ABSTRACT
Objective
This study aims to characterize the adverse events (AEs) following the administration of the mRNA-1273 COVID-19 vaccine from the Vaccine Adverse Event Reporting System (VAERS) data.
Methods
In this case/non-case analysis, reports between 1 January 2021, and 27 October 2022, were extracted from VAERS. AEs were defined as preferred terms (PTs) by Medical Dictionary for Regulatory Activities (MedDRA) terminology. Disproportionality analyses were conducted to calculate the reporting odds and proportional reporting ratios. The Bayesian approach was used to calculate information component (IC) values and Empirical Bayesian Geometric Mean scores for all the AEs detected.
Results
186 MedDRA PTs compromising 702,495 AEs associated with the mRNA-1273 vaccine were identified. Three statistically significant signals were identified for general and systemic AEs, administration site conditions, and product issues. Cardiac disorders were rarely reported, the most common being; 489 reports for ‘myocarditis’ (19.44%), 475 for ‘acute myocardial infarction’ (18.88%), 457 for ‘myocardial infarction’ (18.16%), 290 for ‘bradycardia’ (11.53%) and 281 for ‘pericarditis’ (11.17%).
Conclusions
The most frequently identified AEs following mRNA-1273 vaccination agree with those listed within the Summary of Product Characteristics. In addition, disproportionality analysis did not find any statistically significant signals for myocarditis or pericarditis.
1. Introduction
The World Health Organisation (WHO) declared the coronavirus disease 2019 (COVID-19) outbreak a global pandemic on 11 March 2020. This disease has since emerged as a significant threat to public health worldwide [Citation1,Citation2]. By December 2022, the European Union (EU) alone reported over two million COVID-19-related deaths, underscoring the profound impact of the virus within just the EU [Citation1].
The infection is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The virus’s spike (S) protein binds to the angiotensin-converting enzyme 2 (ACE2) receptor in human cells, specifically type II pneumocytes in the lungs, facilitating viral entry [Citation2]. Transmission primarily occurs through respiratory droplets directly from close contact with an infected individual or indirectly by touching surfaces contaminated with these droplets [Citation2,Citation3].
The high transmissibility of SARS-CoV-2 poses a significant threat, especially to vulnerable populations. Individuals with underlying health conditions, such as hypertension, diabetes, preexisting respiratory infections, cardiovascular disease, and cancer, are at an elevated risk of experiencing severe COVID-19 complications [Citation2]. Given these challenges, there’s an urgent need for preventive strategies, like vaccines, to mitigate the risks associated with COVID-19, including complications, disease spread, and infections from emerging variants [Citation4].
1.1. Moderna COVID-19 Vaccine
Following rigorous assessment, the mRNA-1273 COVID-19 vaccine was approved for use in adults over 18 years by the Medicines and Healthcare Products Regulatory Agency (MHRA) in January 2021 [Citation5]. The mRNA-1273 vaccine consists of messenger ribonucleic acid (mRNA) strands that encode for the viral spike (S) glycoprotein of SARS-CoV-2. This protein plays a crucial role in the initial transmission and ongoing infection of COVID-19 [Citation6]. The strands of mRNA are encapsulated within lipid nanoparticles (LNPs), which enable uptake into the cytosol of host cells, where the translation of the mRNA sequence into the S protein occurs. As a result, the body can produce an immune response and generate memory cells [Citation6]. Phase three randomized controlled trials (RCTs) have established a 94.1% effectiveness of the mRNA-1273 vaccine at preventing COVID-19 illness, including severe disease. As of November 2022, around 161 million doses of the mRNA-1273 vaccine have been administered within the EU/European Economic Area (EEA) [Citation7,Citation8]. However, safety data regarding possible adverse events (AEs) following the mRNA-1273 vaccine is limited across diverse populations.
1.2. Safety
As of November 2022, 68.2% of the global population has received at least one dose of a COVID-19 vaccine, leaving 31.8% still vulnerable to COVID-19 infection [Citation9]. Vaccine hesitancy has been growing at a higher rate since the beginning of the pandemic and is a barrier to obtaining herd immunity. A notable factor contributing to vaccine hesitancy is the concern over potential adverse events (AEs) associated with vaccination [Citation10,Citation11]. An Oxford Coronavirus Explanations, Attitudes, and Narratives Survey (OCEANS) found the public wanted reassurance that ‘safety had not been sacrificed for speed’ [Citation12]. This signifies a gap in public knowledge of the safety surrounding COVID-19 vaccines, such as the mRNA-1273 vaccine. A strategy to target this uncertainty among populations is to increase the availability of accurate guidance on the potential AEs and benefits of the vaccine to allow Health Care Professionals (HCPs) and the public to make informed decisions.
Randomized controlled trials (RCTs) are the main source of safety data available for the mRNA-1273 vaccine. A phase three RCT has concluded that aside from transient local and systemic reactions, no serious adverse events were identified for the mRNA-1273 vaccine [Citation7]. The most common local AE was pain at the injection site following both vaccine doses (93% after dose 1 and 95% after dose 2). The prevalent systemic reactions were headache, fatigue, myalgia, chills, and fever, generally occurring within a day following vaccine administration and resolving within two days [Citation7,Citation13,Citation14]. However, an increase in the severity of systemic events was identified following the second dose of mRNA-1273, possibly due to the substantially increased anti-SARS-CoV-2-spike binding antibody (bAb) levels after the second vaccination leading to a more robust immune response [Citation7,Citation15]. The occurrence of both injection-site and systemic AEs was shown to be more frequent among younger participants (18 to <65 years of age) than among older participants (≥65 years of age) [Citation7]. This can be attributed to the general decline in immune function observed within older populations, resulting in different reactogenicity profiles to the vaccine [Citation16]. Although RCTs are considered the gold standard for evaluating the safety of new treatments, they have several limitations. Including healthy adults alone and lacking data on minority ethnic groups within RCTs reduces the generalizability of results. Additionally, due to the short follow-up periods (ranging from 28 to 82 days) assessed within RCTs, evaluation of the long-term safety profile of the mRNA-1273 vaccine and detection of rare AEs is not possible [Citation13,Citation14]. Thus, the need for continued safety monitoring.
