ABSTRACT
Introduction
Approximately half of the 13.4 billion COVID-19 vaccine doses administered globally were inactivated or viral vector platforms. The harmonization and optimization of vaccine regimens has become a key focus of policymakers and health-care providers and presents an opportunity to reassess the continued use of pandemic-era vaccines.
Areas covered
Immunological evidence from studies of various homologous and heterologous regimens has been rapidly published; however, interpretation of these data is complicated by the many vaccine types and highly variable participant viral exposure and vaccination histories. Recent studies demonstrate that after primary series doses of inactivated (i.e. BBV152, and BBIBP-CorV), and viral vector (ChAdOx1 nCov-2019) vaccines, a heterologous boost with protein-based NVX-CoV2373 elicits more potent ancestral strain and omicron-specific antibody responses compared to homologous and heterologous inactivated and viral vector boosts.
Expert opinion
While mRNA vaccines likely yield similar performance to protein-based heterologous booster doses, the latter offers notable advantages to countries with high uptake of inactivated and viral vector vaccines in terms of transportation and storage logistics and can potentially appeal to vaccine hesitant individuals. Moving forward, vaccine-mediated protection in inactivated and viral vector recipients may be optimized with the use of a heterologous protein-based booster such as NVX-CoV2373.
Pivoting to Protein
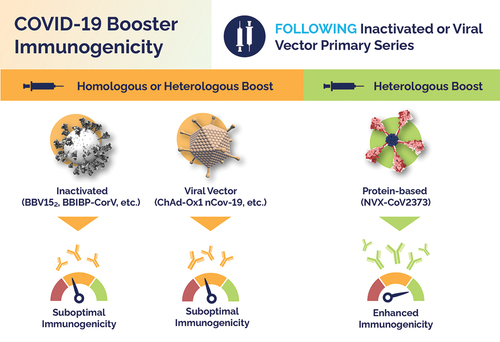
The Immunogenicity and Safety of Protein-based NVX-CoV2373 as a Heterologous Booster for Inactivated and Viral Vector COVID-19 Vaccines. Inactivated or viral vector primary series following a booster dose with homologous or heterologous inactivated vaccines (i.e., BBV152, BBIBP-CorV), and homologous or heterologous viral vector vaccines (i.e., ChAd-Ox1 nCov-19) induces suboptimal immunogenicity compared to the enhanced immunogenicity of heterologous protein-based vaccine NVX-CoV2373.
1. Introduction
Since the COVID-19 outbreak began in Wuhan, Hubei Province, China, in December 2019, the disease has rapidly spread worldwide with a relatively high mortality rate. In response, countries each implemented methods to control the disease outbreak, such as public health measures and lockdowns. Within a year, the first vaccines were deployed under various emergency use authorizations. The platforms included both traditional approaches (i.e. inactivated and protein-based) and newer technologies with minimal prior use (i.e. mRNA and viral vectors). As of mid-2023, over 13.4 billion doses of COVID-19 vaccines have been administered, over half of which have been inactivated or viral vector vaccines [Citation1]. The utilization of many different types of vaccines and the recent inclusion of additional and updated options has created a complex environment highlighted by numerous scientific, medical, and public health uncertainties. In parallel, public trust in vaccine information, vaccine hesitancy, and vaccine fatigue are cause for concern as the need for seasonally boosted immunity is projected to increase. As we transition to a post-pandemic environment, vaccine regimens must be optimized to improve protection and continue to limit the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The expected harmonization of vaccine type, design, and schedule stands to benefit global equity, limit misinformation, and simplify logistical issues that disproportionately impact lower- and middle-income countries (LMICs). While the increasingly rapid availability of vaccine efficacy, effectiveness, and immunogenicity data is a welcome boon to policymakers and health-care providers, it can lead to an overload of information that may bring about challenges in the interpretation, communication, and implementation of key research findings.
Here, we narrowly focus on the use of NVX-CoV2373 as a heterologous booster of inactivated and viral vector COVID-19 vaccines which have been administered to billions of people [Citation2]. We aim to summarize the results of four recent studies identified by a targeted literature review to inform future vaccine decisions in regions which relied heavily on non-mRNA vaccines for primary series doses and are now considering protein-based options for the upcoming seasonal vaccination campaigns.
2. Overview of inactivated, viral vector, and protein-based COVID-19 vaccines
Inactivated vaccines are created by rendering a whole pathogen unable to replicate, which prevents its propagation and the development of disease. The nonpathogenic killed pathogen retains its structure and antigens (i.e. SARS-CoV-2 spike (S) protein) which can stimulate adaptive immune responses. Generally, inactivated vaccines, which have been used for decades to protect against polio, influenza, hepatitis A, and other pathogens, are thought to be poorly immunogenic (less inflammatory cytokine activation and reduced vaccine-mediated humoral and cellular immunity) compared to live replicating vaccines, which more closely mimic natural infection [Citation3]. To overcome poor immune activation, some inactivated vaccines will incorporate the use of adjuvants to activate stimulatory cytokine and chemokine signaling molecule production, or create a ‘depot effect,’ believed to enhance vaccine-mediated responses [Citation4]. Authorized/approved inactivated COVID-19 vaccines include BBV152 (COVAXIN), Alhydroxiquim-II adjuvanted, developed in India by Bharat Biotech in collaboration with the Indian Council of Medical Research and National Institute of Virology [Citation5]; BBIBP-CorV (Corvilo) aluminum-hydroxide adjuvanted inactivated whole-virus COVID-19 vaccine developed in China by Sinopharm (the China National Pharmaceutical Group Corporation), Beijing Institute of Biological Products, and Wuhan Institute of Biological Product [Citation6]; CoronaVac, aluminum-hydroxide adjuvanted, developed in China by SinoVac, VLA2001, adjuvanted with Cytosine phosphoguanine (CpG) 1018 and alum, developed in France by Valneva SE in collaboration with US-based Dynavax Technologies [Citation7] ().
