ABSTRACT
Background
Pneumococcal diseases have a clinical and economic impact on the population. Until this year, a 10-valent pneumococcal vaccine (PCV10) used to be applied in Colombia, which does not contain serotypes 19A, 3, and 6A, the most prevalent in the country. Therefore, we aimed to assess the cost-effectiveness of the shift to the 13-valent pneumococcal vaccine (PCV13).
Research design and methods
A decision model was used for newborns in Colombia between 2022–2025 and adults over 65 years. The time horizon was life expectancy. Outcomes are Invasive Pneumococcal Diseases (IPD), Community-Acquired Pneumonia (CAP), Acute Otitis Media (AOM), their sequelae, Life Gained Years (LYGs), and herd effect in older adults.
Results
PCV10 covers 4.27% of serotypes in the country, while PCV13 covers 64.4%. PCV13 would avoid in children 796 cases of IPD, 19,365 of CAP, 1,399 deaths, and generate 44,204 additional LYGs, as well as 9,101 cases of AOM, 13 cases of neuromotor disability and 428 cochlear implants versus PCV10. In older adults, PCV13 would avoid 993 cases of IPD and 17,245 of CAP, versus PCV10. PCV13 saves $51.4 million. The decision model shows robustness in the sensitivity analysis.
Conclusion
PCV13 is a cost-saving strategy versus PCV10 to avoid pneumococcal diseases.
1. Introduction
Streptococcus pneumoniae (S. pneumoniae), also known as pneumococcus, causes pneumococcal diseases [Citation1]. There are two main types of pneumococcal infections: non-invasive and invasive (IPD). Non-invasive pneumococcal diseases are less severe than IPD and occur outside significant organs such as the circulatory system or central nervous system (CNS). IPD is less frequent but more severe [Citation2] and is more frequent in young children and older adults, with a high lethality rate [Citation3]. Although most pneumococcal infections are mild, they can sometimes be deadly or produce severe sequelae, such as brain damage or hearing loss.
As part of IPD, invasive meningitis is most lethal in toddlers; about 1 in every 15 children under five years of age with pneumococcal meningitis die from this cause [Citation1]. Some survival toddlers could remain with long-term problems, such as hearing loss or delayed neuromotor development [Citation4]; besides, it is highly lethal in older adults [Citation5]. Likewise, the case fatality rate for those with pneumonia complicated by bacteremia is approximately 20 %, and their lethality increases in older adults [Citation6].
Pneumococcus spreads from the nasopharynx (nose and throat) to the upper and lower respiratory system, which can result in AOM or pneumonia [Citation7]. Although the lethality of pneumococcal pneumonia in the general population is 5 % [Citation8], whereas AOM infections are mild and are the most common form of pneumococcal disease [Citation1], some children who develop recurrent infections may need ear tubes [Citation9].
According to the World Health Organization (WHO), given that pneumococcal infection is a vaccine-preventable disease that causes more deaths in infants and children under five years [Citation10], it recommends the use of conjugate vaccines in this condition [Citation11]. In 2007, the Ministry of Health of Colombia included the pneumococcal conjugate 7-valent vaccine (PCV7) in the National Immunization Plan (PAI, acronym in Spanish). Economic studies from the Pan American Health Organization (PAHO) and Fedesarrollo (a Colombian non-profit entity dedicated to research on economic and social policy issues) concluded that vaccination with PCV7 would be a cost-saving strategy versus not vaccinating [Citation12,Citation13]. Therefore, coverage of the vaccinated population increased gradually in the following years after its implementation. In September 2011, PAI changed to the pneumococcal 10-valent conjugated vaccine (PCV10) based on a cost-effectiveness analysis that concluded that routine vaccination with this health technology for S. pneumoniae in Colombia would save costs because it would avoid more AOM of all causes. However, the pneumococcal 13-valent conjugated vaccine (PCV13) would avoid more disease and deaths and will produce higher life years gained (LYG) [Citation14].
Ten years since the introduction of PCV10, serotype redistribution has been one of the most noticeable changes, highlighting a decrease in serotypes in PCV10 and a significant increase in serotype 19A, which is not in that vaccine. Serotype 19A has presented substantial relevance in the last years, according to SIREVA II reports [Citation15,Citation16]. Furthermore, invasive and non-invasive pneumococcal diseases in Colombia continue to generate a significant disease burden for the child population, despite the reduction in the number of cases of AOM in children under five years of age after the implementation of PCV10 [Citation17].
Recently a cost-effectiveness model was published evaluating the economic impact of the switch to PCV13 versus continuing PCV10 in Colombia children under the epidemiological scenario of 2014 [Citation18] and included outcomes of a clinical trial published the same year [Citation19]. It showed that switch-to PCV13 has a good value for money and prevents additional pneumococcal disease burden, saving additional treatment costs; in the same way, the WHO has stated that PCV13 may have an additional benefit in settings where disease attributable to serotypes 19A or 6C are significant [Citation20]. Recently, PCV13 has again become the only vaccine used in Colombia for mass vaccination programs in infants.
Considering all these factors, such as serotype dynamics, vaccine costs, and pneumococcal disease incidence, we must continually reassess economic evaluations for this program. So that it allows decision-makers to identify the most cost-effective strategy given the current scenarios of this disease, incorporating direct and indirect benefits that different alternatives may have. We aim to estimate the value of PCV13 versus PCV10 to calculate its cost-effectiveness in preventing IPD and AOM in children under five years old vaccinated between 2022 and 2025, as well as the herd effect in adults and older adults.
2. Methods
2.1. Target population
Newborns in Colombia between 2022 and 2025, adults older than 18, and the population will turn 18 years between 2022 and 2025. The estimated number of births during this period is 3 million, approximately. Therefore, in 2022, in Colombia, there will be 32,284,009 people between 18 and 64 years old, and it will rise to 33,349,829 people by 2025. Finally, in 2022, the country will have 5,103,168 older people over 64, which will increase to 5,735,600 in 2025.
