ABSTRACT
Introduction
Invasive meningococcal disease (IMD) is a major health concern which can be prevented through vaccination. Conjugate vaccines against serogroups A, C, W, and Y and two protein-based vaccines against serogroup B are currently available in the European Union.
Areas covered
We present epidemiologic data for Italy, Portugal, Greece, and Spain using publicly available reports from national reference laboratories and national or regional immunization programs (1999–2019), aiming to confirm risk groups, and describe time trends in overall incidence and serogroup distribution, as well as impact of immunization. Analysis of circulating MenB isolates in terms of the surface factor H binding protein (fHbp) using PubMLST is discussed as fHbp represents an important MenB vaccine antigen. Predictions of potential reactivity of the two available MenB vaccines (MenB-fHbp and 4CMenB) with circulating MenB isolates are also provided as assessed using the recently developed MenDeVAR tool.
Expert opinion
Understanding dynamics of IMD and continued genomic surveillance are essential for evaluating vaccine effectiveness, but also prompting proactive immunization programs to prevent future outbreaks. Importantly, the successful design of further effective meningococcal vaccines to fight IMD relies on considering the unpredictable epidemiology of the disease and combining lessons learnt from capsule polysaccharide vaccines and protein-based vaccines.
1. Introduction
Invasive meningococcal disease (IMD) mainly present as septicemia and/or meningitis [Citation1], which can develop within 24 hours [Citation2], and may result in substantial morbidity and mortality, with long-term debilitating sequelae such as neurologic complications, limb loss or hearing loss, and a case-fatality rate (CFR) of 10–15% in developed countries [Citation3,Citation4]. The reported incidence of IMD per 100,000 population varies considerably among geographic regions, ranging from 0.0079 in China during 2015–2017 [Citation5], to 0.13 and 0.62 in 2017 in the United States and Europe [Citation5], respectively, and 10–25 in the African meningitis belt in sub-Saharan Africa between outbreaks or even up to 1000 during large-scale epidemics [Citation5,Citation6].
In most countries, incidence of IMD is highest in infants aged <1 year, while in most geographic regions high incidence is also reported among young children (1–4 years), with a second small peak in adolescents and young adults (15–24 year-olds) [Citation5,Citation7]. Household crowding, close relationships, passive smoking, and recent respiratory tract infections have been identified as important risk factors for IMD in children and adolescents [Citation8]. Factors associated with risk of hospitalization for IMD also include medical conditions such as immunodeficiency, asplenia/hyposplenia, autoimmune diseases, hemophilia and severe chronic respiratory disorders, as well as low household income status [Citation9].
The human-specific Gram-negative diplococcus Neisseria meningitidis (N. meningitidis) is the etiologic agent of IMD. IMD-causing strains of N. meningitidis are almost always enclosed in a capsule and are categorized by their capsular polysaccharides into 12 serogroups [Citation1,Citation10]. The majority of IMD cases worldwide are caused by serogroups A, B, C, W, X, and Y, but serogroup prevalence differs by geographic region. Historically, B and C have been the predominant serogroups in the Americas (also Y in Northern America), Europe, Australia and New Zealand, and serogroup A has been the predominant serogroup associated with outbreaks in the African meningitis belt [Citation6]. More recent data estimate that serogroup B is predominant, accounting for 48.5% of N. meningitidis strains globally, while X represents the least common serogroup, accounting for 0.7% of all [Citation11]. Serogroup B also shows increasing rates over time in developing countries of the Western Pacific, Eastern Mediterranean, and African countries, while a decrease in serogroup C was observed in European and American countries [Citation11].
Besides their use in serogroup classification, capsule polysaccharides of N. meningitidis [Citation1] were also the first targets for vaccines developed for this microbe. Polysaccharide vaccines against serogroups A and C were the first efficacious meningococcal vaccines, developed in the 1960s, later on licensed as quadrivalent (A, C, Y, and W) vaccines as well, which were however poorly immunogenic in infants [Citation12]. This limitation was subsequently addressed by the introduction of polysaccharide-protein conjugate vaccines [Citation12], which were also superior than polysaccharide vaccines in terms of conferring immunologic memory [Citation13] and resulted in herd effects from immunization of adolescents [Citation14,Citation15]. Conjugate vaccines have become widespread in the developed world since the late 1990s and are also available as monovalent (A or C) and as quadrivalent (A, C, W, and Y) vaccines [Citation12].
On the other hand, vaccines against Serogroup B Meningococcal (MenB) strains have been difficult to produce due to antigenic mimicry of group B polysaccharide with human neurologic tissues, which results in either failure to induce effective responses or increased risk of auto-immune responses [Citation16,Citation17]. In search for a MenB vaccine, the outer membrane protein factor H binding protein (fHbp; also known as LP2086) was discovered [Citation18]. fHbp is an important virulence factor [Citation19] present in most meningococcal isolates [Citation20,Citation21] and expressed in sufficient levels to be targeted by vaccine-induced antibodies [Citation22]. fHbp is classified into two subfamilies, A and B, with only 60–75% amino acid sequence similarity between them [Citation18,Citation21]. fHbp has been used to develop two different vaccines [Citation23], namely MenB-fHbp (Trumenba, Pfizer) [Citation24–27] and 4CMenB (Bexsero, GlaxoSmithKline) [Citation28,Citation29]. The MenB-fHbp vaccine is composed of two recombinant lipidated fHbp variants, one from each of the two immunologically distinct subfamilies A and B [Citation30,Citation31], while the 4CMenB contains a nonlipidated fHbp variant from subfamily B in addition to recombinant proteins Neisserial heparin-binding antigen (NHBA) and Neisseria adhesin A (NadA), combined with outer membrane vesicles from the MeNZB vaccine [Citation32].
Both protein-based MenB vaccines have demonstrated bactericidal activity in a serum bactericidal assay with human complement (hSBA). However, this assay is impractical for testing vaccine activity against large numbers of isolates. As a surrogate to provide estimates of susceptibility of MenB isolates to MenB-fHbp-induced antibodies, the MEASURE assay (meningococcal antigen surface expression) has been used [Citation22]. Moreover, for predicting reactivity and potential coverage of 4CMenB, the MATS (Meningococcal Antigen Typing System) assay was originally used, assessing the potency of one or more surface proteins in individual MenB strains cross-reacting with a vaccine component [Citation33–35]. These assays however are not widely or immediately available in clinical settings. In the attempt of predicting the vaccines reactivity, genotypic methods have also been developed, such as genetic MATS (gMATS) and the Bexsero antigen sequence type (BAST) scheme, both 4CMenB-specific and the Meningococcal Deduced Vaccine Antigen Reactivity (MenDeVAR), which also enables genotypic analysis of strain coverage by MenB-fHBP vaccine. Both gMATS and MenDeVAR tools have been developed to predict strain coverage based on genotypic data alone, in the attempt of combining multiple and complex data to inform the reactivity of 4CMenB and MenB-fHbp vaccines against specific variants. There are limitations to using a genomic approach, such as the lacking of protein expression level information or the fact that, in some countries, genetic information are available only for a fraction of the total IMD cases [Citation36].
Though the current incidence of IMD is at historically low levels, time trends indicate small-scale outbreaks can occur across all geographical regions, and serogroup prevalence is ever-changing. In light of the unpredictable nature of IMD outbreaks and historical success in controlling epidemics with immunization strategies, accurate epidemiological data are paramount for the initiation of appropriate prevention strategies. This is also highlighted by the fact that World Health Organization has established the first-ever global strategy to defeat meningitis by 2030, recognizing this target as critical for achieving universal health coverage [Citation37]. This review describes the epidemiology of IMD in Southern European countries, which have similar climate conditions, socioeconomic characteristics and similar healthcare systems but have applied different vaccination strategies. The review has a particular focus on MenB disease, with prospects to inform national vaccination policies.
2. Methods
We used publicly available annual surveillance reports from national reference laboratories for the period 1999–2019 and we reviewed national or regional immunization programs from 1999 until 2022 to retrieve IMD incidence and serogroup distribution through time as well as dates of introduction of meningococcal vaccines in Italy [Citation38–45], Portugal [Citation46–48], Greece [Citation49–52] and Spain [Citation53–60]. IMD has been monitored in Italy since 1993 and is part of the National Surveillance System which is active since 2007 [Citation61]. Ιn Portugal, mandatory clinical notification of IMD has been in effect since 1927, and a laboratory report has been mandatory since 2002 [Citation62]. Notification of IMD is mandatory in Greece while the national reference laboratory has been in operation since 1993 [Citation63,Citation64]. In Spain, IMD has been mandatory notifiable since 1901, with a requirement of immediate reporting to the National Epidemiological Surveillance Network (i.e. RENAVE; Red Nacional de Vigilancia Epidemiológica), which has kept laboratory records since its creation in 1996 [Citation65].
Temporal trends of IMD epidemiology are presented using data as available in the annual reports, i.e. per calendar year for Italy, Portugal and Greece, and per epidemiological year for Spain (from week 41 to week 40 of the following year). The age distribution of IMD incidence was assessed as annual incidence in each age group, further categorized into mean annual incidence of distinct periods roughly representing late 2000s, early 2010s and late 2010s.
This review also made use of the Neisseria PubMLST database [Citation66], which is a publicly accessible online sequence repository available at https://pubmlst.org/organisms/neisseria-spp/. The database was accessed on 15 July 2021 to extract information about the prevalence of factor H-binding protein (fHbp) subfamilies among MenB isolates in Italy and Portugal and Clonal complex distribution as well as prevalence of fHbp, NadA, NHBA and PorA variants among MenB isolates in the four countries. Vaccine antigen fHbp variants among MenB isolates in Italy for the period 2014–2019 have also been reported by Carannante et al., 2020 [Citation67]. Published data and surveillance reports were used for information on fHbp subfamily distribution across MenB isolates in Greece [Citation64,Citation68] and Spain [Citation69]. Data on antigenic variant prevalence was retrieved as available from the sources for the period 1999–2019 and cover the period 2014–2019 for Italy, 2012–2019 for Portugal, 2010–2019 for Greece, 2001–2006, 2009–2010, and 2015–2018 for Spain.
The potential cross-reactivity of MenB vaccine components with MenB isolates in Italy, Portugal, Greece, and Spain was assessed using the MenDeVAR Index (Meningococcal Deduced Vaccine Antigen Reactivity) [Citation36], publicly accessible at https://pubmlst.org/organisms/neisseria-spp/mendevar. Using genotypic and published experimental data, MenDeVAR can estimate vaccine reactivity based on the protein variants of an inserted meningococcal genome assembly. The output includes a classification of the MenB isolate either as an ‘exact match’ (contains ≥1 exact sequence match to vaccine antigenic variants), ‘cross-reactive’ (contains ≥1 antigenic variant deemed cross-reactive to vaccine variants through experimental studies), ‘none’ (all the isolate’s antigenic variants have been deemed not cross-reactive to vaccine variants through experimental studies), and ‘insufficient data’ (contains antigens for which there is insufficient data from or are yet to be tested in experimental studies). Herein, the MenDeVAR Index was applied to MenB isolates as available in PubMLST to assess the potential reactivity of the two MenB vaccines licensed in Europe.
