ABSTRACT
Background
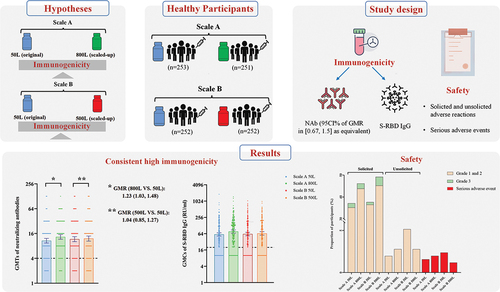
The certification of immunogenicity consistency at different production scales is indispensable for the quality control of vaccines.
Research design and methods
A randomized, double-blind immunobridging trial in healthy adults aged 18–59 was divided into Scale A (50 L and 800 L) and Scale B (50 L and 500 L) based on vaccine manufacturing scales. Eligible participants in Scale A were randomly assigned to receive the single-dose recombinant adenovirus type-5 vectored COVID-19 vaccine (Ad5-nCoV) of different scales at a 1:1 ratio, as was Scale B. The primary endpoint was the geometric mean titer (GMT) of anti-live SARS-CoV-2-specific neutralizing antibodies (NAb) 28 days post-vaccination.
Results
1,012 participants were enrolled, with 253 (25%) per group. The post-vaccination GMTs of NAb were 10.72 (95% CI: 9.43, 12.19) and 13.23 (11.64, 15.03) in Scale A 50 L and 800 L, respectively; 11.64 (10.12, 13.39) and 12.09 (10.48, 13.95) in Scale B 50 L and 500 L, respectively. GMT ratios in Scale A and B have a 95% CI of 0.67–1.5. Most adverse reactions were mild or moderate. 17 of 18 participants reported non-vaccination-related serious adverse reactions.
Conclusions
The Ad5-nCoV in the scale-up production of 500 L and 800 L showed consistent immunogenicity with the original 50 L production scale, respectively.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was detected for the first time in Wuhan, Hubei Province, China, in December 2019. The virus was highly contagious and spread rapidly among the population, leading to the global pandemic of coronavirus disease 2019 (COVID-19) and causing significant disruptions to society, education, and the economy [Citation1,Citation2]. The COVID-19 vaccination could dramatically change the course of the COVID-19 pandemic, reducing severe illness, hospitalizations, and deaths worldwide. An estimated 19.8 million deaths averted by vaccinations from December 2020 to December 2021 were observed [Citation3].
Recombinant adenovirus type-5 vectored COVID-19 vaccine (Ad5-nCoV) from CanSinoBIO has been included in the Emergency Use Listing (EUL) authorized by the World Health Organization (WHO), which was the first domestically approved adenovirus vector COVID-19 vaccine in China [Citation4,Citation5]. The results of the phase 3 clinical trial of Ad5-nCoV carried out in many countries showed that healthy adults over 18 years old were immunized with a single dose of Ad5-nCoV after 28 days, the protective effect on all symptomatic cases was 57.5%, and the protective effect on severe cases was 91.7% [Citation6]. In the background of the COVID-19 pandemic in 2021, the WHO stated that in order to substantially increase the immunity of the global population, all countries should achieve 70% full coverage of the COVID-19 vaccination population by mid-2022, which required at least 11 billion doses of COVID-19 vaccines [Citation7]. Besides, WHO pointed out that to maintain and enhance the momentum of reducing mortality and morbidity with existing vaccines, all countries should further expand coverage and strengthen coverage of medium-priority groups (remaining adults, adolescents and children with comorbidities) [Citation8], which required more doses of COVID-19 vaccines. However, the estimated production capacity of Ad5-nCoV in 2021 was 500–600 million doses [Citation9], which was much lower than the estimated production capacity of 3 billion doses of ChAdOx1-S from AstraZeneca [Citation10] and 1 billion doses of Ad26.COV2.S from Johnson & Johnson [Citation11]. The production capacity of the original 50 L scale was too low to meet the demand for enhanced vaccination across the globe, so expanding the production scales of Ad5-nCoV is necessary. Therefore, the objective of the trial was to evaluate the similarity or consistency of immunogenicity induced by Ad5-nCoV after expanding production scales compared with the original scale, which was crucial for further improving vaccine coverage and providing immunological protection. And the large-scale production of Ad5-nCoV can provide a technical basis for future vaccine research and development.
