ABSTRACT
Background
Adults with chronic or immunocompromising conditions have an elevated risk of invasive pneumococcal disease, yet their pneumococcal vaccination rates remain low.
Methods
This retrospective cohort study used the IBM MarketScan® Multi-State Medicaid database to examine pneumococcal vaccination uptake among adults 19–64 years of age with underlying conditions. Gompertz accelerated failure time model was used to examine factors associated with vaccination.
Results
In the study population of 108,159 adults, the vaccination rate was 4.1% after 1 year of follow-up and 19.4% after 10 years. The mean time from initial diagnosis to vaccination was 3.9 years. Adults aged 35–49 and 50–64 years (relative to 19–34) or those receiving an influenza vaccination were more likely to receive a pneumococcal vaccination. Adults with HIV/AIDS were more likely, while adults with chronic heart or lung disease, alcohol or tobacco dependence, or cancer were less likely to be vaccinated than adults with diabetes mellitus. Adults diagnosed by specialists were less likely to be vaccinated than those diagnosed by primary care providers.
Conclusions
The rates of pneumococcal vaccination among adults with Medicaid plans and underlying conditions were well under Healthy People Initiative targets. Insights into factors associated with vaccination can inform efforts to improve vaccination rates among this population.
1. Introduction
Pneumococcal diseases are caused by infection with Streptococcus pneumoniae and include sinusitis, otitis media, pneumonia, meningitis, and bacteremia. The latter two conditions are classified as invasive pneumococcal disease (IPD) and are a major cause of mortality and morbidity among US adults: a recent meta-analysis estimated an overall mortality rate of 17.2% [Citation1]. The rate of IPD was estimated to be 8 per 100,000 persons or 94.6 per 100,000 hospitalizations [Citation2,Citation3]. Adults with underlying chronic or immunocompromising conditions are at a higher risk of developing IPD and have higher hospitalization and mortality rates once infected than the general adult population [Citation4,Citation5].
Effective vaccines are available to prevent pneumococcal disease [Citation6]. The 2019 Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC) guidelines recommended administration of pneumococcal 23-valent polysaccharide vaccine (PPSV23, trademark Pneumovax23, manufactured by Merck & Co., Inc) for adults 19–64 years of age with chronic medical conditions, and sequential administration of pneumococcal 13-valent conjugate vaccine (PCV13, trademark Prevnar 13, manufactured by Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc) and PPSV23 for those with immunocompromising conditions [Citation7]. In 2021, the US Food and Drug Administration approved 15-valent (PCV15, trademark Vaxneuvance, manufactured by Merck & Co., Inc) and 20-valent (PCV20, trademark Prevnar 20, manufactured by Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc) pneumococcal conjugate vaccines for use in adults 18 years of age or older. The updated 2021 ACIP guidelines recommend either PCV20 alone or PCV15 followed by PPSV23 for adults 19–64 years of age with underlying medical conditions and for adults 65 years or older [Citation7].
The CDC’s Healthy People 2020 initiative set a target of 60% pneumococcal vaccination among high-risk adults 18–64 years of age (the corresponding 2030 goals are yet to be established) [Citation8]. However, the CDC reported that the 2020 pneumococcal vaccination rate was 29.2%, well below the target [Citation8,Citation9]. The literature has also consistently reported low rates of pneumococcal vaccination among adults with underlying conditions, ranging from 4.9 to 30.5% [Citation10–13].
The rate of IPD among US adults has decreased since the introduction of effective vaccines, but IPD still represents a major health and economic burden. An analysis of the economic burden of IPD and nine other vaccine-preventable diseases in US adults estimated that about 80% of total health care costs for these diseases are spent on treating unvaccinated individuals [Citation14]. Individuals with underlying medical conditions incur substantially higher costs for treatment of IPD than do adults without such conditions [Citation5,Citation14,Citation15]. There are numerous missed opportunities in the US healthcare system for pneumococcal vaccination of adults with underlying medical conditions [Citation16]. Understanding factors associated with pneumococcal vaccination among adults with underlying conditions can inform efforts to increase vaccination uptake and reduce the health and economic burden of IPD.
Most studies of pneumococcal vaccination among adults 19–64 years of age with underlying conditions have focused on people enrolled in commercial health plans [Citation11–13]. In contrast, adults in this age range enrolled in Medicaid are rarely studied. It is important to understand pneumococcal vaccination rates among adults enrolled in Medicaid, as of 2020, 18% of the total US population were covered by Medicaid [Citation17]. This study examines the rates of pneumococcal vaccination among US adults 19–64 years of age enrolled in Medicaid plans with newly diagnosed chronic or immunocompromising conditions, and identifies factors associated with pneumococcal vaccination among this population. It further compares pneumococcal vaccination rates among adults with underlying conditions covered with Medicaid versus commercial health plans [Citation13].
