ABSTRACT
Background
Achieving polio eradication requires ensuring the delivery of sufficient supplies of the right vaccines to the right places at the right times. Despite large global markets, decades of use, and large quantity purchases of polio vaccines by national immunization programs and the Global Polio Eradication Initiative (GPEI), forecasting demand for the oral poliovirus vaccine (OPV) stockpile remains challenging.
Research design and methods
We review OPV stockpile experience compared to pre-2016 expectations, actual demand, and changes in GPEI policies related to the procurement and use of type 2 OPV vaccines. We use available population and immunization schedule data to explore polio vaccine market segmentation, and its role in polio vaccine demand forecasting.
Results
We find that substantial challenges remain in forecasting polio vaccine needs, mainly due to (1) deviations in implementation of plans that formed the basis for earlier forecasts, (2) lack of alignment of tactics/objectives among GPEI partners and other key stakeholders, (3) financing, and (4) uncertainty about development and licensure timelines for new polio vaccines and their field performance characteristics.
Conclusions
Mismatches between supply and demand over time have led to negative consequences associated with both oversupply and undersupply, as well as excess costs and potentially preventable cases.
KEYWORDS:
1. Introduction
Stockpiles provide useful tools for ensuring the availability of vaccines, particularly when timely immunization is required to meet public health objectives [Citation1–6]. For example, when the United States (US) decided to end its use of oral poliovirus vaccine (OPV) in 2000 [Citation7] and shift to exclusive use of inactivated poliovirus vaccine (IPV), it explored options for stockpiling polio vaccines in the event of outbreaks [Citation8–10]. However, creating and maintaining stockpiles requires substantial planning and financial investment. For products that expire, including vaccines, stockpiles provide a form of insurance that makes vaccine doses available to manage surges in demand in the event of unexpected needs. For a vaccine whose use is anticipated to end after disease eradication, a stockpile produced prior to the cessation of its use can provide doses in the event of disease recurrence, and ensure the ability to respond to outbreaks rapidly and/or potentially restart the vaccine in routine use. However, if disease eradication and cessation of the vaccine use succeed, then the stockpile doses will expire and eventually be destroyed, with the costs of the option they represented written off (as insurance). As with any investment, the objective of a stockpile determines its design and operation, and good management is essential to minimize inefficiencies [Citation3].
1.1. Challenges for poliovirus vaccine forecasting
For polio, forecasts of vaccine need for stockpiles must consider the complexity of the different types of polioviruses and vaccines [Citation11,Citation12]. Both OPV and IPV induce protection from paralysis, but the vaccines differ substantially in other aspects. IPV requires an injection and costs much more than OPV to produce and administer. IPV primarily induces humoral immunity that protects the recipient against paralysis. In contrast, OPV induces both humoral and mucosal immunity as it replicates in the nasopharynx and gastrointestinal tract of the recipients, which offers more robust protection against paralysis as well as infection. In addition, the replicating OPV can spread secondarily to close contacts of vaccine recipients, inducing an immune response and thus increasing the effective immunity in the population (i.e. population immunity above the coverage level achieved by individual vaccinations). However, as an attenuated live poliovirus, OPV can, in very rare instances, lead to vaccine-associated paralytic polio (VAPP) in recipients or close contacts [Citation13,Citation14]. In addition, in populations with low OPV coverage, if secondary spread continues instead of dying out, the OPV-related viruses can lose their attenuating mutations, and become circulating vaccine-derived polioviruses (cVDPVs) that clinically behave like wild polioviruses (WPVs) [Citation13,Citation15]. Genetically engineered novel OPV (nOPV) formulations promise to substantially reduce these risks [Citation16], but the risks are not zero [Citation17,Citation18]. As such, ending all cases of poliomyelitis requires cessation of all OPV use.
Clinical and public health decision making about the use of various polio vaccines becomes further complicated by the existence of three stable types of polioviruses (types 1, 2, and 3), each with their distinct phenotype, as well as different potential vaccine formulations. Currently, all IPV products include all three poliovirus types in all formulations. IPV can be used standalone or in combination with other antigens as a multivalent or combination vaccine. Standalone IPV administration with fractional dosing (off-label) occurs in some countries (e.g. India [Citation19] and Sri Lanka [Citation20]) and may use innovative delivery technologies to facilitate delivery, save costs, or extend existing supplies [Citation21,Citation22].