3. Results
3.1. IMD incidence by age groups
Based on available data from the past 9–18 years in the four analyzed countries, incidence of IMD is highest in infants and young children, with rates remaining as high as 3.57, 14.76, 5.44, and 9.65 per 100,000 for ages <1 year in Italy, Portugal, Greece, and Spain over the late 2010s () [Citation38,Citation46,Citation52,Citation53]. A secondary peak is observed in adolescents and young adults (age group 15–24 years) in Greece, Spain and Italy. The above age distribution is similar in all four countries yet pronounced differences in mean annual incidence can be observed among countries using data from overlapping calendar periods.
Figure 1. Annual IMD incidence by age group in Italy (2005–2019), Portugal (2003–2019), Greece (2010–2019), and Spain (2012–2019) IMD, invasive meningococcal disease.
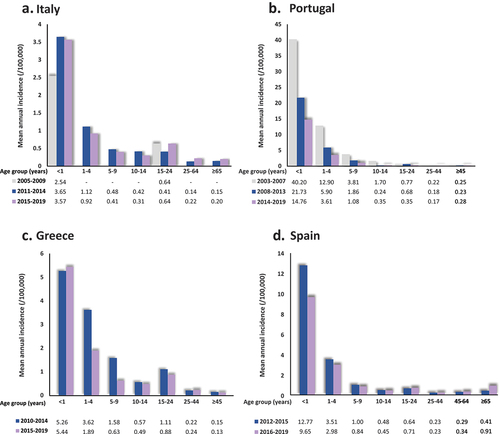
In all four countries, incidence seems to generally have declined in most age groups in the second half of the past decade compared to the first half. On the other hand, incidence has increased in Spain for the age groups 15–24, 45–64, and ≥65 years, and in Italy for the age groups 15–24, 25–64, and ≥65 years. Small increases are also observed over the second half of the past decade in Portugal in the age groups 10–14 and ≥45 years, as well as in Greece for the age group < 1 and 25–44 years.
3.2. IMD incidence and serogroup distribution in the four countries
The annual number of laboratory confirmed IMD cases per serogroup as well as the annual IMD incidence over the past two decades in the four countries are presented in . All four countries display dynamic incidence rates of IMD across the years, with differences observed among them for any specific year. In general, Spain reports the highest and Italy the lowest incidence rates on most years throughout the examined period. More specifically, in Italy, with available data from 2008–2019 (excluding 2009 and 2010) the incidence of IMD per 100,000 persons ranged from a highest of 0.37 cases in 2016 to a lowest of 0.23 cases in 2012 [Citation38]. In Portugal, over the period 2003–2019, annual incidence per 100,000 persons ranged from a highest of 1.40 in 2003 to a lowest of 0.42 in 2016 [Citation46], while in Greece, where available data cover the entire period from 2000 to 2019, annual incidence ranged from a highest of 2.4 cases per 100,000 persons in 2000 to a lowest of 0.29 cases per 100,000 persons recorded in 2019 [Citation52]. For Spain, data are presented per epidemiological season, which extends from week 41 of a calendar year until week 40 of the following. Based on available data from 1999–2020, annual incidence per 100,000 persons in the country ranged from a highest of 4.07 in 1999–2000 to a lowest of 0.53 recorded for 2013–2014 [Citation53].
Figure 2. Annual IMD incidence and number of laboratory confirmed IMD cases by serogroup and evolution of National Immunization Programmes in Italy (2000–2019), Portugal (2003–2019), Greece (2000–2020), and Spain (1999–2000–2019–2020). IMD, invasive meningococcal disease; MenACWY, serogroup a, c, W and Y meningococcal (vaccine); Menb, serogroup B meningococcal (vaccine); Menc, serogroup c meningococcal (vaccine); NIP, National Immunization Program.
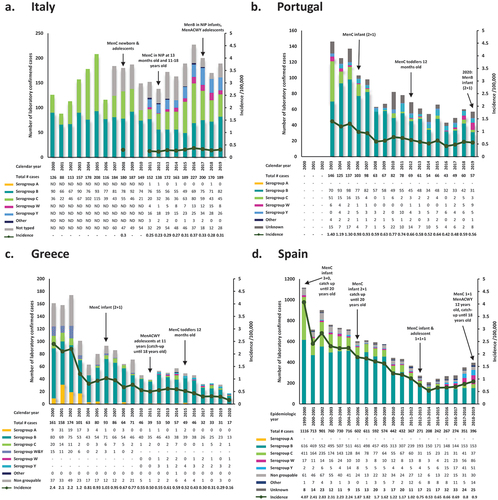
Geographic variations are also observed in the temporal pattern of IMD annual incidence and number of reported cases. In particular, over the examined period, a continuous decline can be observed for Portugal, Greece, and Spain, with only small fluctuations. Thus, over the past two decades, three waves of IMD can be assumed in Portugal and Greece, with decreasing peak height. In Spain, the decrease in the number of annual reported cases of IMD is almost uninterrupted from 2000 to 2013, while a small increase is discerned thereafter. In contrast, Italy shows a net increase in annual number of reported cases between 2000 and 2019, though with pronounced fluctuations [Citation38,Citation46,Citation52,Citation53].
Interestingly, besides fluctuations in the total number of IMD cases, the absolute and relative contribution of each serogroup also displays temporal as well as geographic variations. In general, serogroup B predominates throughout the past two decades in all four countries. In Italy, the proportion of confirmed laboratory cases attributable to serogroup B ranges from 25.9% (2015) to 75.0% (2001) [Citation39,Citation40], while in Portugal, Greece, and Spain, the respective ranges are from 47.9% (2003) to 90.5% (2008) [Citation46,Citation47], 43.1% (2002) to 89.7% (2011) [Citation53], and 38.6% (2018–2019) to 81.5% (2006–2007) [Citation54].
Cumulatively, throughout the two decades, the second most prevalent serogroup is C in all four countries, accounting for a range of 13.2% (2011) to 57.3% (2004), 0.0% (2008, 2016) to 34.9% (2003), 0.0% (2004, 2012, 2015, 2016) to 15.2% (2018), and 7.2% (2013–2014) to 36.8% (1999–2000) of annual cases in Italy, Portugal, Greece, and Spain, respectively. Importantly, for the periods with available data, serogroup A is only confirmed in Greece, with highest numbers of the past twenty years reported in 2001 (31 cases; 19.6% of total) and a total of eight cases reported since 2007.
The other two typed serogroups recorded in the four countries over the examined period are W and Y, with documented cases dating back to the early 2000s in Portugal, Greece and Spain, and only after 2011 in Italy. The combined number of W and Y has decreased over the course of the past two decades in Greece (from 15 in 2000 to 3 in 2019), as opposed to the other three countries, where numbers have increased from 20 cases in 2011 to 34 in 2019 in Italy [Citation38,Citation41,Citation42], from 6 cases in 2003 to 14 in 2019 in Portugal [Citation46,Citation47], and from 19 cases in 1999–2000 to 137 in 2018–2019 in Spain [Citation53]. Over the same period, relative contribution of serogroups W and Y to laboratory confirmed cases shows a net increase from 13.2% to 18.0%, 4.1% to 24.6%, 9.3% to 17.6%, and 1.7% to 25.4%, in Italy, Portugal, Greece and Spain, respectively. Finally, a considerable proportion of isolates is recorded as ‘other,’ ‘unknown,’ ‘not typed,’ or ‘non groupable,’ especially in Italy and Spain.
The evolution of immunization programs is shown in relation to regional epidemiology of IMD in and is provided along with dose schedules in . In addition to the national and regional recommendations for the general pediatric and adolescent population, in all of the countries there are also recommendations for specific groups at increased risk of IMD.
Table 1. Evolution of meningococcal vaccination strategies in Italy, Portugal, Greece and Spain for the general pediatric and adolescent population.
3.3. Molecular characterization and distribution of MenB vaccine antigen variants across MenB isolates
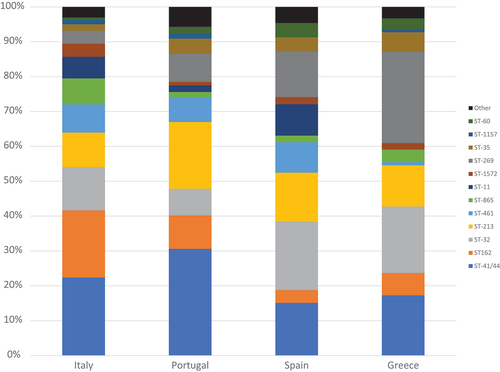
Clonal complex information was retrieved from PubMLST for n = 161 isolates from Italy (2014–2019), n = 209 isolates from Portugal (2012–2017), n = 110 isolates from Greece (2010–2019) and n = 688 isolates from Spain (2001–2018). The most common clonal complexes across countries were ST−41/44, ST−162, ST−32, ST−269 and ST−213, together accounting for more than 65% of isolates in all countries ().
Figure 3. Clonal complex distribution across MenB invasive isolates in Italy (2014–2019), Portugal (2012–2019), Greece (2010–2019) and Spain (2001–2018).
To evaluate potential reactivity of the two available MenB vaccines (4CMenB and Menb-fHbp) [Citation29,Citation70] against circulating meningococci in the four countries, we initially evaluated the distribution of MenB vaccine antigen types with respect to the two fHbp subfamilies across invasive MenB isolates identified in each country.
Of the total laboratory confirmed MenB cases isolated during the period 2014–2019 (401 cases) in Italy, 43.4% (174 cases) had available information on fHbp subfamily () [Citation66]. fHbp peptides belonging to subfamily A were found in nearly half of MenB cases (45%) across the calendar period examined, and subfamily B peptides in the remaining 55%.
Figure 4. Distribution of fHbp subfamily a and b antigens across MenB isolates in (a) Italy (2014–2019), (b) Portugal (2012–2019), (c) Greece (2010–2019), and (d) Spain (2001–2018). fHbp, factor H binding protein; MenB, serogroup B meningococcal (isolate).
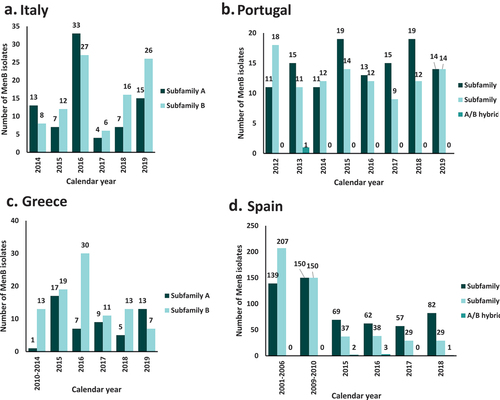
In Portugal, 70% (220/313) of MenB laboratory confirmed cases during the period 2012–2019 had available information on fHbp subfamily () [Citation66], with 53% of the cases belonging to subfamily A and 46% in subfamily B (). No specific trends in time were observed. On the other hand, subfamily B peptides prevailed over subfamily A peptides during the period 2010–2019 in Greece [Citation64,Citation68], representing > 61% of MenB cases overall, and > 70% in 2016 and 2018 (). Noteworthy, the inverse ratio is observed in 2019, with subfamily A peptides accounting for 65% of MenB isolates in Greece. In Spain, over the period 2001–2018 [Citation69] overall 53% (559 cases) of MenB isolates carried subfamily A peptides, while subfamily B peptides were present in 46% (490 cases) (). However, after 2015 more MenB isolates harbored subfamily A peptides compared with subfamily B.
