ABSTRACT
Introduction
Vaccination is an effective, relatively inexpensive, and easy to deliver approach to combating infectious diseases. Widespread vaccination of children has led to the eradication of smallpox and allowed for regional elimination or control of diseases like polio, measles, mumps, tetanus, diphtheria, and whooping cough. But, as we learned from efforts to combat the COVID-19 pandemic, a successful global vaccination program must overcome several hurdles. Failure at any stage can limit vaccine uptake and disease control.
Areas covered
In this review, we break down the vaccine journey from research and development to delivery into several steps. We also list all the important international organizations trying to support this ecosystem. Then we identify the role of each of these organizations in supporting each of the necessary steps for a successful vaccination program.
Expert opinion
The bottlenecks in vaccination can be different for different countries, based on their income and geography. Policy makers need to identify the weaknesses of this ecosystem in different regions of the world and make sure there is adequate global and local support to fill the gaps in the system.
1. Introduction
Economics has long recognized that vaccine markets will ‘fail’ in the absence of public funding [Citation1,Citation2]. Without government support, there may be insufficient private R&D expenditure toward the development of new vaccines and insufficient numbers of people will elect to use vaccines that are developed. For instance, a vaccine for the Ross River virus successfully completed phase III clinical trials in 2015, but it remains unlicensed due to the lack of demand for its use in preventing sporadic cases [Citation3]. As another example, LYMERix, the sole vaccine for Lyme disease available at the time, was discontinued by the manufacturer in 2002 due to inadequate consumer demand [Citation4].
The market failure arises from the large gap between an individual’s willingness to pay to get vaccinated and the social value of the vaccination. Public funding ideally fills this gap. This explains why governments in the United States (US) and other high-income countries support the vaccine sector, typically by supporting vaccine R&D and by subsidizing the cost of vaccination, thereby encouraging their use. Not all countries, however, have the means to do so. Thus, in the absence of external financial assistance from foreign governments or philanthropic organizations, a large share of the population in lower income countries will go unvaccinated, and this increases the burden of infectious disease in those countries.
Infectious diseases, by nature, do not adhere to international boundaries; hence, a disease prevalent in one country can potentially infect vulnerable populations in others. For example, the H5N1 avian influenza continues to pose a global threat due to insufficient international efforts to contain its spread. Furthermore, for some viruses, high rates of infection can accelerate the development of mutations and variants, which can spread across countries and prolong a pandemic universally [Citation5–7]. While certain high-income countries have started investing in global vaccination efforts to mitigate these cross-border risks, these investments are neither universal nor adequate.
Thus, in light of these spillovers, ideally high-income countries would subsidize vaccination programs both domestically and in low-income countries as well. However, the COVID-19 pandemic illustrated that international vaccine aid sometimes falls short. Vaccine companies were able to develop COVID-19 vaccines in record time but faced production capacity constraints. Available vaccine supplies were allocated primarily to jurisdictions most willing to pay [Citation8,Citation9]. As a result, high-income countries, which had the resources to purchase the vaccines and the infrastructure to distribute them, vaccinated their population in record time. By the end of 2021, more than 70% of the population in these countries were fully immunized. However, some low-income countries did not even reach a 10% vaccination rate by that date [Citation10].
The low vaccine uptake rates in some low-income countries were not simply a matter of insufficient doses being available. These countries also had trouble in distributing and administering vaccines to residents, especially those that required ultra-cold refrigeration. For instance, Nigeria destroyed one million doses of COVID-19 vaccine in December 2021 because it was unable to administer these vaccines prior to their expiration date. Only 2% of the Nigerian population were fully vaccinated by that date and the country faced a surge in new COVID-19 cases [Citation11]. Although international assistance plays a vital role in increasing vaccination rates in low-income nations, it is not a sufficient condition. The absence of local political commitment to successful vaccination programs can obstruct the effectiveness of international efforts, thus emphasizing the importance of local political buy-in for successful outcomes.
The COVID-19 pandemic spread to all countries, low- and high-income alike. Other infectious diseases are prevalent mostly in lower income countries. For these infectious diseases, the potential sales revenues earned from a newly developed vaccine may be insufficient to cover expected development costs. In such cases, high-income countries need to contribute some, or all the funds needed to support their development. Unfortunately, there are many infectious diseases prevalent in low-income regions for which there are no vaccines available; these include vaccines for the Zika, Leprosy, and Chikungunya viruses [Citation12,Citation13].
The flow of funds and in-kind support from high-income countries to the global vaccine ecosystem occurs through a complicated web of governmental agencies, international organizations, donor agencies, and non-governmental organizations [Citation14]. The goal of this paper is to describe the objectives and responsibilities of the main players in this ecosystem. Having a clear map of their responsibilities can be helpful for keeping them accountable for the shortcomings or steering more support for the bottlenecks in the system. Our review builds on the work by Bernasconi and colleagues, who described the role of the Coalition for Epidemic Preparedness Innovation (CEPI) in filling gaps in the vaccine ecosystem [Citation15]. They divided the vaccine development pipeline into five different phases and identified the phases in which CEPI was active. They also enumerate several important organizations active in each phase. But, to our knowledge, there is no comprehensive study that reviews all the necessary steps for a successful global vaccination program and that maps the responsibility of all the important players in the ecosystem. In section 2, we introduce a framework for better understanding of the vaccine ecosystem. In section 3, we break down the vaccine pathway from basic research to delivery into detailed steps. For each step, we provide a general overview; identify the main organizations within countries responsible for supporting those steps; provide a detailed description of some of the important international players trying to fill the gaps in the global level. In section 4, we provide a comprehensive list of players in the ecosystem and map their responsibilities onto different steps that we defined earlier.
2. Framework
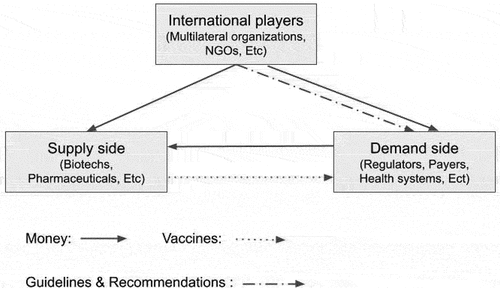
High-income countries provide both financial and in-kind support for vaccination efforts in low-income foreign countries through intermediary organizations, which we call ‘international players.’ These international players include multilateral organizations and philanthropic non-governmental organizations (NGOs), and government agencies. Multilateral organizations and some NGOs identify the needs in the system, provide guidance, and invest directly to facilitate transactions between vaccine developers and producers (the ‘supply side’ players) and groups that procure and administer vaccines to individuals (‘demand side’ players). Identifying and acknowledging the need for immunization is a critical aspect of initiating demand for vaccines, as they define the landscape of the vaccination market and shape the focus of vaccine development.
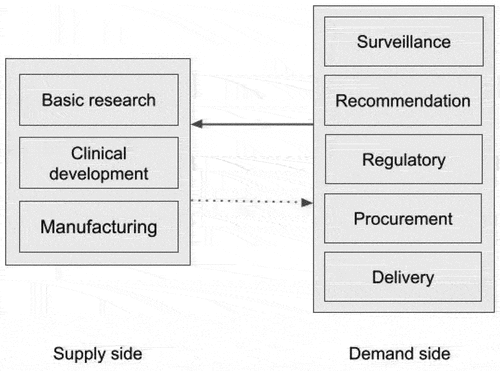
The flow of resources (financial and in-kind) and vaccines between these three groups, international payers, demand side and supply side players, is illustrated in . Money flows from the demand side to the supply side of this market, while vaccines flow in the opposite direction. Players on the supply side include (i) academic and research institutions, which are typically focused on fundamental research and early development of vaccines, (ii) biotechnology firms, and pharmaceutical companies, which mostly engage in clinical development and manufacturing, and (iii) state-owned enterprises (SOEs), and contract manufacturing organizations (CMOs), which focus on vaccine manufacturing.