OPV is typically not combined with any other antigens, although it may be administered at the same time as some other vaccines. Manufacturers historically only licensed and produced trivalent OPV formulations (tOPV), containing all three types of attenuated Sabin strains. However, starting in the mid-2000s, manufacturers licensed monovalent Sabin-strain OPV (mOPV) for types 1, 2, and 3 (i.e. mOPV1, mOPV2, and mOPV3). In 2009, licensure of bivalent Sabin-strain OPV (bOPV, containing types 1 and 3 OPV) further expanded the Sabin OPV options. Most recently, accelerated efforts led to the development, emergency use licensure, large-scale production, and use of a genetically engineered ‘novel’ type 2 OPV vaccine (nOPV2), designed for greater genetic stability and thus lower risk of reversion to neurovirulence and seeding of new cVDPVs [Citation16,Citation23]. Efforts to develop other poliovirus vaccines also continue, including accelerating nOPV for types 1 and 3 with numerous potential combinations of different types of OPV formulations [Citation24,Citation25]. New poliovirus vaccine development efforts could also potentially use vaccine-like particles (VLPs) [Citation26,Citation27], which would not require any live poliovirus replication for vaccine production.
Adding further complexity, all countries deliver polio vaccines (IPV-only or IPV and OPV) in their national routine immunization (RI) programs, and some countries also deliver OPV in supplemental immunization activities (SIAs), either preventively to boost immunity in populations with low RI coverage to avoid outbreaks or reactively in response to outbreaks [Citation11]. The large and expanding number of products and their different costs and properties creates substantial complexity for decision making [Citation24] and therefore for short- and long-term forecasting.
1.2. Stockpiles to manage OPV cessation risks
OPV-related risks led to the recognition of the need to end all OPV use after successful WPV eradication to end all cases of poliomyelitis [Citation28]. From the beginning, discussions of managing the risks of OPV cessation included the development of an OPV stockpile, with the first estimate of 500 million doses needed to cover immunization of three global birth cohorts and with 100 million of these filled and readily available [Citation1]. The Global Polio Eradication Initiative (GPEI) globally coordinated cessation of type 2 OPV (OPV2) use for preventive immunization in April-May 2016 [Citation29]. In preparation for OPV2 cessation, often referred to as the ‘tOPV-bOPV switch’ or simply ‘the switch,’ the GPEI worked with manufacturers to create a global stockpile of Sabin-strain type 2 OPV (mOPV2) for outbreak response and formalized policies for its use [Citation29]. While the GPEI plans in the early 2000s and the 2009 UNICEF tender for the OPV stockpile requested 750 million doses of each mOPV type [Citation3,Citation30], financial resource constraints led to awards to OPV manufacturers in 2013–2014 for 519 million doses of mOPV2 and 300 million doses each of mOPV1 and mOPV3 [Citation31,Citation32].
At the global level, achieving polio eradication and successful OPV cessation requires ensuring the delivery of sufficient supplies of the right formulations of vaccines to the right places at the right times, sometimes with short notice in response to outbreaks. Prior to the mid-2010s, the GPEI considered the information from integrated modeling that incorporated a system dynamics approach and stochastic risks to estimate dynamic and probabilistic vaccine needs from a future OPV stockpile [Citation3,Citation33–36]. These estimates explicitly sought to align with contemporaneous GPEI strategic plans [Citation37] and corresponding budgets to model expected vaccine needs based on stated intentions, and they included the simulation of many possible futures with full consideration of dynamic uncertainties associated with prospective risks and potential exportation events [Citation3,Citation33–36]. For example, modeling highlighted the different objective functions that decision makers might choose in designing OPV stockpiles after globally-coordinated OPV cessation to support emergency outbreak response activities [Citation3], and estimated vaccine requirements to meet an objective of having high confidence about sufficient supplies in the context of recommended outbreak response campaigns to support OPV2 cessation [Citation34–36]. Additional modeling published between 2000 and 2019 [Citation38] and later studies further characterized vaccine stockpile needs for different prospective outbreak response strategies [Citation39–42] and for different timings of OPV cessation [Citation43–45]. These studies provided a wide range of estimates depending on prospective GPEI strategies and their implementation.
National immunization programs and GPEI partners independently develop internal (typically unpublished) vaccine demand forecasts, which may include anticipated stockpile needs for countries that self-produce or self-procure their vaccine supplies. At the global level, the United Nations Children’s Fund (UNICEF) communicates expected demand for the global OPV stockpile (and for RI for the countries that it supports) to vaccine manufacturers during consultations, e.g., [Citation46], tenders (i.e., bidding opportunities), and contracts. Since 2017, GPEI subgroups tasked with forecasting OPV stockpile needs shifted to exclusively considering scenario-based estimates that that included only specific countries, largely based on historical OPV2 use [Citation31,Citation47]. Since this approach focuses on specific scenarios, by design, it does not consider the potential vaccine needs of the countries excluded by the scenarios or provide implied relative probabilities of different possible future trajectories [Citation31].