Vaccines at different commercial production scales need to be clinically evaluated to further verify the consistency of the vaccine production process and quality. The study applied an immunobridging trial to verify and support the immunogenicity consistency and safety of Ad5-nCoV at different production scales.
2. Methods
2.1. Study design and participants
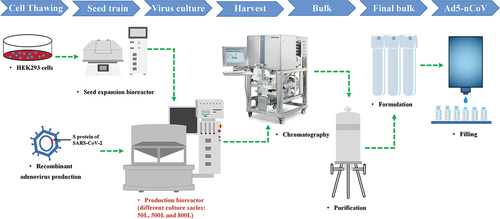
The study was performed as a single-center, randomized, double-blind, immunobridging, and equivalence-designed phase IV clinical trial with Ad5-nCoV from 3 June 2021 to 23 August in Funing County, Jiangsu Province, China. The vaccine was prepared by virus culture, harvest, concentration, purification, and addition of various accessories (containing 5 × 1010 viral particles per 0.5 ml expressing the S protein of SARS-CoV-2 by using the replication-defective human adenovirus type 5 as a vector) (). The production process of Ad5-nCoV was identical, but the bioreactor capacities for cultivating replication-defective human type 5 adenovirus expressing SARS-CoV-2 S protein were 50 L, 500 L, and 800 L.
Figure 1. The production process of Ad5-nCov. The harvest was obtained by amplifying and culturing cells of replication-deficient human adenovirus type 5 expressing the S protein of SARS-CoV-2 in the bioreactor. The bulk was obtained after chromatography and purification of the harvest. The final bulk was prepared from the bulk plus various accessories. The final Ad5-nCov preparation was qualified for marketing after filling in the aseptic state and inspected release. The production process of Ad5-nCov with different production scales is the same, the main difference is that the replication-defective human type 5 adenovirus expressing the S protein of SARS-CoV-2 is cultivated in bioreactors of different scales (50 L, 500 L and 800 L). Ad5-nCoV=recombinant adenovirus type-5 vectored COVID-19 vaccine.
The entire trial was divided into two sub-cohorts, which were the Scale A cohort (50 L and 800 L) and the Scale B cohort (50 L and 500 L), based on the two independent hypotheses that the Ad5-nCoV in the scaled-up production of 500 L, and 800 L is immunogenic equivalence to the original 50 L production, respectively. No differences between the vaccines used in the Scale A 50 L group and the Scale B 50 L group. Participants in the Scale A 50 L group and the Scale B 50 L group were immunized with the same batch of Ad5-nCoV (NCOV202103006 produced in 50 L bioreactors). The Scale A cohort started on 3 June 2021, and the Scale B cohort started on 4 June 2021. On day 0, participants aged 18–59 years and never receiving any type of COVID-19 vaccine were eligible for enrollment after confirmation of good general health through medical history and physical examination at the screening visit. Participants were enrolled if they tested negative for SARS-CoV-2 for immunoglobulin (Ig)M or IgG antibodies via a rapid fingertip blood test; had no history of epidemiological exposure to COVID-19; had no symptoms of COVID-19. Female participants in childbearing should have a negative pregnancy test before vaccination and use contraception during the trial. Participants with severe cardiovascular disease or chronic diseases such as uncontrolled diabetes and hypertension, acquired immune deficiency syndrome, hepatitis B, and hepatitis C were excluded. A complete list of the inclusion and exclusion criteria is provided in Supplementary.
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. The trial protocol was reviewed and approved by the Chinese National Medical Products Administration (NMPA) and the institutional review board of the Jiangsu Provincial Center of Disease Control and Prevention (JSJK2021-A010-01). This trial was registered with Clinicaltrials.gov, NCT04916886. Written informed consent was obtained from each participant before the start of the study.
2.2. Randomization and blinding
The vaccine of Scale A (50 L and 800 L) and Scale B (50 L and 500 L) injected in the adults aged 18–59 years old were randomized by using an interactive web response system (IWRS). The random code was generated by an independent statistician using SAS version 9.4 with block randomization (4 as the block size) and imported into the central IWRS in a ratio of 1:1 for the random assignment of the Scale A cohort as well as the Scale B cohort. Eligible participants were assigned study numbers according to the screening sequence.
Authorized unblinded pharmacists prepared the different scales of vaccines according to the allocation of participants through the IWRS, and nurses injected investigational vaccines into participants. The unblinded staff were not allowed to participate in other trial processes and were prohibited from disclosing allocation information to others. All the other investigators, participants, and laboratory staff remained blinded throughout the trial.
