ABSTRACT
Introduction
Despite children aged 6–35 months developing more severe influenza infections, not all countries include influenza vaccines in their national immunization programs.
Areas covered
This literature review examines the efficacy, immunogenicity, and safety of seasonal trivalent influenza vaccines (TIVs) and quadrivalent influenzae vaccines (QIVs) in children 6–35 months old to determine if greater valency promotes greater protection while maintaining a similar safety profile.
Expert opinion
TIVs and QIVs are safe for children under 3 years old. TIVs and QIVs provided good seroprotection, and immunogenicity (GMT, SCR, and SPR) meeting recommended levels set by CHMP (European) and CBER (USA). However, as QIVs carry two influenza B strains and TIVs only one, QIVs has an overall higher seroprotection against particularly influenza B. Vaccines containing adjuncts had better immunogenicity, particularly after the first dose. Seroprotection of all vaccines lasted 12 months. Increasing the dosage from 0.25 mL to 0.5 mL did not cause more systemic or local side-effects. Further comparisons of efficacy, and wider promotion of influenza vaccines in general are required in preschool children.
1. Introduction
Influenza viruses are enveloped RNA viruses with a viral genome that encodes two major antigenic glycoproteins, hemagglutinin and neuraminidase (NA) [Citation1,Citation2]. Attachment and penetration of the virus is facilitated by hemagglutinin. Neuraminidase promotes viral spread as it is responsible for cleaving terminal sialic acid residues on the cell surface to release virion particles [Citation2]. Patients develop immunity by recognizing strain-specific hemagglutinin and neuraminidase. Major changes in Hemagglutinin and neuraminidase antigenicity reduce host immunity and lead to influenza epidemics [Citation3,Citation4]. Development of more effective vaccines to influenza A is of great importance to global health [Citation5].
Influenza is a highly contagious viral infection circulating each winter, manifesting as an acute febrile respiratory disease [Citation1]. Most patients have mild coryzal symptoms that resolve within a week. However, influenza may cause severe disease in pregnant women, the immunocompromised, the elderly, and children [Citation2]. Due to the devastating effects of the 1918/19 pandemic, the pressure to identify the responsible pathogen increased and it was successfully isolated in 1933 [Citation4], then, during the following decade, the first vaccine was produced [Citation5]. The World Health Organization (WHO) estimates the annual influenza infection rate for severe illness to be 4–5 million cases and 500,000 deaths/year [Citation1]. Epidemics and outbreaks are not predictable since their timing, predominant circulating strains, duration, and intensity all depend on geographical location and vary each year. Furthermore, incidence rates differ between populations depending on age and health status. The highest rates of infection are observed in young children 5 years and below (20–30%), compared to adults (2–10%) [Citation3]. Hardelid et al. (2015) [Citation6] reported on the influenza-like Illness (ILI) rates in the UK from 1995 to 2013. ILI consultation rates were highest among individuals aged 15–44 years [Citation6]. The UK National Health Service 111 service monitors call for flu symptoms daily to survey the disease trends. demonstrates the calls flagged for flu or cold reports for the 2021–2022 season [Citation7]. Children under 4 years of age represented 45%, 33%, and 24% of all NHS 111 calls flagged for flu in Nov 21, Jan 22, and Jul 22, respectively. Additionally, seroepidemiological studies show that children are at much higher risk of symptomatic infection than adults [Citation6]. This inconsistency is explained by the observed reluctance of General Practitioners (GPs) to attribute ILI to children with these clinical manifestations, since ruling out other diagnoses is more difficult for that age group. Hence, it is crucial to take the coding pattern of GPs into consideration when discussing the outcomes of these data.
Figure 1. Daily UK national health service 111 calls flagged for flu or cold for children younger than 1 year (left) and children aged 1–4 years (right) [Citation7].
![Figure 1. Daily UK national health service 111 calls flagged for flu or cold for children younger than 1 year (left) and children aged 1–4 years (right) [Citation7].](/cms/asset/2d8c78d7-52c9-45f4-8afd-b1fc6da22534/ierv_a_2181797_f0001_oc.jpg)
As children are at high-risk for severe influenza illness, vaccination is a priority in this age group. Governmental programs should be encouraged to increase the vaccination levels in children. In the 2014/15 seasons, the influenza vaccine uptake in the UK for children aged 2–4 years was reported. Out of 57,545 participants, 39% received one dose and only 1% of those participants received two doses, 83% of the live attenuated vaccine [Citation8]. There is evidence that vaccine uptake in children 4–16 years old has increased post-COVID (2020–2022) [Citation9,Citation10].
1.1. Influenza vaccine types
There are three types of seasonal influenza vaccines available: (i) live attenuated influenza vaccines (LAIVs), (ii) inactivated influenza vaccine (IIVs) – which include whole inactivated vaccines, split-virion vaccines, virosome vaccines, and subunit vaccines, and (iii) recombinant live viral-vectored vaccines [Citation2].
1.1.1. Live attenuated influenza vaccines
Initially developed after the identification of influenza viruses, LAIVs were considered of interest due to the potential for mimicking immune responses of natural infection and to create long and stable immunity [Citation3]. In 1987, the first LAIV was licensed for use in children 3 years of age and older in Russia (former United Socialist Soviet Republic). The second LAIV was developed and approved in the United States of America (USA) in 2006 for people 2–49 years old, later followed by Mexico, Macau, the United Arab Emirates, the Republic of Korea, and Israel for the same age groups, while it was approved in the EU for people aged 2–17 years and in Canada for people aged 2–59 years [Citation3]. There are four ways to attenuate live influenza viruses: mutations that occur naturally, passage of wild-type viruses through chicken eggs/cell culture, use of chemical methods or ultraviolet light to induce mutations, and the sequential passage of wild-types through decreasing temperatures [Citation2,Citation3].
LAIVs currently licensed for use are produced using the sequential passage of wild-types through decreasing temperatures from two master donor viruses (MDVs) [Citation3]. MDVs were developed in the 1960s at the University of Michigan and consist of the MDV-A (strain A/H2N2/Ann Arbor/6/60) against influenza A viruses and MDV-B (strain B/Ann Arbor/1/66) against influenza B viruses [Citation11]. Wild-type influenza viruses replicate well at higher temperatures, but not at 25°C (similar temperature to the nasopharynx) and can infect the lungs better than the nasal cavity, however, this is prevented in MDVs.