Since 2018, the GPEI has released several additional strategic plans [Citation48–50] in response to ongoing delays in global eradication of WPVs and control of cVDPV outbreaks. The GPEI partners also ordered new production of OPV2 for the vaccine stockpile, and accelerated development of novel OPV2 for emergency response. For this study, we sought to explore the extent of mismatches between supply and demand of the OPV stockpile, explain the motivation for recent decisions for the OPV2 stockpile, and highlight challenges for polio vaccine stockpile managers and forecasters.
2. Methods
2.1. Literature and data reviews
We sought to identify all published quantitative estimates of polio vaccine stockpile needs (i.e., forecasts) and actual use. We began by retrieving all publicly available resources related to the design, management, and/or evaluation of the global OPV stockpiles following OPV2 cessation, including published manuscripts, reviews, reports, and presentations. We searched PubMed® on 11 May 2023, for articles on prospective estimates of poliovirus vaccine needs and/or actual reported vaccine needs. The PubMed search strategy (Supplemental Table S1) retrieved 54 manuscripts related to poliovirus vaccine ‘forecasting,’ 31 manuscripts related to poliovirus vaccine ‘stockpiles,’ and 110 manuscripts coded by PubMed as ‘poliovirus vaccines/supply and distribution.’ Additional manual searches through inspection of cited literature resulted in 5 references not identified through the structured search strategy. After the exclusion of duplicate references (n = 13), references published before 1996 (n = 27), and references in languages other than English (n = 13), the remaining 148 references were subject to review. We chose 1996 as the beginning for the search as it represents the year when discussions of potential cessation of OPV use began at the global level (Supplemental Table S2).
In addition to searching for published manuscripts, we searched GPEI and UNICEF websites to identify any documents related to vaccine stockpiles and/or forecasting. GPEI documents were retrieved from the ‘GPEI Library’ [Citation51] and by searching Google for combinations of search terms supply, forecast and stockpile as well as the ‘site:polioeradication.org’ operator. We retrieved UNICEF reports [Citation52] using combinations of search terms polio, supply, demand, and stockpile. Additionally, we attempted to locate UNICEF ‘Vaccine Industry Consultation’ and ‘Vaccine Supply Outlook’ reports using Google searches for ‘polio industry’ and ‘polio outlook’ and the ‘inurl:unicef’ operator. For all Google searches, we manually screened up to 50 top hits to identify relevant documents. Based on the literature review and additional searches, we compare forecasts to actual demand, and describe the evolution of GPEI policies related to the procurement and use of type 2 OPV vaccines.
2.2. Projections of vaccine needs
Using the United Nations population projections for 2023–2035 for individual countries [Citation53], we estimate the annual expected number of surviving infants by World Bank Income Level (WBIL) [Citation54]. We separated self-procuring high-income and large middle-income countries that self-produce polio vaccine (e.g., China, the Russian Federation, Indonesia, and India) from other countries that rely on external procurement. We use these estimates of surviving infants to demonstrate the calculations to characterize current RI polio vaccine demand. For well-established national immunization programs with relatively stable populations, immunization policies, and markets, forecasts of vaccine demand simply depend on multiplying the estimated birth cohort sizes, the number of doses in the RI schedule, and coverage for each country using the vaccine (adjusted for wastage) [e.g., Citation55] (which notably excluded polio vaccines due to limited data availability). In addition to regular demand for RI, in some cases, national demand estimates also need to cover (1) potential out-of-schedule demand (e.g., travelers, immigrants), (2) maintenance of a buffer stock to cover short-term supply chain disruptions, and/or (3) SIAs performed to increase immunity in under-vaccinated communities – either preventively or reactively (or both for major public health goals [e.g., Citation56]). For SIAs, estimates of vaccine needs primarily involve estimating the number of children in the target populations (e.g., under 5-year-olds) in specific countries, coverage, and timing and number of campaigns for a specific scenario or simulated prospectively. Preventive SIAs (pSIAs, or planned campaigns) offer the benefit of anticipating demand in advance that support larger production runs and benefit from economies of scale, which allows for procurement as part of overall demand. However, for vaccine used from a stockpile exclusively for outbreak response, such use represents unplanned campaigns or outbreak response SIAs (oSIAs).