For Italy, available information in PubMLST does not allow to present fHbp distribution by age group. Αccording to published data for the period 2013–2017, the majority of subfamily A variants were detected in samples collected from young adults and adults (age group 25‒64 years, 40.8%), followed by infants less than one year of age (18.3%) and children 1–4 years old (11.3%). Subfamily B appears to be more distributed across all age groups with slightly different in percentage by variant [Citation67]. In Greece, subfamily A was most prevalent in infants (<1 year) and cases 25 years or older and subfamily B prevalent in toddlers, children, adolescents and young adults (1 to 24 years) [Citation64]. For Portugal, approximately half of the isolates from infants, children and adolescents, as well as people 45–64 years old harbor subfamily A peptides, while in adults 25–44 and 65 years old and older, subfamily A is more prevalent (73% and 87% respectively) [Citation66]. For Spain, a higher fHbp subfamily A proportion was observed in all age groups. This difference was more noticeable in the 10–14 (76.5%) and > 64 (84.6%) age groups. However, similar fHbp subfamilies distribution was observed in the 5–9 and 25–44 (54.2% and 53.6% subfamily A) age groups [Citation69].
The most prevalent subfamily B fHbp peptides in Italy were peptide 390 (12%), 13 (9.1%), and 19 (8.6%) and the most prevalent subfamily A peptides were 45 (6.3%) and 119 (5,7%). In Portugal, most common fHbp peptides were 19 (11,1%), 45 (6,7%) and 21 (5,8%) belonging to subfamily A and peptides 13 and 14 (7.6% each) from subfamily B. For Spain, the 4 most prevalent fHbp peptides were subfamily A: peptide 45 (15.6%), peptide 47 (8.1%), peptide 16 (6.9%) and peptide 19 (6.3%), followed by subfamily B peptides 13 (5.6%) and 1 (5%). In Greece, most prevalent fHbp peptides belong to subfamily B: peptide 15 (21%) and peptide 1 (19%), followed by subfamily A peptide 45 (8.6%) (Supplementary Table S1).
For the rest of the vaccine antigens included in 4CMenB, a full coding NadA gene was present in 10–18% percent of isolates. Most prevalent NHBA variants across the four countries, were peptides 20, 2, 24 and 18 accounting for approximately 50% of isolates from Italy and Portugal, 43% in Spain and 35% in Greece. Peptides 21 and 376 were highly prevalent in Greece (37%) in contrast to the rest of the countries, while peptides 118 and 17 were more prevalent in Spain (23%) (Supplementary Table S2). PorA variants P1.22,14 and P1.7–2,4 were among the most common in all four countries, accounting for 29.9% in Italy, 33.8% in Portugal, 22% in Greece and 21,4% in Spain. In Greece, P1.19–1,15–11 was the most prevalent porA variant (21.2%) and in Spain P1.22,9 was also very common and detected in 12.2% of isolates (Supplementary Table S3).
Next, the reactivity of MenB vaccines in the four countries was evaluated by assessing the cross-reactivity of both 4CMenB and MenB-fHbp vaccines with available isolates from PubMLST using the MenDeVAR Index. From 175 MenB cases in Italy isolated overall during the period 2014–2019 for which genomic data was available, 54.3% (95 cases) were considered to be potentially reactive with MenB-fHbp (i.e. either an exact sequence match or a cross-reactive antigen) (). From 221, 97, and 157 MenB isolates with genomic data in Portugal, Greece and Spain overall during the periods 2012–2019, 2010–2019, and 2011–2020, respectively, the corresponding proportions of cases potentially reactive with MenB-fHbp were 62.4%, 75.3%, and 60.5% (). None of the MenB isolates was considered to be non-reactive with the MenB-fHbp vaccine, while the remaining isolates had insufficient data for the assessment.
Figure 5. Reactivity of MenB-fHbp and 4CMenB vaccines with invasive MenB isolates in (a) Italy (2014–2019), (b) Portugal (2012–2019), (c) Greece (2010–2019), and (d) Spain (2011–2020). MenB, serogroup B meningococcal (isolate).
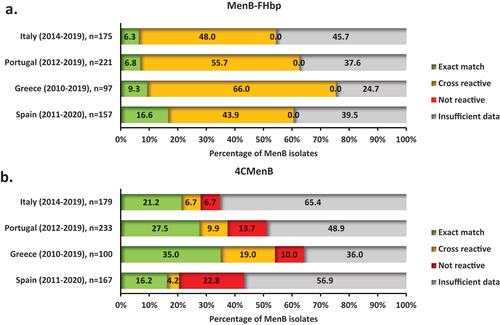
During the aforementioned periods, overall, 27.9%, 37.3%, 54.0%, and 20.4% of MenB cases in Italy (), Portugal (), Greece (), and Spain (), respectively, were potentially reactive with the 4CMenB vaccine according to the MenDeVAR Index. Isolates non-reactive with the 4CMenB vaccine accounted for 6.7%, 13.7%, 10.0%, and 22.8% of MenB cases, while the remaining cases had insufficient data for the assessment.
In total, 61.7% (401/650) of MenB isolates across the four countries were potentially reactive with MenB-fHbp (based on either exact sequence match or presence of a cross-reactive antigen), and none of the MenB isolates were non-reactive. For the 4CMenB vaccine, MenDeVAR estimated 33.1% (225/679) of MenB isolates with ≥ 1 exact sequence match or ≥ 1 cross-reactive antigenic variant, and 13.5% (92/679) as being non-reactive.
4. Discussion
4.1. Incidence of IMD and distribution by age group and serogroup
The age distribution in Italy, Portugal, Greece and Spain observed in the present review is typical of meningococcal disease, irrespective of period assessed, with infants aged <1 year and young children being the most affected age groups, followed by adolescents and young adults, in line with previously published literature [Citation5,Citation7,Citation86]. In 2018, based on available data from the surveillance atlas of the European Centre for Disease Prevention and Control (ECDC), infants aged <1 year accounted for 62% of IMD notified cases in Europe, children aged 1–4 years for 18%, and adolescents/young adults of 15–24 year of age for 7%, leaving less than 5% of IMD notified cases for each of the remaining age groups [Citation87]. A possible explanation for the increased risk of IMD in infants and toddlers compared with other age groups lies in the potential immunity buildup conferred by experience with many episodes of asymptomatic carriage of N. meningitidis throughout the lifetime of an individual, which may also offer protection against heterologous strains [Citation88–90]. Additionally, increased transmission due to changes in life-style, such as close social contacts and active smoking, may be responsible for peaks observed in adolescence and young adulthood [Citation91–94]. Importantly, the incidence per 100,000 among infants and young children has been much higher in Portugal compared with the other three Southern European countries, followed by Spain, Greece and Italy, aligned with the range in incidence across all ages in the respective periods. Overall, incidence of IMD has been declining in the last two decades for Portugal, Greece and Spain, though small outbreaks continue to appear. On the other hand, Italy has had the lowest incidence of IMD and rates have remained stable from 2011 onwards.
Previous literature has shown that the introduction of MenC vaccination into routine childhood immunization at a national level parallels the decreasing trends of IMD since the early 2000s, evidenced also by country-specific studies [Citation95]. Temporal trends presented in this review support this pattern. Spain, where MenC vaccination was introduced before 2001, along with Portugal and Greece, where MenC vaccine was available and used since 2001 [Citation96,Citation97] showed downward trends in overall incidence, especially in serogroup C and its relative contribution to the total confirmed cases. Italy, on the other hand, where MenC immunization was not introduced until 2005, showed a delayed drop in serogroup C cases compared to the other three countries. The number of MenC cases in Italy increased in 2015, coinciding with an unusual extended outbreak of MenC cases reported in Tuscany, Italy, from 2015 to 2016 [Citation98]. MenC cases start to decrease again in 2017, the year when the MenACWY vaccine was also introduced for adolescents. However, any interpretation of vaccination strategy for Italy is complicated by the already low incidence of IMD. In addition, it should be kept in mind that data for Italy was available only for Serogroup B and C for the first years examined (2000–2010). Since 2013 data from the national surveillance system have been also integrated with data from the regional notifications systems, minimizing any underreporting [Citation99]. Although MenC vaccination alone cannot explain the decline of IMD in Southern Europe, the data presented herein suggest that MenC vaccination is associated with reduced disease rates.
Epidemiological studies have shown that type of vaccination policy and implementation strategy may affect IMD trends. MenC vaccination is available to adolescents and young adults in some countries, either as a catch-up or routine immunization, in addition to childhood vaccination [Citation100]. This has led to decreases in MenC in all age groups, as a result of both long-term effective immunity and herd protection [Citation14,Citation15,Citation101–105], in contrast with declines being limited to toddlers by 2010 in Germany for example where catch-up was not routinely conducted [Citation106].
Along these lines, the MenACWY conjugate vaccine became available as routine immunization for adolescents (11–12 years of age) in Greece in 2011 as part of a proactive strategy, and cases of MenW and Y have remained low. In contrast, an increase in number and proportion of all IMD cases caused by these two serogroups was observed in Italy, and Spain, where MenACWY vaccine was introduced later or in Portugal, where it hasn’t been introduced yet. Overall trends in Europe have shown an increase in the notification rates of MenY since 2011 and MenW since 2012, the latter of which has been associated with a hypervirulent cc11 clone [Citation107,Citation108]. Both MenY and MenW affect older age groups than those expected according to the typical age distribution [Citation87,Citation95,Citation107]. This may also explain the small increases observed herein in IMD incidence among older age groups in the late 2010s. As a response measure to the increased prevalence of MenW and Y, the MenACWY vaccine has been offered to adolescents in Italy since 2017 and in Spain since 2019. The effects of the MenACWY vaccine targeting four out of five major disease-causing serogroups in Europe are already seen in the United Kingdom [Citation109], while the continued uptake and epidemiologic monitoring globally will provide further insight into its effectiveness [Citation110].
Serogroup A is not common in Europe, apart from incidences reported in Romania [Citation111], Russia [Citation112] and Greece [Citation113]. As also evidenced by other epidemiological analyses [Citation113], our data indicated a surge of serogroup A in Greece occurring between 2001 and 2003 possibly related to an earlier immigration inflow [Citation114]. Incident cases of serogroup A in Greece have since been nearly non-existent.
Overall, the most relevant rate of IMD is related to serogroup B. Though numbers show downward trends, our review suggests that over the examined period, B is the most prevalent serogroup across years and countries, consistent with trends and distribution among notification rates reported by others [Citation5,Citation95]. Control of meningococcal disease will hence not be achieved until a broadly effective MenB vaccine becomes widely available.