Figure 1. This diagram shows how money, vaccine, and guidelines and recommendations move between international players and players in supply and demand sides of the vaccine ecosystem. International players are the intermediary organizations that provide both financial and in-kind support for vaccination efforts in both high- and low-income countries.
The major players on the demand side are regulators, bodies that review and recommend the adoption of vaccines for use in a jurisdiction, as well as payers, health systems, and civil societies. These players are public institutions that represent the consumer (i.e. the general public), subsidizing the cost of vaccines and recommending the use of specific vaccines. The private vaccine market, i.e. vaccines that are marketed to, and purchased directly by consumers, is quite small in most countries.
The main goal of this project is to describe the major players in each side of the global vaccine ecosystem, in both high-income and low-income countries. To do that, in the next section we go into more detail about different steps in the supply side and demand side of this market.
3. Components of vaccine ecosystem
The pathway from vaccine research and development (R&D) to immunization is illustrated in . The health of the ecosystem – including both the demand and supply side – requires that each step in the pathway be operational. For each of the steps in the pathway, we provide a general overview; identify the main organizations within countries responsible for supporting those steps; and finally provide a detailed description of some of the important international players trying to fill the gaps at the global level. We also use the COVID-19 vaccines to illustrate the pathway from basic research to ‘shots in arms’ and the international players that support the steps along this pathway.
3.1. Basic research
Modern vaccine development requires a firm scientific foundation, based on an understanding of the pathogen and the host (human) response to it, as well as the biotechnologies that are used to construct the vaccine. Vaccine development, in general, is conditioned on successes in basic research in the areas of chemistry, physics, biology, and computational sciences. Innovations in these basic research fields improve the odds of success in vaccine development. The first stage of developing a vaccine is preclinical development which starts by studying the pathogenesis mechanism; an understanding of these mechanisms allows scientists to screen and select appropriate target antigens (parts of the virus/bacteria to target). Second, researchers must develop appropriate biotechnology processes to produce vaccine candidates. The third stage involves studying candidates in in vitro assays (which need their own development) with successful candidates moving on to toxicity and clinical efficacy testing in animal models (which also need to be developed to reflect the vaccine’s efficacy in humans). Success in this intricate and risky scientific endeavor creates the basis for a clinical trial application and the potential for testing in human participants [Citation15]. The rapid development of COVID-19 vaccines was the result of years of previous research on related viruses and earlier investments into new vaccine platforms [Citation16].
Although big pharmaceutical companies spend some resources on basic research and preclinical development, most of this work is done in universities, research institutions, and smaller firms, often referred to as biotechnology companies or ‘biotechs.’ The biotechs are often started by academic scientists who may receive venture capital funding for early-stage R&D. These companies usually do not have enough capital for the later stages of vaccine development. Therefore, biotechs with promising technology often partner with large pharmaceutical firms or are acquired by these large firms as they possess the resources and expertise to carry out the later stages of vaccine development and bring them to the market. For example, the large pharmaceutical firm Pfizer partnered with the biotech firm BioNTech to develop and market BioNTech’s novel mRNA-based COVID-19 vaccine candidate; the vaccine was eventually sold under the brand name Comirnaty [Citation17].
Most of the financial support for early-stage vaccine R&D comes from governmental institutions in the US. The National Institutes of Health (NIH) in the US is one of the major funding sources of academic programs for basic research. According to the G-FINDER project, which tracks annual investment in R&D for new products and technologies, almost half of the R&D funding for vaccines globally is provided by the NIH [Citation18]. The US Biomedical Advanced Research and Development Authority (BARDA), the European Commission (EC), and the US Department of Defense (DOD) are other important governmental organizations that sponsor basic research in vaccines [Citation18]. Indeed, the COVID-19 vaccine developer Moderna received significant financial support through a partnership with BARDA and the NIH [Citation19].
It is not surprising that most government funding is directed toward R&D for vaccines against viruses that pose a domestic threat. But some governments support vaccine R&D initiatives that benefit primarily other countries. These efforts are complemented by funding from philanthropic organizations, such as Bill & Melinda Gates Foundation (BMGF). For example, the BMGF funded BioNTech to develop HIV and tuberculosis vaccine programs, further expanding the company’s infectious disease portfolio [Citation20].
Some organizations provide in-kind support for international vaccine R&D efforts. The WHO has created an R&D blueprint, listing diseases, and pathogens that should be prioritized for R&D in public health emergency contexts. These diseases pose the greatest public health risk due to their epidemic potential and/or whether there are no or insufficient countermeasures [Citation21]. Diseases in this list receive priority financial support for basic research. The Programs for Appropriate Technology in Health (PATH) are another nonprofit group that builds private sector partnerships to develop vaccine technologies suitable for the developing world [Citation22].
3.2. Clinical development
The next phase in the vaccine journey is clinical development, which involves studying vaccine candidates in humans to explore their safety, immunogenicity, and efficacy. This is done through a staged process: phase 1 includes early safety and immunogenicity in a small number of participants; phase 2 involves safety, dose-ranging, and immunogenicity in a larger number of participants (usually a few hundred); and phase 3 includes safety and usually clinical efficacy trials needed for licensure, which generally involves thousands of participants [Citation23].
In the past, clinical trials were predominantly carried out in North America, Europe, and other developed regions. There has been increasing recognition of the importance of testing vaccines in populations that will use them, should the trials be successful. Consequently, in recent years, there has been a rise in the number of vaccine clinical studies conducted in lower income countries. These trials are designed to address diseases such as malaria and dengue fever, which are prevalent in many low- and lower-middle-income countries [Citation24]. Yet, there is also a significant need to ensure diverse representation in trials for vaccines against diseases that are common across different income countries. Encouraging and funding such inclusivity in clinical trials can lead to more effective and equitable vaccine outcomes globally.
The role of large pharmaceutical companies in the vaccine innovation process is predominantly in development and clinical trials. These companies have both the in-house expertise and retained earnings to mount the complex, large-scale trials of vaccine candidates needed to satisfy so-called ‘stringent’ regulators, such as the US Food and Drug Administration (FDA) and European Medicines Agency (EMA). These agencies have relatively high standards for both vaccine safety and efficacy and vaccine production facilities. Meeting these standards requires that vaccine companies have commensurate levels of in-house expertise in clinical research, regulatory affairs, data management, statistics, project management, manufacturing, and quality assurance. They also are structured to make rapid go/no-go decisions on the development strategy required to minimize regulatory risk while assessing the performance of vaccine candidates [Citation25].
Governmental institutions, such as NIH and BARDA, have increased their role in clinical development domestically and internationally in recent years. For example, BARDA has since 2006 supported the advanced development of six cell-based influenza vaccines. This support led to the licensing of two seasonal vaccines in Europe from Novartis and Baxter in 2009, and one seasonal vaccine in the US from Novartis in 2012 [Citation26]. Also, the National Institute of Allergy and Infectious Diseases (NIAID), which is one of the institutes and centers that make up the NIH, launched a phase 2 clinical trial of a universal influenza vaccine called M-001 in 2018. This vaccine is developed and produced by BiondVax Pharmaceuticals, a company based in Israel [Citation27]. Additionally, the clinical trials for Moderna’s COVID-19 vaccine were conducted in close collaboration with NIAID. Most of the funding for clinical development of that vaccine has been provided by BARDA [Citation28].
Non-governmental organizations (NGOs) are the other main international supporters of this component of the vaccine ecosystem. The Bill and Melinda Gates Foundation and Wellcome Trust support several other intermediary NGOs who invest in the development of vaccines that can have the greatest impact on diseases of developing countries [Citation29,Citation30]. For example, the world’s first malaria vaccine was developed by GlaxoSmithKline in a 30-year collaboration with the nonprofit organization PATH; this venture received support from the Bill & Melinda Gates Foundation and a network of African research centers [Citation31].