2. Methods
2.1. Study design and data
This was a retrospective observational cohort study using data from the IBM MarketScan® Multi-State Medicaid database [Citation18]. The database reflects the pooled health care service use of individuals covered by Medicaid programs (managed care and fee-for-service plans) in numerous geographically dispersed states. It includes records of inpatient services and admissions, outpatient services, prescription drug claims, and information on long-term care for more than 48 million individuals. The database is formally certified as deidentified, and all study procedures were compliant with the US Health Insurance Portability and Accountability Act. No Institutional Review Board approval or specific informed consent was required for this study.
The comparison with commercial plan enrollees used vaccination rates from a previous study by the authors [Citation13].
2.2. Study population
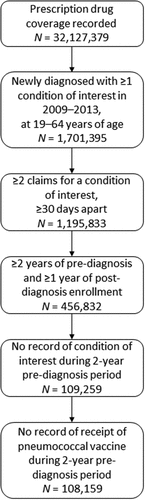
This study population comprised adults 19–64 years of age with Medicaid coverage who had been newly diagnosed with one or more chronic or immunocompromising condition between 1 January 2009 and 31 December 2013. Chronic conditions included chronic heart disease (cardiomyopathy; chronic hypertensive, ischemic, pulmonary, or rheumatic heart disease; heart failure; and others), chronic liver disease (all forms, including cirrhosis and liver abscess), chronic lung disease (asthma, chronic obstructive pulmonary disease, pneumoconiosis, and others), diabetes mellitus (all subtypes), and alcohol or tobacco dependence. Immunocompromising conditions included asplenia (functional or anatomic), cancer (carcinoma in situ, Hodgkin disease, leukemia, malignant neoplasm, and others), chronic renal disease, HIV/AIDs, and organ transplantation (cornea, heart, kidney, liver, lung, pancreas, thymus, and others). Conditions were identified by the presence of one or more relevant International Classification of Diseases, Clinical Modification version 9 or 10 (ICD-9-CM/ICD-10-CM) or Current Procedural Terminology version 4 (CPT4) codes (, Appendix) in any field of an inpatient or outpatient medical claim. At least two administrative claims ≥30 days apart for a condition of interest were required, as were continuous health plan enrollment for ≥2 years before and ≥1 year after the initial diagnosis date (an enrollment gap of ≤45 days was permitted), and a prescription drug benefit. Exclusion criteria were pneumococcal vaccination or any evidence of the condition(s) of interest in the 2-year period prior to date of initial diagnosis. Follow-up was until the date of pneumococcal vaccination, death, or 31 December 2019, whichever came first. The length of the follow-up period ranged from 1 to 10 years.
2.3. Study measures
The following variables potentially associated with pneumococcal vaccination were defined at the time of initial qualifying diagnosis: sex, age, race, health plan type, initial qualifying diagnosis, and type of provider assigning the initial diagnosis. The following time-varying variables were recorded during the entire follow-up period: number of accumulated chronic and/or immunocompromising conditions, average annual number of outpatient and inpatient visits, and receipt of an influenza or pneumococcal vaccine. Receipt of an influenza and pneumococcal vaccine (PPSV23 or PCV13) was defined as the presence of a relevant CPT4 or national drug code (, Appendix). Pneumococcal vaccines PCV15 and PCV20 were approved in the US after the study period ended and were therefore excluded.
2.4. Statistical analysis
Descriptive statistics – percentages and frequencies for categorical variables and means and standard deviations (SDs) for continuous variables – were used to characterize the study population. Bivariate analyses of associations between individual characteristics and pneumococcal vaccination were conducted using a chi-square test with Bonferroni correction for categorical variables and a t test for continuous variables. A multivariable Cox proportional hazards model was first used to examine factors that are associated with pneumococcal vaccination. However, the assumptions that underpins the Cox proportional hazard model, proportionality of hazard function over time, did not hold based on information from the Kaplan-Meier curve and test using Schoenfeld residuals. Therefore, we resorted to parametric approaches, accelerated failure time (AFT) models, which do not require proportional hazard assumption [Citation19]. A number of distributions (exponential, Weibull, Gompertz, Lognormal, Log-logistics, and gamma) of the AFT were considered Based on Akaike’s information criterion (AIC), a Gompertz AFT model was used to assess factors associated with pneumococcal vaccination. Pneumococcal vaccination rates were compared between Medicaid and commercial plan enrollees using a two-sample z test. A P value of < 0.01 was deemed significant in tests of difference. Data analyses were conducted using R version 4.0.
