ABSTRACT
Introduction
Large-scale vaccination campaigns can benefit from using digital health tools, particularly in low- and middle-income countries (LMICs). Selecting the best tool to fit into a pre-existing digital landscape can be challenging.
Areas covered
We conducted a narrative review in PubMed and the grey literature for data available within 5 years to provide an overview of digital health tools used in large-scale vaccination campaigns for outbreak response in LMICs. We discuss tools used along the typical steps of a vaccination process. Digital tool functionalities and technical specifications, open-source options, data privacy and security concerns, and lessons learned from the use of these tools are discussed.
Expert opinion
The landscape of digital health tools for large-scale vaccination processes in LMICs is growing. For efficient implementation, countries should prioritize the appropriate tool(s) depending on their needs and available resources, develop a robust framework around data privacy and security, and select sustainable features. Improving internet connectivity and digital literacy in LMICs will facilitate adoption. This review may aid LMICs still needing to prepare large-scale vaccination campaigns in the selection of supporting digital health tools. Further research on impact and cost-effectiveness is needed.
1. Introduction
Global research and innovation efforts are playing a central role in the international response to pandemics and epidemics, such as the coronavirus disease-19 (COVID-19) pandemic and Ebola outbreaks [Citation1,Citation2]. Through cooperation between governments, patient groups, the pharmaceutical industry, and research communities, vaccines and therapeutics to fight emergent outbreaks are being developed in record time. These collaborations have allowed for successful large-scale vaccination campaigns to be implemented in response to disease outbreaks in many countries upon the availability of vaccines [Citation3,Citation4]. The speed of launch and scaling of these campaigns present challenges in all countries, but this can be particularly challenging in low- and middle-income countries (LMICs) which may have less financial, technical, and trained human resources for quick operationalization [Citation5–7]. To implement effective and fast large-scale vaccination campaigns, countries need to develop national vaccination plans, establish vaccine safety surveillance, set up vaccine distribution infrastructure (including cold chain capabilities), expand and train the healthcare personnel involved in administering the vaccines, strengthen monitoring and evaluation systems to track vaccine roll-out, and build public confidence in vaccines [Citation5,Citation6].
Digital health technology has become increasingly important in the response to infectious disease outbreaks [Citation8–10]. Innovative digital technologies were used in the response to the 2014–2016 Ebola outbreak across West Africa [Citation11,Citation12] and in the response to the 2015–2016 Zika outbreak in Latin America and the Caribbean [Citation13]. The COVID-19 pandemic has further spurred the growth of digital innovations at an unprecedented pace across the world [Citation14]. Countries have applied digital health technologies for pandemic response planning, surveillance, testing, contact tracing, quarantine, and health care [Citation8,Citation11,Citation12,Citation14,Citation15].
Large-scale vaccination campaigns provide opportunities for LMICs to develop and implement digital health tools to track vaccine delivery, create individual vaccination records, record vaccination status, provide digital vaccine certificates, and report adverse reactions [Citation5]. Vaccination campaigns can further leverage mobile phone technology in large-scale health interventions due to the surge in mobile phone use and improved telecom coverage in LMICs [Citation16]. In fact, it is reported that the access to mobile phones in LIMCs is often better than the access to basic services such as electricity, safe drinking water, or sanitation [Citation17]. Further supporting the implementation of digital health tools, the WHO has published their Global Strategy on Digital Health 2020–2025 [Citation18] encouraging the use of digital technologies based on a common framework to accelerate global attainment of health. It highlights a need for ensuring these tools are ethical, secure, equitable, and sustainable with development being conducted with data principles in mind (transparency, accessibility, scalability, replicability, interoperability, privacy, security, and confidentiality).
In this narrative review, we provide an overview of digital health tools that LMICs have used to support large-scale vaccination campaigns in response to outbreaks. We focused on those tools used along the typical steps of a large-scale vaccination process. The global spread of the COVID-19 pandemic and the resulting preparation for large-scale vaccination campaigns allowed us to do a broader assessment of digital health tool use in LMICs and to compare tools implemented in multiple countries. We review the functionalities and technical specifications of the tools identified along the vaccination process. Hereby, we aim to provide a resource for LMICs that consider introducing the use of digital tools health tools in future large-scale vaccination campaigns for outbreak response based on the experience in similar settings. Additionally, we consider topics such as data security and safety, tool availability, costs, challenges specific to LMICs, and options in ensuring sustainability of tools.
2. Methodology
To identify digital health tools used in large-scale vaccination campaigns for outbreak response in LMICs (low- and middle-income countries as defined by the World Bank [Citation19]), we conducted a narrative review of the published literature, websites, and other online resources in October–November 2022 for data available within the last five years. Sources included but are not limited to PubMed, BioMed Central, ScienceDirect, JSTOR, IOS Press, organization-specific releases (e.g. Gavi, The Rockefeller Foundation, etc.), government websites, and news and tech journals (e.g. TechCabal, etc.). Within these data sources, article searches were conducted in English using combinations of various keywords, related to (1) resource-limited settings, (2) digital platforms, (3) use in vaccination campaigns, (4) and tool capabilities aligning with our scope (examples include but are not limited to: “immunization digital tools,” “digital health and vaccination campaigns in LMICs,” “COVID-19 vaccination digital certificate Africa,” “scheduling COVID vaccine platform LATAM,” etc.). Some digital platforms were found through sponsors/partners or projects affiliated with previously found tools. To maintain our focus on large-scale vaccination campaigns for outbreak response, we excluded digital tools employed in routine childhood vaccination programs or preventive vaccination campaigns. After identifying the digital tools in this way, we performed additional searches in PubMed in January 2023 using the name of each tool or the name of each tool combined with “COVID-19” or with “vaccination,” with the aim of finding more peer-reviewed literature related to the use of these tools in large-scale vaccination campaigns in response to outbreaks. From all the available data, we extracted various functionalities and technical specifications of the tools.
3. Vaccination process
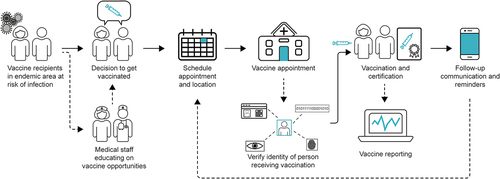
There are well-defined steps for a typical vaccination process at a healthcare center facility delivering a large-scale vaccination campaign in response to outbreaks, which are mostly consistent across countries [Citation20]. These common steps include appointment scheduling, personal identification, vaccine verification (manufacturer, expiry date, and lot number), vaccine administration, issuance of a vaccination certificate, vaccine reporting, follow-up communication and appointment reminders for multi-dose vaccine regimens or booster doses (). We will focus on the use of digital health tools along these steps. We recognize that there are additional functions possible within a vaccination campaign such as tracking adverse events, symptom tracking and risk-assessments, managing non-compliance with additional or booster doses, and determining eligibility for vaccination. For the purpose of this review, we determined that these and other additional steps were out of scope.
4. Implementation models and approaches
For LMICs looking to integrate and implement new digital health tools into their existing systems, it is important take into account the appropriateness of the tool in addressing their need(s), and the associated costs [Citation21]. Factors to consider in the evaluation process include, among others, functionalities, ease of use, integration with existing systems, community access to infrastructure and ability to scale. Cost components include the cost of software, hardware, hosting and support. Open-source tools are free of license cost; however, this does not equate to no-cost solutions [Citation22]. Open-source tools still require investments in software customization, personnel training, and resources, including data management and hosting.