1.1.2. Inactivated influenza vaccines
IIV development began in the late 1930s and only for administered to military personnel initially, until 1945, when the USA licensed IIVs for civilian use [Citation2,Citation3]. The first was a bivalent vaccine containing AH1N1/PR8 and a B-strain. The composition of IIVs changed a multitude of times following the ‘over-take’ of A/H1N1 by A/H2N2 in 1958 and then the appearance of A/H3N2 in 1968 (wiping out A/H2N2) and finally the reappearance of A/H1N1 in the population leading to a trivalent vaccine in 1978 (A/H1N1, A/H3N2, and B) [Citation12].
Early IIVs were grown in fertilized chicken eggs, where the allantoic fluid (filled with viral particles) would be collected, purified, then inactivated. However, there are some disadvantages to growing viruses in egg cultures. From the manufacturing standpoint, it is difficult to attain the quantity of appropriate eggs for mass production and many strains do not grow as efficiently in egg cultures, especially highly pathogenic strains [Citation13]. Hence, high-growth reassortant vaccine viruses have been developed to increase the yield of viral particles to ensure enough supply for the vaccines. High-growth reassortant viruses are the result of genetic reassortment between a high-yield laboratory strain (A/Puerto Rico/8/34) and the wild-type strain contained desired hemagglutinin/neuraminidase proteins [Citation3]. Another undesirable outcome of viral passages through an embryonic egg is the changes in amino acids, which reduce vaccine immunogenicity and efficacy, leading to lower levels of protection elicited and the production of mismatched strains. Finally, an increase in allergies to eggs and egg-derived products in children causes a decrease of egg-derived vaccine administration [Citation14]. Newer IIVs are grown in continuous cell lineages, such as the Madin-Darby Canine Kidney cells (first approved in the EU followed by the USA) and insect cells (approved in Mexico and the USA), to counter the disadvantages of using egg cultures [Citation12]. The process of developing vaccines using Madin-Darby Canine Kidney cells, and insect cells is similar to egg-based techniques, whereby viral cells are grown in the cultures, recovered, purified and inactivated, and then the strains are mixed to produce the completed product. It should be noted that the method of growing influenza strains in insect cells via DNA technology only produces Hemagglutinin proteins without any other proteins [Citation3].
IIVs differ depending on the steps taken after purification of the viral load from the culture. The first-generation IIVs were whole-virus/virion vaccines that used ß-propiolactone or formalin to chemically inactive the virus, attenuating its virulence but retaining its antigenicity [Citation2,Citation12]. Split-virion IIVs were developed to reduce the potential side effects caused by unnecessary viral particles (present in the whole-virus vaccines). After chemical inactivation, the virus is subjected to further disruption of its envelope and the non-antigenic products – nucleic acids, proteins of higher molecular weight, and the splitting agents – are discarded leaving only the Hemagglutinin, Neuraminidase, M, and NP particles [Citation2]. Furthermore, subunit IIVs are derived from split IIVs, where the hemagglutinin and neuraminidase proteins are further concentrated. This construction process has been made easier due to the advancement of molecular cloning techniques; genes encoding the antigenic proteins are inserted into an expression plasmid, which is transferred into a cell (prokaryotic or eukaryotic) to clone and produce the Hemagglutinin and Neuraminidase proteins at larger yields [Citation2]. Virosome IIVs were developed to retain the cell-binding ability of the virus and induce cytotoxic T-cell responses by including the viral phospholipids in addition to the antigenic Hemagglutinin/Neuraminidase proteins [Citation2].
1.1.3. Viral-vectored influenza vaccines
Live-vectored vaccines, most notably against influenza viruses, have become a major research topic in the vaccine developing field due to their advantages. The production of these vaccines relies on genetic engineering methods that integrate the antigen protein-encoding gene segments into a ‘recombinant’ vector to increase proliferation and influenza-protein expression in the host [Citation2]. For influenza viral-vectored vaccines, the most used recombinant vectors are adenoviruses, arenaviruses, baculoviruses, herpesviruses, and Newcastle-disease viruses. These vaccine types efficiently elicit cellular and humoral immune responses (like LAIVs), inducing rapid and long-lasting antibody production. They are also favorable in the manufacturing process as they simplify and reduce the time of vaccine production and have shown improvements in cross-protection between genotypically-different strains [Citation2].
1.2. Influenza vaccine lineages and recommendations
1.2.1. Trivalent and quadrivalent influenza vaccines
First-generation vaccines were bivalent but changed into trivalent vaccines in 1978, when there was a lack of cross-protection between the influenza A viruses A/H1N1 and A/H3N2 lineages. Trivalent Influenza Vaccines (TIVs) contain three antigenically different influenza strains: two influenza A strains from the A/H1N1 and A/H3N2 lineages and a single influenza B strain from the relevant circulating lineage.
In 1985 two separate B lineages, B/Yamagata and B/Victoria have been identified in the global population [Citation15,Citation16]. Quadrivalent Influenza Vaccines (QIVs) contain two influenza A lineages (A/H1N1 and A/H3N2) and two influenza B strains from the dominant B/Yamagata and B/Victoria lineages. Influenza B is particularly concerning in children as it was observed at higher rates compared to influenza A. A 24-year observational study in Japan noted that influenza B caused the largest outbreaks in schoolchildren, even in seasons where most were vaccinated [Citation17]. Furthermore, influenza B is responsible for a disproportionate amount of mortality cases in pediatric populations. The Center for Disease Control and Prevention (CDC) stated that an average of 34% of pediatric influenza deaths (0–18 years old) were caused by influenza B from 2004/05 to 2010/11, excluding the pandemic in 2009/10 [Citation15,Citation18]. Lastly, Bodewes et al. (2011) [Citation19] conducted a study reporting that natural immunity against influenza B developed much slower in children when compared to influenza A, which explains the higher rates of influenza B in children. Hence, health agencies globally are recommending a shift from trivalent to quadrivalent vaccines, which incorporate both dominant circulating B strains.
1.2.2. Recommendations
‘Recommendations for Influenza Vaccine Composition’ consists of two lists, one for each geographical hemisphere, divided into the influenza seasons and updated annually. Each season includes the recommended strains for different vaccines, trivalent, and quadrivalent, selected by the WHO Global Influenza Surveillance and Response System (GISRS). Virological monitoring of specimens is conducted annually by the National Influenza Centers of the GISRS and the data is sent to experts at Collaboration Centers for isolation and candidate selection [Citation20].
The administration of seasonal influenza vaccines is dependent on vaccine type and age. UK NICE guidelines recommend two doses, 4 weeks apart, for patients who have not been previously vaccinated, and a single dose for primed individuals. The guidelines also discuss dosage of administration: 0.5 mL per dose by intramuscular injection (IIVs) for children over 6 months and adults, and a 0.2 mL per dose (0.1 mL per nostril) by intranasal administration (LAIVs) for children 2–17 years of age [Citation21]. The quantity of antigen in IIVs is calculated on the basis of HA content irrespective of the presence of other components. A single vaccine dose contains ≥15 µg of each hemagglutinin protein: three for trivalent and four for quadrivalent vaccines. Additionally, it is recommended that children <17 years are administered two half-doses – 7.5 µg of each Hemagglutinin, 4 weeks apart [Citation12].