Table 1. Inactivated, viral vector, and protein-based COVID-19 Vaccines.
Viral vector vaccines use a nonpathogenic virus as a delivery system to introduce a piece of DNA encoding the protein antigen into the cells of the vaccinated individual. Similar to the mechanism of the widely utilized COVID-19 mRNA vaccines, inoculated cells translate proteins, which can trigger the development of adaptive immunity. Compared to inactivated vaccines, nonpathogenic viral vectors can be more immunogenic as the vector itself or the translation of foreign proteins inside the cell can activate local innate immune responses that enhance vaccine-mediated responses [Citation17,Citation18]. Evidence from non-COVID-19 vaccines suggests that individuals who receive multiple doses or booster vaccinations with the same vector, or who have natural preexisting adenovirus immunity, may have reduced vaccine efficacy due to the development of vector-specific immunity [Citation19–22]. Viral vector COVID-19 vaccines include ChAdOx1 nCov-19 (Vaxzevria [Citation23] and COVISHIELD [Citation24]), an adenoviral vector developed in the UK by the University of Oxford and AstraZeneca (also licensed to Serum Institute of India) [Citation25], and Ad26.COV2.S (Jcovden) an adenoviral vector developed in the US and Belgium-based Janssen Pharmaceutical Company (Johnson & Johnson) [Citation15] ().
Protein-based vaccines contain a select piece or pieces of the pathogen and come in different forms. Protein-vaccines can include full-length antigens, subunits (partial proteins), or be constructs of proteins that form virus-like nanoparticles. Generally, protein-based vaccines are poorly immunogenic compared to other vaccine platforms, and often require multiple doses and/or the use of adjuvant to induce sufficient immunity [Citation17,Citation26]. Variations of protein-based vaccines have been used with great success since the 1980s against influenza, hepatitis B, and other diseases [Citation27]. Authorized/approved protein-based COVID-19 vaccines include the NuvaxovidTM (NVX-CoV2373) full length recombinant S protein nanoparticle adjuvanted with Matrix-MTM [Citation28] developed in the US by Novavax, Inc. (also licensed to Serum Institute of India and Takeda Pharmaceutical Company) [Citation9,Citation29]; VidPrevtyn Beta, a monovalent, recombinant SARS-CoV-2 Beta variant S protein adjuvanted with AS03 and developed in the EU by Sanofi and GSK [Citation17,Citation30]; Corbevax, an S protein receptor-binding domain (RBD) adjuvanted with aluminum-hydroxide gel and CpG 1018 developed in the US by Texas Children’s Hospital, Baylor College of Medicine and Dynavax technologies, and licensed to Biological E. Limited (India) [Citation31]; MVC-COV1901, a recombinant S-2P protein adjuvanted with CpG 1018 developed by Medigen Vaccine Biologics Corporation in Taiwan, Dynavax Technologies, and the US National Institutes of Health [Citation12]; IndoVac, an S protein RBD derived from a yeast-based expression system adjuvanted with Alum and CpG 1018 developed by Indonesian pharmaceutical company Bio Farma and Baylor College of Medicine; ZF2001, a recombinant RBD protein adjuvanted with aluminum hydroxide was developed by Anhui Zhifei Longcom in collaboration with the Institute of Microbiology at the Chinese Academy of Sciences [Citation10,Citation32]. ()
3. Uptake of inactivated, viral vector, and protein-based COVID-19 vaccines [Citation33,Citation34]
There have been over 13 billion doses of COVID-19 vaccines administered globally, potentially half of which are likely to have been inactivated or viral vector vaccines [Citation2]. The majority of these included 5–6 billion doses of China’s CoronaVac and BBIBP-CorV vaccines, and nearly 2.5 billion of ChAdOx1 nCov-19. China is believed to have administered a large amount of their inactivated vaccines domestically with estimations around 3.5 billion in mainland China but also donated to more than 100 countries including Indonesia, Brazil, Pakistan, Turkey, Iran, the Philippines, Morocco, Thailand, Argentina, and others [Citation35]. In terms of breadth of use, ChAdOx1 nCov-19 has been used across more countries (185) than any other vaccine, followed by BNT162b2 (Pfizer/BioNTech) (165), mRNA-1273 (Moderna) (114), Ad26.COV2.S (103), BBIBP-CorV (72), and CoronaVac (41) [Citation36]. Amid the earlier pandemic stages (mid-2021) ChAdOx1 nCov-19 was the most widely recognized COVID-19 vaccine for cross-border travel [Citation37]. As of late 2022, India had administered nearly 1.7 billion doses of ChAdOx1 nCov-19 and 351 million doses of BBV152 [Citation38]. The extensive use of these vaccines was in part due to their timely availability in densely populated regions through the Asia-Pacific, India, the Middle East, and many LMICs. Despite their widespread use, challenges remain in ensuring equitable access for all global populations, particularly in the LMICs where supply chain and logistical challenges persist.
