ABSTRACT
Introduction
Invasive meningococcal disease (IMD) is a leading cause of life-threatening bacterial meningitis and septicemia. Evidence points to a knowledge gap among parents, teenagers, and healthcare providers (HCPs) regarding IMD and available vaccines, including those against the highly prevalent serogroup B.
Areas covered
An online survey was conducted between March 27 and 12 April 2019, to gather insights into the knowledge that parents/guardians have about IMD vaccines. The children were aged 2 months to 10 years in Australia, Brazil, Germany, Greece, Italy, and Spain, 5–20 years in the UK, and 16–23 years in the USA. The findings were discussed in the context of the available literature and solutions were proposed to minimize the knowledge gap and the barriers to vaccination against IMD.
Expert opinion
The survey demonstrated that parents have a good understanding of IMD but a limited understanding of the different serogroups and vaccines. The available literature highlighted multiple barriers to IMD vaccine uptake; these may be reduced through education of HCPs, clear recommendations to parents by HCPs, the use of technology, and disease-awareness initiatives that engage parents through physical and digital channels. Further studies are warranted to assess the impact of the COVID-19 pandemic on IMD vaccination.
1. Introduction
Invasive meningococcal disease (IMD), caused by Neisseria meningitidis (meningococcus), is a leading cause of life-threatening bacterial meningitis and septicemia in most developed countries, with a fatality rate of 8–15% [Citation1–5]. IMD is uncommon, with reported case rates ranging from 0.1 to 2.4 cases per 100,000 in Europe [Citation6]. However, long-term sequelae occur in approximately 10–20% of survivors and include physical, neurological, and psychological phenomena [Citation5]. Amputations and skin scarring are the most common physical sequelae [Citation7–9]. Hearing loss, seizures, cognitive impairment, motor deficits, and visual impairment are the most frequently reported neurological sequelae [Citation5]. Psychological sequelae include post-traumatic stress symptoms, anxiety, and attention-deficit/hyperactivity disorder [Citation5]. These sequelae contributed to the recognition of meningitis as the fourth largest contributor of neurological disability-adjusted life years in 2016 [Citation10]. In addition to the impact of IMD on patients, caring for individuals with IMD has an impact on the health-related quality of life of the caregiver [Citation5,Citation11].
The immunological reactivity of capsular polysaccharides is used to group N. meningitidis into serogroups [Citation12]. The vast majority of infections are attributed to the six most common serogroups (A, B, C, W, X, and Y). Serogroup B is the most common serogroup in many countries in the Americas, Europe, and the Western Pacific, whereas serogroup W is the most common serogroup in most countries in Africa [Citation13]. Although not the most common serogroups in their respective geographic regions, serogroup A is prevalent in Africa, serogroup C in the Americas, and serogroup Y in Europe. Serogroup X is prevalent in some countries in Africa and is the most recent serogroup to cause outbreaks of IMD [Citation13,Citation14].
Effective vaccines have been developed against several meningococcal serogroups and are widely approved and recommended to prevent IMD [Citation15]. These include quadrivalent vaccines that target serogroups A, C, W, and Y and monovalent vaccines that target serogroups A, B, or C [Citation15]. The development of meningococcal polysaccharide-conjugate vaccines to serogroups A, C, W, and Y (MenACWY, MenACWY-CRM, MenACWY-TT) made it possible to help protect children and adolescents (who are at highest risk of IMD), prevent carriage, and induce herd protection [Citation15]. Different MenACWY formulations are available, and their use is recommended or offered in national immunization programs (NIPs) in many countries worldwide [Citation15].
The development of vaccines against serogroup B has been and remains a considerable challenge due to the biological characteristics of this serogroup [Citation15]. Two vaccines against serogroup B are currently available: 4CMenB (Bexsero®, GSK), which was developed through reverse vaccinology, and lipidated MenB-factor H binding protein (FHbp) (Trumenba®, Pfizer), which was developed using a combination of biochemical and immunologic methodologies [Citation15–17]. The vaccines are not interchangeable due to the different formulations and administration schedules [Citation15–17]. The four main antigenic components in 4CMenB also present in non-B meningococcal and gonococcal strains [Citation15,Citation18]. MenB-FHbp is approved for individuals aged 10–25 years in the USA, Canada, and Brazil and those aged ≥ 10 years in Europe and a number of other countries. 4CMenB is additionally licensed for individuals aged ≥ 2 months in Europe, whereas it is only approved for individuals aged 10–25 years in the USA [Citation15–17].
The first introduction of 4CMenB into a NIP occurred in the UK in 2015, with subsequent implementation in some countries (e.g. Czech Republic, Italy, Spain) but not others (e.g. Germany), as of current date [Citation15]. MenB-FHbp is not currently included in any NIP [Citation15]. In the USA, the Advisory Committee on Immunization Practices (ACIP) recommends 4CMenB or MenB-FHbp in individuals aged 16–23 years on the basis of shared clinical decision-making and in individuals aged ≥ 10 years who are at increased risk of MenB disease [Citation19].
While the use of meningococcal vaccines has led to substantial declines in both carriage and incidence of disease globally, IMD remains a global public health concern [Citation15]. For example, by the end of 2020, the conjugate vaccine for N. meningitidis serogroup A (MenAfriVac) had been administered to almost 350 million people in 24 of the 26 countries in the African meningitis belt [Citation20]. Prior to the introduction of this vaccine, serogroup A caused 80–85% of meningitis epidemics in the African meningitis belt [Citation20]. Surveillance data showed that MenAfriVac introduction led to substantial reductions in the incidence of suspected meningitis (57%) and epidemic risk (59%) in vaccinated vs unvaccinated populations, as well as a > 99% reduction in confirmed serogroup A disease in fully vaccinated populations within nine countries between 2005 and 2015 [Citation21]. For adolescents aged 13–17 years in the USA in 2020, the estimated meningitis A, C, W, and Y (MenACWY) vaccine coverage was 89% for ≥ 1 dose and 54% for ≥ 2 doses [Citation22]. For adolescents aged 17 years in the USA in 2020, only 28% had received ≥ 1 dose of the MenB vaccine [Citation22]. As such, much remains to be done in terms of improving IMD awareness and vaccine coverage globally.
Surveys reveal a knowledge gap among some parents regarding IMD, vaccines against IMD, and the vaccination status of their children [Citation23–27]. Some parents are unaware of the potential threat that IMD poses to their children and that their children may not be fully protected against all meningococcal serogroups even when they follow local vaccine recommendations. It has been shown that when parents are provided with accurate information, they are generally willing to vaccinate their children [Citation25,Citation28,Citation29]. However, for a multitude of reasons, including accessibility, lack of healthcare provider (HCP) recommendation, and lack of time to discuss vaccines during HCP visits, many parents who are aware of vaccines against IMD do not vaccinate their adolescents [Citation30]. In the USA, only 28% of adolescents aged 17 years received ≥ 1 dose of MenB vaccine [Citation22]. Of those parents who were willing to accept MenB vaccination, the choice was driven by many different factors, including their perceptions of the severity of the disease, a desire to protect their child, and their doctors” recommendations [Citation25].