A cohort was chosen until 2025 because that year, in the country, two new conjugated polysaccharide vaccines would be available, a 20-valent (PCV20) as well as a PCV15.
2.2. Comparators
The strategies evaluated are the two pneumococcal conjugate vaccines currently licensed in scheme 2 + 1: PCV13 and PCV10. The first one owns to Pfizer and contains thirteen serotypes of the pneumococcus (1, 3, 4, 5, 6A, 6B, 7 F, 9 V, 14, 18C, 19A, 19 F, and 23 F), conjugated to the CRM197 protein of diphtheria as a carrier protein. The other vaccine, PCV10, developed by Glaxo Smith-Kline, differs from PCV13. It does not contain the serotypes 3, 6A, and 19A, and the carrier protein is protein D from non-typeable Haemophilus influenzae (PD) (NTHi), Tetanus Toxoid (TT), and Diphtheria Toxoid (DT).
We did not include the Serum Institute of India Pvt Ltd PCV candidate (SIIPL-PCV), pre-qualified by WHO, and with immunogenicity data [Citation21] in the analysis due to the absence of an efficacy trial. Besides, National Institute for Surveillance on Drugs and Foods (INVIMA –Spanish acronym) has not approved that vaccine, and the price of this vaccine is not available, considering that for the national program, the purchase of vaccines from the producer is via tender to the PAHO.
2.3. Time horizon
The relevant impact on public health of pneumococcal conjugate vaccines (PCVs) has been their high clinical efficacy and protective effect during their lifetime; for this reason, the time horizon for this cost-effectiveness analysis was the life expectancy.
2.4. Study perspective
This analysis has a social perspective, with the direct costs of resources associated with these technologies and pneumococcal disease treatment costs and their aftermath, included or not in the Plan of Benefits of Health of Colombia [Citation22]. In the same way, indirect costs by loss of productivity in people with neurological sequelae caused by pneumococcal meningitis and the health outcomes perceived by patients.
2.5. Discount rate
We used a discount rate of 5 % for costs and benefits, as recommended by the Institute of Technological Evaluation in Health in Colombia [Citation23].
2.6. Choice of health outcomes
We measure these outcomes: avoided cases of IPD (invasive pneumonia, pneumococcal sepsis, and meningitis), community-acquired pneumonia (CAP), deaths by IPD, both children and adults, and pneumococcal AOM in infants. Similarly, sequels derived from pneumococcal diseases such as neuromotor disability, cochlear implants, and LYG in children and adults. We estimated avoided deaths and IPD cases in unvaccinated adults because of the herd effect [Citation24].
2.7. Clinical parameters
We carried out a search strategy to identify the clinical efficacy of pneumococcal vaccines, including only phase III and IV randomized controlled trials (RCTs) in infants and excluding those that have included polysaccharide vaccines. Finally, we looked for RCTs that met the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) bias control criteria [Citation25]. Finally, we adjusted the effectiveness for the national distribution of pneumococcal serotypes in Colombia between 2017 and 2018 (64.4 % PCV13 and 4.27 % PCV10) based on the information reported in SIREVA II [Citation15]. This procedure is the most used approach to calculate the direct effect of each vaccine [Citation16].
The vaccination coverage was 89 % in each birth cohort [Citation26], and when adjusting herd effect among infants, coverage was 93.6 % [Citation27]. Also, during this time, the adults are at risk of invasive pneumococcal disease; hence herd immunity assumed in the model as 22.1 % [Citation28], and we adjusted serotypes coverage in this group [Citation15] ().
Table 1. Pneumococcal conjugate vaccines efficacy adjusted by pneumococcus serotype distribution in Colombia.
We found two RCTs that met the search criteria, one for each vaccine. For PCV10, Tregnaghi et al. RCT outcomes were taken, carried out in Colombia, Argentina, and Panama, and reported the efficacy results for AOM, IPD, and consolidated pneumonia [Citation19]. For PCV13, Black et al. RCT outcomes were taken, which also reported the efficacy results for AOM, IPD, and pneumonia [Citation29]. Still, this study was from 2000, and at that time, the WHO did not have radiographic criteria to diagnose consolidated pneumonia. So, in 2006 same authors again evaluated the chest X-rays of children included in the original study to classify them based on consolidated pneumonia criteria and to estimate PCV13 efficacy [Citation30] better. Therefore, the way of measuring outcomes of both RCTs is comparable.
For children older than five years old, we took the incidence of IPD, bacterial CAP, pneumococcal AOM, and mortality rate by pneumococcal diseases from the control arm of PCV10 RCT [Citation19] and observational studies. For the other age groups, we took incidence rates from Colombian Health Services Delivery Records (RIPS, acronym in Spanish) during the vaccinal period with PCV10. In addition, we implemented mortality for all causes as age-dependent according to data from Colombia’s National Administrative Department of Statistics (DANE, acronym in Spanish) [Citation31–35] ().
Table 2. Epidemiologic input parameters for a model decision of pneumococcal vaccines in Colombia.
2.8. Currency, price data, and conversion
We calculated costs in Colombian pesos (COP). Later, we converted it into USD, based on the average exchange rate reported between July 2022 and November 2022 from the Colombian central bank (Banco de la República, in Spanish): COP 4,492 for USD 1 [Citation36].