In contrast to the rapidly available inactivated and viral vector vaccines that were developed, authorized, and administered with unprecedented speed, protein-based COVID-19 vaccines have been developed and authorized/approved at a slower pace. To date, the most widely used protein-based option may be Corbevax, of which roughly 70 million doses were utilized in India [Citation38]. Thus far, there has been lower global uptake of protein-based vaccinations compared to other options, most likely attributable to their late availability. However, they are now quickly gaining global authorizations and could potentially challenge the pandemic-era vaccines as the boosters-of-choice in future vaccination campaigns.
4. Homologous and heterologous boosting of inactivated and viral vector primary series regimens
4.1. Safety and immunogenicity of the NVX-CoV2373 vaccine as a booster in adults previously vaccinated with the BBIBP-CorV vaccine: an interim analysis [Citation39]
A phase 3 observer-blind, randomized, controlled study (NCT05249816) investigated the use of NVX-CoV2373 as a heterologous booster compared to BBIBP-CorV utilized as a homologous booster (). The study, conducted at two sites in the United Arab Emirates from March 2022 to June 2022, randomized 1000 participants (NVX-CoV2373, n = 499; BBIBP-CorV, n = 501). An interim analysis of the per-protocol analysis group found that IgG antibody levels and seroconversion rates were higher following heterologous NVX-CoV2373 compared to homologous BBIBP-CorV boosting; however, these quantifications were hindered by the upper limit of quantification of the chosen assay (chemiluminescence immunoassay, CLIA) which NVX-Cov2373 met or exceeded in 91% of subjects compared to 20% in BBIBP-CorV. Plaque reduction neutralization test (PRNT) demonstrated that participants who received heterologous NVX-CoV2373 had 5.9 times (Day 14) and 4.0 times (Day 28) greater neutralizing antibody geometric mean titers (GMTs) compared to those who received homologous BBIBP-CorV. The occurrence of adverse events (AEs) was similar between groups, though slightly higher in the NVX-CoV2373 group, correlating to the observed higher immunogenicity. No new safety concerns of either vaccine were observed in the study.
Table 2. Studies of heterologous NVX-CoV2373 boost after inactivated and viral vector COVID-19 primary series vaccination.
4.2. A randomized, controlled study to evaluate the safety and immunogenicity of a heterologous booster dose of an adjuvanted SARS-CoV-2 recombinant spike protein vaccine in adults [Citation40]
A Phase 3, observer-blind, randomized, active controlled study investigated the use of SII-NVX-CoV2373, BBV152, and ChAdOx1 nCov-19 as boosters following ChAdOx1 nCov-19 or BBV152 primary series vaccinations in 372 adults ≥18 years old (). The study, which was conducted at eight centers in India from May 2022 to December 2022, randomized the primary series (ChAdOx1 nCov-19, n = 186; or BBV152, n = 186) cohorts 1:1 to receive SII-NVX-CoV2373 or a homologous booster control. The study found that anti-S IgG for Wuhan, Omicron BA.1, and Omicron BA.2 antibodies GMTs/geometric mean enzyme-linked immunosorbent assay units (GMEUs) after heterologous SII-NVX-COV2373 boost were approximately 2.0 times higher than homologous ChAdOx1 nCov-19 boost, and approximately 5.0–6.0 times higher than homologous BBV152 boost. In both cohorts, GMTs were highest for Wuhan and lowest for Omicron BA.5, though the GMT ratio of heterologous NVX-CoV2373 to homologous boost remained similar for each. Similarly, neutralizing antibody GMT ratio of heterologous SII-NVX-COV2373 boost was approximately 1.9 times higher than homologous ChAdOx1 nCov-19 boost, and 4.8 times higher than homologous BBV152 boost. In terms of safety, all vaccines used in the study appeared safe and well tolerated. Heterologous SII-NVX-CoV2373 displayed lower local reactogenicity and similar systemic reactogenicity compared to homologous regimens, although the study was not powered for these safety comparisons.
4.3. Safety and immunogenicity of a third dose of COVID-19 protein subunit vaccine 1 (Covovax™) after homologous and heterologous two-dose regimens [Citation41]
A cohort study investigated the use of a heterologous Covovax third-dose booster after a two-dose primary series of BBIBP-CorV, ChAdOx1 nCov-19, BNT162b2, CoronaVac, or a mixed primary series of CoronaVac (1 dose) and ChAdOx1 nCov-19 (1 dose) (). The study included 210 adults ≥18 years old and was conducted in Bangkok, Thailand, from May 2022 to July 2022, with 10 vaccination sites in the Chonburi province. Dependent on primary series vaccine, NVX-CoV2373 elicited high levels of anti-S receptor RBD Ig titers (50.0–100.0 times higher) compared to baseline at 3–10 months after primary series vaccination. A surrogate virus neutralizing antibody assay showed that NVX-CoV2373 induced a 70–95% increase in Wild-type and Omicron variant neutralizing antibodies compared to baseline, dependent upon primary series vaccine; however, this result was complicated by the assay reaching the upper ceiling limitation of >95% inhibition. Neutralizing antibody responses against Omicron BA.2 were slightly lower than those to the ancestral strain. Interestingly, a subgroup analysis showed little difference in NVX-CoV2373-mediated antibody responses between participants with evidence of past infection (anti-nucleocapsid (N) positive) compared to those without (anti-N negative). Evidence of T cell responses was observed as interferon-γ levels were significantly increased 14-days post vaccination regardless of primary series vaccine. Solicited adverse events were similar among groups. Though no comparator vaccine was utilized in this study, the authors note that NVX-CoV2373 boost had a lower frequency of pain (32.5% vs 71–87%), myalgia (21.9% vs 34–62%), and headache (16.8% vs 39–50%) and similar-to-lower incidence of fever than was observed for viral vector or the mRNA COVID-19 vaccines in similar studies [Citation42,Citation44,Citation45].