2.3. Study procedures
We recruited participants for the Scale A cohort (50 L versus 800 L) and the Scale B cohort (50 L versus 500 L). A single intramuscular injection of 0.5 ml of Ad5-nCoV of different production scales was administered to each participant at the lateral deltoid muscle of the upper arm on day 0. We took 7.0 ml of blood samples from each participant before and 28 days after the vaccination to evaluate the immunogenicity consistency of the Ad5-nCoV vaccine at different production scales. After vaccination, all participants were monitored at the clinics for at least 30 minutes for any adverse reactions. Participants reported any injection-site and systemic solicited adverse events within 7 days and unsolicited adverse events 28 days after vaccination in the paper diaries. Serious adverse events (SAEs) and adverse events of special interest (AESI) up to 12 months after vaccination were monitored.
2.4. Outcomes
The primary immunogenicity endpoint was the geometric mean titer (GMT) of the specific neutralizing antibody (NAb) against live SARS-CoV-2 at 28 days after injection. The secondary immunogenicity endpoints included GMT ratio, seroconversion rate (SCR) and geometric mean fold increase (GMFI) of specific NAb against live SARS-CoV-2, and geometric mean concentration (GMC), SCR and GMFI of anti-SARS-CoV-2 spike protein receptor binding domain (S-RBD) antibody on day 28 postvaccination. And the stratified analysis of GMT was carried out 28 days after vaccination with the baseline level of the anti-Ad5 NAb as the stratified condition. The primary endpoint for safety was the incidence of adverse reactions within 7 days after a single dose. The secondary safety endpoints included adverse events (AEs) within 28 days after a single dose, SAEs and AESIs during the study (within one year).
2.5. Statistical analysis
The sample size was estimated by using the equivalence tests for the ratio of two means of PASS 16.0. According to the GMT of the live SARS-CoV-2-specific NAb on Day 28 in the phase II clinical trial, which was 18.4, and the standard deviation (SD) of 3.8, the coefficient of variation of GMT in each batch group was estimated to be 2.2 [Citation12]. The sample size was 230 in each group, with the GMT ratio between [0.67, 1.5] [Citation13,Citation14] and a two-way 5% significance level and 80% power, assuming that the GMT ratio was 1.0, and the ratio between the 50 L and 800 L groups was 1:1. Moreover, since the participants were only inoculated with single-dose Ad5-nCoV, the degree of completion after enrollment was relatively high. Therefore, considering group randomization, the dropout rate was finally set at 8.73%, which the Center for Drug Evaluation in China has agreed to. So the final sample size was 504 on the Scale A cohort (50 L and 800 L) and the Scale B cohort (50 L and 500 L), respectively, with 252 in each group. The sample size met the requirement of no less than 500 cases per experimental vaccine group in the Chinese regulation [Citation14].
Immunogenicity analysis was performed on the 29th day after a single injection. The live SARS-CoV-2-specific NAb levels and the S-RBD antibody levels of the groups before and after immunization with different scales of vaccines, were expressed as the GMT or GMC, GMFI, standard difference and 95% confidence interval (CI). One-Way ANOVA was used to calculate the difference in the GMT value between the two groups after log-transformation. The confidence interval method was used to conduct the equivalence test of the batch consistency study, which was preset at 0.67 to 1.5 [Citation13,Citation14]. When the 95% CI for the GMT ratio fell within the range of 0.67 to 1.5, it was considered that the different production scales of Ad5-nCoV vaccine were consistent. The initial dilution reported as lower than the detection value was replaced by the half value of the detection value (4 for the specific NAb against live SARS-CoV-2, 20 for S-RBD IgG antibodies, and 12 for preexisting Ad5 NAb titers). While the initial dilution greater than the highest dilution was converted to the highest dilution. SCR was defined as the proportion of participants who were negative at baseline were positive after immunization, or whose antibody titers of participants who were positive at baseline increased at least 4-fold from baseline after immunization. The chi-square test and Fisher’s exact test were used to compare the difference in SCR between groups. Categorical variables were compared between groups using the chi-square test and Fisher’s exact test.
The primary immunogenicity analysis was based on the per-protocol set (PPS), with all participants who have completed single-dose Ad5-nCoV immunization within the window period, completed the collection of blood samples before and after immunization, and can provide all effective antibodies. And the intention-to-treat (ITT) population included all randomized participants who received the vaccination and had at least one antibody available. The safety evaluation was based on the safety set (SS), including participants who received the vaccine on a different scale and participated in safety follow-up. All statistical tests were two-way with 5% of the default significance level. And all CIs were reported as 95% unless otherwise stated in the analysis description. GraphPad Prism 9.0 and SAS 9.4 were used for statistical analysis.