Open-source and proprietary options might be part of the evaluation to select a fit-for-purpose tool. Digital Public Good (DPG) labeled tools may be good options to consider. The DPG standard, maintained by the Digital Public Goods Alliance [Citation23], assesses tools across multiple factors, including being open-source. Investments in DPGs are promoted by global development agencies and many governments in LMICs as DPGs are designed to contribute to the achievement of the Sustainable Development Goals by creating inclusive and equitable digital health solutions [Citation24].
5. Overview of the digital health tools supporting different steps of the vaccination workflow
Among the digital health tools identified along the vaccination workflow in LMICs, some are very specific and aim to optimize a single step in the vaccination process, while others offer a broader solution and address multiple steps in the vaccination process. Some are utilized in a single country while others are broadly used ().
Figure 2. Low- and middle-income countries using the capabilities of digital health tools along the vaccination process.
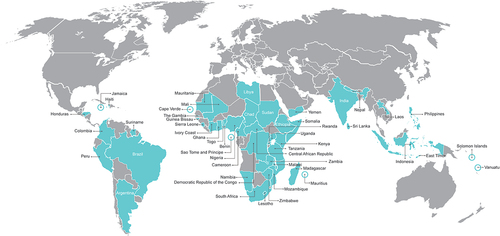
Table 1. Functionalities of the digital health tools used across the large-scale vaccination process in low- and middle-income countries.
Out of the 18 tools we identified, 9 were developed specifically for COVID-19, and others have been used in previous large-scale immunization campaigns in emergency settings (, Supplemental Table S1). For example, the Oracle Health Management System was used in a yellow fever vaccination campaign in Ghana [Citation26] and Vxnaid was used in a large-scale, multi-dose, Ebola vaccination campaign in Rwanda [Citation27]. The functionalities of the digital health tools that we identified are summarized in and their technical specifications in . Additional details about tool functionalities can be found in Supplemental Table S1.
Table 2. Technicalities of the digital health tools used across the large-scale vaccination process in low- and middle-income countries.
5.1. Appointment scheduling
Vaccine scheduling tools offer virtual avenues to conveniently register for and schedule a vaccination appointment. This enables the healthcare staff to efficiently manage appointments and assess if vaccine supply can meet the needs for each site. It is one of the most common features in the identified digital health tools, offering numerous ways that this step can be realized. Tools can allow individuals to schedule their own appointment, allow healthcare staff to schedule appointments, or can depend on a pre-issued appointment in the vaccination system (at the direction of the health authority). Most tools need internet connectivity for scheduling an appointment online. mVacciNation [Citation44] offers self-registration for an appointment via unstructured supplementary service data (USSD), short messaging service (SMS), WhatsApp, or web application. Sometimes additional features are incorporated together with appointment scheduling. For example, India’s Co-WIN system allows users to select convenient vaccine clinic locations and vaccine type or brand [Citation36]. Trusted Vaccines incorporates automated vaccine allotment and appointment scheduling on an organized and priority basis as determined by the health authorities [Citation50]. Some tools have been integrated into government vaccine initiatives. For example, Mi Argentina/Mi Salud has been rolled out by the Argentinian Ministry of Health [Citation41], South Africa’s Electronic Vaccination Data System (EVDS) by the Department of Health [Citation48], using mVacciNation’s tools as a backbone, and Co-WIN by the Ministry of Health and Family Welfare in India [Citation36]. The possibility to schedule follow-up appointments (for a multi-dose vaccine or booster dose) can also be offered; this can either be done automatically (e.g. mVacciNation [Citation44], District Health Information Software 2 [DHIS2] COVID-19 [Citation34]) or in-person (by the vaccinee or healthcare staff). Via M-Vaccin, borne from a partnership between the telecommunications service provider Orange, and the Vaccine Alliance, Gavi, healthcare workers can schedule follow-up appointments with the specific goal of increasing vaccination rates (e.g. a full COVID-19 series) of mothers and their children [Citation42,Citation43]. Many tools that offer appointment scheduling also generate appointment confirmations or reminders as discussed in section 5.6, and some may track and follow-up defaulters who have missed a vaccination appointment (e.g. DHIS2 COVID-19 [Citation34,Citation35], mVacciNation [Citation44], Vxnaid [Citation27]).
5.2. Personal identification
Many people worldwide, especially those living in LMICs, lack an official proof of identity [Citation63]. For vaccine delivery, it is critical to reliably verify the identity of the individuals to avoid inaccurate vaccine tracking and delivery and to reduce duplicate medical records and record falsification [Citation64,Citation65]. Digital health tools help provide reliable identification in a safe way in a large-scale vaccination campaign in emergency settings. Biometrics are commonly used for identification with various technologies developed and implemented to identify individuals using fingerprints, iris, face, and voice recognition [Citation66]. Based upon the learnings from recent Ebola outbreaks, it is important that biometric tools can be contactless, like iris scanning or facial recognition [Citation27]. In the case of highly transmissible infectious pathogens, fingerprinting can represent a risk of transmission and may require additional hygiene measures to reduce this risk [Citation67]. Numerous biometric-based identification methods exist and could be integrated into a tool for a vaccine campaign.
Several tools used for vaccination campaigns (e.g. Vxnaid [Citation27], Simprints [Citation49], Open-source Smart Register Platform [OpenSRP] FHIR Core [Citation45,Citation46], Co-WIN [Citation36]) employ biometric identification based on iris scanning, fingerprint scanning, or facial recognition to identify unique individuals. VaxiGlobal supports biometrics via Simprints [Citation51], highlighting the benefit of interoperability between the identified tools. If biometric identification is not possible, alternative identification modalities can include the manual entry of a unique identifier, QR/barcode scanning of a person’s campaign card or voucher supported by some tools (e.g. DHIS2 COVID-19 [Citation34], Vxnaid [Citation27,Citation53], OpenSRP FHIRCore [Citation45,Citation46], mVacciNation [Citation44]). DHIS2 COVID-19 sends unique SMS codes to registered mobile phone numbers to verify persons for vaccination [Citation34]. The Oracle Health Management System [Citation26], developed by Oracle in collaboration with the Tony Blair Institute, AfriDoctor [Citation28], Simprints [Citation49], and Vaxiglobal [Citation51] can link identified vaccinees with their health records. The Oracle Health Management System creates an electronic health record in a cloud database for every person vaccinated, highlighting the usefulness of a cloud-based tool [Citation26]. Digital health records are beyond the scope of the present review, although they provide an opportunity for sustainable use of health information.
5.3. Vaccination verification
Documentation of vaccine product identification and vaccine lot number data is routinely tracked. Tracking the manufacturer and lot of vaccines provided to individuals is important, as various vaccines can be tested or deployed at the same time, and many vaccines require a multi-dosing regimen. During the COVID-19 pandemic, vaccines from different manufacturers were rolled out over a relatively short period of time, leading countries to recommend mixing different vaccine products for priming and boosting [Citation68]. Accurate tracking of heterologous vaccines, like an Ebola vaccine [Citation69], is important to ensure that effective inoculation against the target virus is maintained. Efficiently recording such information can be of added value to subsequently build an electronic vaccination database, serving as a starting point for generating digital certificates for proof of vaccination, and to potentially link any reported adverse reactions to a particular lot of vaccine. Few of the digital health tools we identified are able to address vaccine verification. For example, Vxnaid can scan a vaccine vial barcode to document the manufacturer and lot number with a built-in warning system if a healthcare worker accidentally selects an unscheduled vaccine for use [Citation53], COVID-19 DHIS2 has a barcode/QR code scanning module [Citation34], and Co-WIN can track doses given [Citation36]. This functionality can replace manual entry to improve data accuracy and completeness and to support safety of vaccine recipients if there are concerns with lot numbers administered, and support vaccination reporting as discussed in section 5.5.