3. Results
3.1. Study population characteristics
A total of 108,159 adults met all study criteria (). The characteristics of the study population are described in . Two-thirds (67.0%) were female, and the mean age was 40.1 (SD = 13.1) years. More than half of the study population (53.7%) were White, 37.1% were Black, 1.0% were Hispanic, and 8.2% were of other or unknown race. A majority (55.1%) of the study population had comprehensive health plans, around one-third (32.2%) had health maintenance organization (HMO) plans, and the remainder (12.7%) had point-of-service (POS) plans. The most diagnosed chronic condition was tobacco dependence (36.9%), followed by chronic lung disease (25.3%) and diabetes mellitus (12.8%); the most diagnosed immunocompromising conditions were cancer (3.0%) and chronic renal disease (1.9%); and 5.7% of the study population had more than one initial condition of interest. Influenza vaccination during the follow-up period was identified for 16.4% of the study population.
Table 1. Characteristics of adults 19–64 years of age newly diagnosed with chronic or immunocompromising conditions in 2009–2013 in the US.
3.2. Pneumococcal vaccination rates by condition and years of follow-up
A total of 11,385 adults (10.5% of the study population) received a pneumococcal vaccine during the follow-up period (). The overall vaccination rate ranged from 4.1% among adults with 1 year of follow-up data available to 19.4% among those with 10 years of follow-up ( & ). Adults initially diagnosed with HIV/AIDS had the highest rate of pneumococcal vaccination (37.2%), followed by those initially diagnosed with chronic renal disease (16.7%) or diabetes mellitus (14.3%; ). The pneumococcal vaccination rate was lowest among adults initially diagnosed with tobacco dependence (7.3%), alcohol dependence (8.7%), or cancer (10.2%). The overall mean time interval between initial diagnosis and subsequent pneumococcal vaccination was 3.9 (SD = 2.6) years and ranged from 2.9 (SD = 2.6) years for adults initially diagnosed with HIV/AIDS to 4.4 (SD = 3.0) years for those initially diagnosed with asplenia (, Appendix).
Figure 2. Pneumococcal vaccination rates among adults 19–64 years of age newly diagnosed with chronic or immunocompromising conditions, by condition and years of follow-up available.
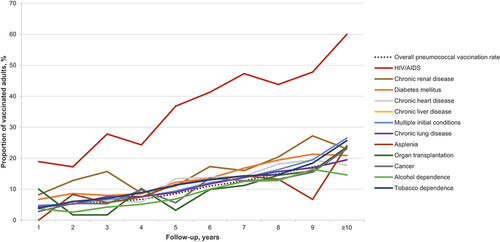
Table 2. Pneumococcal vaccination rates among adults 19–64 years of age newly diagnosed with chronic or immunocompromising conditions, by condition and years of follow-up data available.
The pneumococcal vaccination rates for adults with Medicaid were compared to rates for adults with commercial plans [Citation13] (, Appendix). The overall vaccination rate was statistically significantly lower in the Medicaid than in the commercial population (10.5% versus 13.7%). Further, the rates of vaccination were significantly lower in the Medicaid than in the commercial population for adults initially diagnosed with diabetes mellitus (14.3% versus 19.5%), chronic lung disease (11.5% versus 14.6%), tobacco dependence (7.3% versus 10.7%), HIV/AIDS (37.2% versus 48.3%), or multiple initial conditions (11.6% versus 14.0%). The rates of vaccination were significantly higher in the Medicaid than in the commercial population for adults initially diagnosed with alcohol dependence (8.7% versus 6.3%), chronic kidney disease (17.0% versus 13.7%), or organ transplantation (10.8% versus 7.4%).
3.3. Factors associated with pneumococcal vaccination
The bivariate analysis showed that being male, older, Hispanic (compared with White), initially diagnosed with HIV/AIDs or chronic renal disease (compared with diabetes mellitus), or covered under other types of health plans (compared with HMO), were associated with a higher likelihood of pneumococcal vaccination (). Accumulating two or more conditions (including the initial diagnosis), receiving an influenza vaccination, having one or more inpatient visit per year, or having more annual outpatient visits over the follow-up period were also associated with higher likelihood of pneumococcal vaccination. Being Black (compared with White), or initially diagnosed with chronic liver or lung disease, alcohol dependence, tobacco dependence, asplenia, cancer, organ transplantation, or multiple initial conditions (compared with diabetes mellitus) were associated with lower likelihood of vaccination.