2.9. Estimating resources and costs
We made a top-down costing approach to calculate hospital care costs for children with IPD and bacterial CAP, outpatient healthcare for children with neuromotor disabilities, and adults with IPD. First, we searched for individual costs of all interventions, procedures, and drugs from a Health Maintenance Organization (HMO) database in Colombia with a national presence. This database includes all health resources and healthcare costs for patients with pneumonia, sepsis, and meningitis made in 2022. Then, using descriptive statistics, we estimated the mean, standard deviation (SD), and minimum and maximum values.
We used a bottom-up cost approach to estimate outpatient treatment costs in children with pneumococcal AOM and adults with CAP. For this purpose, we identified drugs used to treat these patients based on HMO clinical practice guidelines. We took the cost of drugs from the Drug Price Information System (SISMED, an acronym in Spanish) [Citation37]. In addition, we took cochlear implant total costs for treating profound sensorineural hearing loss in children from an economic analysis published in Colombia in 2012 [Citation38]. The prices were adjusted for 2022 based on the accumulated consumer-price index for 2012–2021.
Healthcare direct costs for children with neuromotor disability were $ 938 per year, including outpatient healthcare by medical specialists, physiotherapists, and medicines, during their lifetime horizon. We calculated the indirect costs of these patients, assuming that they need 24/7 on-site care, usually by a family member (most of the time, the mother) who cannot produce income at home or pay caregivers 24/7. We estimated the caregiver salary as a mean salary in Colombia during 2022, during the lifetime horizon of the patient. Finally, costs of productivity because of neuromotor disability in children were calculated based on Colombia’s mean salary, assuming most people work between 25 and 65 years of age (age of economic productivity) [Citation39] and adjusted by the national unemployment rate during 2022 [Citation40].
We took vaccine costs from the expanded price list of the immunization program on the PAHO website, last updated in 2019, which were the costs of vaccines in 2022. For example, PCV10 was $ 12.85 per dose, and PCV13 was $ 14.50 per dose [Citation41]. We assumed an administration cost of $ 1 per dose and the implementation of a 2 + 1 dose vaccination schedule at 3, 5, and 12 months of age. We used the 2019 vaccine list price because prices on that list have stayed the same since then ().
Table 3. Costs of pneumococcal diseases by groups of age, Colombia, 2022.
2.10. Choice of model
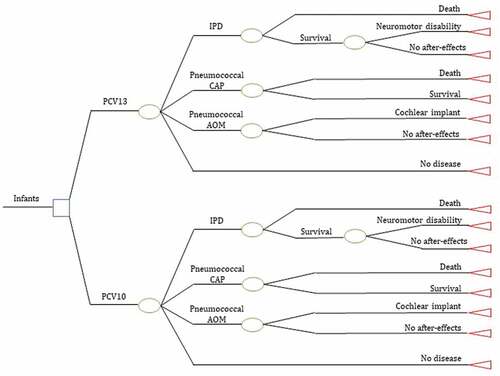
We designed a dynamic cohort model to estimate costs and long-term health outcomes (). We based the model structure on the natural history of pneumococcal diseases (AOM, CAP, and IPD) and their complications in infants and children (neuromotor disability and cochlear implant), considering the period PCV10 post-vaccinal. In addition, we included clinical outcomes in the model from PCVs clinical trials and after-effects as most severe related to pneumococcal infections [Citation42]. Finally, the model consists of vaccinated children (< 5 years) and unvaccinated adults (> 18 years) who benefit from the PCVs herd effect [Citation24].
Given that the model time horizon is population lifetime, we analyzed with cycles based on the annual probability of developing pneumococcal diseases. The model censures every year those patients who develop a pneumococcal disease or die and calculates the probabilities again until the lifetime horizon of each cohort.
We divided the population into three groups according to the risk level of becoming ill and dying from pneumococcal diseases: children vaccinated under five years of age, adults not vaccinated between 18–64 years, and unvaccinated adults > 64 years old. This economic model follows the newborn cohort born between 2022–2025 until the expectancy of life of the first cohort (80 years) expectancy, which integrated the unvaccinated population to measure the indirect vaccination effect.
2.11. Assumptions
This decision model considers the following assumptions concerning comparators:
Herd effect on unvaccinated children and adults is the same for both PCVs; only PCV13 has been shown to protect unvaccinated individuals against vaccine-type pneumococcal infections across all age groups [Citation43–46], but not PCV10 [Citation47–49].
The great migration of Venezuelans that Colombia has received in recent years (2.5 million people, approximately) would imply a change in the frequency distribution of pneumococcal serotypes. However, since SIREVA II records [Citation15] do not identify nationality, the model assumes that such migration would not affect the effectiveness of either of the two PCVs.
The definition of an older adult may differ among published studies; some include adults > 60 years [Citation29] or > 50 years [Citation50]. However, the model assumes an older adult definition as those > 65 years of age because most manuscripts have accepted such a report [Citation28]. For this reason, the model has two different age groups to determine herd effect: 18–64 years and > 65 years.
A life expectancy of 80 years in Colombia is slightly higher than what the country currently has. However, given that model evaluates a dynamic cohort of newborns until 2025, a life expectancy of 80 years was assumed because it can achieve in the following years since it is a life expectancy like that currently reported by countries of the region, such as Chile [Citation51].
The direct efficacy of vaccination decreases in both vaccines; we based this assumption on data from The Community-Acquired Pneumonia Immunization Trial in Adults (CAPiTA) trial (efficacy of PCV13 in older adults), in which researchers observed that efficacy had a 3 % efficacy attenuation annual rate [Citation52]. For this reason, sensitivity analysis of this model will evaluate results with different values of effectiveness attenuation.
2.12. Sensitivity Analysis
We performed sensitivity analyses to consider the uncertainties associated with incidence rates, outcomes, and costs. First, we conducted a sensitivity analysis to determine the influence of a specific parameter on the incremental cost-effectiveness value.