For demonstration, and to help fill the gap noted by other authors [Citation55], we use the current RI schedules to demonstrate the estimation of OPV and IPV RI demand for different WBIL and separating out the large self-procuring countries without adjustment for wastage. For outbreaks of type 1 poliovirus e.g [Citation57], to date the bOPV forecasted demand for use in RI and pSIAs led to sufficient annual production to also provide sufficient vaccine for oSIAs. However, this may not continue prospectively, and would not continue in the event of globally-coordinated use of bOPV for all preventive immunization (i.e., RI and pSIAs).
3. Results
3.1. Reviews
Our literature review identified few peer-reviewed studies relevant to the design and management of polio vaccine stockpiles or estimating vaccine needs. Our PubMed search primarily retrieved editorials, commentaries, and studies with no specific estimates, policy guidelines, or recommendations relevant to polio stockpiles (n = 107). Of the remaining 41 references, 9 provided quantitative estimates of polio vaccine stockpile needs or modeling insights, 23 offered extended commentaries or perspectives with insights relevant to polio vaccine stockpiles, costs, and/or OPV cessation, and 9 discussed field logistics and/or polio vaccine storage, distribution, or administration not related to stockpiles. We retrieved multiple additional relevant reports from the World Health Organization (WHO, including GPEI) and UNICEF from direct search of the respective organizational archives. Instead of focusing on the literature review, we incorporated the key information and insights from the peer-reviewed literature and the organizational documents we retrieved throughout.
Among the 9 papers that provided quantitative estimates of polio vaccine stockpile needs, 2 papers explored US stockpile needs [Citation9,Citation10]. The 7 studies focused on global stockpile needs started with a 2001 extensive discussion and simple estimate of 500 million doses [Citation1]. Modeling of OPV stockpile needs in 2008 suggested that a 500 million dose stockpile could meet needs for outbreak response after OPV cessation [Citation58]. A 2010 study highlighted different potential objectives and their consequences for polio vaccine stockpile design [Citation3]. Although early discussions of the OPV stockpile assumed ongoing OPV production for replenishment [Citation1], by 2016 two major European OPV manufacturers anticipated declining demand and sunsetting production for all OPV, and increased demand for their production of IPV. Modeling prior to OPV2 cessation assumed the creation of the entire fixed stockpile prior to OPV cessation, and that OPV cessation would either succeed with a within 5 years, or the GPEI and OPV-countries would restart OPV production and its use in RI [Citation33,Citation34,Citation59]. These assumptions implied a time-limited global OPV stockpile. Modeling after OPV2 cessation explored different characteristics of polioviruses and vaccines as well as risk tolerance for stockouts and their effects on vaccine stockpile needs [Citation35].
3.2. OPV2 stockpile experience
The OPV2 cessation experience evolved from pre-2016 creation of an OPV2 stockpile as a short-term resource (i.e., insurance) to a necessary tool for dealing with ongoing expected demand due to cVDPV2 outbreaks and reestablished endemic transmission in some areas. This led management and modeling to adapt to the long-term programmatic challenges, and requirement of ongoing manufacturing and release of OPVs. shows the timeline of risk management landmarks for OPV2 cessation, including the creation of a Sabin mOPV2 stockpile prior to OPV2 cessation and ending the production of new bulk mOPV2 (2015–2016). The timeline also shows the acquisition of additional stored OPV2 bulk from the two original manufacturers that supplied bulk for the stockpile, and from one other manufacturer with stored OPV2 bulk, in response to ongoing cVDPV2 outbreaks (2018–2020) [Citation47], and the tender for more potential production of bulk mOPV2 (2020). Notably, the timeline includes the addition of tOPV to stockpiles (2020) for response to outbreaks of cocirculating polioviruses of different types, and accelerated development, production, and deployment of nOPV2 (2018-present). In parallel, modeling shifted the allowable use of OPV2 (from the stockpile for outbreak response) from 5 years to 8 years (i.e., from 2021 to 2024) [Citation60] and explored the use of both Sabin and novel OPVs [Citation40–42]. More recently, given that the resumption of new OPV2 bulk production (for nOPV2), filling (for mOPV2, tOPV, and nOPV2), and extensive use of OPV2 () did not lead to restart of OPV2 use in RI to date, recent modeling studies assumed prospective OPV2 use for outbreak response without restriction and removed OPV restart in RI as a risk mitigation strategy [Citation44,Citation45].