4.4. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomized, controlled, phase 2 trial [Citation42]
COV-BOOST is a randomized, controlled, phase 2 trial of third-dose booster vaccination using various homologous and heterologous COVID-19 vaccines, including NVX-CoV2373, ChAdOx1 nCov-19, the Ad26.COV2.S, VLA2001, as well as mRNA vaccines BNT1622b, mRNA-1273 (used at 100 µg, twice the authorized 50 µg booster), and CVnCov (CureVac) (). All participants received primary vaccinations with ChAdOx1 nCov-19 or BNT1622b. The study, led by the University of Southampton, took place at 18 sites throughout the UK in June 2021, and was funded by the UK Vaccine Task Force and included 2,883 adults ≥30 years old. Importantly, the study was not powered to make comparisons between vaccines, and all vaccines were compared to the active control, MenACWY meningitis vaccine. Among the inactivated, viral vector, and protein-based boosters administered after ChAdOx1 nCov-19 primary series the hierarchy of anti-S IgG titers were determined to be NVX-CoV2373 (6975 ELU/mL) > Ad26.COV2.S (5517 ELU/mL) > ChAdOx1 nCov-19 (2457 ELU/mL) > VLA2001 (1835 ELU/mL). All regimens had similar reactogenicity profiles, though heterologous NVX-CoV2373 was slightly more reactogenic than the inactivated and viral vector vaccinations (but generally lower than the mRNA boosts), correlating to higher immunogenicity. The authors noted that serious adverse events were uncommon, similar in active vaccine and control groups.
4.5. Persistence of immune responses after heterologous and homologous third COVID-19 vaccine dose schedules in the UK: eight-month analyses of the COV-BOOST trial [Citation43]
In a recent long-term follow-up analysis, the kinetics of humoral and cellular immune responses from the COV-BOOST trial were measured from day 28 though day 242 (8 months) post-third-dose vaccination. Among the inactivated, viral vector, and protein-based boosters administered after ChAdOx1 nCov-19 primary series, NVX-CoV2373 promoted higher anti-S IgG titers and cellular responses than ChAdOx1 nCov-19 and Ad26.COV2.S at Day 28, 84 and 242 post-boost. Decay rates following ChAdOx1 nCov-19 primary series (referenced to BNT-162b2 boost, wherein the higher geometric mean ratio (GMR) favors the comparator) were similar and slower than those of mRNA boosters for anti-S IgG (GMR = NVX-CoV2373, 1.49; Ad26.COV2.S, 3.05; ChAdOx1 nCov-19, 2.37), live virus neutralizing antibody (GMR = NVX-CoV2373, 2.22; Ad26.COV2.S, 1.88; ChAdOx1 nCov-19, 2.05), and cellular responses (GMR = NVX-CoV2373, 1.42; Ad26.COV2.S, 0.90; ChAdOx1 nCov-19, 1.19). These findings suggest that protein or viral vector boosters may enhance the persistence and durability of vaccine-mediated immunity compared to mRNA options.