1.3. Immunoassays
Three immunoassays are used to detect immunity to hemagglutinin: virus neutralization (VN), single-radial hemolysis (SRH), and hemagglutination inhibition (HI) assays [Citation22,Citation23]. VN is specific in detecting antibodies, which inhibit Hemagglutinin-mediated entry of the virus into cells [Citation23]. SRH measures immunodiffusion of antibodies through a gel that contains complement factors and red blood cell (RBC) bound influenza viruses [Citation22]. Developed in 1941 by George Hirst, when he noted that RBCs agglutinated upon the addition of influenza viruses, HI assays are the most used vaccine evaluation method [Citation24]. Pre- and post-vaccination geometric mean HI titers (GMTs) compare circulating and vaccine strains to check for vaccine-virus matching [Citation22].
1.4. Correlates of protection
Through studying the natural immune responses in humans or animal models, correlates of protection have been obtained and used to define vaccine targets [Citation25]. In 1972, high levels of protection were found to correlate with HI titer, and HI antibody titer is accepted as the correlate of protection against influenza viruses, although this is not always the case with LAIVs [Citation25–27].
1.5. Vaccine criteria
Regulatory criteria of influenza vaccines are based on the annual requirements of seasonal influenza vaccines set by the European Medicines Agency committee for Medical Products for Human use (CHMP criteria) in 1996. The same criteria were also adopted by the US Food and Drug Administration’s Center for Biologics Evaluation and Research (CBER criteria). Yearly clinical trials are to be conducted to evaluate seasonal influenza vaccine immunogenicity using the HI assay. Three criteria must be met to achieve ‘protective titers’ [Citation27]. Firstly, HI titers (seroprotective rate, SPR) must be ≥40 in more than 60% of people over 60 years old and more than 70% of 18–60-year-olds. Secondly, the seroconversion rate (SCR; a fourfold increase in titer) must be >30% in those over 60 years of age and >40% in 18–60-year-olds. Thirdly, the geometric fold rise increase (GFRI)/geometric mean ratio (GMR) must be >2 in those over 60 years and >2.5 in 18–60-year-olds. The studies to produce these baselines are generally in healthy adults. In children, a HI titer of 330 achieves 80% seroprotection and a HI titer of 110 50% seroprotection [Citation27].
1.6. Study aims
This review aims to evaluate the efficacy, immunogenicity, and safety of TIVs and QIVs in protecting against seasonal influenza vaccines in children aged 6–35 months, and to determine if greater valency promotes greater protection while maintaining a similar safety profile.
2. Methods
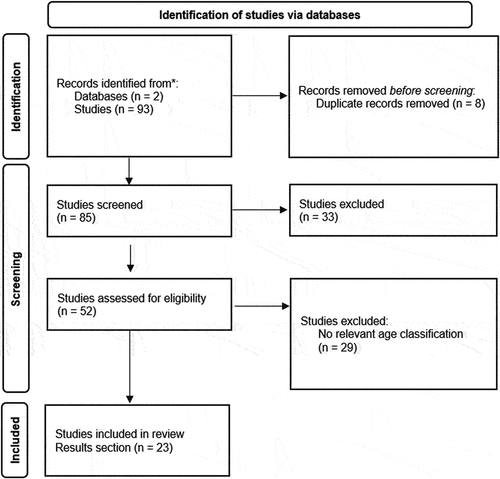
PubMed and Clinical Trials.gov were the search engines used for this literature review. The searches were from 2006 and included the following key terms: ‘seasonal influenza,’ ‘vaccines,’ ‘vaccine,’ ‘vaccination,’ ‘efficacy,’ ‘immunogenicity,’ ‘safety,’ ‘reactogenicity,’ ‘children,’ ‘under two years,’ ‘under three years,’ ‘6–35 months,’ ‘seroprotection,’ ‘seroconversion,’ ‘GMT,’ ‘HI titer.’ Most papers were randomized control trials, specifically phase III clinical trials, along with observational studies. Inclusion criteria were papers written in English and healthy children aged 6–35 months old.
3. Results
A total of 23 articles were reviewed, 11 discussed efficacy, 15 immunogenicity, and 18 safety of seasonal influenza vaccines (). The age group was 6–35 months old, unless stated otherwise.
3.1. Efficacy
Efficacy of vaccines measured the ability of the vaccine to reduce infection rates in healthy children. Reduction in influenza rates in vaccinated children were compared to unvaccinated children. Eleven studies reporting the efficacy of TIVs and QIVs are discussed below and presented in , and .
Table 1. Studies categorized by vaccine type (TIV, QIV, TIV vs QIV).
Table 2. Efficacy, immunogenicity, and safety of different vaccine types in children aged 6–35 months.
Table 3. Summary of the efficacy, immunogenicity, and reactogenicity of different vaccine types in children aged 6–35 months.
3.1.1. Trivalent Influenza Vaccines (TIVs)
Five studies described the efficacy of TIVs compared with either placebo or NIV [Citation28–32]. Two studies comparing CAIV-T to a placebo (saline) showed that CAIV-T demonstrated better vaccine efficacy (VE) over 2 and 3 years. Tam et al. [Citation28] reported that VE of two doses of CAIV-T against influenza strains was 70. A booster shot the following year increased protection and durability (VE = 85% 12 months post-vaccination) [Citation28]. A second study conducted in 70 multinational centers showed that in year 1, VE was 86% against all strains, and in year 2 VE was 86% [Citation29]. A phase 3 study carried out in Germany and Finland compared two IIV3, one TIV adjuvanted with MF59, and non-adjuvanted split TIV (nTIV). VE of the adjudicated MF59-TIV was significantly higher in the 6–23 months olds (79%) compared to control vaccines (40%) [Citation30].
3.1.2. Quadrivalent Influenza Vaccines (QIV)
Five studies concluded that IIV4 QIVs reduced the risk of influenza infection in children 6–35 months old [Citation33–37]. A study of 12,018 participants comparing IIV4 and NIVs found that the VE was higher for A/H1N1, A/H3N2, and B/Yamagata strains than for B/Victoria [Citation34]. VE against moderate-severe influenza was 20% higher in children aged 18–35 months (69%) than in those 6–17 months old (49%). Dbaibo et al. [Citation37] and Esposito et al. [Citation35] studied VE in several countries in both the Northern and Southern hemispheres. Both reported that IIV4s protected children in this age group, but the VE were higher in countries in the Northern hemisphere (73%) and lower (53%) in subtropical/Southern hemisphere countries [Citation35,Citation37].