Later, sensitivity analysis was probabilistic to capture uncertainties of all parameters, running 1 000 Monte Carlo simulations. Finally, we adjusted incidence rates to beta distributions and the costs to gamma distributions.
3. Results
By vaccinating all neonates in Colombia with PCV13 for four years, compared to PCV10, there would be 44,204 additional LYG because PCV13 would avoid 1,399 more deaths than PCV10 in the entire population. Avoided deaths are this way: 1,123 deaths were avoided from pneumococcal pneumonia, 245 deaths were avoided from pneumococcal sepsis, and 31 deaths were avoided from pneumococcal meningitis ().
Table 4. Estimated pneumococcal disease cases by vaccinating newborns in Colombia between 2019–2022 with PCV13 vs. PCV10.
The incremental cost of vaccination with PCV13 of all newborns between 2022 and 2025 was estimated to be $ 13,3 million. Due to the reduced pneumococcal disease burden, we calculated the potential cost savings for all neonate cohorts to $ 36.6 million, direct costs were $ 34.7 million, and indirect costs were $ 1.9 million. Considering the herd effect, the cost saving for the cohorts rises to $ 51.4 million (). As a result, PCV13 is a cost-saving strategy even without herd protection outcomes in the analysis.
Table 5. Estimated costs of health care resources used in all neonates in Colombia between 2022 and 2025 vaccinated with PCV13 vs. PCV10 (in USD million).
3.1. Probabilistic results
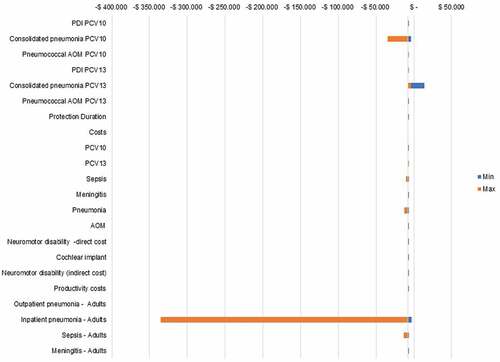
Probabilistic sensitivity analysis showed that variations in probabilities, clinical outcomes, and costs were relatively insensitive to variable changes. The parameter with the most significant impact in this sensitivity analysis was the “cost of inpatient pneumonia in adults,” reflecting that PCV13 is a cost-saving strategy. None of the parameters change these results (). In probabilistic analysis, PCV13 is a cost-effective strategy in all thresholds compared with PCV10.
4. Discussion
PCV13 is a cost-saving strategy to avoid pneumococcal diseases in children and adults in Colombia compared to PCV10. The PCV13 inclusion would prevent many pneumococcal AMO in infants, sepsis, consolidated pneumonia, deaths, and LYG in adults and children. In terms of financial results, this vaccine would save $ 49,5 million associated with outcomes healthcare and $ 1,9 million in indirect costs because of healthcare of main sequels of pneumococcal disease during the horizon time of the study.
These results are mainly because of the difference in pneumococcal serotypes covered by both technologies: PCV10 contains less than 5 % of isolated serotypes of the IPD cases reported by the SIREVAII network. In comparison, PCV13 has almost 65 % of those serotypes, directly affecting both vaccines’ effectiveness. Serotypes 19A and 3 are entirely responsible for the difference in vaccinal serotypes. Carrasquilla et al. reported a positive trend of these serotypes in pneumococcal disease over time [Citation17]. Raw data comparing 19A and 3 serotypes Distribution in 2011–2014 with the 2015–2019 period reported a notable increase, especially serotype 19A (30 % to 44 % and 8 % to 9 %, respectively [Citation15].
Camacho et al. highlight the role of S. pneumoniae serotype 19A in the pediatric population of Bogotá, Colombia, as a leading cause of IPD after PCV10 introduction. Furthermore, they described an essential association with multidrug resistance in patients with a complete vaccination schedule with 3 doses of PCV10, suggesting that the appearance of serotype 19A occurs in children vaccinated with PCV10, and PCV10 does not generate cross-protection against serotype 19A [Citation53]. Therefore, a frequency increase of serotype 19A is a substantial change in epidemiology, and the WHO’s position is to change the product [Citation20].
Other Latin-American countries have observed a similar phenomenon: non-vaccinal serotypes grow in isolated IPD. For example, a study in Porto Alegre (Brazil) reported changes in the percentage of 19A serotypes from 1,2 % in 2011 to 18,5 % in 2014 [Citation54]. Another study in Brazil analyzing laboratory-based surveillance reported changes from 3,2 % in the period early-post -PCV10 to 9,3 % five years after [Citation55]. In Chile, the 19A serotype increased relative frequency in the general population from 3,3 % in pre-PCV10 to 12 % in 2014 and 13 % in 2015. In infants, the changes were more notable, starting at 5,8 %, reaching 25 % and 19 % in 2014 and 2015, respectively [Citation56].
This decision model has two characteristics, thus complying with WHO recommendations [Citation57]: the herd effect in adults and indirect costs and loss of productivity of children who develop neuromotor disabilities. Tsaban et al. published a systematic review, from which it was possible to estimate an indirect effect of PCV in adults that could vary according to absorption rates and time of implementation of PCV (approximately 22 % (range: 0% - 70%) [Citation28]. They found that the population that most benefits from the herd effect are adults > 65 years. It means that a significant proportion of the people at risk of developing pneumococcal diseases, especially CAP, could be protected and reduce health care costs associated with this disease.