5.4. Vaccine certification
A vaccine certificate is an immunization record that can be used for continuity of care as part of an individual’s medical record or to provide proof of vaccination for purposes not related to health care (e.g. travel) [Citation70]. Typically, vaccine records and certifications that stay with the individual have been paper-based, with the International Certificate of Vaccination or Prophylaxis (ICVP), or the “yellow card” routinely being used to track travel-related vaccinations [Citation71,Citation72]. However, with the requirement to provide proof of COVID-19 vaccination status for international travel during the height of the pandemic, digital vaccine certification tools were rapidly developed to provide quickly scannable certifications that are challenging to fake [Citation70]. Digital tools can facilitate the delivery of a vaccine certificate by providing digital proof of vaccination or generate a paper card with a bar code or QR code to link it to the digital record. Vaccine certification tools offer a wide range of features, including storage of vaccine records on- and offline; generating, signing, and verifying vaccine certificates from a health authority; and linking certificates to individuals via secure, confidential methods (biometrically, unique ID codes, QR codes, etc.). For example, DIVOC has reportedly issued 2 billion COVID-19 vaccine certificates globally and uses a QR code system that is uniquely designed to reduce falsification; DIVOC can be integrated with other platforms (e.g. DHIS2 COVID-19, Co-Win) to generate vaccine certificates [Citation40]. Some tools (e.g. mVacciNation [Citation44], Trusted Vaccines [Citation50]) highlight providing proof of vaccination at point-of-entry (country borders, airports, etc.), with plans to expand as the tools grow and coverage broadens.
5.5. Vaccination reporting
Monitoring vaccination coverage and evaluation of a campaign’s performance at a national level can help track vaccination campaign progress and improve efficiency of the campaign. To this end, healthcare workers need to report the vaccination status of the persons vaccinated or the number of vaccinated persons on a given day in a national database or registry. The recording of such information is frequently performed on paper and then digitized post-factum. Digital health tools can ease the work of healthcare workers or data entry clerks by making vaccination status and numbers of individuals vaccinated directly available via dashboard reports. This type of digital solution can prevent double entries or errors and can allow staff to focus on critical healthcare needs. Tools as DHIS2 COVID-19 [Citation34], OpenSRP FHIR Core [Citation45,Citation46], and Trusted Vaccines [Citation50] can collect and track individual immunization data in national electronic immunization registries. Additionally, some tools provide data visualizations, allowing campaign management and field teams to make timely data-driven decisions to meet coverage targets and optimize operations, for example Vxnaid [Citation27,Citation53], Co-WIN [Citation36], DHIS2 COVID-19 [Citation34], and CommCare [Citation31]. Vxnaid [Citation27,Citation53] has the option to generate customizable reports with flexible export capability that can be exported to databases, including DHIS2. It is worth noting that DHIS2 is distinctly different from the DHIS2 COVID-19 tool discussed here, as DHIS2 represents a platform enabling health data collection, validation, analysis, and visualization of individual and aggregated data. DHIS2 COVID-19 has alternate configurations or functionalities specific to the needs of managing data from COVID-19 cases and/or vaccinations.
5.6. Follow-up communication and reminders
Communication with vaccinated individuals keeps them informed, up-to-date, and may improve adherence to their immunization schedule [Citation29]. For example, a study assessing the benefits of WelTel implementation in non-vaccine related healthcare programs in Rwanda, Kenya, and Canada, found appointment reminders increased appointment attendance [Citation73]. Digital health tools that offer follow-up communication primarily focus on sending appointment reminders for scheduled vaccines or alerts to schedule an appointment for an additional or booster dose. This occurs primarily through SMS (AfriDoctor [Citation28], DHIS2 COVID-19 [Citation34], mVacciNation [Citation44], South Africa’s EVDS [Citation48], WelTel Health Platform [Citation54]). Vxnaid can utilize SMS, WhatsApp messages, or pre-recorded voicemail messages in any language [Citation27]. With this platform’s flexibility, additional content can also be created and shared with users to address health and vaccine misinformation, update on travel restrictions, and inform on practical changes to vaccine appointments (i.e. change of clinic opening hours). As mentioned in Section 5.3, some tools can automatically schedule follow-up appointments and send unique codes or vouchers to each person via SMS (e.g. mVacciNation [Citation44], COVID-19 DHIS2 [Citation34]). SMS text messaging capabilities can support other steps in the vaccination process. For example, COVID-19 DHIS2 [Citation34] uses SMS communication for follow-up visits and to print proof of vaccination. The WelTel Health Platform lists reminders and can broadcast informational health videos; reminders serve as a call-to-action and its educational videos aid in follow-through [Citation54]. This tool allows real-time communication between vaccine recipients and healthcare providers.
6. Considerations for data privacy and ethics
Data privacy and security measures are essential for the ethical use of digital health technology [Citation18]. A large amount of health data and sensitive, personally identifying information is collected with the implementation of digital health tools and must be sufficiently protected [Citation18]. Maintaining high levels of data safety and security is especially true for the use of biometric data for which additional privacy concerns exist [Citation74]. The WHO Global Strategy on Digital Health [Citation18], the Principles for Digital Development [Citation21], the DPG standard from the DPG Alliance [Citation23], and the Digital Global Good (DGG) standard maintained by Digital Square [Citation75] all emphasize the need to be transparent about how data will be collected and used, to plan for and thwart security breaches, and to protect data against harmful or inappropriate use. These guidelines recommend addressing the protection of privacy and data security of digital tools already at the design stage [Citation18,Citation21]. These considerations are critical, in light of data breaches in health data, including electronic medical record systems, and the increasing cost of recovering data and system access [Citation76]. Some of the tools identified in this review have DPG or DGG labels (), with the possibility that additional tools are undergoing the review process to receive these labels.
To protect data privacy and ensure data security, countries need to create and implement a robust legal and regulatory framework at national and international levels around the use of personal data with clearly defined roles for data governance for equitable data sharing [Citation18,Citation21], which does not yet exist in all LMICs [Citation77]. Of the 55 African nations, only 31 have specific data protection laws in place, but not all countries enforce them [Citation78]. Another 24 nations are currently preparing legislations or offer no or very limited protection with only generalized rights to privacy and freedom in their constitution. Where laws have been enacted, they tend to follow the African Union Convention on Data Protection and Cybersecurity and the EU General Data Protection Regulation, but how these are enacted varies among countries. In some African countries, the existing legislation is outdated or too constrained for the innovations offered by digital health technologies [Citation79].