3.1.3. TIV vs QIV
Only one Finnish register-based study of 60,000 children under 2 years of age compared the efficacy of TIVs (IIV3s) to QIVs (LAIV4) across three seasons [Citation38]. VE varied with the strain and the season. In 2015/16 both vaccines reduced the incidence of influenza by about half, and for the following two seasons by less than a third. In 2015/16, VE of IIV3 against Influenza A strains was 90%, and for LAIV4 only 46%. This contrasts VE of IIV3 against B strains of 35%, compared with 83% for LAIV4. Thus, in the first season, TIVs provided better protection against A strains, while LAIV4 provided better protection against B strains. In 2016/17, neither vaccine, LAIV4 (20%) and IIV3 (24%) effectively protected against the A/H3N2. Likewise, in the 2017/18 season, neither IIV3 nor LAIV4 provided good protection against A strains (42% and 22%, respectively), and IIV3 failed to protect against B strains (0.2%), while LAIV4 provided significant protection against B strains (75%).
3.2. Immunogenicity
Immunogenicity of a vaccine is the quantification of the elicited responses that increase protection against infection caused by the vaccine. Fifteen studies discussing immunogenicity of TIV and QIV demonstrating a robust response after the second dose are presented below and in .
3.2.1. TIV
Four of the five TIV studies compared adjuvanted and non-adjuvanted IIV3s, while one reported on CAIV-T. Tam et al. [Citation28] showed that the administration of two doses of CAIV-T elicited a good SCR and GMFI compared to placebo for all three strains (CAIV-T vs Placebo; A/H1N1 60% vs 11%; A/H3N2 61% vs 4%; B 57% vs 4%). SCR and GMFI were still higher in the second year [Citation28]. MF59-TIVs were compared to split non-adjuvanted TIVs (nTIV) in two studies. Both studies reported higher HI titers, SPR, GMT, and GMR after two doses of adjuvant MF59-TIVs than nTIV. A single dose of MF59-TIV provided significantly higher seroprotection than two doses of nTIVs (A/H1N1 51% vs 18%, P < 0.001; A/H3N2 91% vs 49%, P < 0.001). After two doses, MF59-TIV, only A/H1N1 and B strain responses were significantly higher than nTIV [Citation39]. MF59-TIV exhibited durability against A/H3N2, demonstrated by SPR; 100% and 88% after 6 and 12 months, respectively, compared to A/H1N1 (48%) and B strains (22%) after 6 months [Citation39]. Three weeks after the booster shot, MF59-TIV and nTIV achieved elevated and similar SPR (100%) and GMT (1027–1248) against A strains, also MF59-TIV SPR (100%) and GMT (182) against B strains were significantly higher than nTIV SPR (68%) and GMT (41) [Citation1]. Vesikari et al. [Citation30] concluded that MF59-TIV elicited significantly higher responses against A and B strains in vaccine-matched and vaccine-mismatched strains. Carmona et al. [Citation40] compared two AS03 adjuvanted vaccines containing varying levels of the adjuvant α-tocopherol: 1.48 mg in AS03D and 2.97 mg in AS03C. The influenza vaccine adult licensure criteria were met at day 42 for SCR, SPR, and GMFR for both vaccines against A/H1N1 and A/H3N2 (all at 100%) and against B strains for AS03D (all at 100%) but not for AS03C (73%, 75%, 73%, respectively) [Citation40]. Six months after primary vaccination, SPR of AS03C were much lower (26%–68%) than AS03D (95%–100%). GMT values of two doses of AS03D were higher than that of one dose of AS03C, 42 days after the initial vaccination for all strains, AS03D-AS03C, A/H1N1 905.2–617.1, A/H3N2 722.5–160, B 460.5–63.1 [Citation40]. A booster dose of a non-adjuvanted TIV administered at 6 months exceeded the licensing criteria for immunogenicity against homologous A/H1N1 and B lineages and against heterologous A/H3N2 strains in both groups. The last study compared the removal of thimerosal from IIV3s in two doses (0.25 mL and 0.5 mL) to an active IIV3. SCR levels of all three vaccination groups against the three influenza strains met CBER and CHMP criteria (>40%), similarly SPR CBER criteria were met against all strains for the 0.5 mL dose (>70%) [Citation41]. SPR CHMP criteria were exceeded by the control IIV3 against all strains but not by the 0.25 mL dose against A/H1N1.
3.2.2. QIV
The studies reporting immunogenicity of QIVs, have compared IIV4s to NIVs, other IIV4s, and in one study different vaccine doses. One study compared pre-/postvaccination titers of an IIV4 and recorded adequate CBER against all strains, except for SPR against A/H3N2 [Citation42]. Robertson et al. [Citation43] conducted a randomized trial comparing half (0.25 mL) and full (0.5 mL) IIV4 doses in 1,460 subjects. A full dose of IIV4 was non-inferior against all four strains to the half-dose in children aged 6–35 months [Citation43]. Two studies showed that IIV4s are highly immunogenic against all four strains of influenza compared to NIVs. Claeys et al. (2018) [Citation34] reported that the immunogenicity of IIV4s was higher in the 18–35 months old group compared to the younger 6–12 months old. A randomized controlled trial in over 56 Eurasian centers consisting of 2,000 participants reported a good response against all strains after primary vaccination with IIV4 (SCR > 65%) [Citation35]. Furthermore, GMFI rates were higher in IIV4 groups compared to NIVs against B strains (2.1 IIV4 vs 1.0 NIVs) and significantly higher against A strains (17.6 IIV4 vs 1.15 NIVs). IIV4s provided immunity for up to 12 months against three strains [Citation35]. Upon, revaccination at 12 months, titers against all four strains increased to significantly higher levels than before boosters. Statler et al. [Citation44] conducted a phase III observer-blind trial comparing two IIV4s. The test IIV4 (T-IIV4) was split with a higher detergent concentration than the control (C-IIV4). The results demonstrated non-inferiority of T-IIV4 to C-IIV4 exhibited by similar SCR, SPR, and GMT values between both vaccines, with values differing between strains.