The Gompertz AFT model identified factors associated with pneumococcal vaccination; the results were consistent with the findings of the bivariate analysis for most variables (). Adults 35–49 years of age (hazard ratio (HR) = 1.94, 95% confidence interval (CI) = 1.84–2.05) or 50–64 years of age (HR = 2.98, 95% CI = 2.82–3.14) were more likely to receive a pneumococcal vaccination than those 19–34 years of age. Hispanic adults (HR = 1.42, 95% CI = 1.20–1.68) were more likely to be vaccinated than White adults. Adults with an initial diagnosis of HIV/AIDS (HR = 3.80, 95% CI = 3.32–4.35) were more likely to be vaccinated than those with diabetes mellitus.
Table 3. Gompertz Accelerated failure time model results on factors associated with pneumococcal vaccination in adults 19–64 years of age newly diagnosed with chronic or immunocompromising conditions.
Black adults (HR = 0.88, 95% CI = 0.84–0.92) were less likely to be vaccinated than White adults. Being covered under a comprehensive or POS plan (HR = 0.92, 95% CI = 0.88–0.97 and HR = 0.70, 95% CI = 0.66–0.75, respectively) was associated with a lower likelihood of pneumococcal vaccination than being covered with an HMO plan. Adults with an initial diagnoses of chronic heart disease (HR = 0.79, 95% CI = 0.73–0.85), chronic lung disease (HR = 0.90, 95% CI = 0.85–0.96), alcohol dependence (HR = 0.75, 95% CI = 0.67–0.84), tobacco dependence (HR = 0.73, 95% CI = 0.69–0.77), or cancer (HR = 0.77, 95% CI = 0.69–0.87) were less likely to be vaccinated compared to adults with an initial diagnosis of diabetes mellitus. Adults who accumulated two or more qualifying conditions during the follow-up period (HR = 0.83, 95% CI = 0.80–0.87) were less likely to be vaccinated than adults with one condition. Adults whose initial condition was diagnosed by a specialist (HR = 0.86, 95% CI = 0.79–0.93) were less likely to be vaccinated than those initially diagnosed by primary care providers.
4. Discussion
This study found that the overall rate of pneumococcal vaccination among US adults 19–64 years of age with underlying conditions and enrolled in Medicaid plans was 10.5%, substantially lower than the CDC’s target of 60%. The rate varied by initial diagnosis, ranging from 7.3% for adults initially diagnosed with tobacco dependence to 37.2% for those initially diagnosed with HIV/AIDS. Adults were vaccinated, on average, 4 years after receiving an initial diagnosis for a chronic or immunocompromising condition that indicated a need for pneumococcal vaccination. The low vaccination rate and the long duration between initial diagnosis and vaccination are concerning given that the included study population is particularly vulnerable to IPD.
This study identified factors associated with pneumococcal vaccination in the regression analysis. Adults who were younger or Black (compared to White) were less likely to receive a pneumococcal vaccination, in line with a previous study on racial differences in pneumococcal vaccination coverage, while Hispanic adults were more likely to be vaccinated than White adults, which is counter to the previous study which utilized survey data [Citation20]. Racial disparities in adult vaccination rates can be partially explained by inequities between races in the social determinants of health (e.g. education and income), barriers to accessing health care, and potential missed opportunities for a provider recommendation [Citation20–25]. Identification of additional drivers of racial disparity remains an active area of research. Interventions to increase awareness of the benefits of vaccination and to prompt providers to offer pneumococcal vaccination to underserved populations may be needed [Citation26,Citation27].
Our findings also highlight a discrepancy between the relative risk of IPD and the likelihood of pneumococcal vaccination. For example, among adults with chronic conditions, the risk of IPD is highest for those with tobacco dependence, followed by those with chronic lung, liver, or heart disease and diabetes [Citation28]. However, we found that adults with tobacco dependence, chronic lung, or heart disease were less likely to be vaccinated than adults with diabetes. Tobacco dependence perhaps is not viewed by adults or providers as a medical condition which might contribute to the low rate of vaccination among adults with tobacco dependence [Citation29]. Further, we found that adults initially diagnosed with HIV/AIDS were more likely to be vaccinated than those with diabetes. Adults with HIV/AIDS are often treated through comprehensive and coordinated programs, which sometime include pneumococcal vaccination as a measure of quality of care [Citation29–31]. Similarly, multidisciplinary care and patients’ participation in diabetes education programs may contribute to the higher likelihood of pneumococcal vaccination among adults with diabetes compared with other conditions [Citation32]. Further investigation is warranted to determine whether implementation of similar comprehensive programs for other conditions would improve the rate of pneumococcal vaccination.