The World Health Organization has developed a roadmap that aims to defeat epidemics of bacterial meningitis, to reduce cases of vaccine-preventable bacterial meningitis by 50% and deaths by 70%, and to reduce disability and improve quality of life after meningitis of any cause [Citation31]. To help achieve these goals, we undertook an online parent/guardian survey to better understand the knowledge gap and barriers regarding vaccination against IMD and to inform solutions to these challenges.
2. Identifying the vaccine knowledge gap: a parent/guardian survey
An IMD awareness survey was conducted by Ipsos on behalf of GSK to understand and gather insights into the knowledge that parents have about IMD, vaccines against IMD, and more specifically the MenB vaccine. The survey was conducted online between March 27 and 12 April 2019. Screening criteria included being a parent or legal guardian of a child aged 2 months to 10 years in Australia, Brazil, Germany, Greece, Italy, and Spain; child, teenager, or young adult aged 5–20 years in the UK; or teenager or young adult aged 16–23 years in the USA (the age of participants targeted in each country was dependent on the approval, recommendation, or provision of 4CMenB within the NIP at the time the survey was conducted). A total of 3600 parents/guardians (70% were female) of 6702 children participated in the research. Respondents were from Australia (n = 500), Brazil (n = 500), Germany (n = 500), Greece (n = 100), Italy (n = 500), Spain (n = 500), the UK (n = 500), and the USA (n = 500). The survey comprised questions and response fields only and included no additional information.
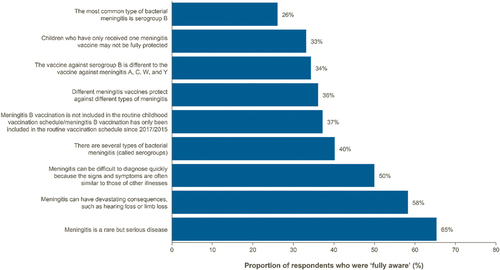
Full data stratified by country are included in the Supplementary Tables. The survey demonstrated that, generally, parents are aware of IMD and its associated sequelae (65% of parents were fully aware that IMD is a rare but serious disease and 58% were fully aware that it can have devastating consequences; ). The proportion of parents/guardians aware that IMD is a rare but serious disease was highest in Italy (80%) and the UK (72%), and lowest in Germany (52%) (Supplementary Table S1). However, only 36% of parents were fully aware that different types of vaccines provide immunity to different IMD serogroups; only 34% were fully aware that vaccines against serogroup B were different from vaccines against serogroups A, C, W, and Y; and only 33% were fully aware that their children/teenagers who had received only one vaccine against IMD may not be protected against all IMD serogroups ( and Supplementary Table S1).
Figure 1. Awareness and knowledge of invasive meningococcal disease.
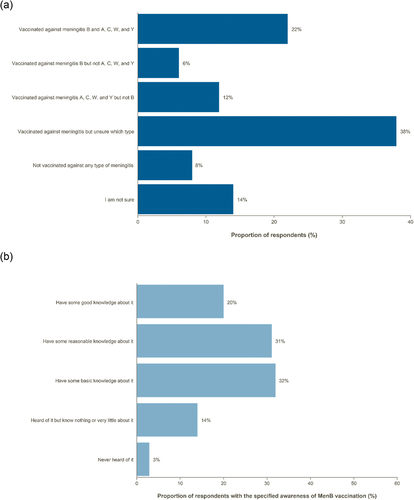
In addition, parents had a low understanding of their children”s vaccination status ( and Supplementary Table S2). In total, 14% of parents were unsure of their children’s vaccination status. Most parents (51%) had good or reasonable knowledge about MenB vaccination ( and Supplementary Table S3). The highest proportion of parents reporting good knowledge about MenB vaccination was in Brazil (26%) and the lowest was in Spain (9%).
Figure 2. Parental knowledge of (a) children’s vaccination status against invasive meningococcal disease serogroups and (b) meningitis B vaccination.
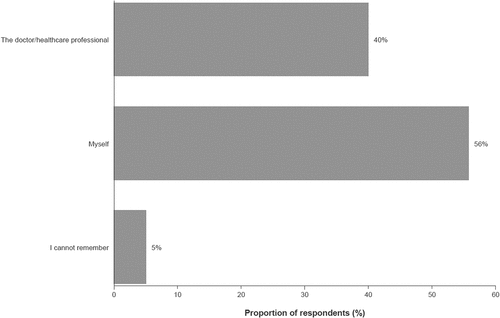
Most parents had discussed vaccination against IMD with an HCP (64%), but a high proportion could not recall whether the discussions were specifically about serogroup B (33%; and Supplementary Table S4). Of those survey respondents who had discussed vaccination against IMD with an HCP, 56% initiated those discussions themselves, and 40% had those discussions initiated by an HCP ( and Supplementary Table S5).
Figure 3. Discussion of meningitis B vaccine with a healthcare professional.
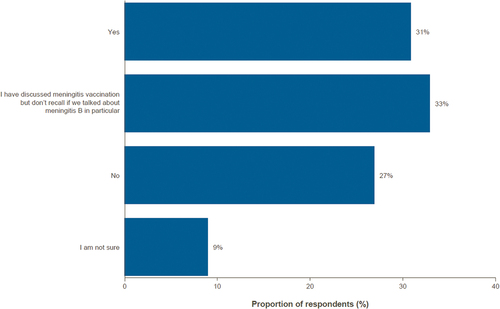
Figure 4. Individual who initiated discussion regarding vaccination against invasive meningococcal disease.
When parents were asked about actions that they will take regarding MenB vaccination after completing the survey, the majority reported that they will ”definitely” or ”probably” talk to their children’s doctor, conduct their own research, or talk with family, friends, or other parents ( and Supplementary Table S6).
Figure 5. Actions following completion of the survey.
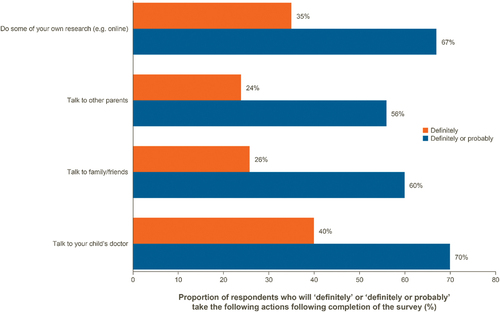
In summary, our research demonstrated that parents generally have a good understanding of IMD, but a knowledge gap exists in their understanding and awareness of vaccines against IMD and that different vaccines against IMD provide protection against different meningococcal serogroups.