3.2.3. TIV vs QIV
Five studies compared the immunogenicity of IIV3s and IIV4s [Citation33,Citation45–48]. Two used different combinations of lineages in the tested TIVs. Three of these studies used TIVs formulated against A/H1N1, A/H3N2, and B/Yamagata lineages excluding protection against B/Victoria. All reported low post-vaccination GMT against B/Victoria compared to B/Yamagata post-vaccination GMT; (Victoria – Yamagata) 21–32 [Citation45], 24–153 [Citation46], 16–107 [Citation47]. Eun et al. [Citation45] concluded that both QIV and TIV met the CHMP criteria against both A lineages and B/Yamagata, while only QIV met them against B/Victoria. Additionally, SCR and SPR values were significantly higher in QIV (SCR, 68%; SPR 76%) compared to TIV (SCR, 58%; SPR, 71%) [Citation45]. Wang et al. [Citation46] demonstrated that post-vaccination GMT and SCR levels between TIV and QIV. Against B/Victoria, TIV was significantly lower (GMT 24; SCR 12% (95% CI 7%–19%)) than QIV (GMT 86; SCR 66% (95% CI 58%–74%)). Both vaccines were highly immunogenic against A/H1N1, A/H3N2, and B/Yamagata, but only QIV achieved CBER criteria for all four strains and was highly immunogenic against B/Victoria [Citation46]. In a third study, GMT and SCR values were higher in QIV participants compared to TIV participants against A strains and B/Victoria but were similar against B/Yamagata [Citation2]. Neither QIV nor TIV met the CBER SPR criteria against A/H3N2, and B/Victoria (<70%), GMT was significantly high in the QIV group against all strains, ranging from 111.4 to 159.4. GMT was highest against B/Yamagata in TIV group (107.2) [Citation47]. This study concluded that QIV demonstrated non-inferiority compared to TIV against shared strains (A/H1N1, A/H3N2, B/Yamagata) and superiority against B/Victoria demonstrated by significantly higher GMT (QIV, 111.4; TIV, 15.6) and SCR (QIV, 74%; TIV, 10%) [Citation47]. The last two studies compared IIV4s to TIVs containing either the B/Victoria strain (TIV-Vic) or the B/Yamagata strain (TIV-Yam) or to the administration of both TIVs (TIV-B). In a study consisting of 2,302 participants, QIV demonstrated a non-inferior GMT to TIV against A/H1N1, A/H3N2, and B/Yamagata, while it was superior against B/Victoria (QIV 623 > 10 TIV) and B/Yamagata (QIV 1010 > 25.3 TIV) [Citation48]. The second study concluded high GMT and SCR by QIV against all four strains, with similar values to TIV for shared A/H1N1, A/H3N2, and B/Yamagata strains [Citation33].
3.3. Safety
The safety of vaccines is measured according to frequency and severity of adverse events. Eighteen studies reviewed the safety and reactogenicity of TIVs and QIVs as discussed below and in .
3.3.1. TIV
One study compared the reactogenicity of IIV3s, two of CAIV-T, and four of adjuvanted IIV3s. The first study comparing four IIV3s stated that all were safe to use in the 6–35 months old children. Injection-site adverse effects (S.AE) were in 10% and systemic S.AE in 19% [Citation49]. CAIV-T also had a low local and systemic reactogenicity [Citation28,Citation29]. In a study of 3,174 participants, the first CAIV-T dose and subsequent dose in year 2 resulted in frequency mild systemic S.AE [Citation28]. Severe adverse effects (SAEs) were uncommon. There were two deaths (one in the vaccine and one in the placebo group) but neither linked to the vaccine. The only discontinuation was caused by a 3-day fever after initial vaccination with CAIV-T in year 1 and was linked to the study medication. Similarly in the second study, the only statistically difference between CAIV-T and the placebo group was nasal congestion after the first dose in year 1: CAIV-T 82%, placebo 75% [Citation29].
Two studies discussed the safety of MF59-adjuvanted TIVs compared to non-adjuvanted TIVs and concluded that the MF59-TIVs is safe for this age group. Vesikari et al. [Citation39] reported an similar reactogenicity (local and systemic), except for mild injection-site swelling which was higher in MF59-TIV (12%) vs 5% in nTIVs. Booster doses of MF59-TIV elicited slightly greater tenderness (45% vs 34%) and irritability (41% vs 33%) [Citation39]. Similarly, the second study reported slightly higher injection-site S.AE with MF59-TIV (54%) compared to nTIVs (46%) and NIVs (52%). Systemic S.AE and U.AE were similar in frequency between all groups [Citation30]. This study demonstrated the safe non-inferiority of MF59-TIVs when compared to nTIVs [Citation30].
In a study comparing α-tocopherol adjuvanted IIV3s in different levels (1.48 mg in AS03D and 2.97 mg in AS03C) to a nTIV booster dose, the reactogenicity of the AS03-TIVs was higher than that of nTIVs. Fever after the booster was statistically lower than after the AS03-TIV (20%) than the second dose of AS03D (35%). There was also a lower frequency of fever after the AS03C booster (21%) than the AS03C (34%) [Citation40]. U.AE and SAE were not thought to be related to the vaccines. Langley et al. [Citation3] compared TF-IIV3s at two doses (0.25 mL and 0.5 mL) with an IIV3s and concluded that there was no statistically significant difference in any safety parameter between the two doses and the comparator. Furthermore, the reactogenicity of all three vaccines decreased with time, as indicated by the lower values of U.AE and medically attended effects (MAE) in the 6 months follow-up [Citation41].
3.3.2. QIV
Three of the six QIV studies contained no control groups and only studied the reactogenicity of IIV4s, concluding these vaccines are safe in children 6–35 months old. Statler at al, 2019 [Citation44] reported a higher frequency of systemic S.AE compared to local/injection-site S.AE in both IIV4s. None of the reported U.AE and SAE were attributed to the vaccines. In another study conducted on 300 participants, four of the ten SAE were vaccine-related, but were moderate and resolved within 1–7 days [Citation42]. The last study recorded no significant difference in S.AE, U.AE, and SAE between the two administered doses of IIV4 [Citation50].
Two studies compared IIV4s to NIVs. A study conducted on 12,018 participants concluded no clinically meaningful differences between the test and control in S.AE, U.AE, and MAE [Citation34]. A second study of 2,000 children, reported that the IIV4 was safe [Citation35]. No significant difference in injection-site S.AE were reported in the IIV4 and the NIV groups [Citation35]. Systemic S.AE levels were lower after the second dose in both groups, compared to the first dose and no statistically significant difference was noted for U.AE, SAE, and MAE [Citation35].
The final study conducted on 1,460 participants compared half (0.25 mL) and full (0.5 mL) doses of IIV4. The frequencies of local and systemic S.AE, U.AE, and SAE were comparable between the two groups. All S.AE resolved within 3 days and only a single case of AE of special interest related to the half-dose vaccine was reported [Citation43].