Adults with one or more annual inpatient visit were more likely to be vaccinated, which may be due to laws in 13 states that require hospitals to offer pneumococcal vaccines to all inpatients [Citation33]. One state (Texas) also has a similar law for ambulatory care [Citation34]. Enacting policies to require and offer pneumococcal vaccination in ambulatory care in more states and during other health care encounters may help improve vaccination uptake. Given that pneumococcal vaccination was associated with influenza vaccination, offering pneumococcal vaccination during seasonal influenza vaccination campaigns may also help increase vaccine uptake [Citation35,Citation36]. We also found that accumulating two or more qualifying conditions during the study period was associated with a lower likelihood of pneumococcal vaccination. The competing demands of treating and managing multiple conditions may take precedence over vaccination and other preventive care for this group [Citation33].
Compared with adults in commercial plans with underlying conditions, this study found that the overall and most of the medical condition-specific pneumococcal vaccination rates were lower among adults with Medicaid. The mean time from initial diagnosis to receiving pneumococcal vaccination was also longer among adults with Medicaid than adults with commercial plans [Citation13]. This finding adds to the literature on lower rates of administration of other adult vaccines among Medicaid enrollees compared to commercial plan enrollees [Citation34–40]. There might be differences in access to and funding for vaccines between Medicaid and commercial plans [Citation33,Citation41,Citation42]. As of 2020, only 24 states included all ACIP-recommended vaccines as a covered benefit for adults with Medicaid, and an even smaller number of states covered all recommended vaccines without cost sharing by patients [Citation43]. In contrast, commercial health plans are required by the Affordable Care Act to provide coverage without out-of-pocket cost for vaccinations recommended by the ACIP. A survey of 242 family medicine and general internal medicine practices showed that more than half of the respondents reported that they lost money from vaccinating adults with Medicaid [Citation44]. With the recent passage of the Inflation Reduction Act in 2022, states are now required to cover, at no cost, all ACIP-recommended vaccines for all Medicaid-eligible adults [Citation45]. While access barriers may still exist, including where a Medicaid enrollee may seek care, this is a promising step to alleviating patient cost barriers to vaccination. In addition, there might be differences between Medicaid and commercial plans in patient and provider awareness of the benefits of pneumococcal vaccination. Patient and provider education, particularly education and support of specialists, given that adults diagnosed by specialists were less likely to be vaccinated, may help to increase vaccination rates.
The uptake of pneumococcal vaccination among adults with Medicaid was higher than that for adults with commercial plans among those with an initial diagnosis of alcohol dependence, chronic kidney disease, or organ transplantation. Providers serving adults with Medicaid may integrate primary care and behavioral health care and receive federal and state grants to improve care for patients with behavioral health issues, including addictions [Citation46]. These programs and incentives may contribute to the higher pneumococcal vaccination rates among adults with alcohol dependence and Medicaid compared with adults with commercial plans. The introduction of the Centers for Medicare & Medicaid Services End-Stage Renal Disease Quality Incentive Program in 2012 may have similarly improved preventive care for individuals with chronic kidney disease and enrolled in Medicaid [Citation47]. Vaccination is not explicitly included in the program’s performance measures but linking payments to dialysis facilities to other measures, such as overall hospitalization rates, may have indirectly incentivized promotion of pneumococcal vaccination. Finally, Medicaid-enrolled organ transplant recipients have been reported to have more severe organ failure than commercially insured individuals [Citation48], which may urge providers to provide more comprehensive care, including pneumococcal vaccination, to these adults. Further study of the reasons for these differences in vaccination uptake may aid in the design of interventions to increase vaccination rates for Medicaid-enrolled adults with other underlying conditions.
The current study found that the pneumococcal vaccination rate varied with Medicaid health plan type. The lower likelihood of vaccination for adults with comprehensive or POS plans (compared with HMO) shown in the regression model may be associated with actual or perceived higher out-of-pocket costs of vaccination among these plans compared with HMO [Citation49,Citation50]. Further research is needed to explain the higher likelihood of vaccination among adults with Medicaid HMO plans and to aid in efforts to increase vaccination rates for all adults with Medicaid.
Study limitations are noted. The observational study design identified associations between variables and pneumococcal vaccination and not causal relationships. In addition, the findings may not be generalizable to adults who are not covered by Medicaid plans. While disease misclassification may be present, we mitigated it by requiring at least two administrative claims for each condition.