3. Barriers to vaccination
Five studies evaluating knowledge gaps in IMD and published up to early 2022 aligned with the findings of our survey, showing that parents have a generally good understanding of IMD and its associated sequelae [Citation23,Citation27,Citation32–34]. In addition, four studies identified a knowledge gap in parental understanding and awareness of vaccines against IMD and that different vaccines against IMD provide protection against different meningococcal serogroups, which was also consistent with our research [Citation26,Citation33–35]. Collectively, these findings suggest that there is an unmet need for education and awareness initiatives to close the knowledge gap in parental understanding of vaccines against IMD and the different meningococcal serogroups.
To further understand and address suboptimal vaccination rates, it is important to understand the barriers to vaccination at the level of parents and teenagers, HCPs, and healthcare organizations.
3.1. Barriers for parents and teenagers
Parents are generally aware of IMD and the availability of vaccines against IMD, yet many of these parents do not vaccinate their children [Citation30]. This highlights a need to understand the parent journey that encompasses disease awareness and the intention to vaccinate their children as well as the barriers that prevent parents from vaccinating their children. Parental awareness of the available meningococcal vaccines needs to be considered in the context of the variations in epidemiology, public health policies, funding, history, and traditions across countries. The lack of inclusion of meningococcal vaccines in some NIPs may affect public and HCP awareness. In our survey, the proportion of parents/guardians aware that IMD is a rare but serious disease was highest in Italy and the UK, where both MenACWY and 4CMenB are included in the respective NIPs, and lowest in Germany, where MenB vaccines are not currently included [Citation36]. In Brazil, the incidence and mortality of meningitis C declined substantially in children aged<5 years due to vaccination, but disease due to serogroup B, against which the population is not protected as the MenB vaccine is not included in the Brazilian NIP, remains a concern [Citation37]. Notably, Brazil was the country with the highest proportion of parents reporting a good knowledge about MenB vaccination in our survey.
A systematic literature review of barriers to vaccination in Latin America, where no country includes the MenB vaccine in their respective NIPs, found that ”individual/group influences” were the most frequently reported barrier to vaccination followed by ”contextual influences” [Citation38]. Individual/group influences include personal perceptions of the vaccine or influences of the social environment, whereas contextual influences reflect the historical, sociocultural, health system, economic, or political environment. Adverse socioeconomic factors, including a low level of education, lack of awareness of diseases and vaccines, and religious and cultural beliefs, are commonly cited as obstacles to vaccination acceptance [Citation38]. Parent- and child-specific barriers to vaccination against IMD include lack of knowledge, low perceived value of vaccines, and misperceptions about the health threats posed by vaccine-preventable diseases [Citation23–27]. However, when parents perceive IMD to be a serious threat to their child’s health, the perceived value of vaccines that prevent IMD is increased [Citation25,Citation26,Citation32,Citation39]. Accordingly, publicization of a mass MenB vaccination campaign during an outbreak in Quebec, Canada, resulted in better parent and teen knowledge of disease severity and vaccine efficacy [Citation40].
The absence of a recommendation from an HCP to be vaccinated against IMD or other diseases is associated with non-receipt of vaccinations and low parental uptake of vaccines [Citation41–50]. In addition, parental intentions to vaccinate against IMD or other diseases are increased after receiving a clinician recommendation, and vaccination rates are higher among children/adolescents whose parents receive such recommendations from a clinician [Citation42,Citation46–50]. Parents may associate the lack of a recommendation with safety issues or lack of experience, usefulness, or necessity.
Misinformation from sources other than HCPs, particularly on the internet and via social media, may influence vaccination decisions [Citation51]. Although the majority (62%) of adolescents (aged 14–18 years) consider HCPs to be the most trusted source of vaccine information, more than half of the information they receive about vaccination comes from other sources [Citation52].
Concerns about vaccine safety are cited by parents as barriers to vaccination. In a survey of French parents’ attitudes about the MenB vaccine, the short history of experience with the vaccine and their fear of side effects were the most frequently cited reasons for refusing vaccination [Citation25]. In a separate French survey of 1000 mothers of children aged 24–35 months, the most frequently reported barriers to meningitis C vaccination were a fear of side effects and a lack of information about the vaccine [Citation53].
Cost and reluctance to pay have been shown to be barriers to vaccination against IMD and other diseases [Citation54–56]. Parents may be unable to afford the vaccinations, may be unwilling to pay, or may not understand why they should spend money to protect their children from a disease that they perceive to be rare. Some vaccines against IMD are not mandatory as part of national vaccination schedules, which may lead to parents perceiving these vaccines to be of low value to their children's health.
Vaccine hesitancy, a concept that encompasses lack of confidence, complacency, and inconvenience associated with vaccines, is a general barrier to vaccination [Citation57–60] and is considered to be one of the top 10 threats to global health according to the World Health Organization [Citation61]. Low vaccine confidence has been shown to be related to loss of trust in the healthcare system, public health organizations, vaccine providers, and vaccines themselves, in addition to misinformation and misconceptions about vaccine-preventable diseases [Citation62].
3.2. Barriers for healthcare providers
In a cross-sectional survey of 293 HCPs in Campania, Italy, the HCPs generally demonstrated good knowledge of the incidence and lethality of IMD but were less informed about the age groups who are at the highest risk of IMD and the serogroups that are most frequently involved [Citation63]. Moreover, HCPs had poor knowledge of the safety profile of the MenB vaccine and what age groups were eligible to receive the MenB vaccine [Citation63]. In addition, a separate cross-sectional survey of 200 pediatricians in Italy demonstrated that 96% of pediatricians were aware of the availability of 4CMenB in Italy, but only 28% were aware of the vaccination schedule for children aged≤2 years [Citation64]. These studies suggest that there may be an educational need for HCPs to fully understand the age groups who are at the highest risk of IMD and are eligible to be vaccinated, the serogroups that are most frequently involved, the safety profile of the MenB vaccine, and the vaccination schedules for different age groups.
A survey of 660 pediatricians and family physicians in the US demonstrated that pediatricians were more likely than family physicians to discuss MenB vaccination during routine visits [Citation65]. The most frequently reported reasons for not recommending MenB vaccination included its non-mandatory recommendation by ACIP, the consistency of reimbursement, and the preexisting ACIP recommendation for another vaccine against IMD (MenACWY) for the same age group [Citation65].
In a cross-sectional survey of 231 pediatricians in Austria, the reasons for a reluctance to co-administer the 4CMenB vaccine with other vaccines were a lack of experience with 4CMenB, an assumption that parents would not provide consent, and the desire for an explicit recommendation on the timing of administration in the Austrian Immunisation Plan [Citation66].
