immunother. 2019 Apr;15(4):850–858.
- Biagini L, Pezzani M, Rojas R, et al. Cost-utility study of PCV13 Versus PPSV23 in adults in Chile. Value Health Reg Issues. 2018;17:194–201.
- Chen C, Beutels P, Newall AT. Evolution over time in the cost-effectiveness of pneumococcal conjugate vaccine (PCV13) in older Australians due to herd protection from infant vaccination. Vaccine. 2018 Apr;36(16):2057–2060.
- Marbaix S, Peetermans WE, Verhaegen J, et al. Cost-effectiveness of PCV13 vaccination in Belgian adults aged 65-84 years at elevated risk of pneumococcal infection. PLoS One. 2018;13(7):e0199427.
- Boccalini S, Bechini A, Gasparini R, et al. Economic studies applied to vaccines against invasive diseases: an updated budget impact analysis of age-based pneumococcal vaccination strategies in the elderly in Italy. Hum Vaccin Immunother. 2017;13(2):417–422.
- Heo JY, Seo YB, Choi WS, et al. Cost-effectiveness of pneumococcal vaccination strategies for the elderly in Korea. PLoS One. 2017;12(5):e0177342.
- Dirmesropian S, Wood JG, MacIntyre CR, et al. Cost-effectiveness of 13-valent pneumococcal conjugate vaccine (PCV13) in older Australians. Vaccine. 2017 Aug;35(34):4307–4314.
- Rodríguez González-Moro JM, Menéndez R, Campins M, et al. Cost effectiveness of the 13-valent pneumococcal conjugate vaccination program in chronic obstructive pulmonary disease patients aged 50+ years in Spain. Clin Drug Investig. 2016 Jan;36(1):41–53.
- Smith KJ, Wateska AR, Nowalk MP, et al. cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with Pneumococcal polysaccharide vaccine. JAMA J Am Med Assoc. 2012 Feb;307(8):804–812.
- zhang H, Lai X, lv Y, et al. Systematic review of economic evaluations of different immunization strategies of pneumococcal conjugate vaccine in China. Chinese Health Economics. 2022;41(2):9–14.
- Slotved HC, Dalby T, Hoffmann S. The effect of pneumococcal conjugate vaccines on the incidence of invasive pneumococcal disease caused by ten non-vaccine serotypes in Denmark. Vaccine. 2016 Feb 3;34(6):769–774.
- Lazarus R, Clutterbuck E, Yu LM, et al. A randomized study comparing combined pneumococcal conjugate and polysaccharide vaccination schedules in adults. Clin Infect Dis. 2011 Mar 15;52(6):736–742.
- Shiramoto M, Hanada R, Juergens C, et al. Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine compared to the 23-valent pneumococcal polysaccharide vaccine in elderly Japanese adults. Hum Vaccin Immunother. 2015;11(9):2198–2206.
- Drost R, van der Putten IM, Ruwaard D, et al. Conceptualizations of the Societal Perspective within Economic Evaluations: a Systematic Review. Int J Technol Assess Health Care. 2017 Jan;33(2):251–260.
- Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics. 1998 Apr;13(4):397–409.
- Hoang VP, Shanahan M, Shukla N, et al. A systematic review of modelling approaches in economic evaluations of health interventions for drug and alcohol problems. BMC Health Serv Res. 2016 Apr;13(16):127.
- Brennan A, Chick SE, Davies R. A taxonomy of model structures for economic evaluation of health technologies. Health Econ. 2006 Dec;15(12):1295–1310.
- Løchen A, Anderson RM. Dynamic transmission models and economic evaluations of pneumococcal conjugate vaccines: a quality appraisal and limitations. Clin Microbiol Infect. 2020 Jan;26(1):60–70.
- Wu DB, Chang CJ, Huang YC, et al. Cost-effectiveness analysis of pneumococcal conjugate vaccine in Taiwan: a transmission dynamic modeling approach. Value Health. 2012 Jan-Feb;15(1 Suppl):S15–9.
- Jmr G-M, Menendez R, Campins M, et al. Cost effectiveness of the 13-valent pneumococcal conjugate vaccination program in chronic obstructive pulmonary disease patients aged 50+years in Spain. Clin Drug Investig. 2016 Jan;36(1):41–53.
- Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMC Health Serv Res. 2022 Jan 27;22(1):114.