3.3.3. TIV vs QIV
Five studies compared TIVs and QIVs to determine where adding an antigen in the QIVs influence reactogenicity [Citation33,Citation45–48]. Three studies compared IIV4s to IIV3s and concluded similar reactogenicity. No statistical difference was observed in S.AEs and U.AE between the two tests groups (IIV4 and IIV3) and no SAE were reported [Citation45]. Similarly, there was no statistically significant difference between S.AEs, U.AE, SAE, and MAE between the two vaccine types, and vaccine-related AE were uncommon; U.AE 7% (IIV4) and 4% (IIV3); SAE 0% in both [Citation46]. Langley et al. (2015) [Citation47] demonstrated the non-inferiority of QIVs compared to TIVs indicated by the similar reactogenicity levels in all parameters. Moreover, systemic and injection-site S.AE were similar between doses, all MAE resolved before the end of the study, and only one SAE was related to IIV4 and was resolved without sequelae [Citation47]. Hence, adding an antigen in QIVs did not negatively impact the safety of the vaccine in children 6–35 months old.
The other two studies used two IIV3s with opposing B strains or their combination: IIV3-Victoria or IIV3-Yamagata. No difference was noted across the three groups for S.AE except for lower rates of diarrhea in the IIV4 group (2%) compared to IIV3-Victoria (3%) and IIV3-Yamagata (4%) [Citation48]. Furthermore, two SAE were recorded after the primary vaccination of IIV4 (0%) recovered within 3 days [Citation48]. The second study conducted on 5,806 participants and included a placebo, showed similar levels in all four groups for systemic S.AE and U.AE, and similarity between the combined-IIV3 group and the placebo for SAE [Citation47]. While IIV4 elicited higher levels of injection-site S.AE (40%) compared to IIV3-1/2 (34%) and placebo (32%) and the only vaccine-related SAE occurred in the IIV4 (quickly recovered) [Citation47].
4. Discussion
Twenty-three articles studying seasonal influenza vaccines in 6–35 months old children were evaluated in this review. Overall, TIVs and QIVs provided good seroprotection and similar immunogenicity (GMT, SCR, and SPR) that met recommended levels of the CHMP (European) and CBER (USA) criteria in several studies. However, VE did vary from season to season, attributable to variable mismatch between the circulating infective strains for that year and vaccine lineage recommended by WHO.
In children under 3 years old, QIVs (IIV4s) were overall, more effective against seasonal influenza B than TIVs as they carried two Influenza B strains and TIVs only one. Immunogenicity of TIVs and QIVs against Influenza A were similar. Vaccines containing an adjuvant (e.g. CAIV-T and MF59-TIV) led to higher VE [Citation38]. Seroprotection was observed for up to 12 months post-vaccination and maintained for longer when a booster was administered. Increasing the dose from 0.25 mL to 0.5 mL resulted in increased immunogenicity of TIVs, but no statistically significant difference in QIVs [Citation41,Citation43].
Trivalent and quadrivalent influenza vaccines are both safe in the 6–35 months age group. Vaccines with adjuvants (CAIV-T and AS03-adjuvanted vaccines) caused milder systemic S.AE than IIV3s [Citation1,Citation5,Citation6]. An increase of dosage from 0.25 mL to 0.5 mL had no effect on systemic and local reactogenicity of TIVs.
A limitation of this review is the small number of studies published on the efficacy of seasonal influenza vaccines in children aged 6–35 months. For instance, there was only one Finnish study comparing TIV and QIV VE. This restricts the ability to assess the relative beneficial effects of the different valent vaccine in this age group. Furthermore, because of antigenic drift and risk of infection – vaccine strain mismatch, longer duration studies are required to determine the annual variation of vaccine efficacy.
5. Summary
Seasonal influenza vaccines are effective and safe for the administration in children 6–35 months old. TIVs and QIVs demonstrate similar efficacy, immunogenicity, and safety profiles against Influenza A lineages. As for Influenza B lineages, QIVs have higher levels of immunogenicity due to the incorporation of the two circulating B lineages – B/Yamagata and B/Victoria, while TIVs only contain one. Valency of vaccines can be increased to include four strains without negatively affecting the reactogenicity profile. Furthermore, the administration of a booster dose 6–12 months following the last vaccination induces longer term protection against the virus in this age group. Finally, selecting vaccine strains that match the expected circulating strains of each season remains challenging and inconsistent at times.
6. Expert opinion
The pediatric population are at risk for an increased burden of influenza infection. Hence, the prevention of seasonal influenza is crucial in this group. Several licensed vaccines can reduce the disease burden. However, healthy children 6–35 months old have relatively low vaccination rates due to their exclusion from national vaccination programs and the lack of widespread knowledge on vaccine safety and efficacy. Whilst some countries recommend influenza vaccine, these are deemed cost-effective to support in national programs [Citation51]. This review highlights the efficacy, immunogenicity, and safety of TIVs and QIVs in this population.
Future studies should evaluate the efficacy of different influenza vaccine types on children aged 6–35 months over longer time periods. Larger multinational comparative studies are needed to provide generalizable reports and detect regional differences in TIVs and QIVs efficacy in the pediatric population. Although, the WHO presents yearly recommendations of the strains to be included in vaccines for both the Northern and the Southern hemispheres, further research is necessary to ensure higher levels of matching. New methods used to identify potential circulating strains must be developed to increase matching-accuracy in vaccines, speed up the production of new seasonal vaccines, and to reduce the burden of influenza in children.
The evidence indicates that young children have a high burden of disease from influenza B strains and that QIVs provide superior VE to TIVs, particularly for these strains without increasing reactogenicity and side-effects, and these quadrivalent vaccines are to be recommended in this age group. Traditional targets groups in the UK and other European nations are high-risk groups – include the elderly, pregnant women, and children with chronic conditions. Baguelin et al. (2013) [Citation52] developed a model to map the transmission patterns of seasonal influenza infection in England and Wales using the surveillance data of fourteen seasons, from 1995/96 to 2008/09. Using this model, they applied various vaccination scenarios to simulate the impacts of the vaccination programs on the population. The results showed that the program encouraging vaccination of all children aged 6–35 months is the most efficient strategy, as they are the group responsible for the highest frequency of transmission [Citation52]. Furthermore, the model shows that an estimated total of 5.3 million annual cases would be prevented if 50% of those aged 2–18 years are vaccinated [Citation53]. The same conclusions have been reached by other studies conducted in Japan, Russia, and the United States [Citation54].