Similarly, considering the social perspective to make this economic evaluation requires us to calculate the indirect healthcare costs for children who develop neuromotor disabilities due to pneumococcal meningitis. These indirect costs are mainly the costs of caregiver-child, which may be his mother or a third party (nurse). If the caregiver is the mother, she must stop working to care for her child and therefore withdraws from the labor market; if the caregiver is a third party, the family must bear the costs of this care. Likewise, economic evaluations of pneumococcal vaccines should include the fees of children who lost productivity with neuromotor disabilities. The main raison d’être of the health systems is to avoid population poverty by reducing out-of-pocket expenditure on health and offering good health to be economically productive. But sometimes are complications, such as neuromotor disability. In that case, the child and his family will have a greater economic vulnerability because the child and his mother or a family member cannot be economically productive for his family and society.
The model has two main features: a dynamic cohort and an economic evaluation of the herd effect in adults. A dynamic cohort is closer to reality in assessing mass vaccination technologies because it allows identifying the economic outcomes of low prevalence and high-cost complications, which are generally minimized in fixed cohorts. After all, they do not allow identifying population risk change over time.
Studies that have monitored vaccination effects in real life have identified the PCV13 impact on herd immunity. In addition, those researchers have shown the extension of benefits of these vaccines beyond the directly vaccinated population, acting as a positive externality because they lower healthcare costs and improve quality of life [Citation58]. The importance of the herd effect is that pneumococcal diseases are common in older adults, and their lethality is high, so it is possible to avoid mortality from pneumococcal infections indirectly. In addition, CAP treatment and invasive pneumonia are expensive in older adults and complicate underlying conditions such as asthma or emphysema. Therefore, the herd effect has been frequently quantified as part of economic assessments of vaccines [Citation59] because it generates clinical and financial outcomes that benefit both the vaccinated population and a proportion of the unvaccinated population.
All these features allow us to identify most health outcomes in vaccinated and unvaccinated people and estimate in a better way the comprehensive health resources used. The vaccination program’s costs are not just vaccine acquisition costs but the potential additional disease burden over time and associated costs because of its implementation. A limitation of this study is that it did not include all indirect effects, such as those generated by antimicrobial resistance. Several studies have demonstrated that PCV13 avoids S. pneumoniae transmission, thereby reducing infections, so PCV13 makes it possible to minimize antibiotics prescription [Citation60–64]. Therefore, there is a broader benefit of vaccination with PCV13, which would be worth incorporating into future studies. Another limitation is related to low precision and lack of statistical power of PCV10 efficacy in Pneumococcal AMO due to the number of isolated cases (56 episodes) according to clinical trials [Citation19]. However, that is the currently available evidence, but separating agents that cause OMA is not shared.
Although some authors have evaluated the efficacy of pneumococcal vaccines specifically for each serotype, our model has a pooled effectiveness estimate. However, it would not make sense to assess the efficacy for each serotype because the three additional serotypes that PCV13 has represented 60% of all pneumococcal diseases in Colombia. So we adjusted the clinical efficacy reported in the clinical trials of both vaccines to the distribution of pneumococcal serotypes in Colombia. Economic evaluations of vaccines should include the distribution of serotypes to adjust efficacy reported in clinical trials to real-world data. In this economic evaluation, we observed that the effectiveness of both vaccines is similar for the serotypes they contain.
Still, given that PCV10 serotypes only represent 5 % of those circulating in Colombia compared to PCV13, which has 65 % of those circulating in the country, it is necessary to make this adjustment in this economic evaluation. In that way, we can establish that this difference in the distribution of serotypes generates significant differences in the clinical and financial results of both vaccines, even though they have similar efficacies (the efficacy is only for the serotypes contained in each of the vaccines).
One problem observed in the long term with the epidemiological behavior of the pneumococcal serotypes is that the arrival of a new vaccine in a country generates a replacement of the circulating serotypes. Therefore, it becomes a challenge for economic evaluations because these serotypes will change in the medium or long term. For this reason, the model has a short time horizon until 2025. After all, when a new pneumococcal vaccine arrives, there is a high level of uncertainty in the long term related to the change in pneumococcal serotypes in that population. So to avoid this uncertainty in the behavior of the pneumococcal serotypes, we made a short time horizon. Based on their observation, ideally, every country would carry out an economic evaluation of pneumococcal vaccines every three or four years, adjusted to the distribution of serotypes existing at the time of the assessment and with short time horizons.
The cost-effectiveness analysis in Colombia has become a key component of decisions on whether to adopt public health policies; the previous cost-effectiveness analyses made in Colombia related to PCV have served as input to decision-makers. One showed the cost-effectiveness of PCV10; ICER per LYG was $ 1,837 to PCV10 and $ 9,514 with PCV13 [Citation14]. However, up-date analysis that incorporates circulating serotypes in Colombia from 2011 to 2014 found PCV13 has a good value for money and avoids the additional burden of pneumococcal disease, saving additional treatment costs when compared with keep-PCV10 in Colombia; the ICER per LGY was $ 2,319 for PCV10, and $ 1,771 for switch-to PCV13 [Citation18]. These results showed ICER of PCV13 compared with PCV10 has changed, and in this analysis, PCV13 is a cost-saving strategy. Although it was clear that these analyses are different because they used their assumptions and estimation parameters, they are evidencing the changes in ICERs, where PCV13 has become the best option.
5. Conclusions
PCV13 is dominant versus PCV10 as a strategy to vaccinate newborns in Colombia because PCV13 avoided more IPD, consolidated CAP, pneumococcal AOM, complications, sequelae, and deaths than PCV10. This result is because serotypes included in PCV13 represent 64,4 % of serotypes isolated in Colombia in children under five years between 2017–2019, while those included in PCV10 represent 4,3 % isolated in the same period.