An intermediary organization in this field is Coalition for Epidemic Preparedness Innovations (CEPI) [Citation32]. Its main responsibility is to facilitate and fund the planning for developing and deploying new vaccines to prevent and reduce the impact of emerging infectious diseases (EID) epidemics. The Coalition is a global partnership between public, private, philanthropic, and civil society organizations. Its mission is to stimulate and accelerate the development of vaccines against EIDs and enable access to these vaccines for people affected by outbreaks. It was founded by the governments of Norway and India, the Bill & Melinda Gates Foundation, the Wellcome Trust, and the World Economic Forum.
3.3. Manufacturing
This stage of vaccine development can be broadly divided into two categories: bulk manufacturing and finishing operations. Bulk manufacturing includes cell culture and/or fermentation-based manufacturing, followed by a variety of separation processes to purify the vaccine. The finishing operations include formulation with adjuvants, stabilizers, and preservatives. This is followed by vial or syringe filling, and then labeling, packaging, and temperature-controlled storage [Citation25]. These processes are scaled up sequentially through clinical development and may be further expanded to meet the market demand of approved vaccines. Therefore, vaccine manufacturing requires major investment in a technologically advanced production plant and the establishment of teams with multidisciplinary expertise in the scale-up and production of biological products.
There are two types of producers in the vaccine eco-system: R&D-based, which are usually located in high-income countries and invest heavily in developing new vaccines or updating existing ones and non-R&D-based producers, which manufacture vaccines developed by others, invest little in R&D and accumulating scientific expertise, and are often based in low- and middle-income countries [Citation33]. The R&D-based producers manufacture the vaccine themselves, or outsource partially or wholly to contract manufacturing organizations (CMOs). The non-R&D-based producers, such as Serum Institute of India and Bio Farma in Indonesia, are the result of a trend among developing countries to build indigenous capabilities and gain control of the vaccine supply chain for use within their own borders, and sometimes to supply other low- to medium-income countries.
In addition to funding research into new vaccines, governments also subsidize the cost of constructing new vaccine manufacturing facilities. Again, most of the funding is used to support domestic manufacturing. For example, the federal government of Canada agreed to provide $415 million in funding for Sanofi’s new egg-based whole virus plant to produce influenza vaccines. Additionally, the Ontario provincial government will contribute $55 million to the project. For its part, Sanofi will contribute more than $455 million [Citation34]. The US government’s Operation Warp Speed (OWS) provided $18 billion to expedite the late-stage clinical development, manufacturing, and distribution of COVID-19 vaccines [Citation35]. The OWS program initially supported eight vaccine candidates, out of which seven progressed to phase 3 testing. These candidates were developed by Pfizer – BioNTech, AstraZeneca – Oxford, Moderna, Johnson & Johnson, Novavax, Merck, and Sanofi – GlaxoSmithKline [Citation35]. At the end of February 2021, the OWS program was assimilated into the White House COVID-19 Response Team. The development and manufacturing of COVID-19 vaccines have seen significant contributions from pharmaceutical companies in China and India. The Serum Institute of India and Beijing Institute of Biological Products are prime examples of companies whose vaccines have received international recognition and have been widely distributed globally [Citation36].
Some governments directly support vaccine manufacturing internationally. As an example, the BARDA International Influenza Vaccine Manufacturing Capacity Building Program was established to raise global pandemic preparedness by helping developing countries build and operate influenza vaccine manufacturing facilities. This program has provided technical and financial support for influenza vaccine production to 14 manufacturers in 13 countries [Citation37]. Additionally, the Bill and Melinda Gates foundation created the NevoLine platform to encourage R&D that can reduce the entry barriers of vaccine manufacturing. The purpose of this platform was to modernize vaccine production through modular manufacturing [Citation36].
3.4. Diagnostic and surveillance
Public health surveillance is the continuous and systematic collection, analysis, and interpretation of data on the circulation of infectious disease [Citation38]. The information gathered is used in a variety of ways: to identify cases requiring investigation, to estimate the magnitude of diseases, to detect outbreaks, to evaluate response and prevention measures such as vaccination, to monitor evolution in infectious agents, to facilitate research, and to measure the impacts of changes in health-care practices [Citation39]. The diseases under surveillance include both unknown pathogens that appear for the first time in a population as well as known pathogens that increase in geographic range or severity, or are reintroduced into the population. Another important category of surveillance within this field is vaccine pharmacovigilance, which encompasses the scientific, methodological, and practical aspects utilized to identify, evaluate, comprehend, mitigate, and report on adverse events related to immunization [Citation40].
There are two types of surveillance systems: passive and active. In passive surveillance systems, medical professionals in the community and at health facilities report suspected cases of infection to the public health agency, which then curates and analyzes these data. As the name implies, active surveillance systems are more pro-active. Public health agency personnel might train medical professionals and health facility personnel to help identify and report infectious disease cases; public health agency may also conduct their own infectious disease testing [Citation41].
Notifiable disease surveillance is an example of passive surveillance. Notifiable diseases are the diseases that have considerable public health importance: they can be a severe risk to human health, outbreak prone, considered to be an emerging or reemerging disease, eradicable diseases, or have a timely intervention available for control of the disease [Citation41]. National notifiable diseases are country-specific. The public health authorities in each country mandate the reporting of these notifiable diseases. For example, in the US, the Centers for Disease Control and Prevention (CDC) and the Council of State and Territorial Epidemiologists (CSTE) compile a list of diseases that have mandated reporting to CDC [Citation42]. The WHO prepares a list of the notifiable diseases in different parts of the world [Citation41].
National immunization programs and national regulatory agencies gather data on adverse events following immunization (AEFI) as a component of their vaccine pharmacovigilance responsibilities. For instance, regulatory agencies such as the European Medicines Agency (EMA) in the EU require companies to submit a risk management plan. This plan must contain post-commercialization studies and be updated with new information about the product during its marketing phase [Citation43]. Additionally, the Immunization Safety Office of the Centers for Disease Control and Prevention (CDC) is responsible for monitoring the safety of vaccines and conducting studies on rare and serious adverse events that may occur following immunization. This work is carried out under the Vaccine Safety Datalink (VSD) project [Citation44]. The World Health Organization (WHO) also extends technical assistance to its Member States to enhance and sustain their capacity for post-licensure vaccine safety monitoring [Citation45].
The CDC works in partnership with the WHO, ministries of health, foundations, development agencies, and other federal agencies to promote national, regional, and international disease surveillance [Citation46]. The CDC also opened regional offices outside the US, mostly in Latin America and Africa, to advance its goal for global health security [Citation47].
Nearly all recent pandemics have a viral etiology with animal origins, and with their intrinsic capacity for interspecies transmission, viral zoonoses are prime candidates for causing future pandemics. In light of this, the US Agency for International Development (USAID) launched PREDICT in 2009. This was the first global scale coordinated program designed to conduct viral discovery in wildlife reservoir hosts and to characterize ecological and socioeconomical factors driving the risks of virus spillover. This project spanned more than 35 countries over 8 years. Another recent initiative, the Global Virome Project, is trying to significantly expand the scale of targeted viral discovery [Citation48].
The World Health Organization (WHO) is the coordinating body for disease surveillance at the international level, but it is dependent on its Member States for reporting. WHO has a number of country and regional representatives that liaise and communicate with national governments [Citation49]. The WHO also coordinates the Global Outbreak and Response Network (GOARN), a network of governments, technical, and academic institutions involved in epidemic surveillance [Citation41]. The main goals of GOARN are to examine and study diseases, evaluate their risks, and improve international capability to deal with them. The International Health Regulations (IHR) are an instrument of international law that is legally binding on 196 countries. This legal framework creates rights and obligations for countries, including the requirement to report public health events. The Regulations also outline the criteria to determine whether a particular event constitutes a ‘public health emergency of international concern.’ At the same time, the IHR requires countries to designate a National IHR Focal Point for communications with WHO and to establish and maintain core capacities for surveillance and response [Citation50].
The WHO remains the main organization responsible for the surveillance of COVID-19 at the international level. It provides technical guidance for domestic surveillance and also encourages member states to report daily counts of cases and deaths, and weekly counts of cases and deaths at different levels of aggregation [Citation51].