In the 2014/15 season, the UK launched a vaccination program, which includes children 2–3 years old. Since then, the program has been extended to children under 17 years old, but excludes healthy children older than 6 months [Citation7]. The WHO recommends that healthy children 6–23 months old be considered target groups for vaccination programs [Citation54]. Many studies and experts recommend that European nations follow the WHO. Canada, and the United States include healthy children above 6 months of age in vaccination programs, with current data supporting the significant protection provided by the influenza vaccines and their safety in this age group [Citation53]. There is a need to raise educational awareness of parents and healthcare professionals to encourage vaccination in healthy children. Several multinational studies have reported either a negative perception of seasonal influenza vaccines or a lack of knowledge of the target groups in healthcare workers and parents [Citation55]. Behavioral models tested in the United States might be applied in Europe to further the education of people on the importance of influenza vaccines – such as providing information to children and their guardians during hospital visits (even those unrelated to influenza) and encouraging pharmacists to do the same for their clients [Citation55].
Article highlights
Two vaccine types are available to protect against seasonal influenza caused by influenza A and B. This systemic literature review compared trivalent (TIV) and quadrivalent (QIV) vaccine immunogenicity and safety in children under 3 years of age.
Twenty-three articles were identified of which 11 discussed efficacy, 15 immunogenicity, and 18 safety of seasonal influenza vaccines.
TIVs and QIVs are effective, immunogenic, and safe in children aged 6–35 months.
QIVs have wider coverage of Influenza B strains than TIVs, as they include two major lineages (B/Yamagata and B/Victoria) compared to TIVs, which carry a single lineage.
Healthy children 6–35 months old have relatively low vaccination rates due to their exclusion from national vaccination programs and the lack of widespread knowledge on vaccine safety and efficacy.
Declaration of interest
R Borrow performs contract research on behalf of UK Health Security Agency for GSK, Pfizer, and Sanofi Pasteur. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Javanian M, Barary M, Ghebrehewet S, et al. A brief review of influenza virus infection. J Med Virol. 2021;93(8):4638–4646.
- Chen J, Wang J, Zhang J, et al. Advances in development and application of influenza vaccines. Front Immunol. 2021;12:711997.
- World Health Organization. (2017). The immunological basis for immunization series: module 23: influenza vaccines [cited 2023 Feb 23]. Available from: https://apps.who.int/iris/handle/10665/259211
- Smith W, Andrewes CH, Laidlaw PP. A virus obtained from influenzae patients. Lancet. 1933;222(5732):66–68.
- Stanley WM. The preparation and properties of influenza virus vaccines concentrated and purified by differential centrifugation. J Exp Med. 1945;81(2):193–218.
- Hardelid P, Rait G, Gilbert R, et al. Recording of influenza-like illness in UK primary care 1995-2013: cohort study. PLoS One. 2015;10(9):e0138659.
- UK Health Security Agency. Weekly national influenza and COVID-19 report: week 27 report. 2022. https://www.gov.uk/government/statistics/national-flu-and-covid-19-surveillance-reports-2021-to-2022-season
- Hardelid P, Rait G, Gilbert R, et al. Factors associated with influenza vaccine uptake during a universal vaccination programme of preschool children in England and wales: a cohort study. J Epidemiol Community Health. 2016;70(11):1082–1087.
- UK Health Security Agency. Seasonal influenza vaccine uptake in children of school age: winter season 2020 to 2021 [cited 23 Feb 2023]. Available from: https://www.gov.uk/government/statistics/seasonal-flu-vaccine-uptake-in-children-of-school-age-monthly-data-2020-to-2021
- UK Health Security Agency. Seasonal influenza vaccine uptake in children of school age. [cited 2023 Feb 23]. Avilable from: https://www.gov.uk/government/statistics/seasonal-flu-vaccine-uptake-in-children-of-school-age-monthly-data-2021-to-2022
- Jin H, Subbarao K. Live attenuated influenza vaccine. In: Oldstone MBA, Compans RW, editors. Influenza pathogenesis and control - volume II. Cham: Springer International Publishing; 2015. p. 181–204.
- Del Giudice G, Rappuoli R. Inactivated and adjuvanted influenza vaccines. In: Oldstone MBA, Compans RW, editors. Influenza pathogenesis and control - volume II. Cham: Springer International Publishing; 2015. p. 151–180.
- Keshavarz M, Mirzaei H, Salemi M, et al. Influenza vaccine: where are we and where do we go? Rev Med Virol. 2019;29(1):e2014.
- Meyer R, De Koker C, Dziubak R, et al. Malnutrition in children with food allergies in the UK. J Hum Nutr Diet. 2014;27(3):227–235.
- Ambrose CS, Levin MJ. The rationale for quadrivalent influenza vaccines. Hum Vaccin Immunother. 2012 Jan;8(1):81–88.
- Baxter D. Evaluating the case for trivalent or quadrivalent influenza vaccines. Hum Vaccin Immunother. 2016;12(10):2712–2717.
- Kawai S, Nanri S, Ban E, et al. Influenza vaccination of schoolchildren and influenza outbreaks in a school. Clin Infect Dis. 2011;53(2):130–136.
- CDC. Past weekly surveillance reports. national center for immunization and respiratory diseases; 2022. [cited 2023 Feb 23]. Available from: https://www.cdc.gov/flu/weekly/pastreports.htm
- Bodewes R, de Mutsert G, van der Klis FR, et al. Prevalence of antibodies against seasonal influenza A and B viruses in children in Netherlands. Clin Vaccine Immunol. 2011;18(3):469–476.
- WHO. Global influenza programme. In: Organization WH, editor. 2022. [cited 2023 Feb 23]. Available from: https://www.who.int/teams/global-influenza-programme/vaccines
- Influenza vaccine. In: Formulary NBN, editor. UK2022. [cited 2023 Feb 23]. Available from: https://bnf.nice.org.uk/drugs/influenza-vaccine/
- Katz JM, Hancock K, Xu X. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Expert Rev Anti Infect Ther. 2011;9(6):669–683.
- Pavlova S, D’Alessio F, Houard S, et al. Workshop report: immunoassay standardisation for “universal” influenza vaccines. Influenza Other Respir Viruses. 2017;11(3):194–201.
- Cann AJ. Chapter 1 - Introduction. In: Cann AJ, editor. Principles of molecular virology (sixth edition). Boston: Academic Press; 2016. p. 1–25.
- Krammer F, Weir JP, Engelhardt O, et al. Meeting report and review: immunological assays and correlates of protection for next-generation influenza vaccines. Influenza Other Respir Viruses. 2020;14(2):237–243.
- Hobson D, Curry RL, Beare AS, et al. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond). 1972;70(4):767–777.
- Cox RJ. Correlates of protection to influenza virus, where do we go from here? Hum Vaccin Immunother. 2013;9(2):405–408.
- Tam JS, Capeding MR, Lum LC, et al. Efficacy and safety of a live attenuated, cold-adapted influenza vaccine, trivalent against culture-confirmed influenza in young children in Asia. Pediatr Infect Dis J. 2007;26(7):619–628.