Declaration of interest
Jaime E. Ordóñez receives payment for consultancies and lectures by AbbVie (Argentina, Colombia, and Costa Rica), AstraZeneca (Argentina, Brazil, Colombia, Costa Rica, Chile, Ecuador, Guatemala, Panama, Peru, the Dominican Republic, and USA), Bayer, Biogen Idec, Bristol Myers-Squibb, Colciencias, Colombian Society of Rheumatology, Corum, Glaukos, Grupo Biotoscana, ISPOR-Colombia, Merck, Metrosalud, Ministry of Health and Social Protection of Colombia, Ministry of National Education of Colombia, Novartis, Novo Nordisk (Colombia, Panama, Paraguay, Uruguay, and Russia), Pfizer (Colombia, Costa Rica, Ecuador, El Salvador, Panama, the Dominican Republic, and USA), Roche (Costa Rica, Panama, Guatemala), Secretariat of Health of Medellín, Secretariat of Social Welfare of Medellín, Stendhal, CES University, University of Antioquia, University of Medellín, University of the Valley of Guatemala. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium for their review work. A reviewer on this manuscript has disclosed Advisory Board membership for PCV, GSK, Pfizer and MSD. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
J Ordóñez and A Ordóñez have substantially contributed to the conception and design of the review paper and interpretation of the relevant literature, and we have been involved in writing the review paper or revising it for intellectual content.
J Ordóñez structured the economic model, searched for epidemiological data, the costs and effectiveness of the vaccines, analyzed the information, and wrote the paper. Angelica Ordóñez programmed the model and collaborated with the writing and revising the article.
J Ordóñez and A Ordóñez have agreed to submit this paper to Expert Review of Vaccines.
J Ordóñez and A Ordóñez have reviewed and agreed on all versions of the paper before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage.
J Ordóñez and A Ordóñez agree to take responsibility and be accountable for the contents of the paper and to share the responsibility to resolve any questions raised about the accuracy or integrity of the published work.
Additional information
Funding
References
- Centers for Disease Control and Prevention. Pneumococcal Disease [Internet]. 2014; cited May 4, 2020. Available from: https://wwwnc.cdc.gov/travel/diseases/pneumococcal-disease-streptococcus-pneumoniae
- Tan TQ. Pediatric invasive pneumococcal disease in the United States in the era of pneumococcal conjugate vaccines. Clin Microbiol Rev. 2012 Jul;25(3):409–419.
- Lepoutre A, Varon E, Georges S, et al. impact of infant pneumococcal vaccination on invasive pneumococcal diseases in France, 2001-2006. Euro Surveill. 2008 Aug 28;13(35):18962.
- Teixeira DC, Diniz LMO, Guimarães NS, et al. Risk factors associated with the outcomes of pediatric bacterial meningitis: a systematic review. J Pediatr (Rio J). 2020 March-April;96(2):159–167.
- Choi C. Bacterial meningitis in aging adults. Clin Infect Dis. 2001 Oct 15;33(8):1380–1385.
- Atkinson W, Hamborsky J, McIntyre L, et al., eds. Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. 9h ed. Washington DC: Public Health Foundation; 2006.
- Karppinen S, Teräsjärvi J, Auranen K, et al. Acquisition and transmission of streptococcus pneumoniae are facilitated during rhinovirus infection in families with children. Am J Respir Crit Care Med. 2017 Nov 1;196(9):1172–1180.
- Henriques-Normark B, Tuomanen EI. The pneumococcus: epidemiology, microbiology, and pathogenesis. Cold Spring Harb Perspect Med. 2013 Jul 1;3(7):a010215.
- Bluestone CD. Clinical course, complications and sequelae of acute otitis media. Pediatr Infect Dis J. 2000 May;19(5 Suppl):S37–46.
- World Health Organization. Global Vaccine Action Plan 2011-2020. Geneva. WHO: 2013 Available from https://www.afro.who.int/sites/default/files/2017-06/9789241504980_eng.pdf
- Destefano F, Pfeifer D, Nohynek H. Safety profile of pneumococcal conjugate vaccines: systematic review of pre- and post-licensure data. Bull World Health Organ. 2008 May;86(5):373–380.
- Instituto de Vacunas Albert B. Sabin, Organización Panamericana de la Salud, Plan para el desarrollo acelerado y la introducción de vacunas neumocócicas de GAVI en John Hopkins, Centros para el Control y la Prevención de Enfermedades. La carga de morbilidad de la enfermedad neumocócica y la rentabilidad de una vacuna antineumocócica en América Latina y el Caribe. Revisión de las pruebas y análisis económico preliminar. Sabin Vaccine Institute: Washington D.C.; 2007.
- María MS, García F, Uribe M. Evaluación económica de la inclusión de la vacuna antineumocócica en el Plan Ampliado de Inmunización. 2008. Available from https://www.repository.fedesarrollo.org.co/bitstream/handle/11445/892/CDF_No_26_Abril_2008.pdf?sequence=1&isAllowed=y
- Castañeda-Orjuela C, Alvis-Guzmán N, Velandia-González M, et al. Cost-effectiveness of pneumococcal conjugate vaccines of 7, 10, and 13 valences in Colombian children. Vaccine. 2012 Mar 2;30(11):1936–1943.
- Instituto Nacional de Salud. Vigilancia por Laboratorio de aislamientos invasores de Streptococcus pneumoniae Colombia 2006-2018. SIREVA II [Internet]. Available from https://www.ins.gov.co/buscador-eventos/Informacin%20de%20laboratorio/Vigilancia%20por%20laboratorio%20S.%20pneumoniae%202006-2018.pdf
- Wasserman M, Sings HL, Jones D, et al. Review of vaccine effectiveness assumptions used in economic evaluations of infant pneumococcal conjugate vaccine. Expert Rev Vaccines. 2018 Jan;17(1):71–78.
- Carrasquilla G, Porras A, Martinez S, et al. Time -trend analysis of pneumococcal disease morbidity in children < 5 years of age after pneumococcal conjugate vaccine introduction in Colombia: An ecological study. 10th Int, Symp Pneumococci Pneumoccal Dis (ISPPD-10), Glasgow Scotland. 2016.