5. The benefits of protein-based NVX-CoV2373 compared to mRNA COVID-19 vaccines
Though the aim of this article is to review recently published data that focused on the use of viral vector, inactivated, and protein-based COVID-19 vaccines, it is also relevant to discuss mRNA options. The results of pivotal phase 3 trials demonstrated similar vaccine efficacy among mRNA vaccines BNT-162b2 and mRNA1273, and protein-based NVX-CoV2373 [Citation29,Citation46,Citation47]. In general, BNT-162b2, mRNA1273, and NVX-CoV2373 have elicited comparable immunogenicity when used in various homologous and heterologous regimes. Though little head-to-head data comparing mRNA and protein-based vaccines as heterologous boosters of viral vector and inactivated vaccines is available, it is reasonable to predict similar performance. However, NVX-CoV2373 offers several distinct advantages. First, there is evidence to suggest that heterologous vaccine regimens, such as the use of NVX-CoV2373 as a booster after inactivated, viral vector or mRNA vaccines, may lead to stronger and longer-lasting immunity [Citation39–43]. Studies have shown that mixing different types of vaccines can lead to higher antibody levels and more robust cellular immune responses, which may provide better protection against SARS-CoV-2 variants [Citation48–55]. The use of different vaccine types can potentially stimulate different parts of the immune system, which may result in a stronger and more diverse immune response overall. It is thought that mRNA vaccines may suffer from poor durability of immune responses compared to other vaccine platforms, which was observed in the COV-BOOST 8-month analysis. Although COV-BOOST participants who received three doses of BNT-162b2 had significantly higher anti-S IgG at all visits except at day 242, those boosted with NVX-CoV2373 demonstrated significantly better persistence shown by a slower decay rate compared to the three dose BNT-162b2 regimen (GMR of D242-to-D28 ratio for NVX-CoV2373/BNT-162b2 = 1.37 [95%CI: 1.04–1.82]). Based upon these observations, the authors suggest that policymakers might want to consider non-mRNA boosters to maintain antibody levels for longer periods. Second, the ancestral spike protein-based NVX-CoV2373 has demonstrated potent cross-protection, particularly against variants of concern such as omicron subvariants [Citation56,Citation57] that has been similar to that of the updated variant-specific mRNA vaccine. In a post hoc analysis of the NVX-CoV2373 phase 3 trial, during which alpha was the predominant SARS-CoV-2 variant, a vaccine efficacy of 100% against COVID-19 associated hospitalization was observed [Citation11]. Expected variant-specific updates of the NVX-CoV2373 vaccine has the potential to improve variant-specific immunogenicity and may offer the broad cross-protection needed to best prepare for the emergence of future variants. Finally, poor uptake of booster doses remains a global concern. Vaccine hesitancy has become prevalent, particularly toward vaccines that utilize new technologies including viral vector or mRNA vaccines [Citation58,Citation59]. Though data have shown these platform-specific concerns are unwarranted, public-trust may be positively influenced by the use of more traditional options with long histories of safe and effective use, such as inactivated and protein-based vaccines [Citation27]. Here, we discuss the lower immunogenicity of inactivated vaccines, which suggests the optimal choice to ensure high uptake of booster doses is the use of protein-based vaccines. Among the most frequently cited reasons for vaccine hesitancy is concern regarding reactogenicity, or vaccine-mediated side effects [Citation60]. A survey of United States (US) healthcare workers indicated that reactogenicity associated with mRNA vaccinations had reduced their future willingness to receive booster vaccinations [Citation61]. Another survey revealed that older adults have been hesitant to receive the updated bivalent mRNA vaccine, as only 42.4% of 1,113 US adults aged 65 years and older indicated that they had received one. Among them, the most cited concerns were “not knowing if the newly formulated vaccine was safe” (40.73%), and “concern over potential side effects” (31.05%) [Citation62]. Notably, the NVX-CoV2373 vaccine has a good safety profile, with fewer serious adverse events reported and potentially reduced reactogenic side effects compared to other options [Citation42,Citation49,Citation63].
6. Conclusion
Among the billions who received inactivated and viral vector COVID-19 vaccines, many may now be eligible for booster vaccinations. As countries plan for mid-to-late year booster campaigns, their policymakers and health-care providers must carefully consider their specific populations and weigh the available data to inform decisions of which vaccine platforms will be most beneficial. When taken separately, these studies paint an incomplete picture; however, together they represent a wealth of data that suggests NVX-CoV2373 is a strongly immunogenic, safe, and well-tolerated booster following inactivated and viral vector primary series COVID-19 vaccines and may elicit greater responses than those of homologous or heterologous inactivated or viral vector boosts. The limitations of the outlined studies should also be acknowledged, specifically the relatively low number of available studies, small study populations, and the narrow focus of immunological analysis on neutralizing antibody data. It is predominantly focused on antibody responses. The complexity of varied dosing number of vaccine boosters, different immunological backgrounds, and changing variant prevalence further limit the interpretation of these findings. Decisions on the rollout of primary series vaccinations were supported by vaccine efficacy estimates from large clinical trials and were heavily impacted by the speed of country-specific availability, which was dependent on manufacturing, storage and handling logistics, and authorization statuses. In contrast, future booster campaigns and the potential recommendations for annual COVID-19 vaccine boosting in some or all populations will likely need to rely on smaller immunogenicity studies and real-world evidence that must contend with the added complexity of past infection and vaccination histories. In the absence of new large-scale efficacy trials or head-to-head studies inclusive of many different vaccine platforms, the identification of clear trends amidst a growing body of published evidence from small immunogenicity studies can support the optimization of booster vaccination regimens. We suggest the consideration of this evidence to support the tailoring of COVID-19 booster recommendations to accommodate global primary series diversity, particularly to improve vaccine-mediated protection in areas with inactivated and viral vector vaccines.
7. Expert opinion
The selective administration of specific heterologous COVID-19 vaccine booster regimens, chosen based on an individual’s prior vaccine history, should be adopted. Across several different studies, the protein-based vaccine NVX-CoV2373 has demonstrated the potential to stimulate enhanced immunity after inactivated and viral vector vaccine primary series doses compared to other homologous and heterologous regimens. In the current COVID-19 landscape, this research should be used to inform the treatment guidelines of policymakers, and the decision-making process of health-care providers regarding vaccine choice. In response to the rapidly changing SARS-CoV-2 virus and COVID-19 disease space, we must exhibit flexibility, adaptability, and a willingness to tailor our approaches to best protect our diverse global populations.