During fast-spreading epidemics and pandemics such as Ebola and COVID-19, early data sharing is critical [Citation80,Citation81]. Being able to do so in an ethical way that protects data privacy remains important. While strides have been made to focus on ensuring data privacy, the COVID-19 pandemic highlighted priorities which were disregarded. At least 27 countries globally relaxed data privacy laws [Citation82], mainly in relation to contact tracing apps, raising concerns about privacy and data protection. Apps used during COVID-19 were rapidly developed and implemented by health officials to track the outbreak without a thorough evaluation of user privacy or security measures [Citation83–85]. Overlooking data privacy and security in a crisis situation is a dangerous precedent which may be difficult to revert in a post-pandemic world [Citation84]. It is critical to ensure that approaches on data privacy will respect ethical fundaments and are prepared well in advance of future emergencies. Health authorities have made strides in addressing data privacy concerns during epidemics and pandemics. This will smooth the rapid implementation of new digital health technologies, while maintaining sufficient standards and ensuring data privacy and security for all individuals.
7. Challenges and opportunities for implementing digital health tools in LMICs
Vaccination campaigns responding to outbreaks face many challenges, regardless of the country. Developing tools with a human-centered design approach [Citation86] allows for local stakeholder input and can develop fit-for-purpose tools that support the local vaccination process and understand interdependencies with other systems. It is critical that the digital tools themselves do not become bottlenecks to efficient vaccination strategies. Modeling vaccination programs and tool use prior to implementation can identify bottlenecks to address for an optimized vaccination process. For example, a recent time-motion study performed at a COVID-19 vaccination center in India measured the time an individual takes to receive a vaccination while utilizing the Co-WIN tool for registration and appointment scheduling [Citation87]. Here, most of the time spent at the center was spent in a queue, waiting for identification verification (average of 34 minutes queuing out of the entire 39-minute process). This bottleneck could be better addressed by more optimal scheduling strategies for booking vaccination appointments. By spreading out appointments and limiting waiting period, social distancing can also be practiced, reducing clinical staff exposure to potentially infected persons. Digital health tools with well-designed, common, and interoperable data capture fields can help address the challenge of capturing high-quality data [Citation88]. High-quality data can be used for informed decision making on bottlenecks and the vaccination program management [Citation89] and to support operational clinical research efforts [Citation88]. Further, the ability to unequivocally and efficiently identify an individual can also enhance the data quality [Citation64] and help address potential wastage of vaccines [Citation65]. Operational research, such as the time-motion study mentioned above [Citation87], illustrates that there are opportunities to learn from simulating the design of the vaccination process in advance so that mitigations can be sought.
Some challenges are more likely to impact LMICs. The availability of a formal means of identification is a known challenge in many countries [Citation63], and biometric identification approaches can be useful. Acceptance of biometric identification tools may be perceived to present challenges, with the idea that local beliefs and (mis)conceptions may erode trust in these identification methods [Citation90]. For example, India’s Aadhaar biometric database, which includes fingerprints and iris scans, has highlighted the importance of securing biometric data so that citizens are not concerned about data leaks or linking to sensitive information and still subscribe to the system [Citation90,Citation91]. Countering these concerns is a growing body of evidence showing there are high levels of acceptance for iris scanning and fingerprinting methodologies [Citation92,Citation93]. It is important to determine the ideal option for a given setting and community, and possibly ensure alternative accepted identification options are available to support the success of a large-scale vaccination campaign.
Although digital health technologies provide opportunities to support inclusiveness and promote health equity, their adoption can be hampered by gender-based, linguistic, and digital literacy barriers [Citation18,Citation84,Citation94]. For example, an analysis of WelTel implementation in clinics in Kenya and Rwanda identified illiteracy as a barrier to the effectiveness of SMS-based communications [Citation73]. Governments should ensure health accessibility and equity for the entire population, including considering using tools that support multiple communication methods. Vxnaid offers both text messages and automated voice messages for reminders and for communicating key information to vaccine recipients [Citation27]. Co-WIN offers an Interactive Voice Response System (IVRS) to register one’s interest to be vaccinated [Citation39] and allows the registration of multiple individuals to a single phone number helping to reach individuals without smartphones or sufficient digital literacy [Citation37].
Vaccination campaigns should always aim to include all populations, even those who have limited or no access to mobile phones and internet, which remains an issue for several communities. The support from telecommunication companies to provide global coverage at affordable rates, even in remote areas, will allow for increased adoption of digital health tools and access to more vulnerable communities with previously limited access to health care. For example, the telecom operator Orange partnered with Gavi to provide off-grid renewable electricity generation, storage systems, and internet connectivity for rural health facilities, addressing a need for infrastructure [Citation42]. Such strategic partnerships can provide access to hard- and software systems needed to successfully launch a digital health tool for large-scale vaccination campaigns responding to a new outbreak.
Despite the fact that many digital tools are already available, they will not solve all of the challenges faced by LMICs in their efforts to set up large-scale vaccination campaigns, such as vaccine hesitancy [Citation95]. Health infrastructure and resources in LMICs are already taxed and many frontline health workers experience high workloads. In times of pandemics, any new digital solutions introduced as part of a vaccine program should endeavor to have quick yet effective training programs to adequately train healthcare workers. This is essential, and a steep learning curve may delay quick adoption of the tools and the critical health impact aimed for [Citation96].
8. Special considerations for forward-thinking and vaccine campaign sustainability
While many digital health tools are developed, not all persist beyond the initial pilot phases and face barriers to scaling and implementation [Citation21,Citation77,Citation97]. Some technologies are not intuitive to use, may not be fit for large-scale deployment and cannot accommodate more users and data over time, lack sustainable funding beyond the pilot program, or do not offer sufficient interoperability with existing digital healthcare systems in LMICs. Therefore, digital tools built with sustainability in mind from the start are necessary to maintain user and stakeholder support and to achieve long-term impact [Citation21]. For example, the successful implementation of DHIS2 to capture health data in Bangladesh greatly benefited from the commitment of international donors, but the country would need to become less dependent on donor funding to ensure long-term sustainability of DHIS2 [Citation96].
When starting from an empty digital space, the probability of being sustainable can be heightened by co-designing innovative digital technologies together with different stakeholders who will be involved in the project implementation, including end users, government and healthcare stakeholders, and telecommunication companies. This ensures that the technologies are fit-for-purpose, enhancing sustainability [Citation98]. Additionally, interoperability plays an important role in increased likelihood of sustainability, with the potential to pick and integrate multiple tools to suit specific needs [Citation98]. This should be at the forefront of the early design phase for digital systems, so that tools can be integrated with each other and existing healthcare systems to avoid duplication and drive efficacy and scale [Citation18,Citation21,Citation98].
In addition to verifying open-source status, the DPG or DGG standards [Citation23,Citation75] also assess maturity, quality, and sustainability aspects of a digital tool, and can be useful when reviewing various tool options. These standards can provide functional guidance for countries looking to use high-quality tools that have sustainable qualities. Digital good registries [Citation99] are an excellent source for identifying such tools. The DPG Alliance also published an assessment of mature DPGs highly relevant to immunization delivery management [Citation100]. In addition, the WHO has developed the Digital Health Atlas [Citation101], where digital health platforms can also be registered with relevant use-case examples offering further information for implementors.
Overall, multiple considerations and tactics need to be considered to ensure that tools being selected or developed keep the long-term future in mind, allowing the tools to be quickly and readily rolled out in response to an outbreak.