- Castañeda-Orjuela C, De la Hoz-Restrepo F. How cost effective is switching universal vaccination from PCV10 to PCV13? A case study from a developing country. Vaccine. 2018 Sep 11; 36(38):5766–5773.
- COMPAS GroupTregnaghi MW, Sáez-Llorens X, López P, et al. Efficacy of pneumococcal nontypable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in young Latin American children: A double-blind randomized controlled trial. PLoS Med. 2014 Jun 3; 11(6):e1001657.
- World Health Organization. WHO Position Paper on Pneumococcal conjugate vaccines in infants and children under 5 years of age. February 2019 [Internet]. Available from https://www.who.int/immunization/policy/position_papers/who_pp_pcv_2019_references.pdf
- Clarke E, Bashorun AO, Okoye M, et al. Safety and immunogenicity of a novel 10-valent pneumococcal conjugate vaccine candidate in adults, toddlers, and infants in The Gambia-Results of a phase 1/2 randomized, double-blinded, controlled trial. Vaccine. 2020 Jan 10;38(2):399–410.
- Ministerio de Salud y Protección Social. Nota técnica unidad de pago por capitación [Internet]. Available from: https://www.minsalud.gov.co/salud/POS/Paginas/unidad-de-pago-por-capitacion-upc.aspx cited October 2022. Consulted in Nov 2022.
- Instituto de Evaluación Tecnológica en Salud. Manual para la elaboración de evaluaciones económicas en salud. Bogotá D.C: IETS; 2014.
- Centers for Disease Control and Prevention (CDC). Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease–United States, 1998-2003. MMWR Morb Mortal Wkly Rep. 2005 Sep 16;54(36):893–897.
- Atkins D, Briss PA, Eccles M, et al. GRADE Working Group. Systems for grading the quality of evidence and the strength of recommendations II: pilot study of a new system. BMC Health Serv Res. 2005 Mar 23;5(1):25.
- Organización Panamericana de la Salud. Indicadores básicos 2019. Tendencias de la salud en las Américas: Washington D.C. Available from: https://iris.paho.org/bitstream/handle/10665.2/51543/9789275321287_spa.pdf?sequence=7&isAllowed=y
- Poehling KA, Talbot TR, Griffin MR, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA. 2006 Apr 12;295(14):1668–1674.
- Tsaban G, Indirect B-S-S. (herd) protection, following pneumococcal conjugated vaccines introduction: A systematic review of the literature. Vaccine. 2017 May 19; 35(22):2882–2891.
- Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19(3):187–195.
- Hansen J, Black S, Shinefield H, et al. effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using World Health Organization standardized interpretation of chest radiographs. Pediatr Infect Dis J. 2006 Sep;25(9):779–781.
- Departamento Administrativo Nacional de Estadística – DANE. Proyecciones de población. Serie nacional de población por área, sexo y edad para el periodo 2018 – 2070 [Internet]. Available from https://www.dane.gov.co/index.php/estadisticas-por-tema/demografia-y-poblacion/proyecciones-de-poblacion cited October 2020. Consulted in Nov 2022.
- National Collaborating Centre for Women’s and Children’s Health (UK). Bacterial Meningitis and Meningococcal Septicaemia: Management of Bacterial Meningitis and Meningococcal Septicaemia in Children and Young People Younger than 16 Years in Primary and Secondary Care. London: RCOG Press; 2010.
- Johnson AP, Waight P, Andrews N, et al. Morbidity and mortality of pneumococcal meningitis and serotypes of causative strains prior to introduction of the 7-valent conjugant pneumococcal vaccine in England. J Infect. 2007 Nov;55(5):394–399.
- Ferreira S, Sant’anna CC, March Mde F, et al. Lethality by pneumonia and factors associated to death. J Pediatr (Rio J). 2014 January-February;90(1):92–97.
- Ministerio de Salud y Protección Social. Registro Individual de Prestaciones de Servicios de Salud - RIPS. [Internet]. Available from: https://www.minsalud.gov.co/proteccionsocial/Paginas/rips.aspx cited March 2021, consulted in Nov 2022.
- Banco de la República de Colombia. Tasa Representativa del Mercado (TRM - Peso por dólar) [Internet]. Available from: https://www.banrep.gov.co/es/estadisticas/trm cited November 2022.
- Ministerio de Salud y Protección Social de Colombia. Sistema de información de precios de medicamentos -SISMED [Internet]. Available from: https://web.sispro.gov.co/WebPublico/Consultas/ConsultarCNPMCadenaComercializacionCircu2yPA_028_2_2.aspx cited November 2022.
- Peñaranda A, Mendieta JC, Perdomo JA, et al. Beneficios económicos del implante coclear para la hipoacusia sensorineural profunda. Rev Panam Salud Publica. 2012;31(4):325–331.
- Departamento Administrativo Nacional de Estadística (DANE). Boletín del Sistema Nacional de Información de Demanda Laboral - SINIDEL. [Internet]. Available from: https://www.dane.gov.co/index.php/estadisticas-por-tema/educacion/boletin-sinidel cited November 2022.
- Departamento Administrativo Nacional de Estadística (DANE). Gran encuesta integrada de hogares (GEIH) Mercado laboral. [Internet]. Available from: https://www.dane.gov.co/index.php/estadisticas-por-tema/mercado-laboral/empleo-y-desempleo/geih-historicos. cited November 2022.
- Pan American Health Organization. Immunization in the Americas. 2020 Summary [Internet]. Available from: https://www.paho.org/en/documents/immunization-summary-2020-summary cited November 2022.
- Gray BM, Dillon HC Jr. Natural history of pneumococcal infections. Pediatr Infect Dis J. 1989 Jan;8(1 Suppl):S23–5.