In contrast, observational studies have found an increased risk of rare AEs such as myocarditis and pericarditis [Citation17–19]. A study by Massari et al. found an increase in the relative incidence (RI) of myocarditis/pericarditis following the first and second doses of mRNA-1273 over a seven-day risk period (RI = 6.55 (95% CI 2.73 to 15.72) and 7.59 (95% CI 3.26 to 17.65) after the first and second doses of mRNA-1273, respectively) [Citation18]. Additionally, the risk of reporting myocarditis/pericarditis was ten times higher in boys compared to girls for the first (ROR 10.1; 95% CI 4.26, 29.6) and second dose (ROR 10.2; 95% CI 4.88, 25.0) of mRNA-1273 [Citation17]. Although rare, these results present a true concern regarding mRNA-1273-related myocarditis/pericarditis within young males. The demographic variability of the sample populations included in these studies further improves the generalizability of these results. However, the large 95% confidence intervals (CI) observed within the results show the need for larger sample sizes to evaluate further the relationship between the mRNA-1273 vaccine and myocarditis/pericarditis. This study aims to characterize the common adverse events experienced within a diverse population following the administration of the mRNA-1273 vaccine from the Vaccine Adverse Event Reporting System (VAERS) data. This research enables the continued monitoring of the AEs associated with the vaccine to detect changes within reporting patterns and allow the identification of rare or unexpected AEs.
2. Materials and method
2.1. Data source
Data regarding AEs associated with the mRNA-1273 vaccine was obtained from the VAERS. Established in 1990, VAERS is a national early warning system designed to detect safety concerns related to United States (US) licensed vaccines. VAERS is co-managed by the Centres for Disease Control and Prevention (CDC) and the US Food and Drug Administration (FDA) [Citation20]. The primary aim of VAERS is to assess the safety of newly licensed vaccines to; detect new or rare adverse events (AEs), monitor changes in known AEs and detect potential patient risk factors for specific AEs [Citation20]. VAERS data is accessible and can be downloaded as raw data in comma-separated value (CSV) files. This allows data importation to different databases for analysis [Citation21]. Each VAERS report is given a unique VAERS ID to allow the anonymity of the recipient. From each report, information regarding the; age, sex, vaccine manufacturer, vaccination data, detailed account of the symptoms, mortality, hospital visits and medical history of the vaccine recipient is available. The Ethical Implications of Research Activity Form to conduct this study was approved by the University of Bath.
2.2. Adverse event definition
Adverse events (AEs) identified from each report were defined as preferred terms (PTs) following the Medical Dictionary for Regulatory Activities (MedDRA) terminology, which is recognized internationally. A preferred term is a ‘single medical concept’ for a symptom, sign, disease, diagnosis, therapeutic indication, investigation, surgical or medical procedure, and medical, social, or family history characteristic. PTs allow for unambiguous and specific definitions for reports made. MedDRA groups each PT under a structural hierarchy which classifies terms into a High-Level Term (HLT), High-Level Group Term (HLGT) and System Organ Class (SOC) [Citation22].
2.3. Study design and participants
In this case/non-case analysis, case reports following any vaccine between 1 January 2021, and 27 October 2022, were extracted from VAERS as CSV files. The analysis included participants of all ages to increase the diversity of the study population and allow for greater generalizability within the results obtained. Cases were reported for the mRNA-1273 vaccine containing a minimum of one AE defined as a PT under a SOC. Non-cases were defined as reports for AEs of mRNA-1273 under other SOCs. The statistical analysis software ‘R’ (version 3.6.1) was used to analyze all the reports obtained to identify the total number of cases and non-cases among mRNA-1273 vaccine recipients and other vaccine recipients [Citation23]. In addition, the ratio of cases/non-cases for AEs associated with the mRNA-1273 vaccine was compared to the ratio of cases/non-cases for all other vaccines within the same study period to allow for disproportionality analysis.
2.3.1. SOC analysis
Reports for the COVID-19 vaccines, BNT162b2 (Pfizer) and Ad26.COV2.S (Janssen), were also obtained for analysis. These reports were classified into SOCs to understand the overall distribution of AEs across the different vaccines.
2.4. Statistical analysis
Disproportionality analyses were conducted to calculate the reporting odds ratio (ROR) and the proportional reporting ratio (PRR) [Citation24]. The Bayesian approach was used to calculate information component (IC) values and Empirical Bayesian Geometric Mean (EBGM) scores for all the AEs detected. The following criteria were applied to determine a disproportionality signal: > 25 reports, lower limit 95% confidence interval (CI) value ≥ 2 for ROR and PRR, > 0 for the IC value and > 1 for the EBGM score. Disproportionality analysis and the detection of statistically significant signals were conducted using R, version 3.6.1 [Citation23].
2.4.1. Age stratification
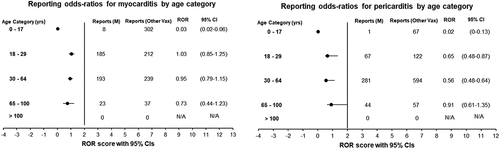
Informed by existing literature, age-stratification was conducted for the PTs ‘myocarditis’ and ‘pericarditis’. Reports for ‘myocarditis’ and ‘pericarditis’ were separated into age categories of; 0–17 years, 18–29 years, 30–64 years, 65–100 years and greater than 100 years of age. The age categories for analyzing reports of myocarditis and pericarditis were carefully chosen based on existing literature. The 0–17 years category represents the pediatric and adolescent population, for whom the risk of myocarditis following vaccination has been a specific concern in the literature. The 18–29 years group encompasses young adults, who have been shown to have a different risk profile than older adults. The 30–64 years and 65–100 years categories were chosen to analyze middle-aged and older adults, which generally have different baseline risks for cardiovascular events. The category of individuals aged greater than 100 years, although comprising a smaller number of individuals, was retained to ensure completeness of the analysis. Disproportionality analyses were conducted for each age category to detect statistically significant signals.
3. Results
869,556 individual case reports associated with COVID-19 vaccines were retrieved between 1 January 2021, and 27 October 2022, from the United States (US). The mean age of all patients included in the reports was 50 years, and 65% were females. Of these, 398,556 (45.8%) reports were from mRNA-1273 recipients with a mean age of 55 years and 67% females. However, the brand of the COVID-19 vaccine was unknown in 2,072 reports. Within the 398,556 case reports for the mRNA-1273 vaccine, the median days to symptom onset were one day (IQR (interquartile range) 0–8). A summary of the study population characteristics can be viewed in . 186 MedDRA PTs compromising a total of 702,495 AEs associated with the mRNA-1273 vaccine were identified from reports. These were divided into appropriate MedDRA system organ classes (SOCs) to allow efficient grouping and analysis of the AEs identified [Citation25]. The analysis found that most AEs identified within case reports are ‘general disorders and administration site conditions’ (n = 401,162) followed by ‘skin and subcutaneous tissue disorders’ (n = 65,010), ‘product issues’ (n = 51,802), ‘gastrointestinal disorders’ (n = 37,460) and ‘respiratory, thoracic, and mediastinal disorders’ (n = 27,251). represents the overall distribution of AEs within SOCs, and summarizes the overall distribution of AEs within SOCs for three COVID-19 vaccines, including mRNA-1273 (Moderna), BNT162b2 (Pfizer) and Ad26.COV2.S (Janssen).