2.6. Role of the funding source
The funders of the study were involved in protocol design, but not in data collection, statistical analysis, data interpretation, or the writing of the report. All the authors had full access to all the data in the study and had final responsibility for the decision to submit it for publication.
3. Results
3.1. Demographic characteristics
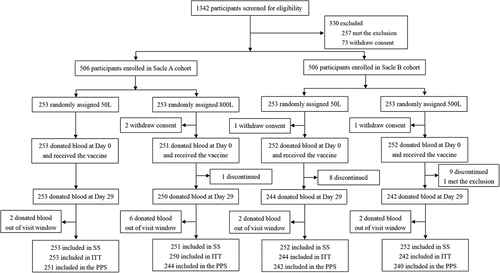
A total of 1342 participants were recruited and screened for eligibility (). 330 participants were excluded, including 257 individuals who met the exclusion criteria and 73 individuals who withdrew consent. 1012 participants who met the inclusion criteria consented to participate in the trial and were randomly assigned, and 4 participants were not vaccinated due to withdrawing consent. Participants who exceeded the window for blood collection or did not complete the blood collection were not included in the PPS. 251, 244, 242, and 240 participants were enrolled in the PPS immunogenicity analysis in Scale A 50 L, Scale A 800 L, Scale B 50 L, and Scale B 500 L, respectively.
Figure 2. Trial profile. The Safety Set (SS) included all participants who received the vaccine with different scale and participated in safety follow-up. The intention-to-treat (ITT) cohort included all randomized participants who received the vaccination and had at least one antibody available. The primary immunogenicity analysis was performed on the per-protocol sets (PPS), including all participants in the immunogenicity subset who received one dose of vaccine and who had available serological test results at baseline, day 29, and at least one later time point.
Demographic characteristics are reported in . The mean age of all participants was 42.29 years (SD 10.04). 539 (53.47%) of the 1,008 participants were male. The mean body-mass index (BMI) of all participants was 25.65 kg/m2 (SD 3.45). 135 (53.36%) participants in Scale A 50 L, 134 (53.60%) in Scale A 800 L, 149 (61.07%) participants in Scale B 50 L, and 126 (52.07%) in Scale B 500 L had preexisting Ad5 NAb titers more than 1:200. The demographic characteristics of the participants and the preexisting Ad5 NAb titers were broadly similar in the Scale A cohort and the Scale B cohort.
Table 1. Baseline characteristics of the subjects in the safety set.
3.2. Immunogenicity
The baseline GMTs of the specific NAb against live SARS-CoV-2 in all participants were reported below detection. On Day 29, a significant increase in the GMTs of the specific NAb against live SARS-CoV-2 was observed in the PPS, showing 10.72 (9.43, 12.19) in Scale A 50 L, 13.23 (11.64, 15.03) in Scale A 800 L, 11.64 (10.12, 13.39) in Scale B 50 L, and 12.09 (10.48, 13.95) in Scale B 500 L, respectively (). The two-sided 95% CI for the GMT ratios calculated in the Scale A cohort and the Scale B cohort were among [0.67, 1.5] intervals (Scale A cohort: 1.23 [1.03, 1.48]; Scale B cohort: 1.04 [0.85, 1.27]). 86.67% ~ 91.80% of SCR in the specific NAb against live SARS-CoV-2 after vaccination were observed, with no statistical differences between the two scale cohorts. The GMFIs of the specific NAb against live SARS-CoV-2 in participants inoculated with high-scale vaccine were higher than those inoculated with low-scale vaccine (6.61 [5.82, 7.52] in Scale A 800 L vs. 5.36 [4.72, 6.09] in Scale A 50 L; 6.05 [5.24, 6.98] in Scale B 500 L vs. 5.82 [5.06, 6.70] in Scale B 50 L). In addition, the GMTs, GMT ratios, GMFIs, and SCR immunogenicity results of postimmunization-specific NAb against live SARS-CoV-2 were similar between PPS and ITT in the Scale A cohort and the Scale B cohort (Table S1).
Table 2. The GMT (GMC), GMT (GMC) ratios, seroconversion and GMFI of neutralizing antibodies to live SARS-CoV-2 and receptor binding domain (RBD)-specific antibodies after immunization based on per-protocol sets.