4.2. MenB vaccination and characterization of MenB isolates in relation to current MenB vaccines
MenB disease-causing strains are very diverse, both in terms of sequence and levels of expressed antigens. As a consequence, the effectiveness of earlier outer membrane vesicle (OMV)-based vaccines has been restricted to specific strains of MenB targeting regional outbreaks [Citation115] and would not have use in larger-scale epidemics and outbreaks. In order to provide a broad protective immune response, the recently developed protein-based MenB vaccines MenB-fHbp and 4CMenB, have been designed to target conserved surface proteins. 4CMenB is indicated for active immunization of individuals from the age of 2 months and older as a 2- or 3-dose schedule with a booster dose [Citation29,Citation116] and Men-fHbp from the age of 10 years under either a 2- or 3-dose schedule [Citation85,Citation116].
Protein-based MenB vaccines have had reasonable efficacy in controlling MenB surges in Canada [Citation117] and the United States [Citation115,Citation118,Citation119]. Nevertheless, experience suggests that the best strategy for outbreak prevention involves proactive, routine immunization [Citation119]. The first country to include the 4CMenB vaccine in the national childhood immunization schedule was the United Kingdom, implementing vaccination at 2 and 4 months of age and as a booster at the age of 12–13 months [Citation120]. This vaccine represented a prophylactic measure taken in the absence of an outbreak, and resulting in substantial declines in MenB incidence among vaccinated individuals (50%; even after adjusting for the decreasing trend), as well as in nonvaccinated individuals [Citation121].
In Italy, the effectiveness and impact of 4CMenB vaccination in two regions using different schedules (2, 4, 6, 12 months in Tuscany vs. 7, 9, 15 months in Veneto) has been evaluated in a recent retrospective study. Vaccine effectiveness was 93.6% [95% confidence limits (CL) 55.4; 99.1] in Tuscany and 91.0% (95% CL 59.9; 97.9) in Veneto, with the overall impact of vaccination being greater where the immunization program starts earlier in life [Citation122].
Since the approval of the first protein-based MenB vaccine (4CMenB) in 2013 in the EU, and as of April 2021, 58 countries had approved at least one of the two protein-based MenB vaccines [Citation123]. Relevant recommendations and practices vary across countries, with evidence of a national MenB vaccination policy in place reported for 24 countries. In Andorra, Austria, Brazil, the Czech Republic, France, Hungary, Ireland, Italy, Lithuania, Malta, Poland, Portugal, San Marino, the United Kingdom, and the United States, MenB vaccination is included in the immunization plans for at least one age-based risk group [Citation100,Citation123]. A fee is required for nationally recommended MenB vaccination of infants in the Czech Republic (unless the immunization starts before 6 months of age), as well as in Austria, Brazil, Hungary, Poland, and San Marino [Citation100,Citation123,Citation124]. In Canada, Finland, Germany, Greece, New Zealand, Norway, and Uruguay, MenB vaccination is recommended only for specific groups, based on underlying medical conditions or increased risk of exposure [Citation100,Citation123,Citation124]. Similarly, in Australia and Spain, MenB vaccination is recommended for risk groups other than age-based ones, yet infant vaccination also applies to certain regions and/or ethnic groups in these countries [Citation123].
With respect to the four countries of interest in this review, the 4CMenB vaccine was implemented in the NIP of Italy in 2017, but regional vaccination policies had been applied earlier in the regions of Tuscany (2014) and Veneto (2015) [Citation122]. The funding of vaccination for early infancy and older infants, respectively, led to a high uptake in the period from 2014/2015 to 2018 and important reductions in disease incidence in both regions, with greater effectiveness when vaccination was started early [Citation122]. In addition to infant vaccination program, six Italian regions decided to introduce MenB-fHbp vaccination for adolescents, extending the free of charge offer providing broader protection [Citation70–76]. In Portugal, MenB vaccination was not publicly funded until 2020 for children born from 2019 [Citation100]. Nevertheless, recommendations by the Portuguese Society of Pediatrics and physician’s education of parents has led to infant immunization [Citation116] allowing for the conduct of a case-control study with data spanning the period between October 2014 and March 2019 [Citation125]. During that period, children with MenB disease were less likely to have been fully immunized than controls without IMD, and had higher rates of death and sequelae compared to no deaths or sequelae among vaccinated individuals [Citation125]. Although 4CMenB is not included in the NIP in Spain, nor is publicly funded, an estimated 34% of infants born in 2015–2016 has been vaccinated, following a recommendation by the Vaccine Committee of Spanish pediatrician Society CAV-AEP [Citation126–129]. Rough estimates comparing pre-vaccination period 2013–2014 with post-vaccination period 2017–2018 suggest reductions in the number of MenB IMD cases. The Castilla y Leon and Canary Islands had already introduced MenB vaccination in their immunization schedules by 2019, followed by Andalusia and Catalonia in 2021 and 2022 [Citation77–81]. Lastly, in Greece, the 4CMenB vaccine is available in the private market since 2014 and MenB-fHbp since 2018, but official data on vaccine uptake are not available [Citation64]. Apart from a study on the predicted reactivity of MenB vaccines based on multi-locus sequence typing (MLST), whole genome sequencing and MEASURE assays [Citation64], there is a paucity of information on vaccine effectiveness and impact.
Owing to the difficulties in targeting MenB, it took 14 years since the availability of the first conjugate vaccine (against MenC) until the approval of the first effective MenB vaccine [Citation130]. Building evidence on real-world use of MenB vaccines will provide more information on the effectiveness and safety. These data will potentially support the widespread use of MenB vaccines to control endemic disease [Citation103]. Though illness can be prevented, challenges remain, considering that impact of MenB vaccines on unvaccinated individuals and carriage rates is not yet established [Citation131–134], meaning that direct protection is necessary.
In view of the low IMD incidence rates and earlier lack of sufficient effectiveness and safety data for the new vaccines, some countries have refrained from implementing routine vaccination against MenB [Citation124]. Taken together with the substantial and increasing body of evidence, the unpredictable and highly variable epidemiology of MenB strains, as well as the high physical and psychological impact of the disease, are likely to drive decision-making in immunization policy. The immune response to MenB is faster and higher in reaction to a booster dose compared to primary vaccination, suggesting that a level of underlying vaccine-induced immunity is desirable [Citation135]. There is an ongoing multi-center, school-based study in the UK assessing the impact of both available MenB vaccines on serogroup B and non-serogroup B disease-causing meningococci and other Neisseria species, as well as their potential for carriage and community protection, the results of which are keenly awaited [Citation136].
The surface proteins belonging to the family of fHbps present immunogenic vaccine antigens which are ubiquitous and segregate into two well-defined subfamilies, A and B [Citation21]. Analysis of MenB strains collected from the US, Europe (Czech Republic, England, Wales, Northern Ireland, France, Norway), New Zealand, and South Africa has previously shown that more than 70% were subfamily B variants and the remainder were subfamily A variants [Citation21]. The respective ranges of subfamily B and A variants across countries were 65% to 77% and 23% to 35% [Citation21]. In contrast, our analysis in Southern European countries revealed that the distribution of subfamily A and B MenB isolates was even, with 50.6% belonging to subfamily A and 48.9% to subfamily B However, variability was seen between countries, with the proportions ranging from 36% to 53% for subfamily A and from 46% to 64% for subfamily B, across the four countries in the overall period examined. Additionally, subfamily distribution varied across years and age groups. Altogether these trends are suggestive of the unpredictable epidemiology of fHbp variants, even among neighboring countries.
The prevalence of fHbp variants varies geographically and temporally, as evidenced by our review of available data and by others [Citation21]. Importantly, epidemiology of fHbp subfamilies has been shown to vary by age group with disease-causing fHbp subfamily A strains being more prevalent among infants than in adolescents [Citation20]. Despite the high intrafamily variant homology, sequence identity between subfamilies is lower. Inclusion of antigens from each of the two phylogenetic subfamilies, i.e. bivalent vaccines, may increase breadth of vaccine coverage, especially considering that expression of fHbp on the surface of MenB isolates is the best predictor of susceptibility to targeting by MenB vaccine-induced antibodies [Citation30]. More than 91% of an extensive collection of invasive MenB isolates were predicted to be potentially susceptible to bactericidal antibodies elicited by the MenB-fHbp vaccine, which contains antigens from one of each of the two fHbp subfamilies [Citation22]. MenB-fHbp vaccination has demonstrated broad activity in individual sera from circulating MenB strains, as well as strains associated with outbreaks [Citation137–139], suggesting this protein-based MenB vaccine offers a breadth of efficacy against diverse invasive MenB strains. When interpreting these results, the limitation of the age indication for MenB-fHbp vaccine (licensed from 10 years of age) should be taken into account. As 4CMenB only contains a non-lipidated fHbp subfamily B antigen, coverage of strains with diverse fHbp variants including those of subfamily A may only be achieved via antibody induced by other antigens. The rest of the antigens included in 4CMenB, also present variability, highlighting the importance of sustained genomic surveillance in each country, to inform immunization policies.
In our review of MenB isolates of Italy, Portugal, Greece and Spain, MenDeVar index analysis showed that among antigens with sufficient data from experimental studies, all were potentially cross-reactive with the MenB-fHbp vaccine, while 29% were non-reactive with the 4CMenB vaccine (19% in Italy, 27% in Portugal, 16% in Greece, and 53% in Spain), in keeping with data from the exemplar studies analyzed by the developers of the platform [Citation36]. The MenDeVAR tool has a unique utilization in estimating cross reactivity by both MenB vaccines, however, especially for the evaluation on 4CMenB, it relays on a limited set of MATS published data and it does not include any potential coverage of the non-PorA components of the OMV [Citation66]. In a recent publication [Citation140], MenDeVAR predictability was compared with gMATS and MATS techniques against the same panel of invasive MenB isolates. Results showed that individual PorA-based predictions were identical between MenDeVAR and gMATS, as expected given identical criteria, and similar for NadA-based predictions. While for fHbp, gMATS and MenDeVAR were in agreement for 53.0% of peptides across isolates and 87.7% for NHBA. Given these discrepancies, some points for MenDeVAR improvement have been suggested [Citation140]. Moreover, only a fraction of the total IMD cases is included in MenDeVAR analysis, which may limit the results representativity. Nevertheless, the presented MenDeVAR results illustrate the potential breadth of protection offered by the two licensed MenB vaccines, and the accessibility of MenDeVAR on PubMLST facilitates assessment on possible immunological coverage or cross-reactivity for public health purpose.
Overall, serogroup B meningococcus is a major cause of IMD in Southern Europe, comprising a group of the most epidemiologically relevant strains. Strains of other serogroups continue to emerge, shifting serogroup and age distribution among disease-causing meningococcal isolates. Multivalent vaccines based on conserved components of meningococcus irrespective of serogroup, such as fHbp, have the potential of inducing bactericidal activity against a breadth of variants regardless of sequence variation and potentially emerging variants. Nonetheless, since N. meningitidis strains are highly diverse, vaccine development and evaluation of vaccination programs should be continuously informed by accurate disease epidemiology and genomic surveillance data.