3.5. Vaccine recommendations
Countries need mechanisms to establish immunization program priorities and identify which new vaccines and immunization technologies should be adopted [Citation52]. Decisions around new vaccine adoption are particularly important in lower income countries as they seek to maximize the value of limited resources. This requires that these decisions be based on deliberate, rational, and evidence-based criteria [Citation53].
Most countries have formally constituted national technical advisory bodies to guide immunization policies. These advisory bodies are often referred to as National Immunization Technical Advisory Groups (NITAGs). They are independent, multidisciplinary expert groups within the national immunization framework. NITAGs provide evidence-based evaluations and recommendations to governmental decision-makers about specific vaccines, vaccine-dosing, vaccine program development, and immunization policy and practice, more generally [Citation54]. In 2011, the WHO recommended that NITAGs be established in each member country [Citation55].
Establishing a well-balanced and institutionalized NITAG can have several advantages for a national immunization program. It can resist pressure from any group with narrow scopes or interests, such as specific disease patient groups or industries. It can create credibility for the process by which major policy decisions are made, thus, in turn, adding credibility to the national immunization program and to the government at large. Additionally, it can add a more comprehensive perspective into immunization programs that can be lacking from a series of disease or vaccine-specific task forces. An indirect benefit of such a group is that it can keep policymakers and health authorities updated about the latest advances in the area of vaccines and vaccine-preventable disease epidemiology and control [Citation52,Citation56].
The Advisory Committee on Immunization Practices (ACIP) is the NITAG in the US [Citation57]. This committee provides evidence-based guidance and recommendations on new vaccines, vaccine formulations, and vaccination schedules. The ACIP also reviews existing vaccines and updates its recommendations as needed. For example, after reviewing available data, ACIP issued an interim recommendation for use of the Pfizer-BioNTech COVID-19 vaccine in persons aged ≥16 years on 12 December 2020 [Citation58], and it issued an interim recommendation for use of the Moderna COVID-19 vaccine in persons aged ≥18 years on 19 December 2020 [Citation59].
The WHO and its global partners have provided leadership and support to countries in the rapid establishment or strengthening of functional, sustainable, and independent NITAGs [Citation60,Citation61]. The WHO also provides global and regional policy recommendations and gives regular updates on the latest developments in the vaccine pipeline. Additionally, it provides guidance about recommended immunization schedules, vaccine delivery technology, vaccine-preventable disease surveillance, and safety and quality data/information [Citation52].
In each of the six WHO regions, Africa, the Americas, the Eastern Mediterranean, Europe, South-East Asia, and the Western Pacific, Regional Technical Advisory Groups on Immunization (RITAG) have been established. They offer suggestions to WHO Regional Directors and countries within their respective areas, regarding the prioritization and planning of regional immunization programs [Citation62].
The Strategic Advisory Group of Experts on Immunization (SAGE) is the principal advisory group to the WHO for vaccines and immunization. It is charged with advising WHO on overall global policies and strategies, ranging from vaccines and technology, research, and development, to delivery of immunization and its linkages with other health interventions. SAGE is concerned not just with childhood vaccines and immunization, but all vaccine-preventable diseases. For the COVID-19 pandemic, SAGE has issued recommendations on Pfizer (8 January 2021), Moderna (25 January 2021), AstraZeneca (21 April 2021), and Janssen (17 March 2021) COVID-19 vaccines. Additionally, SAGE has provided a framework for vaccine access and a roadmap for population prioritization [Citation63].
3.6. Regulatory approval
In most developed countries, a specific vaccine formulation needs a license before distribution. Licensure of the vaccine can occur directly in the country of use if it the country has its own regulatory body. It can also occur in the manufacturing country; in this case, the country of use will review the decision of the foreign regulatory body and provide final approval. To obtain licensure, manufacturers submit detailed information regarding their manufacturing processes, along with results of preclinical and clinical trials to health authorities, who will examine the evidence for quality, safety, and effectiveness of the vaccine. The regulator might ask for additional data and even additional clinical trials before making the final decisions [Citation64]. In addition to approving new vaccines and their manufacturing standards, national regulatory authorities in most countries also engage in post-marketing surveillance.
The Food and Drug Administration (FDA) is the regulatory authority overseeing the safety, effectiveness, and quality of vaccines that are used in the US [Citation65] The Center for Biologics Evaluation and Research (CBER), a division of the FDA, is responsible for licensing new vaccines. It establishes standards for manufacturing processes, facilities, and pre- and post-licensing clinical studies. These standards have an important impact on the nature and direction of vaccine development, and they are important factors in the costs of the development. The majority of CBER members are researchers, public health officials, and medical practitioners [Citation25]. Also, the FDA collaborates with partners inside the US government such as the CDC on immunization programs. Finally, it collaborates with international organizations such as the WHO to help strengthen regulatory systems around the world [Citation66].
In the European Union (EU), vaccines are regulated by the European Medicines Agency (EMA). The EMA’s Committee on Medicinal Products for Human Use has oversight for human vaccines through its Vaccine Working Party. Within the EMA’s jurisdiction, vaccines can be licensed at a national level or, thanks to a centralized procedure, within all countries of the EU [Citation67]. The African Vaccine Regulatory Forum (AVAREF) is another regional regulatory network. It comprises a network of African national regulatory authorities and ethics committees. Its objective is to guarantee that the vaccines utilized on the continent adhere to the highest standards of quality, safety, and efficacy. The organization serves as a platform for the dissemination of information and sharing of best practices in vaccine regulation across the continent [Citation68].
The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) brings together the regulatory authorities and pharmaceutical industry to discuss scientific and technical aspects of drug registration. ICH’s mission is to achieve greater harmonization worldwide to ensure that safe, effective, and high-quality medicines are developed and registered in the most resource-efficient manner [Citation67]. Harmonization is achieved through the development of ICH Guidelines via a process of scientific consensus with regulatory and industry experts working side by side [Citation69].
The WHO Listed Authority (WLA) framework is an initiative by the WHO to evaluate and publicly designate regulatory authorities that operate at an advanced level of performance and comply with international standards and good regulatory practices. The WLA framework aims to enhance access and supply of safe, effective, and quality medical products by facilitating reliance on trusted agencies and promoting regulatory cooperation and convergence. The WLA framework is based on the Global Benchmarking Tool (GBT) that classifies regulatory systems according to maturity levels [Citation70].
WHO vaccines prequalification is a service provided to United Nations agencies, such as UNICEF and its partners, that procure vaccines for low-income countries. The goal of WHO vaccines prequalification is to provide the WHO assessment of the safety and effectiveness of specific vaccines. National regulatory agencies and national control laboratories play a vital role in WHO vaccine prequalification since they are responsible for regulatory oversight, testing, and release of WHO-prequalified vaccines [Citation71]. Additionally, the EMA publishes its scientific opinion on some of the vaccines intended for markets outside of the EU. This aims to facilitate prequalification of the medicine by WHO and registration in low- and middle-income countries [Citation72]. The WHO and its regional offices also provide guidelines and necessary knowledge for establishing regulatory agencies in developing countries [Citation73].
The WHO emergency use listing (EUL) procedure assesses the suitability of novel health products during public health emergencies. The objective is to make medicines, vaccines, and diagnostics available as rapidly as possible to address the emergency. It involves a rigorous assessment of late Phase 2 and Phase 3 clinical trial data as well as substantial additional data on safety, efficacy, quality, and a risk management plan. These data are reviewed by independent experts and WHO teams. As part of the EUL process, the company producing the vaccine must commit to continue to generate data needed to determine whether the vaccine should be fully licensed or prequalified. The WHO prequalification process will assess additional clinical data generated from vaccine trials and deployment on a rolling basis to ensure the vaccine meets the necessary standards. During the COVID-19 pandemic, WHO listed the Pfizer/BioNTech vaccine on 31 December 2020, the AstraZeneca/Oxford vaccine on 15 February 2021, and Janssen (Johnson & Johnson) vaccine on 12 March 2021 for emergency use [Citation63].