In the PPS, participants in Scale A 800 L produced S-RBD IgG antibodies with higher GMCs than those in Scale A 50 L (76.98 relative units (RU)/ml [66.86, 88.63] vs 60.15 RU/ml [52.36, 69.09], P = 0.0135), whereas participants in Scale B 500 L were similar to those in Scale B 50 L (62.06 RU/ml [53.40, 72.11] vs 65.52 RU/ml [56.50, 75.98], P = 0.7233) (). The GMC ratio of S-RBD IgG antibodies was not within the equivalency range of 0.67 ~ 1.5 in the Scale A cohort (1.28 [1.05, 1.56]), but it was in the Scale B cohort (1.06 [0.86, 1.30]). 83.27% (78.16, 87.38) of participants in Scale A 50 L, 88.52% (83.91, 92.11) of participants in Scale A 50 L, 81.82% (76.48, 86.17) of participants in Scale B 50 L, and 83.33% (78.10, 87.51) of participants in Scale B 500 L showed seroconversion of RBD specific binding IgG antibodies after vaccination, observing no statistical difference. The GMFIs of S-RBD IgG antibodies after vaccination were observed at 6.00 (5.22, 6.88) in Scale A 50 L, 7.56 (6.57, 8.69) in Scale A 800 L, 6.21 (5.34, 7.21) in Scale B 50 L, and 6.51 (5.62, 7.55) in Scale B 500 L. Furthermore, the PPS and ITT in the Scale A cohort and Scale B cohort showed identical GMCs, GMC ratios, GMFIs, and SCR immunogenicity outcomes of S-RBD IgG antibodies post-immunization (Table S1).
544 (55.01%) of 1008 participants had high preexisting anti-Ad5 NAb levels before vaccination (). The GMTs of the specific NAb against live SARS-CoV-2 and GMCs of S-RBD IgG antibodies were lower in participants with high preexisting anti-Ad5 NAb than in participants with low preexisting anti-Ad5 NAb (Figure S1 and S2). After stratification with age, participants aged 18–44 years immunized by scale-up production of 500 L and 800 L have higher specific NAb of anti-live SARS-CoV-2 and S-RBD IgG antibodies. The stratified analysis based on gender found that male and female participants with different scale productions in the Scale A cohort and the Scale B cohort showed similar specific NAb against live SARS-CoV-2 and S-RBD IgG antibodies post-vaccination. After stratifying by BMI based on the standards of Chinese adults [Citation15], no statistical significance was observed in postvaccination anti-live SARS-CoV-2 specific NAb and S-RBD IgG antibodies among different BMI groups.
3.3. Safety
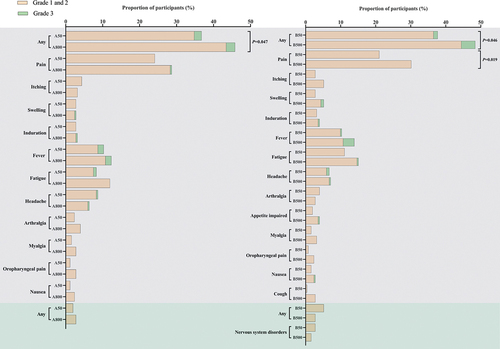
The frequencies of solicited adverse reactions in participants were significantly different among the different scales of the vaccine in Scale A cohort (50 L: 89 [35.18%], 800 L: 110 [43.82%], P = 0.047) and in Scale B cohort (50 L: 92 [36.51%], 500 L: 114 [45.24%], P = 0.046) (, Table S2). The number of participants with unsolicited adverse reactions ranged from 5 (1.98%) to 13 (5.16%), with no difference between different scales of the Ad5-nCoV vaccine. The most common solicited local adverse reactions among the different scales of the vaccine were pain at the injection site, reported by 61 (24.11%) participants in Scale A 50 L, 72 (28.69%) in Scale A 800 L, 53 (21.03%) participants in Scale B 50 L, and 76 (30.16%) participants in Scale B 500 L. The most commonly reported solicited systematic adverse reactions overall were fever (118 [11.71%]), fatigue (115 [11.41%]), and headache (73 [7.24%]), of which no significant difference across different scales was observed. High preexisting Ad5 NAb, aging, and being male were associated with a significantly lower incidence of post-vaccination fever (Table S3). Most of the adverse reactions were mild or moderate. Grade 3 solicited adverse reactions occurred in 24 (2.38%) participants, with 5 (1.98%) in Scale A 50 L, 6 (2.39%) in Scale A 800 L, 3 (1.19%) in Scale B 50 L, and 10 (3.97%) in Scale B 500 L, respectively. But no grade 3 adverse reactions were reported as unsolicited adverse reactions.