Figure 1. Bar chart depicting the total frequency of adverse events reported to VAERS following mRNA-1273 vaccination classed as medical Dictionary of Regulatory Activities system (MedDRA) organ classes.
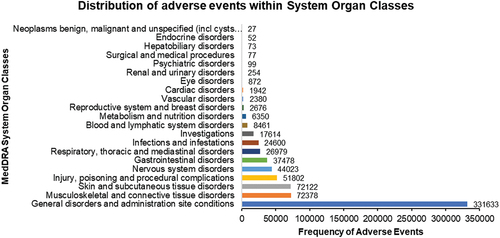
Figure 2. Bubble chart of the overall distribution of AEs within SOCs for three covid-19 vaccines, including mRNA-1273, BNT162b2 and Ad26.COV2.S.
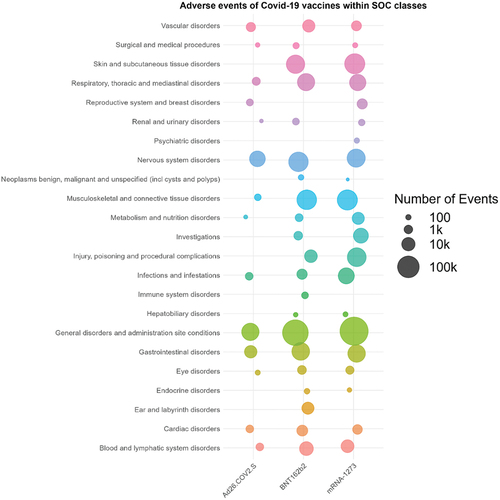
Table 1. Characteristics of VAERS reports received following COVID-19 vaccination between January 1, 2021, and October 27, 2022.
We present data from the clinical trials of the mRNA-1273 vaccine (). Notably, adverse events were reported in both the placebo and vaccine groups. For example, local pain was reported by 17.54% of participants in the placebo group (2658/15155) compared to 83.66% in the vaccine group (12690/15168). Fatigue was another common symptom, with 27.27% of the placebo group (4133/15155) and 37.15% of the vaccine group (5635/15168) reporting this adverse event. Other notable adverse events and their respective rates in the placebo and vaccine groups are detailed in .
Table 2. Nocebo effects of adverse events associated with mRNA-1273 vaccine.
3.1. Commonly occurring AEs according to MedDRA system organ classes
The category of ‘General disorders and administration site conditions’ represented the majority of AEs at 47.25%, with 331,633 AE reports linked to the mRNA-1273 vaccine in the VAERS database. Notably, the most reported AEs in this category were:
– ‘Fatigue’ with 48,750 reports (14.7%)
– ‘Chills’ with 46,217 reports (13.94%)
– ‘Pyrexia’ with 30,060 reports (9.06%)
– ‘Pain’ with 28,890 reports (8.71%)
– ‘Injection site erythema’ with 22,557 reports (6.8%)
Within this organ class, 32.1% of AEs pertained to administration site conditions (n = 106,522), while 58.2% were systemic reactions (n = 192,973). Several statistically significant signals were identified, indicating a heightened likelihood of reporting these AEs for the mRNA-1273 vaccine compared to other vaccines. For instance, the signals for ‘vaccination site erythema’, ‘vaccination site lymphadenopathy’, and ‘injection site warmth’ were particularly notable. Other significant signals were also observed for ‘chills’, ‘vaccination site pain’, and ‘pyrexia’. The ROR’s lower 95% CI limit value exceeding 1 underscores the increased reporting of these AEs for the mRNA-1273 vaccine ().
Figure 3. Forest plots of the ROR and EBGM scores with 95% CIs for the most common mRNA-1273-related adverse events. ROR, reporting odds ratio; EBGM, Empirical Bayesian geometric mean; AE, adverse event; M, mRNA-1273; vax, vaccines; CI, confidence interval.
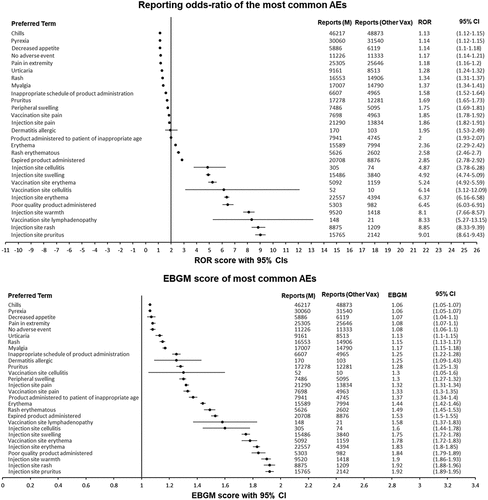
‘Musculoskeletal and connective tissue disorders’ accounted for 72,378 AEs (10.31%). The predominant AEs in this category were ‘pain in extremity’, ‘arthralgia’, ‘myalgia’, and ‘back pain’. Intriguingly, these systemic reactions mirror the symptoms of an active COVID-19 infection.
‘Skin and subcutaneous tissue disorders’ comprised 72,122 AEs (10.27%). The most frequent AEs in this category were ‘pruritus’, ‘rash’, ‘erythema’, ‘urticaria’, and ‘hyperhidrosis’. A significant observation is that nearly all AEs in this System Organ Class (SOC) (99.8%) could be potential hypersensitivity reactions. Several statistically significant signals were identified, such as for ‘rash erythematous’ and ‘erythema’.
‘Injury, poisoning, and procedural complications’ were associated with 51,802 AEs (7.38%). Common issues in this category included reports of ‘expired product being administered’, ‘product storage error’, and ‘products administered to the patient of inappropriate age’. Interestingly, product-related issues haven’t been frequently reported as AEs for the mRNA-1273 vaccine. However, the observed disproportionality signals suggest an increased reporting of these AEs, warranting further investigation.