Though recently published studies provide much clinically useful data, many areas for additional investigation remain. These include the need for more extensive head-to-head studies comparing the immunogenicity, safety, reactogenicity, and durability of the different vaccine platforms being used as boosters. Immunogenicity studies and real-world evidence hold the potential to provide a better understanding of the long-term effectiveness and durability of heterologous vaccine regimens. Ongoing and future cooperative efforts from academic researchers, government groups, and vaccine manufacturers will be necessary to identify and support the use of vaccines in specific populations of interest (i.e. pregnant women, and immunocompromised patients), and the benefits of new vaccination strategies to best combat emerging variants and evolving epidemiological situations.
The future of COVID-19 vaccine research lies not only in the study of heterologous booster regimens but also in the development of next-generation vaccines and the exploration of other promising areas in the field. Technological advancements, including anticipated developments of variant-specific updates to vaccines, could further improve their performance against new SARS-CoV-2 strains. The search for pan-coronavirus vaccines, capable of broad spanning protection against many different coronavirus variants, will be an intriguing field moving forward.
Aside from the need for continued scientific advancement, we must consider the future challenges of social issues, mainly vaccine hesitancy and fatigue. Mandated vaccination requirements are not the answer to positively influence the decision of hesitant or fatigued individuals. Rather, an empathic approach to hear and respond to their concerns should be taken. Fact-based promotion of vaccines that offer reduced reactogenicity or better durability affording longer intervals between boosters, by trusted sources such as community physicians, would appeal to many.
In the coming years, the field of COVID-19 vaccination is likely to evolve significantly. We expect policymakers to promote a more harmonized approach to the next generation of vaccination efforts across the globe. This may include annual vaccination recommendations, similar to that of influenza vaccines, with the potential for seasonally updated boosters based on new variants. Further, the adoption of COVID-19/influenza combination vaccines could streamline the annual vaccination process and promote the global uptake. The implementation of tailored booster recommendations based on individual vaccine histories, such as the receipt of viral vector or inactivated vaccine primary series, should widen the use of heterologous vaccine regimens. In these cases, health-care providers can lean on the wealth of available data to support their patients in making a personalized and optimized vaccine choice.
Article highlights
The optimization of vaccine regimens is necessary to improve protection and continue to limit the spread of SARS-CoV-2
Thus far, there has been lower global uptake of protein-based COVID-19 vaccinations compared to other platforms, but increased global authorizations and positive immunogenicity data could drive protein vaccines to be the boosters-of-choice moving forward
Multiple studies demonstrate that the protein-based COVID-19 vaccine NVX-CoV2373 elicits robust immunogenic responses when utilized as a heterologous booster following primary vaccination with inactivated or viral vector COVID-19 vaccines
Compared to mRNA booster options, NVX-CoV2373 may offer distinct advantages such as lower reactogenicity, which could appeal to vaccine hesitant individuals
Future vaccination campaigns, which may include yearly COVID-19 boosters, will rely on immunogenicity studies to inform on and support the optimization of booster vaccination regimens
Declaration of interest
AM Marchese, R Kalkeri, M Vadivale, and S Toback are employees and shareholders of Novavax, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Our World in Data. Coronavirus (COVID-19) vaccinations: global change data lab [cited 2023 May 8]. Available from: https://ourworldindata.org/covid-vaccinations
- COVID-19 Vaccination Pharmaceutical Technology [updated 2023 Apr 13; cited 2023 Apr 27]. Available from: https://www.pharmaceutical-technology.com/covid-19-vaccination-tracker/
- Sanders B, Koldijk M, Schuitemaker H. Inactivated viral vaccines. Vaccine analysis: strategies, principles, and control. 2014;45–80.
- Awate S, Babiuk LA, Mutwiri G. Mechanisms of action of adjuvants. Front Immunol. 2013;4:114. doi: 10.3389/fimmu.2013.00114
- Ella R, Reddy S, Blackwelder W, et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet. 2021 Dec 11;398(10317):2173–2184.
- Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021 Jul 6;326(1):35–45.
- Tanriover MD, Doganay HL, Akova M, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021 Jul 17;398(10296):213–222.
- COVID-19 Vaccine Tracker [updated 2023 Mar 4; cited 2023 Apr 27]. Available from: https://covid19.trackvaccines.org/vaccines/#progress-meter
- Dunkle LM, Kotloff KL, Gay CL, et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med. 2022 Feb 10;386(6):531–543.
- Dai L, Gao L, Tao L, et al. Efficacy and safety of the RBD-Dimer-based Covid-19 vaccine ZF2001 in adults. N Engl J Med. 2022 Jun 2;386(22):2097–2111.
- Marchese AM, Zhou X, Kinol J, et al. NVX-Cov2373 vaccine efficacy against hospitalization: a post hoc analysis of the PREVENT-19 phase 3, randomized, placebo-controlled trial. medRxiv. 2023. Preprint. doi: 10.1101/2023.03.17.23287306.
- Torales J, Cuenca-Torres O, Barrios L, et al. An evaluation of the safety and immunogenicity of MVC-COV1901: results of an interim analysis of a phase III, parallel group, randomized, double-blind, active-controlled study. medRxiv. 2022. Preprint. doi: 10.1101/2022.07.14.22277617.