3.3. Organizational barriers
Organizational factors can be barriers to vaccination, such as scheduling of appointments, maintenance of immunization records, availability of the vaccine, and coordination with community resources [Citation56]. Moreover, access to healthcare may be influenced by geographical factors and proximity to healthcare facilities. In the US, suboptimal vaccine uptake in adolescents and young adults has been observed for meningococcal diseases among marginalized communities despite the ACIP recommendations, with a systematic review reporting geographic disparities as one of the factors impacting MenACWY and MenB vaccination coverage [Citation67]. Similarly, lower vaccine coverage rates and higher rates of notification and hospitalizations for vaccine preventable diseases have been reported for Aboriginal and Torres Strait Islander people living in rural and remote areas of Australia compared with non-Aboriginal people [Citation68].
4. How to address the challenge in clinical practice
Given the knowledge gap and the role of education in overcoming barriers to vaccination and increasing the intention to vaccinate, HCPs have a crucial role to play in narrowing this gap and ensuring that parents are aware of their options to protect their children against IMD.
HCPs can do numerous things to address the knowledge gap in IMD, overcome barriers to vaccination, and maximize immunization opportunities. First among these is making IMD vaccination education a priority, for parents as well as adolescents [Citation25,Citation33]. Communicating in a confident, concise, and consistent manner is important [Citation69,Citation70]. When discussing IMD vaccination, HCPs should use language that is easy to understand and tailored to individual parents or adolescents, and should be as direct as possible in order to demonstrate their confidence in their recommendations and to build trust [Citation70]. Moreover, the journey from the recommendation to vaccinate by an HCP to receiving the vaccine should be as simple and convenient as possible [Citation71–73].
Several communication strategies are effective in the context of IMD vaccination. Presenting effectiveness in terms of outcomes that have the most impact (e.g. reduced mortality, hospitalization, absenteeism, and disruption to daily life) is recommended when discussing IMD vaccination with parents [Citation74]. Tailoring communications about vaccination to groups who are at risk of low IMD vaccine uptake is potentially useful. For example, using presumptive language that assumes that parents are ready to vaccinate (”announcements”) has been shown to be significantly more effective for increasing vaccination rates than engaging parents in open-ended discussions (”conversations”) and is recommended as a routine practice when discussing IMD vaccination with parents [Citation69,Citation75]. Motivational interviewing techniques, which aim to support decision-making by eliciting and strengthening an individual’s motivation to change their behavior based on their own arguments for change, have also been shown to be significantly effective in overcoming vaccine hesitancy in parents and could be used to increase the uptake of vaccines against IMD [Citation76,Citation77]. In addition, HCPs should be aware of the diverse needs of various communities that may have low trust in HCPs. Listening to their questions and providing appropriate reassurance may be important in improving attitudes toward vaccination [Citation78].
The inclusion and schedule of meningococcal vaccines within NIPs currently varies by country, potentially affecting the extent of public awareness regarding the existing vaccines. The incorporation of IMD vaccines into NIPs will be crucial to addressing the public health need for protection against meningococcal disease, as well as to providing clear guidance to HCPs and parents/guardians, and to reducing inequity of access to IMD vaccines. Beyond NIPs, reimbursement for the cost of vaccination, particularly for disadvantaged individuals, would likely contribute to increasing the uptake of IMD vaccines [Citation78].
Improvements in the use of technology can create immunization opportunities [Citation79,Citation80]. For example, embedding screening tools in electronic medical records could prompt HCPs to discuss IMD vaccination with parents and adolescents [Citation79]. In addition, text messaging has been shown to be an acceptable reminder for childhood IMD vaccinations for a majority of parents [Citation80]. Indeed, a survey in the USA showed that the majority of parents were interested in receiving text or e-mail notifications from their doctor regarding their child’s eligibility for vaccines against IMD [Citation26]. Social media platforms can also be leveraged, such as in the case of the ”Meningitis bewegt” awareness campaign in Germany, which educates the general public on IMD and encourages parents to discuss vaccination with their child’s doctor [Citation81].
5. Conclusion
Our survey demonstrated that parents generally have a good understanding of IMD and its associated sequelae, but that a knowledge gap exists regarding the different serogroups of IMD and the multiple vaccines that are required to provide full protection against all of these serogroups. Many barriers to IMD vaccine uptake currently exist, related to parents, teenagers, HCPs, and organizations. These barriers may be reduced through educating HCPs on the relevant guidelines and the availability of IMD vaccines; confident, concise, and clear communications and recommendations to parents by HCPs about IMD vaccines; and the use of technology and disease-awareness initiatives that engage parents through physical and digital channels. Further studies are warranted to assess the impact of the COVID-19 pandemic on IMD vaccine uptake, including the impact on attitudes toward IMD vaccination. The pentavalent MenABCWY vaccine is currently under development and has the potential to extend the public health benefits through broad IMD protection programs encompassing multiple serogroups [Citation82]. Closing the knowledge gap has the potential to significantly increase IMD vaccine uptake and reduce the burden of IMD across the world.
6. Expert opinion
The results of the survey show that in some high- and middle-income countries, parents/guardians are generally aware of invasive meningococcal disease (IMD) but are less informed on the different serogroups of Neisseria meningitidis and types of meningococcal vaccines available. These findings are cause for both optimism and concern and have important implications for vaccine uptake and protection against IMD, calling for interventions aimed at addressing the knowledge gap.
Interventions aimed at supporting healthcare providers (HCPs) in discussing the different meningococcal serogroups and vaccines with parents/guardians will be key to improving communication and uptake of vaccination to ultimately maximize IMD prevention. It is concerning that some parents/guardians do not report having access to comprehensive information from HCPs about the different serogroups and types of vaccines available. The underlying reasons for this may include limited knowledge and/or reluctance of HCPs to discuss options with parents/guardians, and more practical considerations, such as time constraints and automated healthcare prompts being limited to mandatory vaccines. Additionally, interventions aimed at increasing awareness amongst parents/guardians may prompt more proactive, comprehensive, and bidirectional discussions with HCPs. Accordingly, educational efforts should support both HCPs and patients.
The powerful impact of improving awareness through coordinated efforts between patients, clinicians, and institutions has been shown by organizations such as the Meningitis Research Foundation (MRF) and the Confederation of Meningitis Organisations (CoMO) [Citation83–85]. In the UK, the MRF raises awareness with public distribution of information on the available vaccines and on recognizing the signs and symptoms of meningitis and sepsis [Citation86,Citation87]. Additionally, the MRF collaborated with Meningitis Now to help drive the introduction of the 4CMenB vaccine into the national routine childhood vaccination program by raising awareness of the lifelong impact of meningitis B through the ”Counting the Cost” (MRF), ”Where’s our vaccine?” (MRF), and ”Beat it Now!” (Meningitis Now) campaigns [Citation83,Citation84,Citation88–90]. The campaigns included petitions, cost evaluations, letters from clinicians, scientists, and professional medical bodies to health authorities, social media efforts, and events for Members of Parliament [Citation88,Citation91]. Following the introduction of 4CMenB vaccination in 2015 for infants aged<1 year [Citation92], the advocacy efforts continued with a petition and press releases aimed at extending the age range, including a 10-point action plan for the government [Citation88,Citation91]. Following a debate in Parliament, an ongoing national clinical trial has been funded to evaluate the impact of 4CMenB or MenB-FHbp vaccination on pharyngeal carriage of meningococci in adolescents [Citation91,Citation93]. The UK provided the first evidence of the real-world impact of including 4CMenB in a national routine childhood vaccination program, demonstrating a 75% reduction in incidence of meningitis B in England after 3 years in age groups eligible for vaccination [Citation92,Citation94]. The success of 4CMenB vaccination adds to that of meningitis C vaccination in Australia, Canada, and several countries in Europe, and meningitis A vaccination in Africa [Citation83,Citation92].