5. Conclusions
This study evaluated pneumococcal vaccination among Medicaid enrollees 19–64 years of age with newly diagnosed chronic or immunocompromising conditions. Despite ACIP recommendations, pneumococcal vaccination rates were low, with 4.1% of adults vaccinated after 1 year of follow-up and 19.4% after 10 years of follow-up. The rates are well below the CDC’s Healthy People targets. Vaccination rates were significantly lower for adults with Medicaid compared with commercial health plans. Findings on factors associated with vaccination can inform efforts to improve pneumococcal vaccination rates in adults with Medicaid and underlying conditions. Our findings suggest that patient and provider education tailored to the needs of underserved groups, adults with specific underlying conditions and specific health care plan types may be beneficial. Initiatives such as the implementation of comprehensive, coordinated care programs for adults with specific underlying conditions and the expansion of policies to offer pneumococcal vaccination during ambulatory and other health care encounters may also help to increase the pneumococcal vaccination rate among adults with underlying conditions.
Declaration of interest
Junqing Liu, Kelly D. Johnson, and Linda Shoener Dunham are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, U.S.A and hold shares in Merck & Co., Inc., Rahway, NJ, U.S.A.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Conception and design: Junqing Liu, Kelly D. Johnson, Linda Shoener Dunham. Analysis and interpretation of the data: Junqing Liu, Kelly D. Johnson, Linda Shoener Dunham. Drafting of the paper: Junqing Liu, Kelly D. Johnson, Linda Shoener Dunham. Revising the paper critically for intellectual content: Junqing Liu, Kelly D. Johnson, Linda Shoener Dunham. Final approval of the version to be published: Junqing Liu, Kelly D. Johnson, Linda Shoener Dunham. All authors agree to be accountable for all aspects of the work.
Acknowledgments
The authors thank ScribCo for editorial support; Anna Ostropolets for analytic support; Alexandra Anne Bhatti, Temi Folaranmi, Grace Gregorio, & Eric M. Sarpong for their review of the manuscript.
Additional information
Funding
References
- Demirdal T, Sen P, Emir B. Predictors of mortality in invasive pneumococcal disease: a meta-analysis. Expert Rev Anti Infect Ther. 2021 Jul;19(7):927–944. doi: 10.1080/14787210.2021.1858799
- Centers for Disease Control and Prevention. Pneumococcal disease: surveillance and reporting. Available from: https://www.cdc.gov/pneumococcal/surveillance.html
- Morrill HJ, Caffrey AR, Noh E, et al. Epidemiology of pneumococcal disease in a national cohort of older adults. Infect Dis Ther. 2014 Jun;3(1):19–33.
- Shea KM, Edelsberg J, Weycker D, et al. Rates of pneumococcal disease in adults with chronic medical conditions. Open Forum Infect Dis. 2014 Mar;1(1):ofu024.
- Weycker D, Farkouh RA, Strutton DR, et al. Rates and costs of invasive pneumococcal disease and pneumonia in persons with underlying medical conditions. BMC Health Serv Res. 2016 May 13;16(1):182. doi: 10.1186/s12913-016-1432-4
- Berical AC, Harris D, Dela Cruz CS, et al. Pneumococcal vaccination strategies. An update and perspective. Ann Am Thorac Soc. 2016 Jun;13(6):933–944.
- Centers for Disease Control and Prevention, Advisory committee on immunization practices. Pneumococcal ACIP vaccine recommendations. Available from: https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/pneumo.html#recs
- Healthy People. IID-13.2: increase the percentage of noninstitutionalized high-risk adults aged 18 to 64 years who are vaccinated against pneumococcal disease. Available from. 2020. https://www.healthypeople.gov/2020/data-search/Search-the-Data?nid=4671.
- Centers for Disease Control and Prevention. AdultVaxView: vaccination coverage among adults. Available from: https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/data-reports/general-population/index.html
- Annunziata K, Rak A, Del Buono H, et al. Vaccination rates among the general adult population and high-risk groups in the United States. PLoS One. 2012;7(11):e50553. doi: 10.1371/journal.pone.0050553
- Lu PJ, Nuorti JP. Uptake of pneumococcal polysaccharide vaccination among working-age adults with underlying medical conditions, United States, 2009. Am J Epidemiol. 2012 Apr 15;175(8):827–837. doi: 10.1093/aje/kwr376
- Petigara T, Zhang D. Pneumococcal vaccine coverage in adults aged 19-64 years, newly diagnosed with chronic conditions in the U.S. Am J Prev Med. 2018 May;54(5):630–636. doi: 10.1016/j.amepre.2018.01.033
- Ostropolets A, Shoener Dunham L, Johnson KD, et al. Pneumococcal vaccination coverage among adults newly diagnosed with underlying medical conditions and regional variation in the U.S. Vaccine. 2022 Aug 5;40(33):4856–4863. doi: 10.1016/j.vaccine.2022.06.068
- Ozawa S, Portnoy A, Getaneh H, et al. Modeling the economic burden of adult vaccine-preventable diseases in the United States. Health Aff. 2016 Nov 1;35(11):2124–2132. doi: 10.1377/hlthaff.2016.0462
- Olasupo O, Segal R, Brown J. Missed opportunities for pneumococcal vaccinations in high-risk and older adults in the United States. J Infect Public Health. 2020 Jan;13(1):101–103. doi: 10.1016/j.jiph.2019.06.010
- Schulz PS, Moore SE, Smith D, et al. Missed pneumococcal vaccination opportunities in adults with invasive pneumococcal disease in a community health system. Open Forum Infect Dis. 2022 Apr;9(4):ofac075.