- Vesikari T, Fleming DM, Aristegui JF, et al. Safety, efficacy, and effectiveness of cold-adapted influenza vaccine-trivalent against community-acquired, culture-confirmed influenza in young children attending day care. Pediatrics. 2006;118(6):2298–2312.
- Vesikari T, Knuf M, Wutzler P, et al. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med. 2011;365(15):1406–1416.
- Niang MN, Sugimoto JD, Diallo A, et al. Estimates of inactivated influenza vaccine effectiveness among children in Senegal: results from 2 consecutive cluster-randomized controlled trials in 2010 and 2011. Clin Infect Dis. 2021;72(12):e959–e969.
- Diallo A, Diop OM, Diop D, et al., Effectiveness of seasonal influenza vaccination in children in Senegal during a year of vaccine mismatch: a cluster-randomized trial. Clin Infect Dis. 2019. 69(10): 1780–1788.
- Pepin S, Dupuy M, Borja-Tabora CFC, et al. Efficacy, immunogenicity, and safety of a quadrivalent inactivated influenza vaccine in children aged 6-35 months: a multi-season randomised placebo-controlled trial in the Northern and Southern Hemispheres. Vaccine. 2019;37(13):1876–1884.
- Claeys C, Zaman K, Dbaibo G, et al. Prevention of vaccine-matched and mismatched influenza in children aged 6–35 months: a multinational randomised trial across five influenza seasons. Lancet Child Adolesc Health. 2018;2(5):338–349.
- Esposito S, Nauta J, Lapini G, et al., Efficacy and safety of a quadrivalent influenza vaccine in children aged 6-35 months: a global, multiseasonal, controlled, randomized phase III study. Vaccine. 2022. 40(18): 2626–2634.
- Pepin S, Samson SI, Alvarez FP, et al. Impact of a quadrivalent inactivated influenza vaccine on influenza-associated complications and health care use in children aged 6 to 35 months: analysis of data from a phase III trial in the Northern and Southern Hemispheres. Vaccine. 2019;37(13):1885–1888.
- Dbaibo G, Amanullah A, Claeys C, et al. Quadrivalent influenza vaccine prevents illness and reduces healthcare utilization across diverse geographic regions during five influenza seasons: a randomized clinical trial. Pediatr Infect Dis J. 2020;39(1):e1–e10.
- Baum U, Kulathinal S, Auranen K, et al., Effectiveness of 2 Influenza Vaccines in Nationwide Cohorts of Finnish 2-Year-Old Children in the Seasons 2015-2016 Through 2017-2018. Clin Infect Dis. 2020. 71(8): e255–e261.
- Vesikari T, Pellegrini M, Karvonen A, et al. Enhanced immunogenicity of seasonal influenza vaccines in young children using MF59 adjuvant. Pediatr Infect Dis J. 2009;28(7):563–571.
- Carmona Martinez A, Salamanca de la Cueva I, Boutet P, et al. A phase 1, open-label safety and immunogenicity study of an AS03-adjuvanted trivalent inactivated influenza vaccine in children aged 6 to 35 months. Hum Vaccin Immunother. 2014;10(7):1959–1968.
- Langley JM, Vanderkooi OG, Garfield HA, et al., Immunogenicity and safety of 2 dose levels of a thimerosal-free trivalent seasonal influenza vaccine in children aged 6-35 months: a randomized, controlled trial. J Pediatric Infect Dis Soc. 2012. 1(1): 55–63.
- Langley JM, Carmona Martinez A, Chatterjee A, et al. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine candidate: a phase III randomized controlled trial in children. J Infect Dis. 2013;208(4):544–553.
- Robertson CA, Mercer M, Selmani A, et al. Safety and immunogenicity of a full-dose, split-virion, inactivated, quadrivalent influenza vaccine in healthy children 6-35 months of age: a randomized controlled clinical trial. Pediatr Infect Dis J. 2019;38(3):323–328.
- Statler VA, Albano FR, Airey J, et al. Immunogenicity and safety of a quadrivalent inactivated influenza vaccine in children 6-59 months of age: a phase 3, randomized, noninferiority study. Vaccine. 2019;37(2):343–351.
- Eun BW, Lee TJ, Lee J, et al. A randomized, double-blind, active-controlled Phase III trial of a cell culture-derived quadrivalent inactivated influenza vaccine in healthy South Korean children and adolescents 6 months to 18 years of age. Pediatr Infect Dis J. 2019;38(9):e209–e215.
- Wang L, Chandrasekaran V, Domachowske JB, et al. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine in US children 6-35 months of age during 2013-2014: results from a phase II randomized trial. J Pediatric Infect Dis Soc. 2016;5(2):170–179.
- Langley JM, Wang L, Aggarwal N, et al. Immunogenicity and reactogenicity of an inactivated quadrivalent influenza vaccine administered intramuscularly to children 6 to 35 months of age in 2012–2013: a randomized, double-blind, controlled, multicenter, multicountry, clinical trial. J Pediatric Infect Dis Soc. 2015;4(3):242–251.
- Hu Y, Shao M, Hu Y, et al. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine: a randomized, double-blind, controlled phase III clinical trial in children aged 6-35 months in China. Hum Vaccin Immunother. 2020;16(7):1691–1698.
- Wood NJ, Blyth CC, Willis GA, et al. The safety of seasonal influenza vaccines in Australian children in 2013. Med J Aust. 2014;201(10):596–600.
- Thiem VD, Chabanon AL, Fournier M, et al. Safety of a quadrivalent influenza vaccine in Vietnamese healthy subjects aged 6 months and older. Hum Vaccin Immunother. 2021;17(3):690–693.
- Principi N, Camilloni B, Esposito S. ESCMID Vaccine Study Group (EVASG). Influenza immunization policies: which could be the main reasons for differences among countries? Hum Vaccin Immunother. 2018 Mar 4;14(3):684–692.
- Baguelin M, Flasche S, Camacho A, et al., Assessing optimal target populations for influenza vaccination programmes: an evidence synthesis and modelling study. PLoS Med. 2013. 10(10): e1001527.
- Heikkinen T, Tsolia M, Finn A. Vaccination of healthy children against seasonal influenza: a European perspective. Pediatr Infect Dis J. 2013;32(8):881–888.
- World Health Organization. Influenza Vaccines: WHO position paper 2005. [cited 2023 Feb 23]. Available from: https://www.who.int/publications/i/item/WER8033
- Ramet J, Weil-Olivier C, Sedlak W. Influenza vaccination: the paediatric perspective. Vaccine. 2007;25(5):780–787.