It is hoped that raising awareness of vaccination success with 4CMenB among patients and HCPs will not only increase vaccine uptake but will also contribute to the integration of meningococcal vaccines into national routine childhood vaccination programs. This is particularly relevant following the COVID-19 pandemic, with evidence of reduced rates of uptake of several routine childhood vaccines having been reported by the UK Health Security Agency [Citation95]. The importance of awareness initiatives and advocacy efforts has been acknowledged by the Global Meningococcal Initiative group, with several clinicians and scientists from around the world agreeing that support for such organizations and activities should be included in the Global Recommendations for Meningococcal Disease [Citation83].
To address the knowledge gap, educational interventions aimed at parents/guardians, adolescents, and HCPs will need to be feasible and effective. Awareness and education initiatives should be tailored and targeted, taking into consideration the increased risk for certain serogroups based on sociodemographic and behavioral factors, including the communal lifestyle adopted by many students [Citation83,Citation96,Citation97]. In addition, meningococcal vaccinations should be made available before events that are attended by large numbers of young people [Citation83]. Targeting vaccine misinformation on social media and leveraging the positive effect of mass media coverage may further promote vaccine uptake in adolescents and acceptance in parents/guardians [Citation98,Citation99]. Educational interventions could cover all serogroups simultaneously, introduce individual serogroups successively, or use a combined approach.
Over the next 5 years, further research should focus on recognizing the barriers for HCPs to sharing more comprehensive vaccine information with parents/guardians, understanding the aspects of IMD that parents/guardians and adolescents would like to be better informed about, identifying the most effective formats for delivering educational interventions, and improving HCP education on vaccination, especially in countries where meningococcal vaccines are not currently included in the respective NIP. It is hoped that recognizing the barriers to vaccination and developing feasible and effective awareness and education initiatives will increase acceptance and uptake of meningococcal vaccines. The pentavalent MenABCWY vaccine currently in late-stage development has the potential to provide further public health benefits through broad IMD protection programs encompassing serogroups A, B, C, W, and Y [Citation82]. Collectively, these educational efforts could make a substantial contribution to achieving the World Health Organization’s goals set out by the Defeating Meningitis by 2030 global road map, including a reduction of vaccine-preventable bacterial meningitis by 50% and deaths by 70% [Citation31].
Article highlights
Invasive meningococcal disease (IMD) is a leading cause of life-threatening bacterial meningitis and septicemia
Effective vaccines against several meningococcal serogroups are widely approved and recommended to prevent IMD; this includes vaccines against serogroup B, which is the most common serogroup in many countries in the Americas, Europe, and the Western Pacific
Previous studies revealed a limited understanding among parents/guardians regarding IMD and available vaccines, and this could represent a barrier to vaccination of their children
The findings of this online survey of parents/guardians around the world and the available literature highlighted a knowledge gap among parents/guardians, teenagers, healthcare providers, and organizations regarding IMD and the available vaccines, including vaccines against the highly prevalent serogroup B
We propose solutions to address this knowledge gap, including education of HCPs, clear communication to parents by HCPs, and disease-awareness initiatives
Declaration of interest
I Ballalai has received honoraria from AstraZeneca, GSK, Pfizer, and Sanofi, M Horn has received honoraria from AstraZeneca, Bavarian Nordic, GSK, MSD, Novartis Vaccines, Pfizer, and Sanofi. V Smith has received honoraria from GSK and Sanofi, which was paid to the Meningitis Research Foundation, and travel grants from Pfizer. N Vicic is currently an employee of Moderna (Associate Medical Director, Medical Affairs) and holds stock options. R Bekkat-Berkani and L Soumahoro are employees of GSK and hold stock options. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download (21.3 KB)Acknowledgments
Medical writing support was provided by Matthew Reynolds and Silvia Pregnolato of Apollo, OPEN Health Communications, funded by GSK Biologicals SA, in accordance with Good Publication Practice 3 (GPP) guidelines (www.ismpp.org/gpp-2022).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2023.2211163.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Rappuoli R, Pizza M, Masignani V, et al. Meningococcal B vaccine (4CMenB): the journey from research to real world experience. Expert Rev Vaccines. 2018;17(12):1111–1121.DOI:10.1080/14760584.2018.1547637
- Cohn AC, MacNeil JR, Clark TA, et al. Prevention and control of meningococcal disease: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2013;62(Rr–2):1–28.
- Thigpen MC, Whitney CG, Messonnier NE, et al. Bacterial meningitis in the United States, 1998-2007. N Engl J Med. 2011;364(21):2016–2025. DOI:10.1056/NEJMoa1005384
- Wang B, Santoreneos R, Giles L, et al. Case fatality rates of invasive meningococcal disease by serogroup and age: a systematic review and meta-analysis. Vaccine. 2019;37(21):2768–2782. DOI:10.1016/j.vaccine.2019.04.020
- Olbrich KJ, Muller D, Schumacher S, et al. Systematic review of invasive meningococcal disease: sequelae and quality of life impact on patients and their caregivers. Infect Dis Ther. 2018;7(4):421–438. DOI:10.1007/s40121-018-0213-2
- European Centre for Disease Prevention and Control (ECDC). Surveillance report: invasive meningococcal disease – annual epidemiological report for 2017. Available from: https://ecdc.europa.eu/en/publications-data/invasive-meningococcal-disease-annual-epidemiological-report-2017.
- Buysse CM, Raat H, Hazelzet JA, et al. Surviving meningococcal septic shock: health consequences and quality of life in children and their parents up to 2 years after pediatric intensive care unit discharge. Crit Care Med. 2008;36(2):596–602. DOI:10.1097/01.CCM.0000299740.65484.CA
- Buysse CM, Oranje AP, Zuidema E, et al. Long-term skin scarring and orthopaedic sequelae in survivors of meningococcal septic shock. Arch Dis Child. 2009;94(5):381–386. DOI:10.1136/adc.2007.131862
- Edmond K, Clark A, Korczak VS, et al. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(5):317–328. DOI:10.1016/S1473-3099(10)70048-7
- World Health Organization. Intersectoral global action plan on epilepsy and other neurological disorders 2022-2031. Available from: https://cdn.who.int/media/docs/default-source/brain-health/first-draft-action-plan-on-epilepsy-and-other-neurological-disorders_180621_en.pdf?sfvrsn=16474e26_24&download=true.