‘Gastrointestinal disorders’ were linked to 37,478 AEs (5.34%). The most reported AEs in this SOC were ‘nausea’, ‘diarrhea’, and ‘vomiting’. Additionally, more severe AEs like ‘ulcerative colitis’ and ‘acute pancreatitis’ were identified, though their severity remains unspecified. No statistically significant signals were detected for any AEs in this organ class.
Lastly, ‘infections and infestations’ were associated with 24,600 AEs (3.5%). Most of these AEs were reports of ‘COVID-19’ and ‘asymptomatic COVID-19’. Despite the concerns related to the mRNA-1273 vaccine, no statistically significant signals were identified for these Preferred Terms (PTs), suggesting that the likelihood of these events post-administration of mRNA-1273 aligns with other vaccines. However, significant signals were observed for ‘vaccination site cellulitis’ and ‘injection site cellulitis.
3.2. Rare AEs according to MedDRA system organ classes
A total of 2,676 Adverse Events (AEs), representing 0.38%, were linked to the ‘Reproductive system and breast disorders’ category. Within this category, the following AEs were reported:
– ‘Heavy menstrual bleeding’ with 1,473 reports (55.04%)
– ‘Menstrual disorder’ with 752 reports (28.1%)
– ‘Intermenstrual bleeding’ with 413 reports (15.43%)
– ‘Premenstrual bleeding’ with 38 reports (1.42%)
No statistically significant signals were detected within this SOC.
2,380 AEs (0.34%) were recorded in the’ Vascular disorders’ category. The most frequently reported AEs in this class were:
– ‘Thrombosis’ with 922 reports (38.74%)
– ‘Deep vein thrombosis’ with 869 reports (36.51%)
– ‘Cyanosis’ with 196 reports (8.24%)
– ‘Haematoma’ with 102 reports (4.29%)
For all AEs in this organ class, the 95% CI lower limit for the ROR was below 1. This indicates that the likelihood of these events post-administration of the mRNA-1273 vaccine aligns with the probability observed for other vaccines.
Regarding ‘Cardiac disorders’, 1,942 AEs (0.28%) were identified. The most common AEs in this category included:
- ‘Myocarditis’ with 489 reports (25.18%)
– ‘Acute myocardial infarction’ with 475 reports (24.46%)
– ‘Bradycardia’ with 290 reports (14.93%)
– ‘Pericarditis’ with 281 reports (14.47%)
Again, for all reported AEs in this organ class, the lower limit of the 95% CI for ROR was below 1. Age-specific analyses for ‘myocarditis’ and ‘pericarditis’ revealed that the majority of reports came from individuals aged 18–29 years (with 185 reports for ‘myocarditis’ and 67 for ‘pericarditis’) and those aged 30–64 years (with 193 reports for ‘myocarditis’ and 281 for ‘pericarditis’). However, the disproportionality analysis yielded no statistically significant signals ().
4. Discussion
Between 1 January 2021, and 27 October 2022, 869,556 individual case reports following any COVID-19 vaccine were retrieved from VAERS. Of these, 398,556 (45.8%) reports were from mRNA-1273 vaccine recipients. In this study, reports received following any dose of the mRNA-1273 vaccine were analyzed to identify the common AEs experienced by recipients. In addition, 186 MedDRA PTs, accounting for 702,495 AEs, were obtained. Statistical analysis identified three significant signals for thirteen AEs and two for fifteen AEs. In addition, these adverse events included several general and systemic AEs, administration site conditions and product issues. A summary of all the significant signals can be found in supplementary table S1.
4.1. General and systemic disorders
Systemic adverse events accounted for many AEs identified, the most common being fatigue, chills, pyrexia, pain, and dizziness. Together, these accounted for nearly 20% of all recorded AEs (18.99%) and were the most frequently reported. Furthermore, two statistically significant signals were found for; chills, pyrexia, pain in extremities, peripheral swelling, myalgia and decreased appetite. These findings are consistent with results obtained from randomized control trials (RCTs) and observational studies conducted within diverse populations, which have also concluded that the most common AEs experienced following mRNA-1273 vaccination were transient systemic reactions such as fatigue, headache, myalgia or muscle pain, chills, fever, and nausea [Citation7,Citation13,Citation14,Citation27–29]. Randomized controlled trials are considered the gold standard for evaluating the safety of new treatments since they eliminate the risk of bias within results. This enables a fair comparison between treatment groups, thereby improving the reliability of inferences made regarding causal relationships [Citation30]. The common systemic AEs identified are comparable to symptoms elicited by an active COVID-19 infection and occur as a result of the body’s immune response stimulated by the foreign Spike (S) glycoprotein of SARS-CoV-2 which is synthesized within the body from the mRNA delivered by the vaccine [Citation6,Citation31]. These findings were expected and agreed with the Summary of Product Characteristics (SmPC) for ‘very common’ AEs of the mRNA-1273 vaccine. Therefore, these findings can be used to educate the public regarding the general AEs expected following vaccination [Citation32].
However, it is important to acknowledge that the safety data does not differentiate between symptoms that may be due to anxiety, e.g. headache, fatigue, pain at the injection site and those that are unrelated; hence, it is crucial to consider the psychological factors that may contribute to these reports. As highlighted by Hause et al., symptoms such as headache, fatigue, and pain at the injection site, commonly reported as AEs, may sometimes be attributable to anxiety rather than the vaccine itself [Citation33]. Geers et al. further elucidate the role of psychosocial factors, suggesting that the anticipation of negative effects after vaccination – a phenomenon akin to the ‘nocebo effect’ – can lead to the experience of such effects. For example, common general AEs reported, such as fatigue, chills, pain, and dizziness – except for pyrexia – are symptoms often associated with anxiety [Citation34]. This is a significant consideration, as it suggests that some reported AEs may not be directly driven by the vaccine but rather by the individual’s psychological response. This distinction is important for understanding the patient experience and interpreting safety data, as it underscores the potential for psychological factors to influence the reporting of AEs and, consequently, the perceived safety profile of vaccines.
To effectively educate the public regarding the AEs associated with vaccination, it is essential to communicate the nature and context of these side effects. Firstly, the majority of side effects are short-term, typically resolving within a matter of days without the need for medical intervention. This transient nature of side effects is a critical point that can help to alleviate public concern. Secondly, these side effects are overwhelmingly non-severe. Common reactions, such as soreness at the injection site, fatigue, and mild fever, are generally well-tolerated and far less severe than the potential complications associated with the diseases these vaccines aim to prevent. Thirdly, it is vital to recognize and communicate that some reported side effects may not be directly attributable to the vaccine itself. Instead, they may be influenced by psychological factors, such as an individual’s vaccine anxiety level or attitudes toward the vaccine. For example, heightened anxiety about receiving the vaccine may manifest as physical symptoms, a phenomenon observed in various vaccination contexts [Citation33,Citation34]. By delineating these nuances – emphasizing the short-term, non-severe nature of common side effects and acknowledging the role of psychological factors – healthcare professionals can provide a more accurate and reassuring picture of what individuals can expect after vaccination, thereby fostering informed and confident vaccination decisions.