- Keisler-Starkey K, Bunch LN. Census Bureau Current Population Reports, P60-274, Health Insurance Coverage in the United States: 2020. Washington, DC: US Government Publishing Office. 2021. https://www.census.gov/content/dam/Census/library/publications/2021/demo/p60-274.pdf
- IBM MarketScan research databases for life sciences researchers. IBM Watson Health and MarketScan are trademarks of IBM corporation in the United States, other countries, or both. [Internet]. 2021. Available from: https://www.ibm.com/downloads/cas/OWZWJ0QO.
- Mutua MK, Ochako R, Ettarh R, et al. Effects of low birth weight on time to BCG vaccination in an urban poor settlement in Nairobi, Kenya: an observational cohort study. BMC Pediatr. 2015 Apr 18;15(1):45. doi: 10.1186/s12887-015-0360-5
- Lu PJ, O’Halloran A, Williams WW, et al. Racial and ethnic disparities in vaccination coverage among adult populations in the U.S. Am J Prev Med. 2015 Dec;49(6 Suppl 4):S412–25.
- Centers for Disease Control and Prevention. What is Health Equity? 2022. Available from: https://www.cdc.gov/healthequity/whatis/
- Daniels NA, Gouveia S, Null D, et al. Acceptance of pneumococcal vaccine under standing orders by race and ethnicity. J Natl Med Assoc. 2006 Jul;98(7):1089–1094.
- Granade CJ, Lindley MC, Jatlaoui T, et al. Racial and Ethnic Disparities in Adult Vaccination: a Review of the State of Evidence. Health Equity. 2022;6(1):206–223. doi: 10.1089/heq.2021.0177
- Lindley MC, Wortley PM, Winston CA, et al. The role of attitudes in understanding disparities in adult influenza vaccination. Am J Prev Med. 2006 Oct;31(4):281–285.
- National Vaccine Advisory Committee. A pathway to leadership for adult immunization: recommendations of the national vaccine advisory committee: approved by the national vaccine advisory committee on June 14, 2011. Public Health Rep. 2012 Jan;127 Suppl 1:1–42. doi: 10.1177/00333549121270S101
- Krueger BS, Hutchison ML, Bodo EC, et al. Science-based communication to decrease disparities in adult pneumococcal vaccination rates. J Am Pharm Assoc. 2003 [2020 Nov];60(6):861–867. doi: 10.1016/j.japh.2020.05.020
- Prins W, Butcher E, Hall LL, et al. Improving adult immunization equity: where do the published research literature and existing resources lead? Vaccine. 2017 May 25;35(23):3020–3025. doi: 10.1016/j.vaccine.2017.02.016
- Torres A, Blasi F, Dartois N, et al. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax. 2015 Oct;70(10):984–989.
- Gallant JE, Adimora AA, Carmichael JK, et al. Essential components of effective HIV care: a policy paper of the HIV Medicine Association of the Infectious Diseases Society of America and the Ryan White Medical Providers Coalition. Clin Infect Dis. 2011 Dec;53(11):1043–1050.
- Horberg MA, Aberg JA, Cheever LW, et al. Development of national and multiagency HIV care quality measures. Clin Infect Dis. 2010 Sep 15;51(6):732–738. doi: 10.1086/655893
- Ojikutu B, Holman J, Kunches L, et al. Interdisciplinary HIV care in a changing healthcare environment in the USA. AIDS Care. 2014;26(6):731–735. doi: 10.1080/09540121.2013.855299
- Mendez I, Lundeen EA, Saunders M, et al. Diabetes Self-Management Education and Association with Diabetes Self-Care and Clinical Preventive Care Practices. Sci Diabetes Self Manag Care. 2022 Feb;48(1):23–34.