Figure 3. Adverse reactions occurred within 7 days after the vaccination. A50 = Scale a 50 L. A800 = Scale a 800 L. B50 = Scale B 50 L. B500 = Scale B 500 L. Any = all the participants with any adverse reactions. The analysis was based on the safety set cohort, with all participants receiving vaccines after randomization. The figure only showed symptoms with an incidence rate of adverse reactions over 2% within either scale group in the Scale a cohort and the Scale B cohort. Solicited adverse reactions were on the gray background, and unsolicited adverse reactions were on the green background.
Solicited AEs but not unsolicited AEs in participants receiving a single dose of the Ad5-nCoV vaccine within 28 days were reported, with statistically significant differences in frequency among the different scales of the vaccine (Table S4). Solicited AEs in participants were reported by 90 (35.57%) participants in Scale A 50 L, 113 (44.02%) participants in Scale A 800 L, 95 (37.70%) participants in Scale B 50 L, and 118 (46.83%) participants in Scale B 500 L. The number of participants with unsolicited AEs after vaccination ranged from 13 (5.14%) to 24 (9.52%). 30 grade 3 AEs occurred in 25 participants who reported solicited AEs and 5 participants who reported unsolicited AEs, respectively. The frequencies of grade 3 AEs in participants receiving different scales of the Ad5-nCoV vaccine were not significantly different. In total, 18 (1.82%) participants experienced SAEs (four [1.58%] in Scale A 50 L, five [1.99%] in Scale A 800 L, six [2.38%] in Scale B 50 L, and three [1.19%] in Scale B 500 L), which were determined irrelevant to vaccinations except for one participant in Scale A 50 L who experienced abnormal uterine bleeding and hemorrhagic anemia (Table S5).
4. Discussion
The single-center, randomized, double-blind production-scale immunobridging trial showed that the immunogenicity of Ad5-nCoV produced at different scales was consistent and safe after vaccination in healthy adult populations. And identical findings in the ITT analysis and the PPS analysis were observed.
The COVID-19 pandemic has devastated global health, economies and societies. COVID-19 vaccination remains the mainstay of prevention of SARS-CoV-2 infection. The results of previous phase I, II and III clinical studies showed that Ad5-nCoV has good immunogenicity and safety [Citation6,Citation12,Citation16]. The scale of production directly influenced the availability, promotion and use of Ad5-nCoV, as the first and only adenovirus vector vaccine included in EUL authorized by WHO in China. The current global demand for COVID-19 vaccines is not as high as during the pandemic, which poses a risk of overcapacity of Ad5-nCoV. However, the large-scale production and quality control system established by Ad5-nCoV can provide technical support for subsequent development of vaccines, given that most of the equipment in the vaccine production process can be used universally.
The production process of Ad5-nCoV with different production scales is the same, and the main difference is that the replication-defective human type 5 adenovirus expressing the S protein of SARS-CoV-2 is cultivated in bioreactors of different scales (50 L, 500 L and 800 L). The difference in production scale may have an impact on the consistency of vaccine production, thereby affecting the immunogenicity and safety of Ad5-nCoV. Therefore, the immunobridging trial comparing scale-up production of 500 L and 800 L with the original 50 L production scale is significant for the development and application of vaccines. According to the recommendations of the Food and Drug Administration (FDA), and the Center for Drug Evaluation [Citation14,Citation17,Citation18], the GMT ratio of specific NAb against live SARS-CoV-2 after vaccination is used to evaluate the equivalence of vaccines produced at different production scales, that is, comparison between 800 L and 50 L in the Scale A cohort, as well as between 500 L and 50 L in the Scale B cohort. If the 95% CI of the GMT ratio falls within the preset equivalent range of 0.67–1.50, it can be considered that the immunogenicity equivalence of vaccines of different scales is established. Our clinical study showed that production scales of 50L, 500 L and 800 L have good immunogenicity consistency. All participants vaccinated with a single dose of Ad5-nCoV vaccine produced high levels of specific NAb against live SARS-CoV-2 and anti-RBD antibodies. Previous studies found a strong positive correlation between anti-RBD-binding antibodies and NAb [Citation16,Citation19]. Our study found that scale equivalence held for neutralizing antibodies but only for the Scale B cohort for anti-RBD binding antibodies. This result may be associated with the fact that only neutralizing antibodies against the live viruses but not RBD-binding antibodies were considered in the calculation of the sample. Therefore, this positive correlation does not make anti-RBD-binding antibodies a surrogate for NAb in the immunobridging trial. Moreover, compared with the S-RBD IgG ELISA test, the test of NAb against live virus needs to be performed by qualified personnel in the third-level biosafety laboratory. Considering that detecting anti-live SARS-CoV-2 specific neutralizing antibodies has higher requirements on place and personnel selection and funding, it is possible to try RBD-binding antibodies as an immunological surrogate endpoint for SARS-CoV-2 vaccine immunobridging tests.