4.2. Administration site conditions
Administration site reactions were highly prevalent among reports, with the most common being; injection site erythema, injection site pain, pruritus, and vaccination site pain. Consistent with these results, a randomized control trial conducted with 30,420 participants found that injection site events accounted for 84.2% of AEs after the first dose and 88.6% of AEs after the second dose mRNA-1273 [Citation7]. Additionally, strong disproportionality signals were generated for; vaccination site lymphadenopathy, injection site warmth, injection site swelling, injection site rash, injection site pruritus and injection site erythema. Further, two signals were also found for vaccination site erythema: injection site pain and vaccination site pain. The terms ‘vaccination site’ and ‘injection site’ were acknowledged to be relatively under the same context and, therefore grouped within the same PT; however, this is unlikely to impact the signals found. A study by Creech CB et al. also found that the most common local AE was pain at the injection site, and other local reactions included; erythema, swelling and lymphadenopathy [Citation13]. Injection site reactions are common with many immunizations, and they are generally caused by the body’s immune response to the needle or medicine, which typically manifests as the above symptoms [Citation35]. These findings suggest that following vaccination with mRNA-1273, it is common to expect reactions such as; pain, swelling and redness at the injection site. This has also been reflected within the product literature under ‘very common’ side effects [Citation32].
4.3. Skin disorders
The Moderna mRNA-1273 vaccine, one of the front-runners in the fight against COVID-19, has been associated with various cutaneous manifestations. Comprehensive research, including a systematic review and meta-analysis, has shed light on these reactions [Citation36]. The spectrum ranges from common injection site reactions to rarer manifestations like delayed inflammatory reactions to tissue fillers and flares of preexisting dermatoses. Notably, severe reactions like anaphylaxis have been documented but remain exceedingly rare at 0.05%. While cutaneous adverse reactions are prevalent, especially with mRNA vaccines, the majority are mild. Exceptions like anaphylaxis are rare but crucial contraindications for subsequent doses. Additionally, the vaccine has been associated with exacerbations of existing skin conditions and specific reactions to tissue fillers.
Post-administration of the mRNA-1273 vaccine, inflammatory skin conditions such as erythema, rash erythematous, and induration have been consistently reported. Furthermore, while not directly attributed to the mRNA-1273 vaccine, severe dermatological conditions like pemphigus vulgaris, observed post-BNT162b2 vaccination, highlight the broader dermatological concerns related to COVID-19 vaccines [Citation37].
Research on the skin reactions after the mRNA-1273 booster dose aligns with earlier findings, reinforcing the consistency of observed skin reactions [Citation38]. A notable concern is the potential hypersensitivity reactions linked to the mRNA-1273 vaccine. Studies indicate that the emergence of anti-polyethene glycol (PEG) IgG and IgM antibodies post-vaccination might be the culprits, suggesting hypersensitivity to the PEG components in the vaccine [Citation39]. The actual incidence of clinical PEG hypersensitivity in the general population is not explicitly mentioned in the published literature. Based on the study conducted by Stone et al. [Citation40], the prevalence of PEG hypersensitivity in the general population before the advent of COVID-19 vaccination was estimated to be extremely low, often cited as less than 0.1%. Another study by Richter et al. demonstrated antibodies to PEG in 3.3% of an untreated allergic population and 0.2% of a healthy study population [Citation41]. Notably, these percentages refer to antibodies against PEG and not necessarily the clinical manifestation of hypersensitivity reactions to PEG. Given this extremely low baseline prevalence of PEG hypersensitivity, it is crucial to interpret the emergence of anti-PEG antibodies post-vaccination within this context.
Masuda T et al. found that erythema, induration and swelling were more commonly reported among older participants than in younger participants [Citation14]. Therefore, the results may arise from the prevalence of an older study population within the VAERS reports analyzed (mean age = 55 years).
4.4. Procedural complications
Issues with the product administered were not an expected AE since it is generally not an adverse event analyzed within RCTs or observational studies evaluating the safety of the mRNA-1273 vaccine. Particularly within RCTs, known for their controlled environments, the likelihood of such an event occurring is uncommon. Furthermore, product issues are events more likely to be noted and reported by administrators or storage technicians of the vaccine, a population unlikely to be involved within sample populations. In this study, statistically significant signals were generated for poor-quality products administered and expired products administered. Additionally, two statistically significant signals and a lower limit of 95% CI ≥ 1 for ROR were found; the product administered to the patient of inappropriate age and inappropriate product administration schedule showed an increase in the reporting of these events. These results suggest a lack of compliance with the guidelines for administering the mRNA-1273 vaccine compared to other vaccine reports within VAERS. The CDC has generated strict guidelines regarding the storage and handling of the vaccine, including the schedule and dosing for different age groups [Citation42,Citation43]. Non-compliance with these guidelines can lead to errors such as those found within this study, resulting in serious consequences. For example, providing an expired medicinal product can reduce its effectiveness, thereby exposing the recipient to infection by COVID-19 and its severe outcomes. The lack of literature regarding this area of vaccine safety emphasizes the need for further investigation through continued postmarketing surveillance and retrospective case-control studies to understand the extent of these concerns. These errors can lead to serious patient harm, such as overdoses.
4.5. Rare adverse events
Thrombotic outcomes such as thrombosis comprised a small fraction of the AEs reported, as expected. However, within the blood and lymphatic system disorders, the most common AEs reported were; thrombosis, deep vein thrombosis and anemia. These results are consistent with studies that concluded that thromboembolic outcomes are not a concern for the mRNA-1273 vaccine [Citation44]. Furthermore, the SmPC has not included thrombotic outcomes as a potential adverse vaccine event [Citation32].