- ClinicalTrials.gov. Immuno-bridging study of COVID-19 protein subunit recombinant vaccine (NCT05433285) [cited 2023 Apr 27]. Available from: https://clinicaltrials.gov/ct2/show/NCT05433285
- Lazarus R, Querton B, Corbic Ramljak I, et al. Immunogenicity and safety of an inactivated whole-virus COVID-19 vaccine (VLA2001) compared with the adenoviral vector vaccine ChAdOx1-S in adults in the UK (COV-COMPARE): interim analysis of a randomised, controlled, phase 3, immunobridging trial. Lancet Infect Dis. 2022 Dec;22(12):1716–1727.
- Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose ad26.COV2.S vaccine against covid-19. N Engl J Med. 2021 Jun 10;384(23):2187–2201.
- Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021 Jan 9;397(10269):99–111.
- Vetter V, Denizer G, Friedland LR, et al. Understanding modern-day vaccines: what you need to know. Ann Med. 2018 Mar;50(2):110–120.
- Chang J. Adenovirus vectors: excellent tools for vaccine development. Immune Netw. 2021 Feb;21(1):e6. doi: 10.4110/in.2021.21.e6
- Shirakawa T. The current status of adenovirus-based cancer gene therapy. Mol Cells. 2008 Jun 30;25(4):462–466.
- Douglas JT. Adenoviral vectors for gene therapy. Mol Biotechnol. 2007 May;36(1):71–80. doi: 10.1007/s12033-007-0021-5
- Campos SK, Barry MA. Current advances and future challenges in Adenoviral vector biology and targeting. Curr Gene Ther. 2007 Jun;7(3):189–204. doi: 10.2174/156652307780859062
- Goncalves MA, de Vries AA. Adenovirus: from foe to friend. Rev Med Virol. 2006 May;16(3):167–186. doi: 10.1002/rmv.494
- Vaxzevria, COVID-19 Vaccine (ChAdox1-S [recombinant]) SmPC.
- COVSHIELD (ChAdOx1 nCoV-19 Corona Virus Vaccine [Recombinant]) Fact Sheet.
- Falsey AR, Sobieszczyk ME, Hirsch I, et al. Phase 3 safety and efficacy of AZD1222 (ChAdox1 nCoV-19) Covid-19 vaccine. N Engl J Med. 2021 Dec 16;385(25):2348–2360.
- Di Pasquale A, Preiss S, Tavares Da Silva F, et al. Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines (Basel). 2015 Apr 16;3(2):320–343.
- Marchese AM, Beyhaghi H, Orenstein WA. With established safe and effective use, protein vaccines offer another choice against COVID-19. Vaccine. 2022 Nov 2;40(46):6567–6569.
- Stertman L, Palm AE, Zarnegar B, et al. The matrix-M adjuvant: a critical component of vaccines for the 21(st) century. Hum Vaccin Immunother. 2023 Dec 31;19(1):2189885.
- Heath PT, Galiza EP, Baxter DN, et al. Safety and Efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021 Sep 23;385(13):1172–1183.
- VidPrevtyn Beta, COVID-19 Vaccine SmPC.
- Thuluva S, Paradkar V, Gunneri SR, et al. Evaluation of safety and immunogenicity of receptor-binding domain-based COVID-19 vaccine (Corbevax) to select the optimum formulation in open-label, multicentre, and randomised phase-1/2 and phase-2 clinical trials. EBioMedicine. 2022 Sep;83:104217.
- Gao L, Li Y, He P, et al. Safety and immunogenicity of a protein subunit COVID-19 vaccine (ZF2001) in healthy children and adolescents aged 3-17 years in China: a randomised, double-blind, placebo-controlled, phase 1 trial and an open-label, non-randomised, non-inferiority, phase 2 trial. Lancet Child Adolesc Health. 2023 Apr;7(4):269–279.
- Chakraborty C, Sharma AR, Bhattacharya M, et al. Asian-origin approved COVID-19 vaccines and current status of COVID-19 vaccination program in Asia: a critical analysis. Vaccines (Basel). 2021 Jun 4;9(6):600.
- Dolgin E. Omicron thwarts some of the world’s most-used COVID vaccines. Nature. 2022 Jan;601(7893):311. doi: 10.1038/d41586-022-00079-6
- Pettersson H, Manley B, Hernandez S, et al. Tracking COVID-19 vaccinations worldwide: cNN Health [updated 2023 Mar 20; cited 2023 Apr 27]. Available from: https://edition.cnn.com/interactive/2021/health/global-covid-vaccinations/
- Holder J Tracking Coronavirus Vaccinations Around the World. The New York Times; [updated 2023 Mar 13; cited 2023 Apr 27]. Available from: https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html
- Which COVID-19 vaccine is the most widely accepted for international travel? The Economist [updated 2021 Jul 20; cited 2023 Apr 27]. Available from: https://www.economist.com/graphic-detail/2021/07/20/which-covid-19-vaccine-is-the-most-widely-accepted-for-international-travel
- Ministry of Health and Family Welfare. Co-win: winning over Covid-19 [cited 2023 Apr 27]. Available from: https://dashboard.cowin.gov.in/
- Toback S, Marchese AM, Warren B, et al. Safety and Immunogenicity of the NVX-CoV2373 vaccine as a booster in adults previously vaccinated with the BBIBP-CorV vaccine: an interim analysis. medRxiv. 2023; Preprint:. doi: 10.1101/2023.03.24.23287658.