4.3. Limitations
When interpreting the data summarized in this review there are three major limitations that need to be considered. The first limitation is related to reporting of cases, i.e. clinical and/or laboratory notification rate and timeliness of notification, which can vary across country regions or years [Citation141]. Based on older data, an estimated 50% of meningococcal cases are confirmed by polymerase chain reaction (PCR) [Citation142]. To date, IMD is a mandatory notifiable disease in all four countries and completeness has been improving in time with the majority of isolates sent to the national reference laboratories for typing and characterization [Citation64,Citation141,Citation143,Citation144]. The second limitation is related to the completeness of data available in PubMLST, which may have affected the results with respect to proteins present in MenB isolates and reactivity with current MenB vaccines; not all MenB isolates had fHbp subfamily information, while 43%, 75%, 31%, and 16% of MenB isolates in Italy (2014–2019), Portugal (2012–2019), Greece (2010–2019), and Spain (2011–2019) had available sequencing data, respectively. Due to the data availability limitations, results should be interpreted with caution. As vaccination coverage is not routinely monitored in all countries, genomic methods of protein characterization and tools such as MenDeVAR are essential for assessing potential reactivity of available vaccines. This would require more extensive use with genomic methods to sequence antigen proteins in PCR confirmed samples. However, MenDeVAR, as any genomic – based approach, cannot predict protein expression, which represents a key element for vaccine efficacy. Finally, predictions of cross-reactivity of MenB isolates with current MenB vaccines may underestimate effectiveness in real-life, especially considering that a large proportion of proteins cannot be assessed due to lack of sufficient experimental data. For MenDeVar results interpretation, the age indication of the two MenB vaccines should also be taken into account, as MenB-fHbp is licensed for use from 10 years of age. In this review it was not feasible to break down MenDeVar results by age due to limited availability of data and small numbers in some age groups. In our analysis, 46.0% of the isolates were classified as ‘insufficient data’ (ranging from 24.7% for MenB-fHbp in Greece to 65.4% for 4CMenB in Italy).
5. Conclusion
Several factors need to be taken into account when informing management of infections in clinical practice, including identification of high-risk groups, epidemiological time trends, heterogeneity of circulating variants, evidence of efficacy derived from existing protective measures and potential coverage of future outbreaks with lessons learnt from the past.
Incidence of IMD based on laboratory-confirmed cases in Italy, Portugal, Greece, and Spain confirmed that the age groups that are at highest risk are infants, followed by toddlers and the age group of adolescents and young adults. Geographic and temporal variations in the rate of incidence are aligned with the overall incidence of the disease, which has been declining in Portugal, Greece, and Spain. Analysis of serogroup distribution revealed that over the past two decades, though variable between countries and across time, most IMD cases have been caused by serogroups B and C, while serogroups W and Y are also emerging. Serogroup A had been notified in early 2000s in Greece but has since disappeared. Success with MenC immunization in the four countries follows global trends, while owing to the delay in developing and introducing effective MenB vaccines, MenB still remains the predominant cause of IMD. Two protein-based vaccines against MenB have shown promising results, and have only started to be integrated in the NIP. The Nm surface factor H binding protein (fHbp) represents important MenB vaccine antigens as they were consistently present across MenB isolates, while the distribution of the two fHbp subfamilies (A and B) showed geographical and temporal variations. PorA and NHBA protein antigens also demonstrated variability across countries and the potential contribution of NadA to vaccine coverage is minimal, as a full coding NadA gene was present in a small proportion of invasive MenB isolates. The predicted vaccine reactivity also ranged, indicating differences in potential cross-reactivity between currently available MenB vaccines.
Continued close monitoring is important to identify increases in prevalence, as well as changes in serogroup epidemiology, prompt reforms in immunization policies and evaluate vaccine effectiveness. Genomic surveillance is essential for identifying changes in N. meningitidis characteristics that can inform vaccine selection and guide development of new vaccines.
6. Expert opinion
Although IMD in Southern Europe is rare, it continues to present a life-threatening disease. Vaccination is the mainstay preventive measure for IMD and continued efforts for prophylaxis against meningococcus rely on identifying which target group would benefit more from immunization programs. Global literature as well as the results of our review indicate that infants and young children have historically but also recently been the groups most at risk. Importantly, IMD incidence rates in the four Southern European countries are low and overall declining with time, yet small unpredictable surges still appear, with MenB remaining the predominant cause. Experience from child and adolescent immunization with conjugate vaccines has been successful in controlling MenC outbreaks, offering either direct or herd protection, also evident from the dramatic decreases presented for Portugal, Greece, and Spain. In spite of reductions in MenB cases with the more recently licensed protein-based MenB vaccines used as reactive and proactive measures, these vaccines, though commercially available, had not been introduced in the NIP before 2020, with the exception of Italy (in 2017). An integrated picture of IMD epidemiology considering also the potential breadth of reactivity of these recombinant protein-based vaccines might improve routine use and result in a reduced disease burden.
In light of the availability of tools for predicting vaccine activity based on meningococcal strain sequences, continued genomic surveillance is warranted to inform vaccine selection. Increasing evidence on the potential cross-reactivity of the new vaccines with many invasive MenB isolates but also considering IMD has great epidemic potential may prompt initiation of immunization campaigns that will help mitigate the risk of outbreaks. Future prospects lie in accurate records of MenB epidemiology and real-life coverage of available MenB vaccines as they become integrated in routine clinical practice. Such data will further inform predictive tools which currently lack in amount of experimental data available and will complement the preliminary research suggestive of effectiveness of protein-based vaccines across strains from different serogroups.
Epidemiologic monitoring is imperative as understanding rates and dynamics of IMD is essential for designing further effective meningococcal vaccines which constitute a key public health priority. The diversity of MenB strains and the potential for emergence of new virulent strains not susceptible to current vaccines remains a concern in the medical and scientific community. Presence of fHbp antigens in most MenB isolates has been previously demonstrated and is also supported by the results of screening MenB isolates of Italy, Portugal, Greece and Spain, highlighting the great potential of fHbp as a broad-activity vaccine antigen, especially if both fHbp subfamilies are represented. Particularly in view of the initial signs of susceptibility in targeting the surface factor fHbp and the success noted with targeting the polysaccharide capsule, a combination of the two methods could be efficacious in the fight against IMD. In order to continue to lower morbidity and mortality associated with IMD, and given the unpredictable epidemiology, proactive prevention strategies targeting all risk groups and all five serogroups are imperative for efficient control of the disease and achievement of the WHO goal to defeat meningitis by 2030.
Article highlights
Based on epidemiological data from the past two decades, infants represent individuals most affected by IMD in Italy, Portugal, Greece and Spain, followed by toddlers and young children. A small yet consistent peak is observed during adolescence and young adulthood in certain countries.
Incidence of IMD in Portugal, Greece, and Spain has been declining, while Italy shows relatively stable trends over the examined period. Small surges in IMD rates continue to appear, with temporal and geographical variation.
Over the past two decades, the most prominent disease-causing meningococcal serogroup in the four Southern European countries is B, while increases in prevalence of serogroup Y and W are also observed.
Since the immunization with MenC vaccines in the early 2000s, MenC cases have been declining, while MenB remains the predominant cause of IMD in all four countries, with two protein-based MenB vaccines, 4CMenB and MenB-fHbp, only recently being introduced in routine practice.
fHbp, generally classified into two immunogenically distinct subfamilies A and B, represents an important MenB vaccine antigen, consistently present across circulating MenB isolates in Italy, Portugal, Greece and Spain. Variants of both subfamilies A and B, but with observed differences in distribution were detected across countries and years.
The 4CMenB vaccine is designed to target fHbp peptides of subfamily B, while peptides of both A and B, are targetable by the MenB-fHbp vaccine. Based on in silico predictions, 54.3-75.3% of circulating MenB isolates across the four countries are potentially reactive with MenB-fHbp and 20.4-54% are potentially reactive with 4CMenB.
Declaration of interest
All authors are Pfizer employees and may hold stock options. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclsed that they perform contract research on behalf of UK Health Security Agency for GSK, Pfizer and Sanofi Pasteur. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Author contributions
All authors have substiantially contributed to to the conception and design of the review article and interpreting the relevant literature and have been involved in writing the review article or revised it for intellectual content.
Supplemental Material
Download MS Word (32.1 KB)Acknowledgments
Medical writing support was provided by Athena Georgilis and Panagiota Karagianni at Qualitis SA and was funded by Pfizer.
Part of this work has been presented as an abstract in the 40th Annual meeting of the European Society of Infectious Diseases (ESPID) 2022.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2023.2225596
Additional information
Funding
References
- Rouphael NG, Stephens DS. Neisseria meningitidis: biology, microbiology, and epidemiology. Methods Mol Biol. 2012;799:1–20.
- Thompson MJ, Ninis N, Perera R, et al. Clinical recognition of meningococcal disease in children and adolescents. Lancet. 2006;367(9508):397–403. DOI:10.1016/S0140-6736(06)67932-4
- Pace D, Pollard AJ. Meningococcal disease: clinical presentation and sequelae. Vaccine. 2012;30(Suppl 2):B3–9. doi: 10.1016/j.vaccine.2011.12.062
- Stein-Zamir C, Shoob H, Sokolov I, et al. The clinical features and long-term sequelae of invasive meningococcal disease in children. Pediatr Infect Dis J. 2014;33(7):777–779. DOI:10.1097/INF.0000000000000282
- Parikh SR, Campbell H, Bettinger JA, et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J Infect. 2020;81(4):483–498. DOI:10.1016/j.jinf.2020.05.079
- Pelton SI. The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. J Adolesc Health. 2016;59(2 Suppl):S3–s11. doi: 10.1016/j.jadohealth.2016.04.012
- Gabutti G, Stefanati A, Kuhdari P. Epidemiology of Neisseria meningitidis infections: case distribution by age and relevance of carriage. J Prev Med Hyg. 2015;56(3):E116–E120.
- Spyromitrou-Xioufi P, Tsirigotaki M, Ladomenou F. Risk factors for meningococcal disease in children and adolescents: a systematic review and Meta-analysis. Eur J Pediatr. 2020;179(7):1017–1027. doi: 10.1007/s00431-020-03658-9
- Taha MK, Weil-Olivier C, Bouee S, et al. Risk factors for invasive meningococcal disease: a retrospective analysis of the French national public health insurance database. Hum Vaccin Immunother. 2021;17(6):1858–1866. DOI:10.1080/21645515.2020.1849518
- World Health Organization, Meningitis. [cited 2021 Oct 25]. Available from: https://www.who.int/news-room/fact-sheets/detail/meningitis.
- Purmohamad A, Abasi E, Azimi T, et al. Global estimate of Neisseria meningitidis serogroups proportion in invasive meningococcal disease: a systematic review and meta-analysis. Microb Pathog. 2019;134:103571.
- Findlow J, Lucidarme J, Taha MK, et al. Correlates of protection for meningococcal surface protein vaccines: lessons from the past. Expert Rev Vaccines. 2022;21(6):739–751. doi: 10.1080/14760584.2021.1940144.