3.7. Procurement
There are two types of vaccine procurement in the ecosystem: direct procurement and pool procurement. Direct procurers are mostly high- and middle-income countries. They define and implement their own vaccination programs and provide their own funding for the procurement. Pooled procurers are usually in low- and middle-income countries. They pool resources to purchase vaccines through a process that is generally supported by subsidies from international donors [Citation33].
Residents of the US receive their routine immunizations through both private and public sectors. The federal government supports vaccination of vulnerable population through programs such as Section 317 of the Public Health Service Act and Vaccines for Children (VFC) program [Citation74]. Using the insights from these programs, CDC purchases vaccines directly from manufacturers at discounted prices, and distributes them to state health departments, local public health agencies, and other partners [Citation75]. Some US states have extended VFC and created ‘Universal Purchase’ (UP) structure. They buy all routinely recommended vaccines through the CDC purchasing contracts and provide them to all children, including those who are privately insured [Citation76]. During the COVID-19 pandemic, US Department of Health and Human Services (HHS) and Department of Defense (DOD) have purchased vaccines from both Pfizer and Moderna to help meet demand for these vaccines in the US [Citation77].
In 2011, Global Alliance for Vaccines and Immunization (Gavi) started providing financial support to poor countries for the procurement of some essential vaccines [Citation14]. This had an important effect on the growth of the worldwide demand for supported vaccines. Now Gavi supports routine vaccination programs, vaccination campaigns, and stockpiles for outbreak responses. Although Gavi provides the financial support for the procurement, it is the United Nations Children’s Emergency Fund (UNICEF) that purchases the vaccines for Gavi-qualified countries and some other middle-income countries [Citation76]. Pan American Health Organization (PAHO) member countries also take advantage of the pooled procurement mechanism. These countries finance and manage their own vaccination programs, but they use pool procurement through the revolving fund mechanism for vaccine purchases [Citation78].
COVAX (COVID-19 Vaccines Global Access) is a worldwide initiative for global equal access to COVID-19 vaccines; it is directed by Gavi, CEPI, and the WHO. COVAX helps 92 low- and middle-income countries get access to COVID-19 tests, therapies, and vaccines [Citation79]. UNICEF is their key partner since it has the experience of being the largest vaccine buyer in the world and can handle procurement logistics [Citation80]. The vaccines procured by COVAX include those produced by AstraZeneca, Moderna, Sinopharm, Sinovac, and Janssen [Citation79]. Implementation of COVAX’s distribution goals turned out to be challenging and not all goals were met [Citation81,Citation82]. Despite the efforts of COVAX to establish itself as a central entity for distributing donated vaccines globally, a significant proportion, approximately 30%, of the donated doses were distributed through bilateral agreements [Citation83].
3.8. Delivery
Vaccine distribution systems vary from country to country. The difference is more pronounced between developed and developing countries. In developed countries, vaccines are usually accessible via primary-care physicians, local public health units, pharmacies, or community health clinics. These providers either order the vaccines directly from a distributor or are supplied by a local public health agency [Citation84]. Inadequate healthcare infrastructure in some developing nations hinders their vaccination efforts. As a result, various local and international organizations have taken on the task of distributing and delivering vaccines directly to the population in need.
UNICEF and its partners supply vaccines for 45% of the world’s children under five. They support immunization programs in over 100 countries. UNICEF’s main activities include engaging communities to define vaccine demand, procuring, and distributing vaccines, and keeping vaccines safe through cold-chain logistics [Citation85]. One of UNICEF’s partners, Gavi, invests in high-performance cold-chain equipment to improve the delivery of vaccines in inaccessible parts of the world [Citation86]. Additionally, in July 2021, UNICEF signed an agreement with the African Vaccine Acquisition Trust (AVAT) to supply COVID-19 vaccines to all 55 member states of the African United (AU) organization. UNICEF will procure and deliver COVID vaccines on behalf of AVAT to the AU [Citation87,Citation88].
One of the main challenges for a successful vaccination campaign is to ensure that a large proportion of people are willing to receive the vaccines that are proven to be safe and effective. However, there is a significant percentage of people who have doubts or fears about vaccines in some countries, reaching up to 17% according to a 2019 survey [Citation89]. This phenomenon is known as vaccine hesitancy and it is defined by the WHO as the delay or refusal of vaccination despite the availability of vaccines [Citation90]. Vaccine hesitancy poses a serious threat to global health, as it reduces the levels of immunization coverage and increases the risk of outbreaks of vaccine-preventable diseases. In fact, vaccine hesitancy was declared 1 of the top 10 global health challenges by WHO in 2019 [Citation91]. Therefore, WHO recommends that countries implement strategies to assess and address vaccine hesitancy within their immunization programs [Citation92].
4. Mapping the responsibilities of the main players
In this section, we first list all the important international players in the vaccine ecosystem and identify their roles. One can see the list of the players in the first column of . Some of these organizations are the top funders of vaccine R&D according to G-FINDER project [Citation91]. We included all organizations that spent more than US$10 m on vaccine development in 2019. The rest of the list has been gathered by consultation with experts in the field.
Table 1. List of important players in vaccine ecosystem. The shaded rectangles in the fifth column indicate if the organization is active in the corresponding stage in the fourth column.
The second column of this table identifies the type of each organization. They can be assigned one of the five types: Multilateral organization, Philanthropic organization, Governmental institutions, NGO, and Partnership. The third column identifies the country or the region on which they are primarily focused. Next, the fourth column lists all the vaccine stages for which each organization provides major support.
Finally, the last column shows the roles of major institutions in the vaccine ecosystem. Depending on its involvement in the overall ecosystem, each institution was assigned one to three of the following general roles: funder, facilitator, and implementer. Funders provide the necessary money for filling the gaps at each stage. Facilitators come up with needed supporting policies and communicate them via guidelines and recommendations. They also connect different organizations who are trying to solve the same problems and coordinate their activities. Implementers bring the supporting policies into action.
5. Conclusion
Economics teaches us that vaccination is a global public good that requires substantial public investment. A global vaccine ecosystem has arisen to meet this challenge. This ecosystem, however, is complex, with many different interacting players both domestic and international in scope. It also appears to be underfunded given the extent of vaccine-preventable mortality still plaguing parts of the globe. In this paper, we reviewed the steps needed to mount a successful vaccination program, from the R&D stage to the vaccination. Then, we listed all the main international organizations active in different steps of the pathway and described their roles. It is important to recognize that while international entities play a crucial role in the global vaccine ecosystem, the future success of vaccination programs is contingent upon the capability of middle- and low-income countries to improve their infrastructure and resources. Thus, emphasis should be placed on strengthening these nations’ capacities in this sector.
6. Expert opinion
Creating a structure for better understanding the vaccine ecosystem and mapping the responsibilities of major players gives us several important insights. One of the most important lessons we can learn from this exercise is that the bottlenecks for vaccines reaching people’s arms can be different for high-income and low-income countries. The bottlenecks can also be different for different regions of the world.
Lack of coordination and free riding problems are fundamental issues on the supply side of the vaccine ecosystem. In the realm of social sciences, the free riding problem represents a form of market failure that arises when individuals are permitted to consume more than their equitable share of common resources or pay less than their fair share of costs, leading to an inefficient allocation of goods or services. Within the context of this paper, if some high-income countries bear the costs of fundamental research and vaccine development, everyone stands to benefit from increased safety. However, this creates an incentive for other high-income countries to engage in free riding, particularly in situations where a disease primarily affects low-income nations. We can see the result of this phenomenon in the share of R&D expenditure of different countries. As we mentioned before, the majority of the funding for supporting this step of vaccine development is provided by governmental institutions in the US. Smaller high-income countries generally have a tendency to free ride. The free riding problem can lead to underinvestment in this sector. Therefore, having a stronger international framework for supporting this stage of vaccine development can be beneficial.