- Shen J, Begum N, Ruiz-Garcia Y, et al. Range of invasive meningococcal disease sequelae and health economic application - a systematic and clinical review. BMC Public Health. 2022;22(1):1078. DOI:10.1186/s12889-022-13342-2
- Rosenstein NE, Perkins BA, Stephens DS, et al. Meningococcal disease. N Engl J Med. 2001;344(18):1378–1388. DOI:10.1056/NEJM200105033441807
- Peterson ME, Li Y, Bita A, et al. Meningococcal serogroups and surveillance: a systematic review and survey. J Glob Health. 2019;9(1):010409. DOI:10.7189/jogh.09.010409
- Xie O, Pollard AJ, Mueller JE, et al. Emergence of serogroup X meningococcal disease in Africa: need for a vaccine. Vaccine. 2013;31(27):2852–2861. DOI:10.1016/j.vaccine.2013.04.036
- Pizza M, Bekkat-Berkani R, Rappuoli R. Vaccines against meningococcal diseases. Microorganisms. 2020;8(10):1521. DOI:10.3390/microorganisms8101521
- Findlow J, Nuttens C, Kriz P. Introduction of a second MenB vaccine into Europe - needs and opportunities for public health. Expert Rev Vaccines. 2019;18(3):225–239.
- Safadi MAP, Martinón-Torres F, Serra L, et al. Translating meningococcal serogroup B vaccines for healthcare professionals. Expert Rev Vaccines. 2021;20(4):401–414. DOI:10.1080/14760584.2021.1899820
- Ruiz García Y, Sohn WY, Seib KL, et al. Looking beyond meningococcal B with the 4CMenB vaccine: the Neisseria effect. NPJ Vaccines. 2021;6(1):130. DOI:10.1038/s41541-021-00388-3
- Mbaeyi SB, Duffy J, Duffy J, et al. Meningococcal vaccination: recommendations of the advisory committee on immunization practices, United States, 2020. (Ed.^(Eds). 2020;69(9):1–41. DOI:10.15585/mmwr.rr6909a1
- World Health Organization. Immunization coverage. Available from: https://www.who.int/news-room/fact-sheets/detail/immunization-coverage.
- Trotter CL, Lingani C, Fernandez K, et al. Impact of MenAfriVac in nine countries of the African meningitis belt, 2010-15: an analysis of surveillance data. Lancet Infect Dis. 2017;17(8):867–872. DOI:10.1016/S1473-3099(17)30301-8
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(35):1183–1190. DOI:10.15585/mmwr.mm7035a1
- Wang B, Clarke M, Afzali HH, et al. Community, parental and adolescent awareness and knowledge of meningococcal disease. Vaccine. 2014;32(18): 2042–2049.
- Jackson C, Yarwood J, Saliba V, et al. UK parents’ attitudes towards meningococcal group B (MenB) vaccination: a qualitative analysis. BMJ Open. 2017;7(4):e012851. DOI:10.1136/bmjopen-2016-012851
- Le Ngoc Tho S, Ader F, Ferry T, et al. Vaccination against serogroup B Neisseria meningitidis: perceptions and attitudes of parents. Vaccine. 2015;33(30):3463–3470. DOI:10.1016/j.vaccine.2015.05.073
- Basta NE, Becker AB, Li Q, et al. Parental awareness of Meningococcal B vaccines and willingness to vaccinate their teens. Vaccine. 2019;37(4):670–676. DOI:10.1016/j.vaccine.2018.11.078
- Drozd-Dabrowska M, Topczewska K, Korzen M, et al. Parental knowledge about meningococcal disease and vaccination uptake among 0-5 years old Polish children. Int J Environ Res Public Health. 2019;16(2):265. DOI:10.3390/ijerph16020265
- Bakhache P, Rodrigo C, Davie S, et al. Health care providers’ and parents’ attitudes toward administration of new infant vaccines–a multinational survey. Eur J Pediatr. 2013;172(4):485–492. DOI:10.1007/s00431-012-1904-4
- Marshall H, Ryan P, Roberton D, et al. A cross-sectional survey to assess community attitudes to introduction of human papillomavirus vaccine. Aust N Z J Public Health. 2007;31(3):235–242. DOI:10.1111/j.1467-842X.2007.00054.x
- Coyne-Beasley T, Reiter PL, Liberty AC, et al. Awareness is not enough: the need to increase meningococcal vaccine uptake. Clin Pediatr (Phila). 2013;52(5):441–450. DOI:10.1177/0009922813481847
- World Health Organization. Defeating meningitis by 2030; 2021. Available from: https://www.who.int/publications/i/item/9789240026407
- Morrone T, Napolitano F, Albano L, et al. Meningococcal serogroup B vaccine: knowledge and acceptability among parents in Italy. Hum Vaccin Immunother. 2017;13(8):1921–1927. DOI:10.1080/21645515.2017.1313940
- Pelullo CP, Napolitano F, Di Giuseppe G. Meningococcal disease and vaccination: knowledge and acceptability among adolescents in Italy. Hum Vaccin Immunother. 2018;14(5):1197–1202. DOI:10.1080/21645515.2018.1436918
- Timmermans DR, Henneman L, Hirasing RA, et al. Attitudes and risk perception of parents of different ethnic backgrounds regarding meningococcal C vaccination. Vaccine. 2005;23(25):3329–3335. DOI:10.1016/j.vaccine.2005.01.075
- Richardson E, Ryan KA, Lawrence RM, et al. Perceptions and knowledge about the MenB vaccine among parents of high school students. J Community Health. 2021;46(4):808–816. DOI:10.1007/s10900-020-00954-1
- Martinón-Torres F, Taha MK, Knuf M, et al. Evolving strategies for meningococcal vaccination in Europe: overview and key determinants for current and future considerations. Pathog Glob Health. 2022;116(2):85–98. DOI:10.1080/20477724.2021.1972663
- Aparecido Nunes A, De Jesus Lopes De Abreu A, Cintra O, et al. Meningococcal disease epidemiology in Brazil (2005-2018) and impact of MenC vaccination. Vaccine. 2021;39(3):605–616. DOI:10.1016/j.vaccine.2020.11.067
- Guzman-Holst A, DeAntonio R, Prado-Cohrs D, et al. Barriers to vaccination in Latin America: a systematic literature review. Vaccine. 2020;38(3):470–481. DOI:10.1016/j.vaccine.2019.10.088
- van Lier A, Ferreira JA, Mollema L, et al. Intention to vaccinate universally against varicella, rotavirus gastroenteritis, meningococcal B disease and seasonal influenza among parents in the Netherlands: an internet survey. BMC Res Notes. 2017;10(1):672. DOI:10.1186/s13104-017-3004-z
- Dube E, Gagnon D, Hamel D, et al. Parents’ and adolescents’ willingness to be vaccinated against serogroup B meningococcal disease during a mass vaccination in saguenay-lac-st-jean (Quebec). Can J Infect Dis Med Microbiol. 2015;26(3):163–167. DOI:10.1155/2015/732464
- Dorell C, Yankey D, Strasser S. Parent-reported reasons for nonreceipt of recommended adolescent vaccinations, national immunization survey: teen, 2009. Clin Pediatr (Phila). 2011;50(12):1116–1124.