- MacDonald NE, Halperin SA, Law BJ, et al. Induction of immunologic memory by conjugated vs plain meningococcal C polysaccharide vaccine in toddlers a randomized controlled trial. JAMA. 1998;280(19):1685–1689. DOI:10.1001/jama.280.19.1685
- Ramsay ME, Andrews NJ, Trotter C, et al. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. BMJ. 2003;326(7385):365–366. DOI:10.1136/bmj.326.7385.365
- Maiden MCJ, Ibarz-Pavon AB, Urwin R, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197(5):737–743. DOI:10.1086/527401
- Finne J, Leinonen M, Mäkelä PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983;2(8346):355–357. doi: 10.1016/S0140-6736(83)90340-9
- Wyle FA, Artenstein MS, Brandt BL, et al. Immunologic response of man to group B meningococcal polysaccharide vaccines. J Infect Dis. 1972;126(5):514–522. DOI:10.1093/infdis/126.5.514
- Fletcher LD, Bernfield L, Barniak V, et al. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect Immun. 2004;72(4):2088–2100. DOI:10.1128/IAI.72.4.2088-2100.2004
- Schneider MC, Exley RM, Chan H, et al. Functional Significance Of Factor H Binding to Neisseria meningitidis. J Immunol. 2006;176(12):7566–7575. DOI:10.4049/jimmunol.176.12.7566
- Hoiseth SK, Murphy E, Andrew L, et al. A multi-country evaluation of Neisseria meningitidis serogroup B factor H-binding proteins and implications for vaccine coverage in different age groups. Pediatr Infect Dis J. 2013;32(10):1096–1101. DOI:10.1097/INF.0b013e31829aa63b
- Murphy E, Andrew L, Lee KL, et al. Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis. J Infect Dis. 2009;200(3):379–389. DOI:10.1086/600141
- McNeil LK, Donald RGK, Gribenko A, et al. Predicting the susceptibility of meningococcal serogroup B isolates to bactericidal antibodies elicited by bivalent rlp2086, a novel prophylactic vaccine. MBio. 2018;9(2):e00036–18. DOI:10.1128/mBio.00036-18
- Findlow J, Bayliss CD, Beernink PT, et al. Broad vaccine protection against Neisseria meningitidis using factor H binding protein. Vaccine. 2020;38(49):7716–7727. doi: 10.1016/j.vaccine.2020.08.031.
- Richmond PC, Nissen MD, Marshall H, et al. A bivalent Neisseria meningitidis recombinant lipidated factor H binding protein vaccine in young adults: results of a randomised, controlled, dose-escalation phase 1 trial. Vaccine. 2012;30(43):6163–6174. DOI:10.1016/j.vaccine.2012.07.065
- Richmond PC, Marshall HS, Nissen MD, et al. Safety, immunogenicity, and tolerability of meningococcal serogroup B bivalent recombinant lipoprotein 2086 vaccine in healthy adolescents: a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2012;12(8):597–607. DOI:10.1016/S1473-3099(12)70087-7
- Nissen MD, Marshall HS, Richmond PC, et al. A randomized, controlled, phase 1/2 trial of a Neisseria meningitidis serogroup B bivalent rLP2086 vaccine in healthy children and adolescents. Pediatr Infect Dis J. 2013;32(4):364–371. DOI:10.1097/INF.0b013e31827b0d24
- Marshall HS, Richmond PC, Nissen MD, et al. A phase 2 open-label safety and immunogenicity study of a meningococcal B bivalent rLP2086 vaccine in healthy adults. Vaccine. 2013;31(12):1569–1575. DOI:10.1016/j.vaccine.2013.01.021
- Frosi G, Biolchi A, Lo Sapio M, et al. Bactericidal antibody against a representative epidemiological meningococcal serogroup B panel confirms that MATS underestimates 4CMenB vaccine strain coverage. Vaccine. 2013;31(43):4968–4974. DOI:10.1016/j.vaccine.2013.08.006
- Bexsero. Summary of Product Characteristics [cited 2021 Oct 25]. Available from: https://www.ema.europa.eu/en/documents/product-information/bexsero-epar-product-information_en.pdf.
- Jiang HQ, Hoiseth SK, Harris SL, et al. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine. 2010;28(37):6086–6093. DOI:10.1016/j.vaccine.2010.06.083
- Brehony C, Wilson DJ, Maiden MCJ. Variation of the factor H-binding protein of Neisseria meningitidis. Microbiology (Reading). 2009;155(Pt 12):4155–4169. doi: 10.1099/mic.0.027995-0
- Serruto D, Bottomley Matthew J, Sanjay R, et al. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine. 2012;30(Suppl 2):B87–B97. DOI:10.1016/j.vaccine.2012.01.033
- Masignani V, Pizza M, Moxon ER. The development of a vaccine against meningococcus B using reverse vaccinology. Front Immunol. 2019;10(751). doi: 10.3389/fimmu.2019.00751.
- Křížová P, Musílek M, Vacková Z, et al. Predicted strain coverage of a new protein-based meningococcal vaccine in the Czech Republic. Epidemiol Mikrobiol Imunol. 2014;63(2):103–106.
- Vogel U, Taha MK, Vazquez J, et al. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect Dis. 2013;13(5):416–425. DOI:10.1016/S1473-3099(13)70006-9
- Rodrigues CMC, Jolley KA, Smith A, et al. Meningococcal Deduced Vaccine Antigen Reactivity (MenDevar) Index: a rapid and accessible tool that exploits genomic data in public health and clinical microbiology applications. J Clin Microbiol. 2020;59(1):e02161–20. doi: 10.1128/JCM.02161-20.
- World Health Organization, Defeating meningitis by 2030: a global road map. [cited 2021 Nov 16]. Available from: https://www.who.int/publications/i/item/9789240026407.
- Instituto Superior di Sanita, Invasive bacterial disease surveillance reports. [cited 2021 Jul 29]. Available from: https://www.iss.it/sn-mbi-rapporti-iss.
- Ministry of Health of Italy, National vaccination plan 2005–2007. [cited 2021 Jul 30]. Available from: https://www.salute.gov.it/imgs/C_17_pubblicazioni_543_allegato.pdf.
- Ministry of Health of Italy, National vaccination plan 2008–2010. [cited 2021 Jul 28]. Available from: http://www.ccm-network.it/documenti_Ccm/convegni/SANIT/materiali2008/25.6/6-Piano_Nazionale_Vaccini_Ricciardi.pdf.
- Ministry of Health of Italy, National vaccination plan 2012–2014. [cited 2021 Jul 30]. Available from: https://www.salute.gov.it/imgs/C_17_pubblicazioni_1721_allegato.pdf.
- Ministry of Health of Italy, National vaccination plan 2017–2019. [cited 2021 Jul 30]. Available from: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf.
- Bollettino Ufficiale della Regione Sicilia D.A n 1965/2017. [cited 2021 Jul 28]. Available from: http://pti.regione.sicilia.it/portal/page/portal/PIR_PORTALE/PIR_LaStrutturaRegionale/PIR_AssessoratoSalute/PIR_Infoedocumenti/PIR_DecretiAssessratoSalute/PIR_DecretiAssessoriali/PIR_DecretiAssessorialianno2017/D.A._n%B0_1965%5B1%5D%20-%20serv.4.pdf.
- Bollettino Ufficiale della Regione Puglia. n. 77 del. 11-Jun-2018[cited 2021 Jun 11]. Available from . http://burp.regione.puglia.it/documents/10192/19676300/Bollettino+numero+77±+Ordinario±+anno+2018/fff50b03-4743-4414-9d5a-d7aee8252ec3;jsessionid=5028E63241FAEC36BC69C395FA33D7D8
- Bollettino Ufficiale della Regione Campania - n. 20 del 08-42019. [cited 2021 Jul 28]. Available from: https://www.aiopcampania.it/public/normativa/4b06bd76312d445d85b9f8caa6082a8b.pdf.
- Simões MJ, BettencourtC, FernandesT. Doença invasiva meningocócica em Portugal - Vigilância epidemiológica integrada, 2003-2020: relatório anual da Rede de Laboratórios VigLab Doença Meningocócica. Instituto Nacional de Saúde Doutor Ricardo Jorge, IP 2022 http://hdl.handle.net/10400.18/8254 .
- Gabinete do Secretário de Estado da Saúde Despacho n.º 12434/2019. Aprova o novo esquema vacinal do Programa Nacional de Vacinação. [cited 2021 Jul 28]. Available from: https://dre.pt/application/conteudo/127608823.
- DGS, Programa Nacional de Vacinação 2006. Circular Normativa 08/DT de 21/12/2005, Direcção Geral de Saúde, Ministério da Saúde; 2005. [cited 2021 Jul 19].
- Ministry of Health and Social Solidarity, National Immunization Programmes. [cited 2021 Jul 30]. Available from: https://www.moh.gov.gr/articles/health/dieythynsh-dhmosias-ygieinhs/metadotika-kai-mh-metadotika-noshmata/ethnika-programmata-emboliasmwn.
- Ministry of Health and Social Solidarity, General directorate of Public Health. Directorate of Public Hygiene, Department of disease epidemiology. Circular Υ1/Γ.Π. 17234, 14/6/2006 New National Immunization Programme. [cited 2021 Jul 19].
- Ministry of Health and Social Solidarity, General directorate of Public Health. Directorate of Public Hygiene, Department of disease epidemiology. Circular Υ1/Γ.Π.οικ.140202, 20/12/2011. New National Immunization Programme for children and adolescents 2011. [cited 2021 Jul 19].
- National Meningitis Reference Laboratory, annual reports 2010-2020. [cited 2021 Jul 28]. Available from: https://eelno.uniwa.gr/monada-ergastiriakis-epitirisis-tis-vaktiriakis-miniggitidas/#apologismoiekam.
- Instituto de salud Carlos III - RENAVE, Enfermedad meningocócica. Resultados de la vigilancia [cited 2021 Jul 29]. Available from: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Paginas/Resultados_Vigilancia_Enfer_Meningo.aspx.
- Ministerio de Sanidad, Calendarios de vacunación en España 1975 - 2015. [cited 2021 Jul 30]. Available from: https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/calendario-y-coberturas/calendario/docs/CalendariosVacunacion1975_2015.pdf.
- Ministerio de Sanidad, C.y.B.S, Recomendaciones de vacunación frente a la enfermedad meningocócica invasiva. 2019. [cited 2021 Jul 30]. Available from: https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/docs/Recomendaciones_Vacunacion_Meningococo.pdf.
- Calendario de vacunación a lo largo de la vida 2021. [cited 2021 Jul 19]. Available from: https://www.sanidad.gob.es/profesionales/saludPublica/.
- Andalucia. Calendario de Vacunaciones. [cited 2021 Jul 30]. Available from: https://www.juntadeandalucia.es/organismos/saludyfamilias/areas/salud-vida/vacunas/paginas/calendario-vacunacion.html.
- Castilla y Leon. Calendario vacunal para toda la vida. [cited 2021 Jul 30]. Available from: https://www.saludcastillayleon.es/profesionales/es/vacunaciones/calendario-vacunal-toda-vida.
- Canarias. Nuevo Calendario vacunal para todas las edades de la vida. [cited 2021 Jul 29]. Available from: https://www3.gobiernodecanarias.org/sanidad/scs/contenidoGenerico.jsp?idDocument=3cc62be0-9746-11e0-ba66-75bd8cf93e41&idCarpeta=f60021f6-6ad8-11e2-bc0c-6512fc1bab5e.
- Boletín Oficial de la Junta de Andalucía. Número 36 - Martes, 23 de febrero de 2021. [cited 2021 Jul 29]. Available from: https://www.juntadeandalucia.es/boja/2021/36/BOJA21-036-00002-2748-01_00186669.pdf.