Within ‘Reproductive system and breast disorders, 55% of AEs accounted for heavy menstrual bleeding (n = 1,473). The recent EMA (European Medicines Agency) COVID-19 vaccine safety update also identified increased heavy menstrual bleeding following mRNA-1273 vaccination [Citation45]. Menstruation is the endometrium-shedding process regulated by levels of estrogen and progesterone hormones [Citation46]. The immune response following mRNA-1273 vaccination can disrupt this regulation leading to abnormal menstrual changes [Citation47]. A cohort study by Wong KK et al. also found that changes in the severity of menstrual bleeding accounted for 67% of responses related to menstruation or vaginal bleeding following vaccination [Citation48]. However, disproportionality analysis did not produce statistically significant results for menstrual bleeding in this study. A possible explanation is the prevalence of menstrual irregularities among women without vaccination. Therefore, establishing a true correlation without considering baseline rates is difficult [Citation48]. Additionally, the generality of this AE within women makes it difficult to differentiate it as an adverse event exclusively caused by the vaccine leading to underreporting. Due to this, the SmPC has also listed ‘heavy menstrual bleeding’ as a side effect of unknown frequency [Citation32].
Cardiac disorders were also rarely reported, the most common being myocarditis, acute myocardial infarction, myocardial infarction, bradycardia, and pericarditis. Disproportionality analysis showed no statistically significant signals. This was unexpected since recent postmarketing surveillance found an increased relative incidence (RI) of myocarditis/pericarditis following the first and second doses of mRNA-1273 over a seven-day risk period [Citation17,Citation18]. The risk of reporting myocarditis/pericarditis was also 10-fold higher among young males aged 18–29 than females [Citation17]. The lack of disproportionality signals in this study for myocarditis/pericarditis may be due to the older population (mean age = 55) included and the overrepresentation of females (67%) within the reports obtained from VAERs. Within studies which detected a higher risk of myocarditis/pericarditis among mRNA-1273 recipients, the mean age was 26 years, with a 68.5% male population [Citation18]. However, age stratification did not yield statistically significant signals which indicate a positive association between individuals ages 18–29 years and myocarditis/pericarditis following vaccination. In contrast, the number of reports of myocarditis/pericarditis was slightly high within this age group. The lack of signals may attribute to underreporting, a major constraint of passive surveillance systems such as VAERS [Citation49].
The SmPC has listed myocarditis/pericarditis as a ‘very rare’ AE that may affect 1 in 10,000 people [Citation32]. This is in conjunction with the results obtained. The biological plausibility for the association of myocarditis/pericarditis with the mRNA-1273 vaccine stems from the occurrence of myocarditis/pericarditis in response to infections or damage to the heart through autoimmune diseases [Citation50]. The immune response stimulated by the mRNA-1273 vaccine (bAb levels exceeding those found in convalescent COVID-19 sera) causes inflammation, which can, in rare cases, affect the heart, leading to myocarditis/pericarditis [Citation15,Citation51]. The absence of statistically significant signals within this SOC is of reassurance to the public that the risk of myocarditis may be low following mRNA-1273 vaccination. However, the association between the risk of mRNA-1273-related myocarditis/pericarditis among young males requires further investigation to establish a true causal relationship.
4.6. Nocebo effects
While our analyses did not specifically investigate nocebo effects, the data from the clinical trials of the mRNA-1273 vaccine () provide valuable insights into the reporting of adverse events in both the placebo and vaccine groups. For example, arthralgia was reported by 11.76% of participants in the placebo group (1783/15155) despite these individuals not receiving the active vaccine. This is noteworthy compared to the 16.55% (2511/15168) in the vaccine group who reported the same symptom. These data raise important considerations regarding the potential influence of nocebo effects – negative side effects induced by patients’ expectations rather than the treatment itself. The rates of adverse events reported in the placebo group may reflect, at least in part, such nocebo effects. The adverse events reported in the placebo group, while not resulting from the active vaccine, illustrate the complex interplay of psychological and physiological factors that can contribute to reporting side effects after medical interventions, including vaccination.
We acknowledge that using the placebo group data to discuss potential nocebo effects has limitations, as our study was not designed to assess these effects directly. Nevertheless, they serve as a poignant reminder of the critical role that psychological factors, such as anxiety and expectations, can play in reporting adverse events following vaccination.
4.7. Strengths and limitations
The VAERS spontaneous reporting system has many strengths. Firstly, the accessibility of VAERS allows anyone among the general public to make a report and enables early detection of rare AEs which may not be detected by small-scale studies such as RCTs [Citation52]. Secondly, the database collects information regarding the vaccine from a diverse population, including those often excluded from clinical trials. This provides researchers with large study populations to improve the generalizability and validity of results obtained further. Finally, the provision of safety data covering the entire life cycle of the drug allows the detection of rare AEs, changes in existing reporting patterns and continued monitoring.
However, this study also has several limitations. First, a causal relationship between AEs and the mRNA-1273 vaccine cannot be established with the data presented since VAERS does not establish the true cause of the AEs reported. Second, a notable limitation is the lack of data on psychological factors, specifically vaccination anxiety, that may influence the reporting and experience of adverse events (AEs) following vaccination. As previously noted, there is a robust correlation between AEs and anxiety, particularly regarding vaccination-related anxiety. The lack of this information in our dataset limits our ability to differentiate AEs that may be more related to psychological factors from those directly resulting from the vaccine. Third, a major limitation of VAERS is underreporting. Reports to VAERS must be personally submitted by someone who has experienced or witnessed the AE. The rate of reporting can, therefore depend on different health behaviors. For example, a patient or physician who understands pain at the injection site is a common AE following vaccination is less likely to report it [Citation49,Citation52]. Fourth, stimulated reporting is another disadvantage of spontaneous reporting systems such as VAERS. Increased media coverage and public awareness of certain AEs can promote increased rates of reporting affecting the validity of results.
Fifth, reports submitted to VAERS can lack details and may contain errors that impact the validity of the data analyzed. Sixth, the results obtained are largely representative of the US population. Seventh, analysis of AEs by dosing schedule was not completed; therefore, comparisons of AE profiles between different doses of the mRNA-1273 vaccine were not possible. Eighth, the rapidly evolving COVID-19 vaccine landscape presents a challenge. Notably, as of 18 April 2023, the FDA updated the Emergency Use Authorization (EUA) for the Moderna COVID-19 Vaccine to a bivalent version. This significant development highlights the swift advancements in vaccine formulations. However, it is crucial to note that our study was conceptualized and commenced before the widespread adoption of this bivalent vaccine, making our findings specific to the monovalent iteration of the vaccine. Finally, it is impossible to calculate the occurrence rate of AEs within a population using VAERS data since it does not collect information regarding the number of vaccines administered.