Free riding is less of an issue in vaccine manufacturing. Most countries have a strong incentive to have an adequate vaccine manufacturing capacity to guarantee their health security. But this capacity is very low in some regions of the world. For example, almost all vaccines used in Africa are imported, despite facing frequent infectious disease threats. Lack of vaccine manufacturing capacity in Africa was highlighted during the COVID-19 pandemic. Therefore, international support is needed to expand manufacturing capacity in low-income regions of the world. This would help the countries in those regions to produce some of the necessary vaccines for their population; this in turn will improve health security in those regions.
High-income countries have well-established national institutions that represent consumers at the demand side of the vaccine ecosystem. As a result, these countries do not need financial support in that side of vaccine ecosystem. Although, global coordination between national institutions in high-income countries can strengthen some of the steps on the demand side, the WHO has already created the necessary framework for this coordination.
However, middle- and low-income countries need more international support for some of the steps on the demand side. For instance, despite the fact that some low-income countries in the region lack strong regulatory capacity, the majority of countries in the Americas region possess adequate regulatory and distribution systems [Citation93]. But, even those countries with well-developed regulatory and distribution systems might struggle with procurement of vaccines. Therefore, the focus of PAHO for supporting the ecosystem in the region is creating a pool procurement and helping with vaccine purchase for member countries. On the other hand, some of the poorer countries in Africa also lack adequate infrastructure for regulatory approval and distribution of vaccines. Therefore, several international organizations are active in supporting those stages in those parts of the world.
This study provides a systematic structure to identify the gaps in the vaccine ecosystem. The COVID-19 pandemic already created major changes in this landscape. A better understanding of the complexity of vaccine ecosystem can help to make sure all the necessary steps for a successful global vaccination program get the adequate support for future pandemics.
Article highlights
The vaccine ecosystem is complex, with many different public and private organizations each attempting to advance vaccines along the different stages of the vaccine development pathway. In this paper, we review these different stages, from basic R&D to ‘shots in arms,’ and identifying all the major organizations globally that are active at each stage.
The vaccine development pathway starts with basic research, proceeding through clinical development for manufacturing, regulatory approval, procurement, and delivery.
There are a large number of international players in the vaccine ecosystem involved at each stage, including multilateral organizations, philanthropic organizations, governmental institutions, non-governmental organizations (NGOs), and vaccine companies. These organizations interact in various ways, including through formal partnerships.
These international players can be grouped into funders, facilitators, or implementers. Funders provide the necessary money for filling the gaps in each stage. Facilitators come up with needed supporting policies and coordination strategies. Implementers bring the supporting policies into action.
Declaration of interest
J Moradpour is supported by a MITACs post-doctoral fellowship which is supported by the Government of Canada and Sanofi. S O. Besada-Lombana Castro and A Chit are employees of Sanofi and may hold shares and/or stock options in the company. P Grootendorst has consulted for both generic and branded drug companies on pharmaceutical intellectual property-related issues. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they are a vaccine scientist and developer for global health COVID vaccines. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Authors contributions
Javad Moradpour wrote the paper. Paul Grootendorst, Sandra O. Besada-Lombana Castro and Ayman Chit helped with designing the research question, and revised the paper for important intellectual content.
Acknowledgments
We warmly thank Emmanuel Audusseau and Alejandra Martinez for their advice and feedback. Moreover, two summer students Abhishek Chopra and Abraam Ghattas, assisted us with collecting information about international organizations. We have these persons’ permission to mention their names in the manuscript for publication.
Data availability statement
All data sources used in this paper are publicly available.
Additional information
Funding
References
- Kremer M. Creating markets for new vaccines. Part I: rationale. Inno Policy Econ. 2000;1:35–72. doi: 10.1086/ipe.1.25056141
- Arnould R, DeBrock L. The application of economic theory to the vaccine market. Supplying Vaccines: An Economic Analysis Of Critical Issues (IOS Press). 1996;101–131.
- Shanks GD. Could ross river virus be the next zika? J Travel Med. 2019;26(5):26. doi: 10.1093/jtm/taz003
- Poland GA. Vaccines against lyme disease: what happened and what lessons can we learn? Clin Infect Dis. 2011;52(Suppl 3):s253–8. doi: 10.1093/cid/ciq116
- Wen FT, Malani A, Cobey S. The beneficial effects of vaccination on the evolution of seasonal influenza. bioRxiv. 2020;162545. doi: 10.1101/162545
- Arinaminpathy N, Ratmann O, Koelle K, et al. Impact of cross-protective vaccines on epidemiological and evolutionary dynamics of influenza. Proc Natl Acad Sci U S A. 2012;109(8):3173–3177. doi: 10.1073/pnas.1113342109
- Cobey S, Larremore DB, Grad YH, et al. Concerns about SARS-CoV-2 evolution should not hold back efforts to expand vaccination. Nat Rev Immunol. 2021;21(5):330–335. doi: 10.1038/s41577-021-00544-9
- Basak P, Abir T, Al Mamun A, et al. A global study on the correlates of Gross Domestic Product (GDP) and COVID-19 vaccine distribution. Vaccines (Basel). 2022;10(2):266. doi: 10.3390/vaccines10020266
- Christopher Auld M, Toxvaerd F. The great covid-19 vaccine rollout: behavioural and policy responses. Natl Inst Econ Rev. 2021;257:14–35. doi: 10.1017/nie.2021.23
- Coronavirus (COVID-19) Vaccinations. Our World In Data [Internet]. [cited 2022 Feb16].Available from: https://ourworldindata.org/covid-vaccinations
- Asadu C. Nigeria to reject vaccine donations with short shelf lives. Associated Press. [cited 2021 Dec 14]. https://apnews.com/article/coronavirus-pandemic-health-africa-nigeria-d924cab165a324604c3b93a28cc4190d
- Hotez PJ, Pecoul B, Rijal S, et al. Eliminating the neglected tropical diseases: translational science and new technologies. PLoS Negl Trop Dis. 2016;10(3):e0003895. doi: 10.1371/journal.pntd.0003895
- Department of Control of Neglected Tropical Diseases Ending the neglect to attain the sustainable development goals: a road map for neglected tropical diseases 2021–2030. Geneva: World Health Organization; 2020. https://www.who.int/publications/i/item/9789240010352
- Shih W. Bill & Melinda Gates Foundation: shaping the vaccine manufacturing ecosystem. Gates Open Res. 2019;3:1619.