- Ministero della Salute, DIREZIONE GENERALE DELLA PREVENZIONE SANITARIA. UFFICIO 5 – PREVENZIONE DELLE MALATTIE TRASMISSIBILI E PROFILASSI INTERNAZIONALE. [cited 2021 Dec 15]. Available from: https://www.iss.it/documents/20126/0/Circolare_su_prevenzione_e_controllo_delle_malattie_batteriche_invasive_prevenibili_con_vaccinazione_2017.pdf/617b3ec6-fddc-fc76-1eda-e9db0700624a?t=1616751347702,2017.
- Simões MJ, Martins JV, Doença invasiva meningocócica em Portugal – Vigilância epidemiológica integrada, 2007-2016. [cited 2021 Dec 15]. Available from: https://www.sip-spp.pt/media/5vwgpwwr/insa_doenca_invasiva_meningococica_2007-2016.pdf.
- Tzanakaki G, ΕΘΝΙΚΟ ΚΕΝΤΡΟ ΑΝΑΦΟΡΑΣ ΜΗΝΙΓΓΙΤΙΔΑΣ. ΠΑΡΕΧΟΜΕΝΕΣ ΥΠΗΡΕΣΙΕΣ ΚΑΙ ΟΔΗΓΙΕΣ ΑΠΟΣΤΟΛΗΣ ΔΕΙΓΜΑΤΩΝ [cited 2021 Dec 15]. Available from: https://eelno.uniwa.gr/wp-content/uploads/sites/388/2020/06/%CE%A5%CE%A0%CE%97%CE%A1%CE%95%CE%A3-%CE%9C%CE%97%CE%9D%CE%99%CE%93%CE%93.pdf.
- Tzanakaki G, Xirogianni A, Tsitsika A, et al. Estimated strain coverage of serogroup B meningococcal vaccines: a retrospective study for disease and carrier strains in Greece (2010-2017). Vaccine. 2021;39(11):1621–1630. DOI:10.1016/j.vaccine.2021.01.073
- Ministerio de Sanidad, Real Decreto 2210/1995, de 28 de diciembre, por el que se crea la red nacional de vigilancia epidemiológica. [cited 2021 Dec 15]. Available from: https://www.boe.es/buscar/act.php?id=BOE-A-1996-1502.
- Jolley KA, Bray JE, Maiden MCJ. Open-access bacterial population genomics: bIGSdb software, the PubMLST.Org website and their applications. Wellcome Open Res. 2018;3:124. doi: 10.12688/wellcomeopenres.14826.1
- Carannante A, Fazio C, Neri A, et al. Meningococcal B vaccine antigen FHbp variants among disease-causing Neisseria meningitidis B isolates, Italy, 2014–2017. PLoS ONE. 2020;15(11):e0241793. DOI:10.1371/journal.pone.0241793
- National Meningitis Reference Laboratory, annual reports 2018-2019. [cited 2021 Jul 28]. Available from: https://eelno.uniwa.gr/monada-ergastiriakis-epitirisis-tis-vaktiriakis-miniggitidas/#apologismoi_ekam.
- Abad R, Garcia-Amil C, Navarro C, et al. Molecular characterization of invasive serogroup B Neisseria meningitidis isolates from Spain during 2015-2018: evolution of the vaccine antigen factor H binding protein (FHbp). J Infect. 2021;82(4):37–44. DOI:10.1016/j.jinf.2021.01.030
- Bollettino Ufficiale della Regione Puglia n. 149 del 30-12-2017. [cited 2022 Sep 12]. Available from: https://www.vaccinarsinpuglia.org/assets/uploads/files/191/legge-regionale-n.67-del-2017-art-60.pdf.
- Sezione Promozione della Salute e del Benessere, Legge regionale n. 67 del 29-122019. [cited 2022 Sep 12]. Available from: https://www.vaccinarsinpuglia.org/assets/uploads/files/191/nota-prot.-aoo152-135.pdf.
- Gazzetta Ufficiale della regione Sicilia. Anno 71°. Numero. 49. [cited 2022 Sep 12]. Available from http://www.gurs.regione.sicilia.it/Gazzette/g17-49/g17-49.pdf
- Decreto N 28 del 25-03-2019. Regione Campania. [cited 2022 Sep 12]. Available from: http://www.aiopcampania.it/public/normativa/4b06bd76312d445d85b9f8caa6082a8b.pdf.
- Delibere della Regione Basilicata Anno 2021. [cited 2022 Sep 12]. Available from: http://opservice.regione.basilicata.it/opendata-cma/ServletDocumentAtti?idAllegato=795801307B5906377C5102&idDocumento=795801307B5903357F5C.
- Decreto della Regione Calabria N. 32 DEL 07 APRILE 2022. https://www.regione.calabria.it/website/portalmedia/decreti/2022-04/DCA-n.32-del-7.4.2022.pdf. [cited 2022 Sep 12]. Available from.
- Delibera Regione Lazio U.0626754.29-07-2019 [cited 2022 Sep 12] Available from https://www.aslroma2.it/attachments/article/311/Prot_0626754_Circolare%20Vaccini_pediatri.pdf
- Leon, J.d.C.y, Calendario vacunal para toda la vida de Castilla y León. [cited 2022 Sep 12]. Available from: https://www.saludcastillayleon.es/profesionales/en/vacunaciones/calendario-vacunal-toda-vida-castilla-leon.
- Salud, S.C.d.l., Nuevo Calendario vacunal para todas las edades de la vida. [cited 2022 Sep 12]. Available from: https://www3.gobiernodecanarias.org/sanidad/scs/contenidoGenerico.jsp?idDocument=3cc62be0-9746-11e0-ba66-75bd8cf93e41&idCarpeta=b25ca6dc-a9a4-11dd-b574-dd4e320f085c.
- Familias, J.d.A.-C.d.S.y, Calendario de Vacunaciones 2022. [cited 2022 Sep 12]. Available from: https://www.andavac.es/calendario-vacunaciones/.
- Catalunya GD, La vacuna contra el meningococo B se incluye en el calendario de vacunaciones. [cited 2022 Sep 12]. Available from: https://web.gencat.cat/es/actualitat/detall/La-vacuna-contra-el-meningococ-B-sinclou-al-calendari-de-vacunacions.
- Catalunya GD, Calendari de vacunacions. [cited 2022 Sep 12]. Available from: https://canalsalut.gencat.cat/ca/salut-a-z/v/vacunacions/calendari/.
- Ciudad Aútonoma de Melilla. [cited 2022 Sep 13]. Available from: https://www.melilla.es/melillaPortal/RecursosWeb/DOCUMENTOS/1/0_26083_1.pdf.
- Murcia Salud. [cited 2022 Sep 13]. Available from: https://www.murciasalud.es/pagina.php?id=399382&idsec=824#.
- Govern Illes Balears. Calendario común de vacunación a lo largo de toda la vida en las Illes Balears 2022. [cited 2022 Sep 13] Available from: https://www.caib.es/sites/vacunacions/es/calendario_de_vacunacion_a_lo_largo_de_toda_la_vida/.
- Trumenba. Summary of product characteristics. [cited 2021 Oct 25]. Available from: https://www.ema.europa.eu/en/documents/product-information/trumenba-epar-product-information_en.pdf.
- ECDC, Invasive meningococcal disease: annual Epidemiological Report for 2017. [cited 2021 Nov 11]. Available from: https://www.ecdc.europa.eu/en/meningococcal-disease/surveillance-and-disease-data/aer.
- ECDC, Surveillance Atlas of Infectious Diseases. [cited 2021 Nov 1]. Available from: https://www.ecdc.europa.eu/en/meningococcal-disease/surveillance-and-disease-data/atlas.
- Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129(6):1327–1348. doi: 10.1084/jem.129.6.1327
- Pollard AJ, Frasch C. Development of natural immunity to Neisseria meningitidis. Vaccine. 2001;19(11–12):1327–1346. doi: 10.1016/S0264-410X(00)00333-9
- Trotter CL, Gay NJ, Edmunds WJ. The natural history of meningococcal carriage and disease. Epidemiol Infect. 2006;134(3):556–566. doi: 10.1017/S0950268805005339
- Bruce MG, Rosenstein NE, Capparella JM, et al. Risk factors for meningococcal disease in college students. JAMA. 2001;286(6):688–693. DOI:10.1001/jama.286.6.688
- Imrey PB, Jackson LA, Ludwinski PH, et al. Outbreak of serogroup C meningococcal disease associated with campus bar patronage. Am J Epidemiol. 1996;143(6):624–630. DOI:10.1093/oxfordjournals.aje.a008792
- Tully J, Viner RM, Coen PG, et al. Risk and protective factors for meningococcal disease in adolescents: matched cohort study. BMJ. 2006;332(7539):445–450. DOI:10.1136/bmj.38725.728472.BE
- van Ravenhorst MB, Bijlsma MW, van Houten MA, et al. Meningococcal carriage in Dutch adolescents and young adults; a cross-sectional and longitudinal cohort study. Clin Microbiol Infect. 2017;23(8):e573.1–.e573.7. doi: 10.1016/j.cmi.2017.02.008
- Whittaker R, Gomez Dias J, Ramliden M, et al. The epidemiology of invasive meningococcal disease in EU/EEA countries, 2004-2014. Vaccine. 2017;35(16):2034–2041. doi: 10.1016/j.vaccine.2017.03.007.
- DGS, Campanha de vacinação contra a doença invasiva por Neisseria meningitidis do serogrupo C. Circular Normativa 09/DT de 22/12/05, Direcção Geral de Saúde, Ministério da Saúde; 2005. [cited 2021 Jul 19].
- Flountzi A, Georgakopoulou T, Balasegaram S, et al. Epidemiology of invasive meningococcal disease in Greece, 2006-2016. Eur J Clin Microbiol Infect Dis. 2019;38(12):2197–2203. DOI:10.1007/s10096-019-03668-y
- Stefanelli P, Miglietta A, Pezzotti P, et al. Increased incidence of invasive meningococcal disease of serogroup C/clonal complex 11, Tuscany, Italy, 2015 to 2016. Euro Surveill. 2016;21(12). doi: 10.2807/1560-7917.ES.2016.21.12.30176
- Pezzotti P, Bellino S, Riccardo F, et al. Vaccine preventable invasive bacterial diseases in Italy: a comparison between the national surveillance system and recorded hospitalizations, 2007-2016. Vaccine. 2019;37(1):41–48. DOI:10.1016/j.vaccine.2018.11.047
- ECDC, Vaccine scheduler. meningococcal disease: recommended vaccinations. [cited 2021 Nov 20]. Available from: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=48&SelectedCountryIdByDisease=-1.