9. Research gaps and limitations
Our review highlights remaining gaps in the current use of digital technology in large-scale vaccination campaigns in emergency settings in LMICs. We noted a gap in finding information on tools that can verify the type and lot of vaccine administered to an individual. It is, however, possible that countries are using non-digital methods for tracking vaccine lot information. The field of digital health tools should endeavor to assess the impacts and cost-effectiveness of these tools in large-scale vaccination campaign settings for outbreak response and produce peer-reviewed publications. Through the sharing lessons learned in various LMICs with different tools, the global community can collectively gain knowledge to be best prepared for a future pandemic response.
This review has several limitations. The data presented here on the functionalities and technicalities of the identified tools was driven by the information available, and include peer-reviewed publication, but also information from websites, promotional and user materials, or news releases. Therefore, we may have missed additional features of the tools, and failed to capture them in our summaries. For certain tools, we were unable to verify whether their code was based on another tool’s code, or whether a support service was available, as this information could not be retrieved. Countries may have adopted additional digital health tools or customized existing ones that were not captured in our search. For example, we are aware of digital tools introduced in Vietnam by their Ministry of Health in partnership with the Ministry of Information and Communication and the company Viettel [Citation102]. These tools support vaccination management, health declaration based upon QR codes, quarantine monitoring, and data analytics, but there is limited publicly available information detailing technical specifications and thus we have not included it in our overview. Our search retrieved only a limited number of peer-reviewed publications, and operational research studies assessing the effectiveness of digital tools to improve large-scale vaccination campaigns in response to outbreaks were very rare, despite the potential to provide critical insights to help improve the effectiveness of such tools in future emergency response health interventions. Finally, we only included digital health tools implemented in large-scale vaccination campaigns in response to outbreaks in LMICs, choosing to omit tools used in routine childhood or preventive immunization campaigns. Tools for these latter use-cases also include digital health technologies that may have the potential to be adapted for large-scale vaccinations in response to outbreaks. Regardless, we believe that the digital health tools presented here provide a useful summary of the current landscape for large-scale vaccine campaigns in emergency settings in LMICs, and to our knowledge, this is the first review exploring this topic.
10. Conclusion
The landscape of digital health technologies in LMICs for large-scale vaccination delivery in response to outbreaks is growing fast with a wide range of digital health tools available from either open or proprietary sources. Each of these tools has a variety of features that need to be assessed against the specific country context and requirements. In LMICs, the need for sustainable solutions and clear regulations about data privacy and security are of particular importance and should be considered at an early stage. Open-source approaches seem to offer more accessibility as a starting point for tool selection. One does, of course, still need to consider the challenge for a country’s health authority in determining the value and potential fit for purpose of what is on offer, and the relative financial cost associated with a given tool. The current review, including the referenced DPG and DGG frameworks, can help organizations and LMICs in identifying appropriate digital tools to support vaccination campaigns in response to outbreaks. This should be augmented with vaccination program modeling and additional research data on impact and cost-effectiveness to ensure that the digital technologies selected can become a lasting component of LMIC emergency response toolkits to prepare for the next pandemic.
11. Expert Opinion
This overview of various digital health tools currently used in LMIC vaccination campaigns, can serve as a useful resource and increase awareness of the potential strength of these tools for those wanting to set up future campaigns. The adoption of digital technology can improve equity for people living in LMICs, from vaccine access and delivery to the ability to travel and cross borders in a global pandemic situation. Technology adoption in countries with limited resources can be hampered by insufficient funding. Therefore, investments in a vaccine-agnostic tool, which can function beyond a single campaign and support multiple vaccination efforts, can be a more sustainable choice.
A key area for improvement in this digital arena is building connections between various digital systems, as not all platforms can address all needs [Citation103], particularly for different countries or regions. Interoperability and the use of international standards can be an optimal way forward to ensure systems can communicate efficiently. Having a data privacy and ethical framework in place prior to any new pandemic is imperative for LMICs to help guarantee protection of individual citizen’s data, to build confidence that data will be adequately protected, and to facilitate data sharing for the benefit of developing scientific solutions to curb outbreaks. Another required improvement is the availability of internet, telecom coverage, and electricity, requiring investments by governments, those responsible for infrastructure and possibly international funding agencies. According to UNICEF, “Today half the world’s population is not online. Africa leads the world in the percentage of the population without connection at 88%. Furthermore, in the countries that are connected, male internet users outnumber their female counterparts in every region of the world” [Citation104], highlighting the social component that must be overcome. Addressing inequities will further the use of digital tools in all aspects of life, including in future health interventions for emergency response. Education will be a key component to address multiple of these shortcomings, including comfort with digital tools, gender equality in access to health care, and development of an expert local IT workforce.
Increased operational research on the implementation of digital health tools is critical to learn what really works in LMIC settings and what can be improved going forward. An expanded pool of scientific literature can allow for better comparisons across digital approaches and perhaps drive better fit-for-purpose choices, even in times of emergency response. A built-in benefit to digital health tools is that they collect and provide data on their use, thereby opening the potential to collect real-world evidence on the impact of their implementation for health interventions. As technology continues to evolve, the recording of the perceptions and experiences of citizens undergoing such digitalized vaccination processes can improve the execution of large-scale vaccination campaigns in emergency settings in the future. We encourage health researchers and health authorities to report and publish on their experiences, to improve the available literature on this topic.
Historically, paper documentation has been used to track vaccination records, and there is no standard protocol used globally. Looking forward, the use of electronic medical records across LMICs and the automatic feed of digital immunization data into these records is likely to become standard. Efforts like internet-enabled balloons [Citation105] or Starlink satellite internet [Citation106] signal that improvements in globally available internet and telecom access are anticipated and will lead to increased smartphone adoption in LMICs. This in turn may stimulate further adoption of digital technology. Biometrics technology is currently employed by many of the digital tools to improve identification of individuals. We foresee the use of blockchain or even self-sovereign identity [Citation107] that links to an individual’s electronic medical records or health system services regardless of the country they are in as a likely means of identification verification in future digital health applications. The global health community should monitor the maturity of these tools.
The global community now has the opportunity to build on the current experience with digital tools and optimize approaches to ensure that plans for future large-scale vaccination campaigns in response to outbreaks are detailed with the learnings garnered from not only the COVID-19 pandemic but also from other outbreaks like Ebola or Zika. Examples of this have been reported already for the DHIS2 COVID-19 tool which was used to respond to Ebola outbreaks in Guinea in 2021 [Citation108] and in Uganda in 2022 [Citation109]. Our ultimate hope is that the digital health tools will be used in a more sustainable way and that this will lead to better outcomes in large-scale health interventions.
Article highlights
The set-up of large-scale vaccination campaigns for outbreak response can be challenging in many countries, but low- and middle-income countries (LMICs) may face additional challenges due to fewer financial and human resources. By taking advantage of a surge in mobile phone use and wireless connectivity in LMICs, the implementation of digital health tools in large-scale vaccination campaigns may help overcome some of these challenges.
This narrative review identifies which digital health tools have been used in LMICs to support large-scale vaccine delivery and provides an overview of their functionalities and technical specifications. We focus on tools used for appointment scheduling, personal identification, verification of vaccination dose, vaccine certification, vaccine reporting, and follow-up communication and reminders.
LMICs use a wide range of digital health tools with open-source or proprietary business models in the context of large-scale vaccination campaigns for outbreak response. When planning the introduction of new digital tools, it is important for countries to evaluate their specific needs, taking into account interoperability with existing digital systems, sustainability, inclusiveness, regulations about data privacy and security, and overall costs.