5. Conclusions
In this study, the most frequently identified AEs following mRNA-1273 vaccination agree with the common side effects listed within the SmPC. However, the disproportionality analysis results for ‘Product issues’ were unexpected due to the absence of these concerns within the existing literature. This signifies a gap in understanding the occurrence of product-related errors and their source. Furthermore, since errors in the handling or quality of the product (mRNA-1273 vaccine) are not typically evaluated within RCTs and other observational studies, data regarding them is insufficient. In addition, disproportionality analysis did not find any statistically significant signals for myocarditis or pericarditis. As more data becomes available, further monitoring of these concerns is required to prevent serious patient harm.
Article highlights
Reports regarding adverse events (AEs) associated with the mRNA-1273 vaccine were obtained from the Vaccine Adverse Event Reporting System (VAERS) from January 1, 2021, and October 27, 2022.
Age analyses for ‘myocarditis’ and ‘pericarditis’ found that the majority of reports were made from individuals aged 18-29 years (185 reports for ‘myocarditis’ and 67 for ‘pericarditis’) and 30-64 years (193 reports for ‘myocarditis’ and 281 for ‘pericarditis’). However, disproportionality analysis did not find any statistically significant signals
Statistical analysis identified three significant signals for thirteen AEs and two for fifteen AEs. These adverse events included several general and systemic AEs, administration site conditions and product issues.
The most frequently identified AEs following mRNA-1273 vaccination agree with the common side effects listed within the Summary of Product Characteristics (SmPC). However, the disproportionality analysis results for ‘Product issues’ were unexpected.
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors have substantially contributed to the conception and design of the review article and interpreting the relevant literature and have been involved in writing the review article or revising it for intellectual content.
Supplemental Material
Download MS Word (38.7 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2023.2260477
Additional information
Funding
References
- World Health Organization. Coronavirus disease (COVID-19) pandemic. WHO; 2022. [cited 2022 Dec 22]. Available from: https://www.who.int/europe/emergencies/situations/covid-19
- Shi Y, Wang G, Cai XP, et al. An overview of COVID-19. J Zhejiang Univ Sci B. 2020 May;21(5):343–360.
- NHS. Coronavirus (COVID-19) symptoms in adults: crown. 2022. [updated 2022 Oct 24; cited 2022 Dec 22]. Available from: https://www.nhs.uk/conditions/coronavirus-covid-19/symptoms/main-symptoms/
- NHS. Coronavirus (COVID-19) vaccine: crown. 2022 [updated 2022 Oct 24; cited 2022 Dec 22]. Available from: https://www.nhs.uk/conditions/coronavirus-covid-19/coronavirus-vaccination/coronavirus-vaccine/
- GOV.UK. Moderna COVID-19 vaccine authorised by UK medicines regulator. Crown; 2021. [cited 2022 Dec 22]. Available from: https://www.gov.uk/government/news/moderna-covid-19-vaccine-authorised-by-uk-medicines-regulator
- Schoenmaker L, Witzigmann D, Kulkarni JA, et al. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int J Pharm. 2021 May 15;601:120586.
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021 Feb 4;384(5):403–416. doi: 10.1056/NEJMoa2035389
- EMA. Safety of COVID-19 vaccines: European Medicines Agency. 2022. [cited 2022 Nov 20]. Available from: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/safety-covid-19-vaccines
- Mathieu E, Ritchie H, Rodés-Guirao L, et al. Coronavirus (COVID-19) vaccinations: our World in data. 2020; [updated 2022 Nov 28; cited 2022 Nov 20]. Available from: https://ourworldindata.org/covid-vaccinations
- Kricorian K, Civen R, Equils O. COVID-19 vaccine hesitancy: misinformation and perceptions of vaccine safety. Hum Vaccin Immunother. 2022 Dec 31;18(1):1950504.
- Falotico JM, Desai AD, Shah A, et al. Curbing COVID-19 vaccine hesitancy from a dermatological standpoint: analysis of cutaneous reactions in the vaccine adverse event reporting system (VAERS) database. Am J Clin Dermatol. 2022 Sep;23(5):729–737. doi: 10.1007/s40257-022-00715-x
- Freeman D. COVID-19 vaccine hesitancy in the. (UK): University of Oxford; 2020. Available from: https://www.ox.ac.uk/news/science-blog/covid-19-vaccine-hesitancy-uk
- Creech CB, Anderson E, Berthaud V, et al. Evaluation of mRNA-1273 covid-19 vaccine in children 6 to 11 years of age. N Engl J Med. 2022 May 26;386(21):2011–2023. doi: 10.1056/NEJMoa2203315
- Masuda T, Murakami K, Sugiura K, et al. A phase 1/2 randomised placebo-controlled study of the COVID-19 vaccine mRNA-1273 in healthy Japanese adults: an interim report. Vaccine. 2022;40(13):2044–2052. doi: 10.1016/j.vaccine.2022.02.030
- Chu L, McPhee R, Huang W, et al. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine. 2021 May 12;39(20):2791–2799. doi: 10.1016/j.vaccine.2021.02.007
- Valiathan R, Ashman M, Asthana D. Effects of ageing on the immune system: infants to elderly. Scand J Immunol. 2016 Apr;83(4):255–266. doi: 10.1111/sji.12413
- Foltran D, Delmas C, Flumian C, et al. Myocarditis and pericarditis in adolescents after first and second doses of mRNA COVID-19 vaccines. Eur Heart J Qual Care Clin Outcomes. 2022 Mar 2;8(2):99–103. doi: 10.1093/ehjqcco/qcab090
- Massari M, Spila Alegiani S, Morciano C, et al. Postmarketing active surveillance of myocarditis and pericarditis following vaccination with COVID-19 mRNA vaccines in persons aged 12 to 39 years in Italy: a multi-database, self-controlled case series study. PLOS Med. 2022 Jul;19(7):e1004056. doi: 10.1371/journal.pmed.1004056
- Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022 Jan 25;327(4):331–340. doi: 10.1001/jama.2021.24110
- VAERS. About VAERS. https://vaers.hhs.gov/about.html
- VAERS. VAERS data. https://vaers.hhs.gov/data.html
- Medical Dictionary for Regulatory Activities. Introductory Guide MedDRA version 23.1: MedDRA. 2020. [cited 2023 Jan 3]. Available from: https://admin.new.meddra.org/sites/default/files/guidance/file/intguide_%2023_1_English.pdf
- Team RC. R: the R project for Statistical computing. The R Foundation; 2020. https://www.r-project.org/
- Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009 Jun;18(6):427–436. doi: 10.1002/pds.1742
- Activities MDfR. Introductory Guide MedDRA version 23.1: MedDRA. 2020. https://admin.new.meddra.org/sites/default/files/guidance/file/intguide_%2023_1_English.pdf
- Amanzio M, Mitsikostas DD, Giovannelli F, et al. Adverse events of active and placebo groups in SARS-CoV-2 vaccine randomized trials: a systematic review. Lancet Reg Health Eur. 2022 Jan;12:100253.