- Gray SJ, Trotter CL, Ramsay ME, et al. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the meningococcal reference Unit. J Med Microbiol. 2006;55(7):887–896. DOI:10.1099/jmm.0.46288-0
- Larrauri A, Cano R, Garcia M, et al. Impact and effectiveness of meningococcal C conjugate vaccine following its introduction in Spain. Vaccine. 2005;23(32):4097–4100. DOI:10.1016/j.vaccine.2005.03.045
- Campbell H, Andrews N, Borrow R, et al. Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin Vaccine Immunol. 2010;17(5):840–847. DOI:10.1128/CVI.00529-09
- Garrido-Estepa M, Nunez OG, Leon-Gomez I, et al. Meningococcal C conjugate age-dependant long-term loss of effectiveness. Vaccine. 2015;33(19):2221–2227. DOI:10.1016/j.vaccine.2015.03.044
- Garrido-Estepa M, Leon-Gomez I, Herruzo R, et al. Changes in meningococcal C epidemiology and vaccine effectiveness after vaccine introduction and schedule modification. Vaccine. 2014;32(22):2604–2609. DOI:10.1016/j.vaccine.2014.03.010
- Hellenbrand W, Elias J, Wichmann O, et al. Epidemiology of invasive meningococcal disease in Germany, 2002-2010, and impact of vaccination with meningococcal C conjugate vaccine. J Infect. 2013;66(1):48–56. DOI:10.1016/j.jinf.2012.09.008
- Krone M, Gray S, Abad R, et al. Increase of invasive meningococcal serogroup W disease in Europe, 2013 to 2017. Euro Surveill. 2019;24(14). doi: 10.2807/1560-7917.ES.2019.24.14.1800245
- Booy R, Gentile A, Nissen M, et al. Recent changes in the epidemiology of Neisseria meningitidis serogroup W across the world, current vaccination policy choices and possible future strategies. Hum Vaccin Immunother. 2019;15(2):470–480. DOI:10.1080/21645515.2018.1532248
- Campbell H, Edelstein M, Andrews N, et al. Emergency meningococcal ACWY vaccination program for teenagers to control group w meningococcal Disease, England, 2015-2016. Emerg Infect Dis. 2017;23(7):1184–1187. DOI:10.3201/eid2307.170236
- Presa J, Findlow J, Vojicic J, et al. Epidemiologic trends, global shifts in meningococcal vaccination guidelines, and data supporting the use of MenACWY-TT vaccine: a review. Infect Dis Ther. 2019;8(3):307–333. DOI:10.1007/s40121-019-0254-1
- Luca V, Gessner BD, Luca C, et al. Incidence and etiological agents of bacterial meningitis among children <5 years of age in two districts of Romania. Eur J Clin Microbiol Infect Dis. 2004;23(7):523–528. DOI:10.1007/s10096-004-1169-6
- Achtman M, van der Ende A, Zhu P, et al. Molecular epidemiology of serogroup a meningitis in Moscow, 1969 to 1997. Emerg Infect Dis. 2001;7(3):420–427. DOI:10.3201/eid0703.017309
- Tsolia MN, Theodoridou M, Tzanakaki G, et al. Invasive meningococcal disease in children in Greece: comparison of serogroup a disease with disease caused by other serogroups. Eur J Clin Microbiol Infect Dis. 2006;25(7):449–456. DOI:10.1007/s10096-006-0155-6
- Kremastinou J, Tzanakaki G, Velonakis E, et al. Carriage of Neisseria meningitidis and Neisseria lactamica among ethnic Greek school children from Russian immigrant families in Athens. FEMS Immunol Med Microbiol. 1999;23(1):13–20. DOI:10.1111/j.1574-695X.1999.tb01711.x
- Balmer P, York LJ. Optimal use of meningococcal serogroup B vaccines: moving beyond outbreak control. Ther Adv Vaccines Immunother. 2018;6(3):49–60. doi: 10.1177/2515135518781757.
- Martinón-Torres F, Banzhoff A, Azzari C, et al. Recent advances in meningococcal B disease prevention: real-world evidence from 4CMenB vaccination. J Infect. 2021;83(1):17–26. doi: 10.1016/j.jinf.2021.04.031.
- De Wals P, Deceuninck G, Lefebvre B, et al. Impact of an immunization campaign to control an increased incidence of serogroup b meningococcal disease in one region of Quebec, Canada. Clin Infect Dis. 2017;64(9):1263–1267. DOI:10.1093/cid/cix154
- Fiorito TM, Bornschein S, Mihalakos A, et al. Rapid response to a college outbreak of meningococcal serogroup B disease: nation’s first widespread use of bivalent rLP2086 vaccine. J Am Coll Health. 2017;65(4):294–296. DOI:10.1080/07448481.2017.1285772
- Capitano B, Dillon K, LeDuc A, et al. Experience implementing a university-based mass immunization program in response to a meningococcal B outbreak. Hum Vaccin Immunother. 2019;15(3):717–724. DOI:10.1080/21645515.2018.1547606
- Joint Committee on Vaccination and Immunisation, JCVI position statement on use of Bexsero® meningococcal B vaccine in the UK. [cited 2021 Nov 2]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/294245/JCVI_Statement_on_MenB.pdf.
- García FJ Á, Cilleruelo Ortega MJ, Alvarez Aldean J, et al. Calendario de vacunaciones de la Asociación Española de Pediatría: recomendaciones 2021. Anales de Pediatría. 2021;94(1):.e53.1–.e53.10. DOI:10.1016/j.anpedi.2020.10.002
- Azzari C, Moriondo M, Nieddu F, et al. Effectiveness and Impact of the 4CMenB vaccine against group B meningococcal disease in Two Italian Regions Using Different Vaccination Schedules: a Five-Year Retrospective Observational Study. Vaccines (Basel). 2014-2018;2020(8):469. DOI:10.3390/vaccines8030469
- Sulis G, Horn M, Borrow R, et al. A comparison of national vaccination policies to prevent serogroup B meningococcal disease. Vaccine. 2022;40(26):3647–3654. DOI:10.1016/j.vaccine.2022.04.101
- ECDC, Expert opinion on the introduction of the meningococcal B (4CMenB) vaccine in the EU/EEA [cited 2021 Nov 3]. Available from: https://www.ecdc.europa.eu/en/publications-data/expert-opinion-introduction-meningococcal-b-4cmenb-vaccine-eueea.
- Rodrigues FMP, Marlow R, Joao Simoes M, et al. Association oF use of a meningococcus group B vaccine with Group B invasive meningococcal disease among children in Portugal. JAMA. 2020;324(21):2187–2194. DOI:10.1001/jama.2020.20449
- Rivero-Calle I, Raguindin PF, Gomez-Rial J, et al. Meningococcal Group B Vaccine for the prevention of invasive meningococcal disease caused by neisseria meningitidis serogroup B. Infect Drug Resist. 2019;12: 3169–3188. doi: 10.2147/IDR.S159952
- Moreno-Pérez D, Alvarez Garcia FJ, Aristegui Fernandez J, et al. Immunisation schedule of the Spanish Association of Paediatrics: 2016 recommendations. An Pediatr (Barc). 2016;84(1):60.e1–13. DOI:10.1016/j.anpedi.2015.10.001
- Calendario de Vacunaciones de la Asociación Española de Pediatría, Razones y bases de las recomendaciones 2022. Madrid: AEP; 2022. [cited 2022 Sep 12]. Available from: https://vacunasaep.org/profesionales/calendario-de-vacunaciones-de-la-aep-2022.
- Parikh SR, Andrews NJ, Beebeejaun K, et al. Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: a national observational cohort study. Lancet. 2016;388(10061):2775–2782. DOI:10.1016/S0140-6736(16)31921-3
- Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine. 2001;20(Suppl 1):S58–67. doi: 10.1016/S0264-410X(01)00299-7
- Deceuninck G, Lefebvre B, Tsang R, et al. Impact of a mass vaccination campaign against Serogroup B meningococcal disease in the Saguenay-Lac-Saint-Jean region of Quebec four years after its launch. Vaccine. 2019;37(31):4243–4245. DOI:10.1016/j.vaccine.2019.06.021
- Marshall HS, McMillan M, Koehler AP, et al. Meningococcal B vaccine and meningococcal carriage in adolescents in Australia. N Engl J Med. 2020;382(4):318–327. DOI:10.1056/NEJMoa1900236
- Soeters HM, Whaley M, Alexander-Scott N, et al. Meningococcal carriage evaluation in response to a serogroup B meningococcal disease outbreak and mass vaccination Campaign at a College-Rhode Island, 2015-2016. Clin Infect Dis. 2017;64(8):1115–1122. DOI:10.1093/cid/cix091
- McNamara LA, Dolan Thomas J, MacNeil J, et al. Meningococcal carriage following a vaccination campaign with MenB-4C and MenB-FHbp in response to a university serogroup B meningococcal Disease Outbreak-Oregon, 2015-2016. J Infect Dis. 2017;216(9):1130–1140. DOI:10.1093/infdis/jix446
- Snape MD, Saroey P, John TM, et al. Persistence of bactericidal antibodies following early infant vaccination with a serogroup B meningococcal vaccine and immunogenicity of a preschool booster dose. CMAJ. 2013;185(15):E715–E724. DOI:10.1503/cmaj.130257
- Carr J, Plested E, Aley P, et al. ‘Be on the TEAM’ Study (Teenagers Against Meningitis): protocol for a controlled clinical trial evaluating the impact of 4CMenB or MenB-fHbp vaccination on the pharyngeal carriage of meningococci in adolescents. BMJ Open. 2020;10(10):e037358. DOI:10.1136/bmjopen-2020-037358
- Harris SL, Donald RGK, Hawkins JC, et al. Neisseria meningitidis Serogroup B vaccine, bivalent rLP2086, induces broad serum bactericidal activity against diverse invasive disease strains including outbreak strains. Pediatr Infect Dis J. 2017;36(2):216–223. DOI:10.1097/INF.0000000000001399
- Lujan E, Partridge E, Giuntini S, et al. Breadth and duration of meningococcal serum bactericidal activity in health care workers and microbiologists immunized with the MenB-FHbp Vaccine. Clin Vaccine Immunol. 2017;24(8):e00121–17. DOI:10.1128/CVI.00121-17
- Taha MK, Hawkins JS, Liberator P, et al. Bactericidal activity of sera from adolescents vaccinated with bivalent rLP2086 against meningococcal serogroup B outbreak strains from France. Vaccine. 2017;35(11):1530–1537. DOI:10.1016/j.vaccine.2017.01.066
- Pollard AJ, MacDonald NE, Dubé E, et al. Presentations at the UK National Immunisation Conference. Hum Vaccin Immunother. 2022;18(7):2087411.
- Andrianou XD, Riccardo F, Caporali MG, et al. Evaluation of the national surveillance system for invasive meningococcal disease, Italy, 2015–2018. PLoS ONE. 2021;16(1):e0244889. DOI:10.1371/journal.pone.0244889
- Fox AJ, Taha MK, Vogel U. Standardized nonculture techniques recommended for European reference laboratories. FEMS Microbiol Rev. 2007;31(1):84–88. doi: 10.1111/j.1574-6976.2006.00048.x
- Simões MJ, Bettencourt C, De Paola R, et al. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Portugal. PLoS ONE. 2017;12(5):e0176177. DOI:10.1371/journal.pone.0176177
- Instituto de salud Carlos III - RENAVE, Informes Anuales de la Red Nacional de Vigilancia Epidemiológica (RENAVE) [cited 2021 Nov 9]. Available from: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Paginas/Informes-anuales-RENAVE.aspx.