The assessment of digital tools through a framework such as the Digital Public Good or Digital Global Good standards may help countries in selecting high-quality, sustainable tools.
The global community can build on the knowledge gained from the current experience with digital tools in various LMICs to better prepare for vaccine delivery in LMICs for future health emergencies.
Declaration of interests
P Mc Kenna, A Willems, S Masyn, T Pattery, and R Draghia-Akli are employees of Janssen and potential stockholders of Johnson and Johnson. L A. Broadfield is an employee of Akkodis Belgium c/o Janssen. The Vxnaid platform described in the current manuscript has been developed by Johnson & Johnson Global Public Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium for their review work. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Author contribution statement
All authors have substantially contributed to the conception and design of the review article and interpretation of the relevant literature. All authors have been involved in writing the review article or revised it for intellectual content.
Trademark section
Vxnaid is a trademark of Johnson & Johnson. All other third-party marks used herein are trademarks of their respective owners or licensors.
Supplemental Material
Download MS Word (45.3 KB)Acknowledgments
We thank Akkodis Belgium for medical writing support (Kristel Vercauteren) and graphic design (Maria Maior), and PharmaValue Partners, Inc., for performing the literature search, on behalf of Janssen Pharmaceutica NV.
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. The data supporting the findings of this study are available within the article or its supplementary materials.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2023.2184091
Additional information
Funding
References
- World Health Organization. COVID-19 Research and Innovation: Powering the world’s pandemic response – now and in the future. [Cited 2022 Dec 5]. Available from: https://cdn.who.int/media/docs/default-source/blue-print/achievement-report-_grif_web_finalversion15.pdf
- Henao-Restrepo AM, Preziosi MP, Wood D, et al. On a path to accelerate access to Ebola vaccines: The WHO’s research and development efforts during the 2014-2016 Ebola epidemic in West Africa. Curr Opin Virol. 2016;17:138–144.
- Watson OJ, Barnsley G, Toor J, et al. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22(9):1293–1302.
- Feldmann H, Sprecher A, Geisbert TW. Ebola. N Engl J Med. 2020;382(19):1832–1842.
- The World Bank. Assessing country readiness for COVID-19 vaccines: first insights from the assessment rollout. [Cited 2022 Dec 20]. Available from: https://documents1.worldbank.org/curated/en/467291615997445437/pdf/Assessing-Country-Readiness-for-COVID-19-Vaccines-First-Insights-from-the-Assessment-Rollout.pdf
- Wouters OJ, Shadlen KC, Salcher-Konrad M, et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021;397(10278):1023–1034.
- World Health Organization. Health workforce requirements for universal health coverage and the Sustainable Development Goals. [Cited 2023 Jan 12]. Available from: https://apps.who.int/iris/handle/10665/250330
- Whitelaw S, Mamas MA, Topol E, et al. Applications of digital technology in COVID-19 pandemic planning and response. Lancet Digit Health. 2020;2(8):e435–e440.
- Wood CS, Thomas MR, Budd J, et al. Taking connected mobile-health diagnostics of infectious diseases to the field. Nature. 2019;566(7745):467–474.
- Ye J. The Role of Health Technology and Informatics in a Global Public Health Emergency: Practices and Implications From the COVID-19 Pandemic. JMIR Med Inform. 2020;8(7):e19866.
- Bempong NE, De Castañeda RR, Schütte S, et al. Precision Global Health - The case of Ebola: a scoping review. J Glob Health. 2019;9(1):010404.
- Tom-Aba D, Olaleye A, Olayinka AT, et al. Innovative technological approach to Ebola virus disease outbreak response in Nigeria using the Open Data Kit and Form Hub technology. PLoS One. 2015;10(6):e0131000.
- Ahmadi S, Bempong N-E, De Santis O, et al. The role of digital technologies in tackling the Zika outbreak: a scoping review. J Public Health Emerg. 2018;2(6):20.
- Budd J, Miller BS, Manning EM, Budd J, Miller BS, Manning EM et al. Digital technologies in the public-health response to COVID-19. Nat Med. 2020;26(8):1183–1192.
- Agarwal S, Pandya JM, Ferguson S, et al. Digital solutions for COVID-19 response: an assessment of digital tools for rapid scale-up for case management and contact tracing. (Johns Hopkins Global mHealth Initiative (JHU-GmI) - Johns Hopkins Bloomberg School of Public Health, Baltimore: Maryland, 2020)
- McCool J, Dobson R, Whittaker R, et al. Mobile Health (mHealth) in low- and middle-income countries. Annu Rev Public Health. 2022;43(1):525–539.
- The World Bank. Year in review: 2019 in 14 charts. [Cited 2022 Dec 1]. Available from: https://www.worldbank.org/en/news/feature/2019/12/20/year-in-review-2019-in-charts
- World Health Organization. Global Strategy on Digital Health 2020-2025. [Cited 2022 Nov 15]. Available from: https://apps.who.int/iris/handle/10665/344249
- The World Bank. New World Bank country classifications by income level: 2022-2023. [Cited 2022 Dec 16]. Available from: https://blogs.worldbank.org/opendata/new-world-bank-country-classifications-income-level-2022-2023
- Department of Health and Social Care. UK COVID-19 vaccines delivery plan. [Cited 2023 Jan 31]. Available from: https://www.gov.uk/government/publications/uk-covid-19-vaccines-delivery-plan
- The Digital Principles community. Principles for digital development. [Cited 2022 Dec 12]. Available from: https://digitalprinciples.org/
- Schneider P Guide to the total cost of ownership of open-source software. [Cited 2023 Feb 3]. Available from: https://www.qt.io/blog/is-open-source-really-free
- Digital Public Goods Alliance. Digital Public Goods Standard. [Cited 2022 Dec 7]. Available from: https://digitalpublicgoods.net/standard/
- Digital Public Goods Alliance. The DPG Charter: key takeaways on norms and principles for safe and inclusive digital public infrastructure. [Cited 2023 Feb 7]. Available from: https://digitalpublicgoods.net/blog/the-dpg-charter-key-takeaways-on-norms-and-principles-for-safe-and-inclusive-digital-public-infrastructure/
- University of Oslo. WHO health data toolkit. [Cited 2023 Feb 14]. Available from: https://dhis2.org/who/
- Oracle. Tony Blair Institute and Oracle launch Africa vaccine management in the cloud. [Cited 2020 Nov 23]. Available from: https://www.oracle.com/news/announcement/tony-blair-institute-oracle-launch-africa-vaccine-management-in-the-cloud-112320/
- Mc Kenna P, Masyn S, Willems A, et al. Leapfrogging with technology: introduction of a monitoring platform to support a large-scale Ebola vaccination program in Rwanda. Hum Vaccin Immunother. 2021;17(9):3192–3202.