In addition, Ad5-nCoV was well tolerated and safe. As with earlier clinical trials, the most commonly observed local adverse reaction among participants was mild or moderate injection site pain, which resolved within a week of vaccination [Citation6,Citation12]. Systemic adverse reactions such as fever, headache, and fatigue were the most commonly reported, and most were mild or moderate, consistent with previous research results. Grade 3 adverse reactions were reported in different scale vaccines, but the frequency of occurrence was infrequent. Participants vaccinated with Ad5-nCoV had 30 SAEs. Abnormal uterine bleeding and hemorrhagic anemia have been observed in association with the vaccine. Other SAEs were judged not to be related to the vaccine. The above results showed that the Ad5-nCoV vaccine has good safety.
This study also found that increasing age and preexisting antibody immunity may affect the immunogenicity and safety of participants after Ad5-nCoV immunization, which was consistent with the findings of previous phase II trials [Citation12]. For each scale, participants with a higher age (45 years and above) and high levels of anti-Ad5 immunization had lower levels of neutralizing and anti-RBD antibodies. This may be related to the history of Ad5 exposure in the elderly. And the decreased occurrence of fever was associated with declining age and low preexisting immunity. Based on previous studies of heterologous and homologous prime-boost immunization with Ad5-vectored Ebola vaccine [Citation20] and immunization with Ad5-nCoV and inactivated COVID-19 vaccine [Citation21], it is recommended that older populations with preexisting antibody immunity receive a second dose of Ad5-nCoV or other types of SARS-CoV-2 vaccine within 3 to 6 months to enhance the immune response.
There are some limitations to our trials. First, there is a lack of protective efficacy data with the disease as the observation endpoint. Participants were not infected with SARS-CoV-2 after vaccination, so the effectiveness of the Ad5-nCoV vaccine could not be evaluated. Secondly, this study only measured the equivalence of NAbs against live SARS-CoV-2, not other variants of concern (VOCs). However, some studies have observed that B.1.1.529 (Omicron), one of the VOCs, has partial NAb escape [Citation22,Citation23]. Whether the vaccine is also immunogenically equivalent to emerging VOCs is open to question. Thirdly, our study only collected immunogenicity and safety data 28 days after immunization, and there is no data on immune persistence or long-term safety. Therefore, the long-term persistence of antibodies in the real world requires further study after the vaccine is marketed. Fourth, due to the single-center clinical trial, recruiting representative research subjects was difficult. And the research population of this study only involves healthy adults aged 18–59. It is not yet known whether vaccines of different production scales are equally effective for individuals in different countries or regions, children, the elderly, and patients with underlying diseases, and additional research is needed to confirm. Fifth, participants in this study received a single dose of the Ad5-nCoV, so the immunogenicity consistency and safety of the second and booster doses of the vaccine haven’t been evaluated. Finally, the relatively small sample size at each scale limited the statistical power of subgroup analyses.
5. Conclusion
The results of this trial confirmed that the scales of 50 L, 500 L and 800 L have immunogenicity consistency and safety, providing a basis for the scale-up application of the vaccine. Aging and preexisting antibody immunity can significantly hinder the humoral immunity and safety of vaccination.
Author contributions
Feng-cai Zhu was the principal investigator of this trial. Feng-cai Zhu and Jing-xin Li designed the trials and the study protocol. Jing-xin Li and Feng-cai Zhu contributed to critical review and revising of the report. Yan-fei Wu, Ming-wei Wei contributed to the data interpretation, drafting, and revising of this manuscript. Rui-jie Wang provided the vaccines of trial and led the trial supervision. Xi-ling Guo led the laboratory tests. Hong-xing Pan contributed to tiral supervision. Ming-wei Wei and Ya-chun Gao led and participated in the site work, including the recruitment, follow-up, and data collection. Xiao-long Li, Xue Wang, Xiao-min Ma, Peng Wan monitored the trial. Li Zhou and Ya-wen Zhu contributed to statistical analysis.