- Bernasconi V, Kristiansen PA, Whelan M, et al. Developing vaccines against epidemic-prone emerging infectious diseases. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2020;63(1):65–73. doi: 10.1007/s00103-019-03061-2
- Ball P The lightning-fast quest for COVID vaccines — and what it means for other diseases. Nature [Internet]. [2020 Dec]. Available from: https://www.nature.com/articles/d41586-020-03626-1
- Pfizer and BioNTech Announce Further Details on Collaboration to Accelerate Global COVID-19 Vaccine Development. Business Wire [Internet]. [2020 Apr 9]. Available from: https://www.businesswire.com/news/home/20200409005405/en/
- Policy Cures Research. G-FINDER: tracking funding for global health R&D. G-FINDER Data Portal [Internet]. [cited 2021 Oct 1]. Available from: https://gfinderdata.policycuresresearch.org/
- Adams B Moderna nabs a BARDA billion as its kick-starts late-stage pandemic vaccine test. Fierce Biotech [Internet]. 2020 Jul 27. Available from: https://www.fiercebiotech.com/biotech/moderna-nabs-a-barda-billion-as-its-kickstarts-late-stage-pandemic-vaccine-test
- BioNTech. BioNTech announces new collaboration to develop HIV and tuberculosis programs. 2019. https://investors.biontech.de/news-releases/news-release-details/biontech-announces-new-collaboration-develop-hiv-and
- Prioritizing diseases for research and development in emergency contexts. World Health Organization (WHO) [Internet]. [cited 2022 Feb17]. Available from: https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts
- Better health moves humanity forward. PATH [Internet]. [cited 2022 Apr]. Available from: https://www.path.org/about/
- Singh K, Mehta S. The clinical development process for a novel preventive vaccine: an overview. J Postgrad Med. 2016;62(1):4–11. doi: 10.4103/0022-3859.173187
- Grenham A, Villafana T. Vaccine development and trials in low and lower-middle income countries: key issues, advances and future opportunities. Hum Vaccin Immunother. 2017;13(9):2192–2199. doi: 10.1080/21645515.2017.1356495
- Douglas RG, Samant VB. The Vaccine Industry. Vaccines. 7th ed. Elsevier; 2018;41–50.e1. doi: 10.1016/B978-0-323-35761-6.00004-3
- U.S. Department of Health & Human Services. Influenza vaccine development programs. Medical Countermeasures [Internet]. [cited 2022 Feb 17]. Available from: https://www.medicalcountermeasures.gov/barda/influenza-and-emerging-infectious-diseases/influenza-vaccine-development-programs/
- Universal Influenza Vaccine Research. National Institute of Allergy and Infectious Diseases [Internet]. [cited2022 Feb 17]. Available from: https://www.niaid.nih.gov/diseases-conditions/universal-influenza-vaccine-research
- Moderna Announces Expansion of BARDA Agreement to Support Larger Phase 3 Program for Vaccine (mRNA-1273) Against COVID-19. Business Wire [Internet]. [cited 2020 Jul 26]. Available from: https://www.businesswire.com/news/home/20200726005025/en/
- How we work. Bill & Melinda Gates Foundation [Internet]. [cited 2022 Feb 17]. Available from: https://www.gatesfoundation.org/about/how-we-work
- Who we are. Wellcome [Internet]. [cited 2022 Feb 17]. Available from: https://wellcome.org/who-we-are
- Somauroo J Malaria vaccine 30 years and $1 billion in the making now deployed in third country. Forbes [Internet]. [cited 2019 Sep 24]. Available from: https://www.forbes.com/sites/jamessomauroo/2019/09/24/malaria-vaccine-30-years-and-1-billion-in-the-making-now-deployed-in-third-country/?sh=96d57ce2583f
- A global coalition for a global problem. CEPI [Internet]. [cited 2022 Feb 17]. Available from: https://cepi.net/about/whoweare/
- Watson M, de Goër E F. Are good intentions putting the vaccination ecosystem at risk? Hum Vaccin Immunother. 2016;12(9):2469–2474. doi: 10.1080/21645515.2016.1172162
- Government, Sanofi officials unveil $925M in vaccine funding in Toronto. CBC News [Internet]. [cited 2021 Mar31]. Available from: https://www.cbc.ca/news/canada/toronto/flu-vaccine-production-north-york-toronto-sanofi-1.5970870
- Kim JH, Hotez P, Batista C, et al. Operation warp speed: implications for global vaccine security. Lancet Glob Health. 2021;9(7):e1017–e1021. doi: 10.1016/S2214-109X(21)00140-6
- World Health Organization. COVID-19 vaccines with WHO emergency use listing. https://extranet.who.int/pqweb/vaccines/vaccinescovid-19-vaccine-eul-issued
- U.S. Department of Health & Human Services. International influenza vaccine manufacturing capacity building program. Medical Countermeasures [Internet]. [cited 2022 Feb]. Available from: https://www.medicalcountermeasures.gov/barda/influenza-and-emerging-infectious-diseases/international-influenza-vaccine-manufacturing-capacity-building-program/
- Surveillance in emergencies. World Health Organization (WHO) [Internet]. [cited 2022 Feb 17]. Available from: https://www.who.int/emergencies/surveillance
- Institute of Medicine (US) Forum on Emerging Infections. Davis JR, Lederberg J, editors. Public health systems and emerging infections: assessing the capabilities of the public and private sectors. Washington (DC): National Academy Press (US); 2000. doi: 10.17226/9869
- Budhiraja S, Akinapelli R. Pharmacovigilance in vaccines. Indian J Pharmacol. 2010;42(2):117. doi: 10.4103/0253-7613.64488
- Murray J, Cohen AL. Infectious Disease Surveillance. International Encyclopedia Public Health. 2nd ed. Oxford: Academic Press; 2017:222–229. doi: 10.1016/B978-0-12-803678-5.00517-8
- Nationally Notifiable Diseases. Centers For Disease Control And Prevention (CDC) [Internet]. [cited 2022 Feb 17]. Available from: https://www.cdc.gov/healthywater/statistics/surveillance/notifiable.html
- European Medicines Agency (EMA). Risk management plans. https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/pharmacovigilance/risk-management/risk-management-plans
- Centers for Disease Control and Prevention. Vaccine safety datalink (VSD). https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html
- CIOMS/WHO Working Group on Vaccine Pharmacovigilance. Definitions and applications of terms for vaccine pharmacovigilance. Geneva: The Council for International Organizations of Medical Sciences (CIOMS); 2012.
- Arthur RR, Leduc JW, Hughes JM Surveillance for Emerging Infectious Diseases and Bioterrorism Threats . Tropical Infectious Diseases Second Edition . 2006 195–200 doi:10.1016/B978-0-443-06668-9.50020-X ;1 (Philadelphia: Churchill Livingstone).
- Where We Work. In: Centers for Disease Control and Prevention (CDC) [Internet]. [cited 2022 Feb17]. Available from: https://www.cdc.gov/globalhivtb/where-we-work/index.html
- Carroll D, Daszak P, Wolfe ND, et al. The global virome project. Science. 2018;359(6378):872–874. doi: 10.1126/science.aap7463
- Morse SS. Global infectious disease surveillance and health intelligence. Health Aff. 2007;26(4):1069–1077. doi: 10.1377/hlthaff.26.4.1069
- International Health Regulations. In: World Health Organization (WHO) [Internet]. [cited 2022 Feb 17]. Available from: https://www.who.int/health-topics/international-health-regulations#tab=tab_1
- Public health surveillance for COVID-19: interim guidance. World Health Organization (WHO) [Internet]. [cited 2022 Feb 17]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-SurveillanceGuidance-2022.1
- Duclos P. National immunization technical advisory groups (NITAGs): guidance for their establishment and strengthening. Vaccine. 2010;28(Suppl 1):A18–25. doi: 10.1016/j.vaccine.2010.02.027
- Piso B, Wild C. Decision support in vaccination policies. Vaccine. 2009;27(43):5923–5928. doi: 10.1016/j.vaccine.2009.07.105
- Harmon SHE, Faour D, MacDonald N. National immunization technical advisory groups (NITAGs): a schema for evaluating and comparing foundation instruments and NITAG operations. Med Law Int. 2021;21(1):69–98. doi: 10.1177/09685332211002594
- Duclos P, Dumolard L, Abeysinghe N, et al. Progress in the establishment and strengthening of national immunization technical advisory groups: analysis from the 2013 WHO/UNICEF joint reporting form, data for 2012. Vaccine. 2013;31(46):5314–5320. doi: 10.1016/j.vaccine.2013.08.084