- AfriDoctor. [Cited 2023 Jan 31]. Available from: https://afridoctor.com/
- Salud digital. Government of Peru enabled the use of mobile app to validate vaccination against COVID. [2023 Jan 31]. Available from: https://saluddigital.com/en/noticias/gobierno-de-peru-habilito-el-uso-de-app-movil-para-validar-vacunacion-contra-covid/
- Ministerio de Salud del Peru. Carné de Vacunación. [Cited 2023 Jan 31]. Available from: https://carnetvacunacion.minsa.gob.pe/#/auth
- Dimagi. Digital solutions for COVID-19 response: CommCare. [Cited 2023 Jan 31]. Available from: https://www.dimagi.com/covid-19/
- Ministério da Saúde do Brazil. Connect SUS Citizen. [Cited 2023 Feb 10]. Available from: https://www.gov.br/saude/pt-br/assuntos/conecte-sus/conecte-sus-english
- Ministério da Saúde do Brazil. Conecte SUS. [Cited 2023 Jan 31]. Available from: https://conectesus.saude.gov.br/home
- University of Oslo. DHIS2 COVID-19 vaccine delivery toolkit. [Cited 2023 Feb 3]. Available from: https://dhis2.org/covid-vaccine-delivery/
- Paton C, Braa J, Muhire A, et al. Open source digital health software for resilient, accessible and equitable healthcare systems. Yearb Med Inform. 2022;31(1):67–73.
- Ministry of Health and Family Welfare. Co-WIN, winning over COVID-19. [Cited 2023 Jan 31]. Available from: https://www.cowin.gov.in/
- Ministry of Health and Family Welfare. Citizen registration and appointment for vaccination - Co-WIN user manual. [Cited 2022 Dec 16]. Available from: https://www.mohfw.gov.in/pdf/UserManualCitizenRegistration&AppointmentforVaccination.pdf
- Singh K, Verma A, Lakshminarayan M. India’s efforts to achieve 1.5 billion COVID-19 vaccinations: a narrative review. Osong Public Health Res Perspect. 2022;13(5):316–327.
- Pivcevic K India says biometrics-backed CoWIN vaccine app will only track relevant vaccine data. [Cited 2022 Dec 15]. Available from: https://www.biometricupdate.com/202101/india-says-biometrics-backed-cowin-vaccine-app-will-only-track-relevant-vaccine-data
- eGov Foundation. DIVOC. [Cited 2023 Feb 1]. Available from: https://divoc.egov.org.in/
- Rizzato Lede DA, Pedernera FA, López E, et al. Mi Argentina/Mi Salud: The Argentinian Citizen Digital Health Portal. Stud Health Technol Inform. 2020;270:1011–1015.
- Gavi. Orange. [Cited 2023 Jan 31]. Available from: https://www.gavi.org/investing-gavi/funding/donor-profiles/orange
- Village Reach. m-Vaccin - Leveraging mobile technology to save lives. [Cited 2023 Feb 13]. Available from: https://www.villagereach.org/wp-content/uploads/2020/03/M-Vaccin_Overview.pdf
- Mezzanine. mVacciNation. [Cited 2023 Jan 31]. Available from: https://mvaccination.com/
- OpenSRP. OpenSRP - The better way to connect frontline health workers to clients and health systems. [Cited 2023 Jan 31]. Available from: https://smartregister.org/
- OpenSRP FHIRCore. OpenSRP FHIR Core Smart Vaccination Certificates. [Cited 2023 Feb 14]. Available from: https://www.fhircore.org/
- Oracle. Oracle Health Immunization Management user guide. [Cited 2023 Feb 13]. Available from: https://docs.oracle.com/en/industries/health/health-immunization-management/dmsug/get-started.html
- Department of Health Republic of South Africa. Electronic Vaccination Data System (EVDS). [Cited 2023 Feb 13]. Available from: https://sacoronavirus.co.za/evds/tscs/
- Simprints. Our biometric solution will help you tackle your greatest impact challenges. [Cited 2023 Jan 31]. Available from: https://www.simprints.com/
- Africa Centres for Disease Control and Prevention. Trusted Vaccines. [Cited 2023 Jan 31]. Available from: https://africacdc.org/trusted-vaccines/
- Vaxiglobal. [Cited 2023 Jan 31]. Available from: https://vaxiglobal.com/
- Global Finder Africa. Vaxiglobal. [Cited 2023 Feb 7]. Available from: https://africa.globalfinder.org/company_page/vaxiglobal
- GitHub. Vxnaid. [Cited 2023 Feb 1]. Available from: https://github.com/johnsonandjohnson/vxnaid/wiki
- WelTel Health. The leading digital health platform delivering better care for all. [Cited 2023 Jan 31]. Available from: https://www.weltelhealth.com/
- GitHub. Commcare. [Cited 2023 Feb 1]. Available from: https://github.com/dimagi/commcare-hq
- GitHub. DHIS2. [Cited 2023 Feb 1]. Available from: https://github.com/dhis2
- GitHub. Co-WIN. [Cited 2023 Feb 1]. Available from: https://github.com/topics/cowin
- GitHub. DIVOC. [Cited 2023 Feb 1]. Available from: https://github.com/egovernments/DIVOC
- GitHub. Mi Argentina. [Cited 2023 Feb 1]. Available from: https://github.com/argob/mi-argentina-distro
- Gavi. Download for life: a mobile app to improve vaccine coverage in Côte d’Ivoire. [Cited 2023 Feb 13]. Available from: https://www.gavi.org/vaccineswork/download-life-mobile-app-improve-vaccine-coverage-cote-divoire
- GitHub. OpenSRP FHIR Core. [Cited 2023 Feb 14]. Available from: https://github.com/opensrp/fhircore
- GitHub. OpenSRP. [Cited 2023 Feb 1]. Available from: https://github.com/OpenSRP
- Desai VT, Diofasi A, Lu J The World Bank Blogs - The global identification challenge: Who are the 1 billion people without proof of identity? [Cited 2022 Dec 8]. Available from: https://blogs.worldbank.org/voices/global-identification-challenge-who-are-1-billion-people-without-proof-identity
- Weintraub R, Yadav P, Berkley S A Covid-19 vaccine will need equitable, global distribution. [Cited 2023 Feb 6]. Available from: https://hbr.org/2020/04/a-covid-19-vaccine-will-need-equitable-global-distribution
- Weintraub R, Plotkin S, Liu M, et al. COVID-19 vaccine delivery: an opportunity to set up systems for the future. Gates Open Res. 2020;4:182.
- Jain AK, Nandakumar K, Ross A. 50 years of biometric research: Accomplishments, challenges, and opportunities. Pattern Recogn Lett. 2016;79:80–105.
- Jacobs JA, Van Ranst M. Biometric fingerprinting for visa application: device and procedure are risk factors for infection transmission. J Travel Med. 2008;15(5):335–343.
- Vogel G. Mixing COVID-19 vaccines appears to boost immune responses. [Cited 2022 Dec 16]. Available from: https://www.science.org/content/article/mixing-covid-19-vaccines-appears-boost-immune-responses
- Pollard AJ, Launay O, Lelievre J-D, et al. Safety and immunogenicity of a two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Europe (EBOVAC2): a randomised, observer-blind, participant-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2021;21(4):493–506.
- World Health Organization. Digital documentation of COVID-19 certificates: vaccination status — technical specifications and implementation guidance, 27 August 2021. [Cited 2022 Dec 15]. Available from: https://apps.who.int/iris/bitstream/handle/10665/343361/WHO-2019-nCoV-Digital-certificates-vaccination-2021.1-eng.pdf
- Centers for Disease Control and Prevention. Traveler’s health [Cited 2022 Dec 15]. Available from: https://wwwnc.cdc.gov/travel/page/icvp
- Centers for Disease Control and Prevention. Vaccine information for adults - Keeping your vaccine records up to date. [Cited 2022 Dec 15]. Available from: https://www.cdc.gov/vaccines/adults/vaccination-records.html
- El Joueidi S, Bardosh K, Musoke R, et al. Evaluation of the implementation process of the mobile health platform ‘WelTel’ in six sites in East Africa and Canada using the modified consolidated framework for implementation research (mCFIR). BMC Med Inform Decis Mak. 2021;21(1):293.