Declaration of interest
RJ Wang, XL Li, X Wang, XM Ma and P Wan are the employees of CanSino Biologics Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Clinical trial registration
www.clinicaltrials.gov identifier is NCT04916886.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. The trial protocol was reviewed and approved by the Chinese National Medical Products Administration (NMPA) and the institutional review board of the Jiangsu Provincial Center of Disease Control and Prevention (JSJK2021-A010-01).
Supplemental Material
Download MS Word (2.7 MB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2023.2234997.
Additional information
Funding
References
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 Feb 20;382(8):727–733.
- Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020 Mar 26;382(13):1199–1207.
- Watson OJ, Barnsley G, Toor J, et al. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022 Sep;22(9):1293–1302.
- WHO. Coronavirus disease (COVID-19): Vaccines 2022 [cited 2022 Nov 21]. Available from: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-covid-19-vaccines
- WHO. Interim recommendations for use of the Cansino Ad5-nCoV-S vaccine (Convidecia ®) against COVID-19 19 May 2022; [cited 2022 Nov 24]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE-recommendation-Ad5-nCoV-Convidecia
- Halperin SA, Ye L, MacKinnon-Cameron D, et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet. 2022 Jan 15;399(10321):237–248.
- Organization WH Strategy to achieve global Covid-19 vaccination by mid-2022 2021 [cited 2021 Jun 23]. Available from: https://www.who.int/publications/m/item/strategy-to-achieve-global-covid-19-vaccination-by-mid-2022
- Organization WH. Global COVID-19 vaccination strategy in a changing world: July 2022 update. 2022 [cited 2022 Nov 24]. Available from: https://www.who.int/publications/m/item/global-covid-19-vaccination-strategy-in-a-changing-world–july-2022-update
- So AD, Woo J. Reserving coronavirus disease 2019 vaccines for global access: cross sectional analysis. BMJ. 2020 Dec 15;371:m4750. doi: 10.1136/bmj.m4750.
- AZD1222 vaccine met primary efficacy endpoint in preventing COVID-19 [Internet]. 2020. Available from: https://www.astrazeneca.com/media-centre/press-releases/2020/azd1222hlr.html#!
- Johnson & Johnson initiates pivotal global phase 3 clinical trial of Janssen’s COVID-19 vaccine candidate [Internet]. 2020. Available from: https://www.jnj.com/johnson-johnson-initiates-pivotal-global-phase-3-clinical-trial-of-janssens-covid-19-vaccine-candidate
- Zhu FC, Guan XH, Li YH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020 Aug 15;396(10249):479–488.
- Organization WH. Guidelines on clinical evaluation of vaccines regulatory expectations 2004 [cited 2022 Jul 11]. Available from https://www.who.int/publications/m/item/guidelines-on-clinical-evaluation-of-vaccines-regulatory-expectations
- Center for Drug Evaluation N. Guidance of clinical comparability research guidelines for preventive vaccines 2019 [cited 2023 Jul 4]. Available from: https://www.cde.org.cn/main/fullsearch/fullsearchpage
- Chen C, Lu FC. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17(Suppl):1–36.
- Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020 Jun 13;395(10240):1845–1854.
- Nauta J. Statistics in clinical vaccine trials. Springer Berlin Heidelberg: 2010. doi:10.1007/978-3-642-14691-6
- FDA. Emergency use authorization for vaccines to prevent COVID-19; guidance for industry Mar 31, 2022 [cited 2022 Nov 17]. Available from: https://www.fda.gov/media/142749
- Salazar E, Kuchipudi SV, Christensen PA, et al. Convalescent plasma anti-SARS-CoV-2 spike protein ectodomain and receptor-binding domain IgG correlate with virus neutralization. J Clin Invest. 2020 Dec 1;130(12):6728–6738.
- Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020 Sep 26;396(10255):887–897.
- Li J, Hou L, Guo X, et al. Heterologous AD5-Ncov plus CoronaVac versus homologous CoronaVac vaccination: a randomized phase 4 trial. Nat Med. 2022 Feb;28(2):401–409.
- Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022 Feb;602(7898):671–675.
- Liu L, Iketani S, Guo Y, et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022 Feb;602(7898):676–681.