- Ali K, Berman G, Zhou H, et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N Engl J Med. 2021 Dec 9;385(24):2241–2251. doi: 10.1056/NEJMoa2109522
- Anderson EJ, Creech CB, Berthaud V, et al. Evaluation of mRNA-1273 vaccine in children 6 months to 5 years of age. N Engl J Med. 2022 Nov 3;387(18):1673–1687. doi: 10.1056/NEJMoa2209367
- Kadali RAK, Janagama R, Peruru S, et al. Non-life-threatening adverse effects with COVID-19 mRNA-1273 vaccine: a randomized, cross-sectional study on healthcare workers with detailed self-reported symptoms. J Med Virol. 2021 Jul;93(7):4420–4429. doi: 10.1002/jmv.26996
- Kabisch M, Ruckes C, Seibert-Grafe M, et al. Randomized controlled trials: part 17 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2011 Sep;108(39):663–668. doi: 10.3238/arztebl.2011.0663
- NHS. Coronavirus (COVID-19) symptoms in adults: crown. 2022. [updated 2022 Oct 24; cited 2022 Nov 15]. Available from: https://www.nhs.uk/conditions/coronavirus-covid-19/symptoms/main-symptoms/
- Moderna Biotech UK Ltd. Spikevax dispersion for injection COVID-19 mRNA vaccine (nucleoside modified). 2021 [cited 2022 Dec 23]. Available from: https://www.medicines.org.uk/emc/product/13982/pil
- Hause AM, Gee J, Johnson T, et al. Anxiety-related adverse event clusters after Janssen COVID-19 vaccination - five U.S. Mass vaccination sites, April 2021. MMWR Morb Mortal Wkly Rep. 2021 May 7;70(18):685–688. doi: 10.15585/mmwr.mm7018e3
- Geers AL, Clemens KS, Faasse K, et al. Psychosocial factors predict COVID-19 vaccine side effects. Psychother Psychosom. 2022;91(2):136–138. doi: 10.1159/000519853
- Galan N. When to call your healthcare provider about an injection side effect: Dotdash media, Inc. [cited 2022 Nov 6th]. Available from: https://www.verywellhealth.com/injection-side-effects-call-doctor-2616542#:~:text=An%20injection%20site%20reaction%20is%20your%20body%27s%20response,response%20to%20the%20needle%2C%20vaccine%2C%20or%20other%20medicine
- Washrawirul C, Triwatcharikorn J, Phannajit J, et al. Global prevalence and clinical manifestations of cutaneous adverse reactions following COVID-19 vaccination: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2022 Nov;36(11):1947–1968. doi: 10.1111/jdv.18294
- Solimani F, Mansour Y, Didona D, et al. Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2. J Eur Acad Dermatol Venereol. 2021 Oct;35(10):e649–e651. doi: 10.1111/jdv.17480
- Potestio L, Villani A, Fabbrocini G, et al. Cutaneous reactions following booster dose of COVID-19 mRNA vaccination: what we should know? J Cosmet Dermatol. 2022 Nov;21(11):5339–5340. doi: 10.1111/jocd.15331
- Carreño JM, Singh G, Tcheou J, et al. mRNA-1273 but not BNT162b2 induces antibodies against polyethylene glycol (PEG) contained in mRNA-based vaccine formulations. Vaccine. 2022 Oct 6;40(42):6114–6124. doi: 10.1016/j.vaccine.2022.08.024
- Stone CA Jr., Liu Y, Relling MV, et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than We have recognized. J Allergy Clin Immunol Pract. 2019 May;7(5):1533–1540.e8. doi: 10.1016/j.jaip.2018.12.003
- Richter AW, Akerblom E. Polyethylene glycol reactive antibodies in man: titer distribution in allergic patients treated with monomethoxy polyethylene glycol modified allergens or placebo, and in healthy blood donors. Int Arch Allergy Appl Immunol. 1984;74(1):36–39. doi: 10.1159/000233512
- CDC. Storage & handling: centers for disease control and prevention 2022. https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/storage.html
- CDC. Vaccine preparation and administration summary: centers for Disease Control and Prevention. 2022 https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/downloads/prep-and-admin-summary.pdf
- Schulz JB, Berlit P, Diener HC, et al. COVID-19 vaccine-associated cerebral venous thrombosis in Germany. Ann Neurol. 2021 Oct;90(4):627–639. doi: 10.1002/ana.26172
- EMA. COVID-19 vaccines safety update: European Medicines Agency. 2022. [cited 2022 Nov 20]. Available from: https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccines-safety-update-10-november-2022_en.pdf
- OASH. Your menstrual cycle: office on women’s health. 2021. [updated 2022 Feb 21; cited 2022 Nov22]. Available from: https://www.womenshealth.gov/menstrual-cycle/your-menstrual-cycle
- Leon R, Richard J, Galea L. Menstrual irregularities and the COVID-19 vaccine. The University of British Columbia; 2021. [cited 2022 Nov 22]. Available from: https://womenshealthresearch.ubc.ca/blog/menstrual-irregularities-and-covid-19-vaccine#
- Wong KK, Heilig CM, Hause A, et al. Menstrual irregularities and vaginal bleeding after COVID-19 vaccination reported to v-safe active surveillance, USA in December, 2020-January, 2022: an observational cohort study. Lancet Digit Health. 2022 Sep;4(9):e667–e675. doi: 10.1016/S2589-7500(22)00125-X
- VAERS. Guide to interpreting VAERS data. https://vaers.hhs.gov/data/dataguide.html
- Wu KC, Deshpande SR Myocarditis: BMJ. 2022. [cited 2022 Nov 26]. Available from: https://bestpractice.bmj.com/topics/en-gb/244
- Gilotra NA. Myocarditis: Johns Hopkins 2022 [cited 2022 Nov 26]. Available from: https://www.hopkinsmedicine.org/health/conditions-and-diseases/myocarditis
- VAERS. Vaccine adverse event reporting system (VAERS): CDC; 2022. Available from: https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vaers/index.html