- Kamiya H, Okabe N. Leadership in immunization: the relevance to Japan of the USA experience of the Centers for Disease Control and Prevention (CDC) and the Advisory Committee on Immunization Practices (ACIP). Vaccine. 2009;27(11):1724–1728. doi: 10.1016/j.vaccine.2009.01.030
- Smith JC. The structure, role, and procedures of the U.S. Advisory Committee on Immunization Practices (ACIP). Vaccine. 2010;28(Suppl 1):A68–75. doi: 10.1016/j.vaccine.2010.02.037
- Oliver SE, Gargano JW, Marin M, et al. The advisory Committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine — United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1922–1924. doi: 10.15585/mmwr.mm6950e2
- Oliver SE, Gargano JW, Marin M, et al. The advisory Committee on immunization practices’ interim recommendation for use of Moderna COVID-19 vaccine — United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69(5152):1653–1656. doi: 10.15585/mmwr.mm695152e1
- Ba-Nguz A, Shah A, Bresee JS, et al. Supporting national immunization technical advisory groups (NITAGs) in resource-constrained settings. New strategies and lessons learned from the task force for global health’s Partnership for influenza vaccine introduction. Vaccine. 2019;37(28):3646–3653. doi: 10.1016/j.vaccine.2019.05.046
- Bell S, Blanchard L, Walls H, et al. Value and effectiveness of national immunization technical advisory groups in low-and middle-income countries: a qualitative study of global and national perspectives. Health Policy Plan. 2019;34(4):271–281. doi: 10.1093/heapol/czz027
- World Health Organization (WHO). Regional technical advisory groups on immunization (RITAG). World Health Organization (WHO) [Internet]. [cited 2023 Feb 8] Available from: https://www.who.int/teams/immunization-vaccines-and-biologicals/regional-technical-advisory-groups-on-immunization-(ritag)
- WHO Lists additional COVID-19 vaccine for emergency use and issues interim policy recommendations. World Health Organization (WHO) [Internet]. [cited 2021 May 7]. Available from: https://www.who.int/news/item/07-05-2021-who-lists-additional-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations
- Sahar N, Lee O, Hoffman SJ, et al. Overview of key legal, political, and social challenges facing global vaccination efforts. Imaginations: Journal Of Cross-Cultural Image Studies/Imaginations: Revue D’études Interculturelles de L’image. 2020;11(2):127–153. doi: 10.17742/IMAGE.IN.11.2.9
- Vaccine Development—101. In: US Food and Drug Administration [Internet]. [cited 2022 Feb17]. Available from: https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/vaccine-development-101
- Partnerships and Collaboration. US Food And Drug Administration [Internet]. [cited 2022 Feb 17]. Available from: https://www.fda.gov/international-programs/partnerships-and-collaboration
- Gomez PL, Robinson JM, Rogalewicz JA. Vaccine manufacturing. Vaccines 6th ed. London: W.B. Saunders; 2013:44–57. doi: 10.1016/B978-1-4557-0090-5.00019-7
- World Health Organization (WHO). AVAREF overview. World Health Organization (WHO) [Internet]. [cited 2023 Feb 8]. Available from: https://www.afro.who.int/health-topics/immunization/avaref/overview
- Mission: Harmonisation for Better Health. International Council For Harmonisation (ICH) [Internet]. [cited 2022 Feb 17] Available from: https://www.ich.org/page/mission
- World Health Organization (WHO). WHO-Listed authority (WLA). [cited 2023 Mar 23]. Available from: https://www.who.int/initiatives/who-listed-authority-reg-authorities
- Welcome to Vaccines Prequalification. World Health Organization (WHO) [Internet]. [cited 2022 Feb 17]. Available from: https://extranet.who.int/pqweb/vaccines
- Medicines for use outside the European Union. European Medicines Agency [Internet]. [cited 2022 Feb 17].Available from: https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/medicines-use-outside-european-union
- Khadem Broojerdi A, Baran Sillo H, Ostad Ali Dehaghi R, et al. The World health Organization global benchmarking tool an instrument to strengthen medical products regulation and promote universal health coverage. Front Med. 2020;7:457. doi: 10.3389/fmed.2020.00457
- Hinman AR, Orenstein WA, Rodewald L. Financing immunizations in the United States. Clin Infect Dis. 2004;38(10):1440–1446. doi: 10.1086/420748
- About the Vaccines for Children Program (VFC). Centers For Disease Control And Prevention (CDC) [Internet]. [cited 2022 Apr6]. Available from: https://www.cdc.gov/vaccines/programs/vfc/about/index.html
- Mulligan K, Snider JT, Arthur P, et al. Examination of universal purchase programs as a driver of vaccine uptake among US States, 1995–2014. Vaccine. 2018;36(28):4032–4038. doi: 10.1016/j.vaccine.2018.05.103
- Biden Administration purchases additional doses of COVID-19 vaccines from Pfizer and Moderna. U.S. Department Of Health And Human Services (HHS) [Internet]. [cited 2022 Apr 6]. Available from: https://www.hhs.gov/about/news/2021/02/11/biden-administration-purchases-additional-doses-covid-19-vaccines-from-pfizer-and-moderna.html
- PAHO Revolving Fund. Pan American Health Organization (PAHO) [Internet]. [cited 2022 Feb17]. Available from: https://www.paho.org/en/revolvingfund
- COVAX. Gavi [Internet]. [cited 2022 Feb 17].Available: https://www.gavi.org/covax-facility?gclid=Cj0KCQjwwNWKBhDAARIsAJ8HkhdpvIUKB83PXcyP5vzZkDDTrQcxhftCAJ1SBBBvjjJOhJvm3WAbzxEaAm60EALw_wcB
- COVAX: ensuring global equitable access to COVID-19 vaccines. UNICEF [Internet]. [cited 2022 Apr]. Available from: https://www.unicef.org/supply/covax-ensuring-global-equitable-access-covid-19-vaccines
- de Bolle M COVAX is failing to halt the COVID-19 pandemic: here’s why, and how to fix it. The Conversation [Internet]. [cited 2021 Jun 8]. Available from: https://theconversation.com/covax-is-failing-to-halt-the-covid-19-pandemic-heres-why-and-how-to-fix-it-162146
- Ducharme J COVAX was a great idea, but is now 500 million doses short of its vaccine distribution goals. What Exactly went wrong? Time[Internet]. 2021. https://time.com/6096172/covax-vaccines-what-went-wrong/
- de Bengy Puyvallée A, Storeng KT. COVAX, vaccine donations and the politics of global vaccine inequity. Global Health. 2022;18(1):26. doi: 10.1186/s12992-022-00801-z
- Smith J, Lipsitch M, Almond JW. Vaccine production, distribution, access, and uptake. Lancet. 2011;378(9789):428–438. doi: 10.1016/S0140-6736(11)60478-9
- Immunization. UNICEF [Internet]. [cited 2022 Feb17]. Available from: https://www.unicef.org/immunization
- Anyiam-Osigwe T. The long view: keeping vaccines cool with cold chain. Gavi [Internet]. [2021 May 31]. Available from: https://www.gavi.org/vaccineswork/long-view-keeping-vaccines-cool-cold-chain?gclid=CjwKCAjwy7CKBhBMEiwA0Eb7agfoM7WSBp-sKmrDHHjfGLtG8g1Ri2aI_jIbgcjaaksF1xrO3HeXCBoCFsYQAvD_BwE
- Africa Centres for Disease Control and Prevention (Africa CDC). African vaccine acquisition trust delivers 12 000 doses of COVID-19 vaccine to the african union. 2021. https://africacdc.org/news-item/african-vaccine-acquisition-trust-delivers-12-000-doses-of-covid-19-vaccine-to-the-african-union/
- The african union’s african vaccine acquisition trust (AVAT) initiative. UNICEF [Internet]. [cited 2022 Feb 17]. Available from: https://www.unicef.org/supply/african-unions-african-vaccine-acquisition-trust-avat-initiative
- de Figueiredo A, Simas C, Karafillakis E, et al. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet. 2020;396(10255):898–908. doi: 10.1016/S0140-6736(20)31558-0
- MacDonald NE; SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161–4164. doi: 10.1016/j.vaccine.2015.04.036
- World Health Organization (WHO). Ten Threats to global health in 2019. 2019. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
- Oduwole EO, Pienaar ED, Mahomed H, et al. Current tools available for investigating vaccine hesitancy: a scoping review protocol. BMJ Open. 2019;9(12):e033245. doi: 10.1136/bmjopen-2019-033245
- Forum on Drug Discovery, Development, andTranslation, Board on Health Sciences Policy, Institute of Medicine (IOM). International regulatory Harmonization amid globalization of Drug development: workshop summary. Weisfeld V, Lustig TA, editors. (WA) (DC): National Academies Press (US); 2013.