- Waight PA, Andrews NJ, Ladhani SN, et al. effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015 May;15(5):535–543.
- Moore MR, Link-Gelles R, Schaffner W, et al. effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015 Mar;15(3):301–309.
- Dagan R, Patterson S, Juergens C, et al. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin Infect Dis. 2013 Oct;57(7):952–962.
- Cohen R, Levy C, Bingen E, et al. impact of 13-valent pneumococcal conjugate vaccine on pneumococcal nasopharyngeal carriage in children with acute otitis media. Pediatr Infect Dis J. 2012 Mar;31(3):297–301.
- Finnish institute for health and welfare. Incidence of invasive pneumococcal disease in Finland [Internet]. Available from https://thl.fi/en/web/thlfi-en/research-and-development/research-and-projects/monitoring-the-population-effectiveness-of-pneumococcal-conjugate-vaccination-in-the-finnish-national-vaccination-programme/incidence-of-invasive-pneumococcal-disease-in-finland Updated in May, 2020. cited Nov 2022.
- van den Bergh MR, Spijkerman J, Swinnen KM, et al. Effects of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D-conjugate vaccine on nasopharyngeal bacterial colonization in young children: a randomized controlled trial. Clin Infect Dis. 2013 Feb;56(3):e30–9.
- Knol MJ, De Melker H, Sanders L, et al. Incidence of invasive pneumococcal disease in the Netherlands up to four years after introduction of 10-valent pneumococcal conjugate vaccination. Poster Present 10th Int Symp Pneumococci Pneumococcal Dis (ISPPD); June 29, 2016; Glasgow, United Kingdom.
- Lexau CA, Lynfield R, Danila R, et al. Active Bacterial Core Surveillance Team. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005 Oct 26;294(16):2043–2051.
- The World Bank. Data. Life expectancy at birth, total (years). Available from https://data.worldbank.org/indicator/SP.DYN.LE00.IN cited November 2022.
- Van WC, Huijts S, Bolkenbaas M, et al. 13-valent pneumococcal conjugate vaccine efficacy is declining with old age: results from an exploratory analysis of the CAPiTA trial. Open Forum Infect Dis. 2014;1(1):S324–5. Available from https://academic.oup.com/ofid/article/1/suppl_1/S324/2339548
- Camacho Moreno G, Imbachi LF, Leal AL, et al. Emergence of Streptococcus pneumoniae serotype 19A (Spn19A) in the pediatric population in Bogotá, Colombia as the main cause of invasive pneumococcal disease after the introduction of PCV10. Hum Vaccin Immunother. 2020 Sep 1;16(9):2300–2306.
- Mott MP, Caierão J, Cunha GR, et al. Emergence of serotype 19A Streptococcus pneumoniae after PCV10 associated with a ST320 in adult population, in Porto Alegre, Brazil. Epidemiol Infect. 2019;Jan 147e93.
- Brandileone MC, Almeida SCG, Minamisava R, et al. distribution of invasive Streptococcus pneumoniae serotypes before and 5 years after the introduction of 10-valent pneumococcal conjugate vaccine in Brazil. Vaccine. 2018 May 3;36(19):2559–2566.
- Potin M, Fica A, Wilhem J, et al. Opinión del Comité Consultivo de Inmunizaciones Sociedad Chilena de Infectología: Vacuna neumocóccica conjugada en niños y la emergencia de serotipo 19A. Rev Chil infectología. 2016 Jun;33(3):304–306.
- World Health Organization. WHO Guide on Standardization of Economic Evaluations of Immunization Programmes. No WHO/IVB/19.10. 2nd ed. Geneva; 2019. Available from https://apps.who.int/iris/bitstream/handle/10665/329389/WHO-IVB-19.10-eng.pdf cited Nov 2022.
- Loo JD, Conklin L, Fleming-Dutra KE, et al. Systematic review of the indirect effect of pneumococcal conjugate vaccine dosing schedules on pneumococcal disease and colonization. Pediatr Infect Dis J. 2014 Jan;33(Suppl 2):Suppl2 Optimum Dosing of Pneumococcal Conjugate Vaccine For Infants 0 A Landscape Analysis of Evidence Supportin g Different Schedules. S161–71.
- Nymark LS, Sharma T, Miller A, et al. inclusion of the value of herd immunity in economic evaluations of vaccines. A systematic review of methods used. Vaccine. 2017 Dec 14;35(49PtB):6828–6841.
- Angoulvant F, Cohen R, Doit C, et al. Trends in antibiotic resistance of Streptococcus pneumoniae and Haemophilus influenzae isolated from nasopharyngeal flora in children with acute otitis media in France before and after 13 valent pneumococcal conjugate vaccine introduction. BMC Infect Dis. 2015 Jun 21;15:236.
- Imöhl M, Reinert RR. van der Linden M. Antibiotic susceptibility rates of invasive pneumococci before and after the introduction of pneumococcal conjugate vaccination in Germany. Int J Med Microbiol. 2015 Oct;305(7):776–783.
- Ben-Shimol S, Givon-Lavi N, Leibovitz E, et al. Near-elimination of otitis media caused by 13-valent pneumococcal conjugate vaccine (PCV) serotypes in southern Israel shortly after sequential introduction of 7-valent/13-valent PCV. Clin Infect Dis. 2014 Dec 15;59(12):1724–1732.
- Tomczyk S, Lynfield R, Schaffner W, et al. Prevention of Antibiotic-Nonsusceptible Invasive Pneumococcal Disease With the 13-Valent Pneumococcal Conjugate Vaccine. Clin Infect Dis. 2016 May 1;62(9):1119–1125.
- Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009 Aug 19;302(7):758–766.