- Kulkarni P, Gunale B, Kohli S, et al. A randomized, controlled study to evaluate the safety and immunogenicity of a heterologous booster dose of an adjuvanted SARS CoV-2 recombinant spike protein vaccine in adults. Res Square. 2023; Preprint. doi: 10.21203/rs.3.rs-2549560/v1.
- Kanokudom S, Chansaenroj J, Suntronwong N, et al. Safety and immunogenicity of a third dose of COVID-19 protein subunit vaccine (Covovax(tm)) after homologous and heterologous two-dose regimens. Int J Infect Dis. 2023 Jan;126:64–72. doi: 10.1016/j.ijid.2022.11.022.
- Munro APS, Janani L, Cornelius V, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021 Dec 18;398(10318):2258–2276. doi: 10.1016/S0140-6736(21)02717-3.
- Liu X, Munro APS, Wright A, et al. Persistence of immune responses after heterologous and homologous third COVID-19 vaccine dose schedules in the UK: eight-month analyses of the COV-BOOST trial. J Infect. 2023 Apr 19;87(1):18–26. doi: 10.1016/j.jinf.2023.04.012.
- Assawakosri S, Kanokudom S, Chansaenroj J, et al. Persistence of immunity against Omicron BA.1 and BA.2 variants following homologous and heterologous COVID-19 booster vaccines in healthy adults after a two-dose AZD1222 vaccination. Int J Infect Dis. 2022 Sep;122:793–801.
- Mallory RM, Formica N, Pfeiffer S, et al. Safety and immunogenicity following a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373): a secondary analysis of a randomised, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2022 Nov;22(11):1565–1576.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020 Dec 31;383(27):2603–2615.
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021 Feb 4;384(5):403–416.
- Garg I, Sheikh AB, Pal S, et al. Mix-and-match COVID-19 vaccinations (heterologous boost): a review. Infect Dis Rep. 2022 Jul 20;14(4):537–546. doi: 10.3390/idr14040057
- Atmar RL, Lyke KE, Deming ME, et al. Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022 Mar 17;386(11):1046–1057.
- Barros-Martins J, Hammerschmidt SI, Cossmann A, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021 Sep;27(9):1525–1529.
- Schmidt T, Klemis V, Schub D, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med. 2021 Sep;27(9):1530–1535.
- Liu X, Shaw RH, Stuart ASV, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021 Sep 4;398(10303):856–869.
- Pozzetto B, Legros V, Djebali S, et al. Immunogenicity and efficacy of heterologous ChAdOx1-BNT162b2 vaccination. Nature. 2021 Dec;600(7890):701–706.
- Tan CS, Collier AY, Yu J, et al. Durability of Heterologous and homologous COVID-19 vaccine boosts. JAMA Netw Open. 2022 Aug 1;5(8):e2226335.
- Assawakosri S, Kanokudom S, Suntronwong N, et al. Neutralizing activities against the omicron variant after a heterologous booster in healthy adults receiving two doses of coronavac vaccination. J Infect Dis. 2022 Oct 17;226(8):1372–1381.
- Food and Drug Administration. Vaccines and Related Biological Products Advisory Committee (VRBPAC) Meeting, Zoom video conference [transcript]. 2023 Jan 26. Accessed 26 Jan 2023. Available from: https://www.fda.gov/media/165307/download
- Raiser F, Davis M, Adelglass J, et al. Immunogenicity and safety of NVX-CoV2373 as a homologous or heterologous booster: a phase 3 randomized clinical trial in adults. medRxiv. 2023. Preprint. doi: 10.1101/2023.03.16.23287030.
- Rubin R. COVID-19 vaccine makers plan for annual boosters, but it’s not clear they’ll be needed. JAMA. 2021 Dec 14;326(22):2247–2249.
- Bergen N, Kirkby K, Fuertes CV, et al. Global state of education-related inequality in COVID-19 vaccine coverage, structural barriers, vaccine hesitancy, and vaccine refusal: findings from the Global COVID-19 Trends and Impact Survey. Lancet Glob Health. 2023 Feb;11(2):e207–e217.
- Beleche T, Ross T, Marus J, et al. COVID-19 vaccine hesitancy and reasons for hesitancy among essential and non-essential workers. 2023 March. Accessed 30 June. Available from: https://aspe.hhs.gov/sites/default/files/documents/f2fbfbd9cdce16032233b6a9ff69f161/aspe-essential-worker-vaccine-hesitancy.pdf
- Chrissian AA, Oyoyo UE, Patel P, et al. Impact of COVID-19 vaccine-associated side effects on health care worker absenteeism and future booster vaccination. Vaccine. 2022 May 20;40(23):3174–3181.
- Costa K Older adults’ intentions and attitudes toward the updated bivalent COVID-19 booster: survey, United States, may 2023 HealthCanal2023 [cited 2023 May 8]. Available from: https://www.healthcanal.com/health/the-bivalent-covid-19-booster-survey
- Sutton N, San Francisco Ramos A, Beales E, et al. Comparing reactogenicity of COVID-19 vaccines: a systematic review and meta-analysis. Expert Rev Vaccines. 2022 Sep;21(9):1301–1318.