- Reiter PL, Gilkey MB, Brewer NT. HPV vaccination among adolescent males: results from the national immunization survey-teen. Vaccine. 2013;31(26):2816–2821.
- Stokley S, Cohn A, Dorell C, et al. Adolescent vaccination-coverage levels in the United States: 2006-2009. Pediatrics. 2011;128(6):1078–1086. DOI:10.1542/peds.2011-1048
- Sonawane K, Zhu Y, Montealegre JR, et al. Parental intent to initiate and complete the human papillomavirus vaccine series in the USA: a nationwide, cross-sectional survey. Lancet Public Health. 2020;5(9):e484–492. DOI:10.1016/S2468-2667(20)30139-0
- Lindley MC, Jeyarajah J, Yankey D, et al. Comparing human papillomavirus vaccine knowledge and intentions among parents of boys and girls. Hum Vaccin Immunother. 2016;12(6):1519–1527. DOI:10.1080/21645515.2016.1157673
- Dorell C, Yankey D, Kennedy A, et al. Factors that influence parental vaccination decisions for adolescents, 13 to 17 years old: national immunization survey-teen, 2010. Clin Pediatr (Phila). 2013;52(2):162–170. DOI:10.1177/0009922812468208
- Perkins RB, Lin M, Silliman RA, et al. Why are U.S. girls getting meningococcal but not human papilloma virus vaccines? Comparison of factors associated with human papilloma virus and meningococcal vaccination among adolescent girls 2008 to 2012. Womens Health Issues. 2015;25(2):97–104. DOI:10.1016/j.whi.2014.12.005
- Rahman M, Laz TH, McGrath CJ, et al. Provider recommendation mediates the relationship between parental human papillomavirus (HPV) vaccine awareness and HPV vaccine initiation and completion among 13- to 17-year-old U.S. adolescent children. Clin Pediatr (Phila). 2015;54(4):371–375. DOI:10.1177/0009922814551135
- Reiter PL, McRee AL, Pepper JK, et al. Longitudinal predictors of human papillomavirus vaccination among a national sample of adolescent males. Am J Public Health. 2013;103(8):1419–1427. DOI:10.2105/AJPH.2012.301189
- Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the US national immunization survey. Am J Public Health. 2013;103(1):164–169.
- Bernstein HH, Bocchini JA Jr, Byington CL. Committee on infectious diseases. The need to optimize adolescent immunization. Pediatrics. 2017;139(3):e20164186.
- Griffin DS, Muhlbauer G, Griffin DO. Adolescents trust physicians for vaccine information more than their parents or religious leaders. Heliyon. 2018;4(12):e01006.
- Gaudelus J, Cohen R, Leboucher B, et al. Meningococcal C vaccine coverage in France in infants, children, and adolescents. Med Mal Infect. 2019;49(3):180–186. DOI:10.1016/j.medmal.2019.01.014
- Marshall HS, Chen G, Clarke M, et al. Adolescent, parent and societal preferences and willingness to pay for meningococcal B vaccine: a discrete choice experiment. Vaccine. 2016;34(5):671–677. DOI:10.1016/j.vaccine.2015.11.075
- Lavelle TA, Messonnier M, Stokley S, et al. Use of a choice survey to identify adult, adolescent and parent preferences for vaccination in the United States. J Patient Rep Outcomes. 2019;3(1):51. DOI:10.1186/s41687-019-0135-0
- Vu M, King AR, Jang HM, et al. Practice-, provider- and patient-level facilitators of and barriers to HPV vaccine promotion and uptake in Georgia: a qualitative study of healthcare providers’ perspectives. Health Educ Res. 2020;35(6):512–523. DOI:10.1093/her/cyaa026
- Bedford H, Attwell K, Danchin M, et al. Vaccine hesitancy, refusal and access barriers: the need for clarity in terminology. Vaccine. 2018;36(44):6556–6558. DOI:10.1016/j.vaccine.2017.08.004
- Roberts JR, Thompson D, Rogacki B, et al. Vaccine hesitancy among parents of adolescents and its association with vaccine uptake. Vaccine. 2015;33(14):1748–1755. DOI:10.1016/j.vaccine.2015.01.068
- Strelitz B, Gritton J, Klein EJ, et al. Parental vaccine hesitancy and acceptance of seasonal influenza vaccine in the pediatric emergency department. Vaccine. 2015;33(15):1802–1807. DOI:10.1016/j.vaccine.2015.02.034
- Williams SE. What are the factors that contribute to parental vaccine-hesitancy and what can we do about it? Hum Vaccin Immunother. 2014;10(9):2584–2596.
- World Health Organization. Ten Threats to global health in 2019. Available from: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
- Badur S, Ota M, Ozturk S, et al. Vaccine confidence: the keys to restoring trust. Hum Vaccin Immunother. 2020;16(5):1007–1017. DOI:10.1080/21645515.2020.1740559
- Ponticelli D, D’Ambrosio A, Cancellieri M, et al. Do HCWs adequately know about meningitis and 4CMenB vaccine and recommend its use to parents? A cross sectional analysis in Campania region, Italy. J Prev Med Hyg. 2019;60(2):E147–157. DOI:10.15167/2421-4248/jpmh2019.60.2.1018
- Ferrara P, Stromillo L, Albano L. Awareness, attitudes, and practices toward meningococcal B vaccine among pediatricians in Italy. Med (Kaunas). 2018;54(6):100.
- Kempe A, Allison MA, MacNeil JR, et al. Adoption of serogroup B meningococcal vaccine recommendations. Pediatrics. 2018;142(3):e20180344. DOI:10.1542/peds.2018-0344
- Wagner A, Kundi M, Zwiauer K, et al. Paediatricians require more information before they routinely co-administer the meningococcal B vaccine with routine infant vaccines. Acta Paediatr. 2015;104(10):e439–447. DOI:10.1111/apa.13100
- Masaquel C, Schley K, Wright K, et al. The impact of social determinants of health on meningococcal vaccination awareness, delivery, and coverage in adolescents and young adults in the United States: a systematic review. Vaccines (Basel). 2023;11(2):256. DOI:10.3390/vaccines11020256
- Middleton BF, Davies J, Webby R. Vaccine success and challenges in northern Australia. Microbiol Aust. 2022;43(3):113–116.