- The World Bank - ID4D. Practioner’s guide: biometric data. [Cited 2022 Dec 13]. Available from: https://id4d.worldbank.org/guide/biometric-data
- Digital Square. Digital health Global Good - Global Goods for health. [Cited 2023 Jan 11]. Available from: https://digitalsquare.org/digital-health-global-goods
- Seh AH, Zarour M, Alenezi M, et al. Healthcare data breaches: insights and implications. Healthcare (Basel). 2020;8(2):133.
- Exemplars in Global Health. Overview digital health tools: COVID-19 solutions. [Cited 2022 Dec 12]. Available from: https://www.exemplars.health/emerging-topics/epidemic-preparedness-and-response/digital-health-tools
- Daigle B Data protection laws in Africa: a pan-African survey and noted trend. [Cited 2023 Jan 12]. Available from: https://www.usitc.gov/publications/332/journals/jice_africa_data_protection_laws.pdf
- Nabyonga-Orem J, Asamani JA, Makanga M. The state of health research governance in Africa: what do we know and how can we improve? Health Res Policy Syst. 2021;19(1):11.
- Lucas-Dominguez R, Alonso-Arroyo A, Vidal-Infer A, et al. The sharing of research data facing the COVID-19 pandemic. Scientometrics. 2021;126(6):4975–4990.
- Yozwiak NL, Schaffner SF, Sabeti PC. Data sharing: Make outbreak research open access. Nature. 2015;518(7540):477–479.
- Futures Centre. Data protection rules are being relaxed across the globe in response to the COVID-19 pandemic. [Cited 2022 Dec 21]. Available from: https://www.thefuturescentre.org/signal/signal-of-change-data-protection-rules-are-being-relaxed-across-the-globe-in-response-to-the-covid-19-pandemic/
- Afroogh S, Esmalian A, Mostafavi A, et al. Tracing app technology: an ethical review in the COVID-19 era and directions for post-COVID-19. Ethics Inf Technol. 2022;24(3):30.
- Arriagada-Bruneau G, Gilthorpe M, Müller VC. The ethical imperatives of the COVID-19 pandemic: an analysis from the ethics of data. Veritas. 2020;46:13–35.
- Fahey RA, Hino A. COVID-19, digital privacy, and the social limits on data-focused public health responses. Int J Inf Manage. 2020;55:102181.
- Norman DA. The design of everyday things. Cambridge, MA: The MIT Press; 2013.
- Alekhya G, Giri PP, CA M, et al. A time-motion study of the COVID-19 vaccination process in an urban primary health center of Odisha, India. Hum Vaccin Immunother. 2022;18(5):2073759.
- Rosenbloom ST, Carroll RJ, Warner JL, et al. Representing knowledge consistently across health systems. Yearb Med Inform. 2017;26(1):139–147.
- Siyam A, Ir P, York D, et al. The burden of recording and reporting health data in primary health care facilities in five low- and lower-middle income countries. BMC Health Serv Res. 2021;21(Suppl 1):691.
- Jones P Lessons from India’s attempt to marry biometric and voter ID databases. [Cited 2023 Feb 6]. Available from: https://www.brookings.edu/techstream/lessons-from-indias-attempt-to-marry-biometric-and-voter-id-databases/
- Bondre A, Pathare S, Naslund JA. Protecting mental health data privacy in India: the case of data linkage with Aadhaar. Glob Health Sci Pract. 2021;9(3):467–480.
- Mistry SK, Akter F, Hossain MB, et al. Exploring factors associated with women’s willingness to provide digital fingerprints in accessing healthcare services: a cross-sectional study in urban slums of Bangladesh. Int J Environ Res Public Health. 2021;19(1):40.
- Zola Matuvanga T, Johnson G, Larivière Y, et al. Use of iris scanning for biometric recognition of healthy adults participating in an Ebola vaccine trial in the Democratic Republic of the Congo: mixed methods study. J Med Internet Res. 2021;23(8):e28573.
- Mitgang EA, Blaya JA, Chopra M. Digital health in response to COVID-19 in low- and middle-income countries: opportunities and challenges. Glob Polic. 2021;12(S6)107–109.
- Larson HJ. Negotiating vaccine acceptance in an era of reluctance. Hum Vaccin Immunother. 2013;9(8):1779–1781.
- Begum T, Khan SM, Adamou B, et al. Perceptions and experiences with district health information system software to collect and utilize health data in Bangladesh: a qualitative exploratory study. BMC Health Serv Res. 2020;20(1):465.
- Jane E, Foutry G, Sanou S. Using digital tools at scale: the integrated e-diagnostic approach in Burkina. Africa Health. 2018;40(4)26–28.
- McCool J, Dobson R, Muinga N, et al. Factors influencing the sustainability of digital health interventions in low-resource settings: Lessons from five countries. J Glob Health. 2020;10(2):020396.
- Digital Public Goods Alliance. Registry. [Cited 2023 Feb 6]. Available from: https://digitalpublicgoods.net/registry/
- Digital Public Goods Alliance. Health DPGs - Immunization delivery management (final report). [Cited 2023 Feb 10]. Available from: https://digitalpublicgoods.net/DPGA_Health-DPG-Technical-Assessment.pdf
- World Health Organization. Digital Health Atlas. [Cited 2022 Dec 7]. Available from: https://www.digitalhealthatlas.org/en/-/
- Vietnamnet Global. National technology platform used to combat COVID-19 for first time. [Cited 2022 Dec 21]. Available from: https://vietnamnet-vn.translate.goog/en/national-technology-platform-used-to-combat-covid-19-for-first-time-758572.html?_x_tr_sl=en&_x_tr_tl=nl&_x_tr_hl=nl&_x_tr_pto=sc
- Silenou BC, Nyirenda JLZ, Zaghloul A, et al. Availability and suitability of digital health tools in Africa for pandemic control: scoping review and cluster analysis. JMIR Public Health Surveill. 2021;7(12):e30106.
- Make4Prosperity. Digital divide and digital literacy. [Cited 2022 Dec 16]. Available from: https://gdc.unicef.org/resource/digital-divide-and-digital-literacy
- International Finance Corporation. Balloons to deliver emergency internet across Kenya. [Cited 2023 Feb 10]. Available from: https://www.ifc.org/wps/wcm/connect/news_ext_content/ifc_external_corporate_site/news+and+events/news/insights/telkom-kenya
- Starlink. World’s most advanced broadband satellite internet. [Cited 2023 Feb 10]. Available from: https://www.starlink.com/technology
- Gilles AS Self-sovereign identity. [Cited 2022 Dec 16]. Available from: https://www.techtarget.com/searchsecurity/definition/self-sovereign-identity
- Eggers C, Martel L, Dismer A, et al. Implementing a DHIS2 Ebola virus disease module during the 2021 Guinea Ebola outbreak. BMJ Glob Health. 2022;7(5):e009240.
- University of Oslo. Uganda responds to an Ebola outbreak using DHIS2 tools and lessons learned from COVID-19. [Cited 2023 Feb 10]. Available from: https://dhis2.org/uganda-ebola-response/