- Rehm SJ, File TM, Metersky M, et al. Identifying barriers to adult pneumococcal vaccination: an NFID task force meeting. Postgrad Med. 2012 May;124(3):71–79.
- Byron SC, Roth L, Acton RM, et al. Harnessing electronic clinical data to report adult and prenatal immunization quality measures. J Am Med Inform Assoc. 2021 Sep 18;28(10):2226–2232. doi: 10.1093/jamia/ocab125
- Ghaswalla P, Poirrier JE, Packnett ER, et al. Maternal Immunization in the U.S.: a nationwide retrospective cohort study. Am J Prev Med. 2019 Sep;57(3):e87–e93.
- Ghaswalla PK, Patterson BJ, Cheng WY, et al. Hepatitis A, B, and A/B vaccination series completion among US adults: a claims-based analysis. Hum Vaccin Immunother. 2018;14(11):2780–2785. doi: 10.1080/21645515.2018.1489189
- Goodman RM, Bridges CB, Kim D, et al. Billing and payment of commercial and Medicaid health plan adult vaccination claims in Michigan since the Affordable Care Act. Vaccine. 2019 Oct 23;37(45):6803–6813. doi: 10.1016/j.vaccine.2019.09.042
- Goudie A, Martin B, Li C, et al. Higher Rates of Preventive Health Care with Commercial Insurance Compared with Medicaid: findings from the Arkansas Health Care Independence “Private Option. Program Med Care. 2020 Feb;58(2):120–127.
- Krishnarajah G, Carroll C, Priest J, et al. Burden of vaccine-preventable disease in adult Medicaid and commercially insured populations: analysis of claims-based databases, 2006-2010. Hum Vaccin Immunother. 2014;10(8):2460–2467. doi: 10.4161/hv.29303
- Moll K, Wong HL, Fingar K, et al. Vaccine exposure during pregnancy among privately and publicly insured women in the United States, 2016-2018. Vaccine. 2021 Oct 1;39(41):6095–6103. doi: 10.1016/j.vaccine.2021.08.091
- Johnson DR, Nichol KL, Lipczynski K. Barriers to adult immunization. Am j med. 2008 Jul;121(7 Suppl 2):S28–35. doi: 10.1016/j.amjmed.2008.05.005
- Shapiro MF, Morton SC, McCaffrey DF, et al. Variations in the care of HIV-infected adults in the United States: results from the HIV cost and services utilization study. JAMA. 1999 Jun 23-30;281(24):2305–2315. doi: 10.1001/jama.281.24.2305
- Park C, Zettle A Medicaid Coverage of Vaccines. Presented by the Medicaid and CHIP Payment Access Commission, September 25, 2020. https://www.macpac.gov/wp-content/uploads/2020/09/Medicaid-Coverage-of-Vaccines.pdf
- Lindley MC, Hurley LP, Beaty BL, et al. Vaccine financing and billing in practices serving adult patients: a follow-up survey. Vaccine. 2018 Feb 14;36(8):1093–1100. doi: 10.1016/j.vaccine.2018.01.015
- 117th Congress. H.R.5376 - inflation reduction act of 2022. 2022.
- Jones EB, Staab EM, Wan W, et al. Addiction treatment capacity in health centers: the role of Medicaid reimbursement and targeted grant funding. Psychiatr Serv. 2020 Jul 1;71(7):684–690. doi: 10.1176/appi.ps.201900409
- Centers for Medicare & Medicaid Services. ESRD quality incentive program. Available from: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/ESRDQIP
- DuBay DA, MacLennan PA, Reed RD, et al. Insurance type and solid organ transplantation outcomes: a historical perspective on how Medicaid expansion might impact transplantation outcomes. J Am Coll Surg. 2016 Oct;223(4):611–620 e4.
- Jetty A, Petterson S, Rabin DL, et al. Privately insured adults in HDHP with higher deductibles reduce rates of primary care and preventive services. Transl Behav Med. 2018 May 23;8(3):375–385. doi: 10.1093/tbm/ibx076
- Singer DC, Davis MM, Gebremariam A, et al. Underinsurance for recently recommended vaccines in private health plans. J Community Health. 2012 Dec;37(6):1164–1167.
Appendix
Table A1. International Classification of Diseases, Current Procedural Terminology, and National Drug Codes used to identify qualifying medical conditions and relevant vaccinations.
Table A2. Time from initial diagnosis to pneumococcal vaccination in adults 19–64 years of age newly diagnosed with chronic or immunocompromising conditions.
Table A3. Proportions of pneumococcal vaccination by condition: comparison of adults with Medicaid and commercial health plansA.