- Brewer NT, Hall ME, Malo TL, et al. Announcements versus conversations to improve HPV vaccination coverage: a randomized trial. Pediatrics. 2017;139(1):e20161764. DOI:10.1542/peds.2016-1764
- Kaufman J, Ryan R, Walsh L, et al. Face-to-face interventions for informing or educating parents about early childhood vaccination. Cochrane Database Syst Rev. 2018;5(5):Cd010038. DOI:10.1002/14651858.CD010038.pub3
- Cox AD, Cox D, Cyrier R, et al. Can self-prediction overcome barriers to hepatitis B vaccination? A randomized controlled trial. Health Psychol. 2012;31(1):97–105. DOI:10.1037/a0025298
- Smedley J, Poole J, Waclawski E, et al. Influenza immunisation: attitudes and beliefs of UK healthcare workers. Occup Environ Med. 2007;64(4):223–227. DOI:10.1136/oem.2005.023564
- Brown K, Fraser G, Ramsay M, et al. Attitudinal and demographic predictors of measles-mumps-rubella vaccine (MMR) uptake during the UK catch-up campaign 2008-09: cross-sectional survey. PLoS ONE. 2011;6(5):e19381. DOI:10.1371/journal.pone.0019381
- Bekkat-Berkani R, Romano-Mazzotti L. Understanding the unique characteristics of seasonal influenza illness to improve vaccine uptake in the US. Vaccine. 2018;36(48):7276–7285.
- Jacobson RM, St Sauver JL, Griffin JM, et al. How health care providers should address vaccine hesitancy in the clinical setting: evidence for presumptive language in making a strong recommendation. Hum Vaccin Immunother. 2020;16(9):2131–2135. DOI:10.1080/21645515.2020.1735226
- Gagneur A. Motivational interviewing: a powerful tool to address vaccine hesitancy. Can Commun Dis Rep. 2020;46(4):93–97.
- Gagneur A, Lemaitre T, Gosselin V, et al. A postpartum vaccination promotion intervention using motivational interviewing techniques improves short-term vaccine coverage: PromoVac study. BMC Public Health. 2018;18(1):811. DOI:10.1186/s12889-018-5724-y
- Taha MK, Martinon-Torres F, Köllges R, et al. Equity in vaccination policies to overcome social deprivation as a risk factor for invasive meningococcal disease. Expert Rev Vaccines. 2022;21(5):659–674. DOI:10.1080/14760584.2022.2052048
- Harris SK, Aalsma MC, Weitzman ER, et al. Research on clinical preventive services for adolescents and young adults: where are we and where do we need to go? J Adolesc Health. 2017;60(3):249–260. DOI:10.1016/j.jadohealth.2016.10.005
- Hofstetter AM, Vargas CY, Kennedy A, et al. Parental and provider preferences and concerns regarding text message reminder/recall for early childhood vaccinations. Prev Med. 2013;57(2):75–80. DOI:10.1016/j.ypmed.2013.04.007
- GSK. Meningitis bewegt. Available from: https://www.meningitis-bewegt.de/
- ClinicalTrials.gov. NCT04502693. Study to assess effectiveness of GlaxoSmithKline’s (Gsk’s) meningococcal group B and combined ABCWY vaccines in healthy adolescents and young adults. Available from: https://clinicaltrials.gov/ct2/show/NCT04502693
- Borrow R, Alarcón P, Carlos J, et al. The global Meningococcal initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 2017;16(4):313–328. DOI:10.1080/14760584.2017.1258308
- Meningitis research foundation. About us. About us: https://www.meningitis.org/about-us
- Confederation of meningitis organisations. About us. About us: http://www.comomeningitis.org/about-us/
- Meningitis research foundation. One life, one shot. Available from: https://www.meningitis.org/our-work/advocacy-and-campaigning/one-life-one-shot.
- Meningitis research foundation. Report highlights meningitis being missed. Available from: https://www.meningitis.org/news/safety-netting
- Meningitis now. Beat it now! campaign timeline. Available from: https://www.meningitisnow.org/support-us/news-centre/public-affairs/campaigns/beat-it-now/campaign-timeline/
- Meningitis research foundation. Where’s our vaccine?. Available from: https://www.meningitis.org/our-work/advocacy-and-campaigning/wheres-our-vaccine
- Meningitis Research Foundation. Counting the costs. Available from: https://www.meningitis.org/our-work/completed-projects/counting-the-costs
- Borrow R, Caugant DA, Ceyhan M, et al. Meningococcal disease in the middle east and Africa: findings and updates from the global Meningococcal initiative. J Infect. 2017;75(1):1–11. DOI:10.1016/j.jinf.2017.04.007
- Sohn WY, Tahrat H, Novy P, et al. Real-world implementation of 4-component meningococcal serogroup B vaccine (4CMenB): implications for clinical practices. Expert Rev Vaccines. 2022;21(3):325–335. DOI:10.1080/14760584.2022.2021881
- Carr J, Plested E, Aley P, et al. “Be on the TEAM“ study (teenagers against meningitis): protocol for a controlled clinical trial evaluating the impact of 4CMenB or MenB-fHbp vaccination on the pharyngeal carriage of meningococci in adolescents. BMJ Open. 2020;10(10):e037358. DOI:10.1136/bmjopen-2020-037358
- Ladhani SN, Andrews N, Parikh SR, et al. Vaccination of infants with meningococcal group B vaccine (4CMenB) in England. N Engl J Med. 2020;382(4):309–317. DOI:10.1056/NEJMoa1901229
- UK Health Security Agency. Impact of COVID-19 on childhood vaccination counts up to week 6; 2021. Available from: https://www.gov.uk/government/publications/covid-19-impact-on-vaccination-programmes/impact-of-covid-19-on-childhood-vaccination-counts-up-to-week-6-2021
- Snyder Bulik B. GSK’s message to college-bound kids and their parents: get meningitis B vaccine; 2018. Available from: https://www.fiercepharma.com/marketing/gsk-push-to-college-bound-kids-and-their-parents-get-men-b-vaccinated
- Blagden S, Seddon D, Hungerford D, et al. Uptake of a new meningitis vaccination programme amongst first-year undergraduate students in the United Kingdom: a cross-sectional study. PLoS ONE. 2017;12(8):e0181817. DOI:10.1371/journal.pone.0181817
- Wilson SL, Wiysonge C. Social media and vaccine hesitancy. BMJ Glob Health. 2020;5(10):e004206.
- Bíró A, Szabó-Morvai Á. Mass media coverage and vaccination uptake: evidence from the demand for meningococcal vaccinations in Hungary. Eur J Health Econ. 2021;22(6):887–903.