Figure 1. Timeline of major stockpile landmarks related to risk management plans for OPV2 cessation. The original plans for creation of an outbreak response vaccine stockpile prior to the 2016 OPV2 cessation are shown in black font. Subsequent decisions and actions taken in response to the ongoing circulation of vaccine-derived viruses are shown in red font. Additional details and specific references for this timeline are provided in Supplemental Table S2.
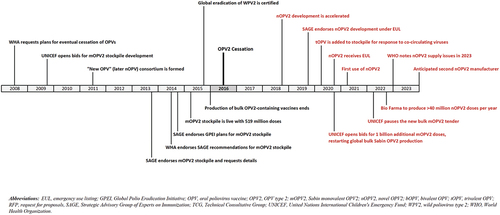
Figure 2. Cumulative number of vaccine doses received (input) and released (output) from oral poliovirus vaccine stockpiles created for response to outbreaks of type 2 circulating vaccine-derived polioviruses after the planned OPV2 cessation in 2016. (Source: WHO, data as of 19 June 2023).
In contrast to pre-OPV2 cessation expectations of supply of a fixed number of mOPV2 stockpile doses for use within 5 years after OPV2-cessation, now 7 years since OPV2 cessation, shows the cumulative supply of all filled OPV2 doses by formulation (i.e. mOPV2, tOPV, nOPV2) and total for the stockpile (panels a-d, solid curves) as of 19 June 2023. also shows the total authorized distributions of the doses for outbreak response (panels a-d, dashed curves). Use of OPV2 from the stockpile exceeded the initial 519 million mOPV2 stockpile doses procured with the initial available financing [Citation32] by early 2020 (, panel a). Actual demand exceeded use, but the GPEI constrained releases from the stockpile for outbreak response due to limited vaccine availability and emphasis on reduced outbreak scope. The manufacturers who contributed these initial doses also retained some additional bulk at the time of OPV2 cessation, as did a third manufacturer, which the GPEI later purchased for the OPV2 stockpile and converted to finished product [Citation61]. Although UNICEF issued a tender for new mOPV2 bulk [Citation47], it did not make any awards. As of June 2023, 39 countries had reported over 3,000 paralytic cases caused by type 2 transmission [Citation62], leading to demand for over 1.5 billion doses of OPV2-containing vaccines for outbreak response (, panel d, dashed curve). These outbreaks included at least 8 emergences seeded by nOPV2 [Citation63]. Thus, instead of a draw down and the end of a centralized OPV2 stockpile for outbreak response, clearly demonstrates ongoing and increasing OPV2 needs from the stockpile. Most notably, the GPEI moved away from the idea of potential OPV2 restart in RI [Citation50], and remains focused on wide distribution and use of nOPV2 for use in outbreak response () with more than 700 million doses released from the stockpile between January of 2021 and December of 2022 [Citation64].
shows the size of the population targeted for OPV2 outbreak response rounds between 2017 and 2022 [Citation65]. Following the emergency use licensure of nOPV2 in late 2020, shows the rapid shift away from Sabin OPV2 (i.e. mOPV2 and tOPV) to nOPV2. The relatively rapid policy shifts manifested as the stockpile management complications seen in . Notably, the de facto abrupt abandonment of Sabin OPV2 beginning around the time of the nOPV2 EUL led to a large excess inventory of mOPV2 and some excess tOPV, which will likely be wasted with product expiration (, difference between solid and dashed curves in panels a, b and d). The notable gaps between mOPV2 and tOPV cumulative supply and release curves in imply a mismatch of nearly 500 million doses, which is almost the size of the initial mOPV2 stockpile. While this supply served as insurance to mitigate the risks of delays in the nOPV2 EUL, some of this excess supply could have enabled more timely outbreak responses in countries that chose to wait for nOPV2 instead of using mOPV2. Not surprisingly, management of supply and demand for nOPV2 presented substantial challenges, with unmet demand partially visible due to the release curve for nOPV2 appearing above the supply curve in . Notably, nOPV2 development and production efforts encountered substantial start-up and/or scale-up challenges and delays; a familiar phenomenon for polio vaccines that the GPEI could have anticipated due to the recent experience with the expansion of IPV use in RI [Citation66,Citation67]. Additional delays in releasing nOPV2 from the single manufacturer in 2022-early 2023 also limited the ability to manage nOPV2 supplies. The curves in do not show the full unmet demand, because decisions to release OPV2 doses from the stockpile included consideration of available supply instead of only reflecting need for outbreak response. The consequences of decisions made by countries to delay responding to outbreaks using available mOPV2 and instead to wait for nOPV2 increased the number of children paralyzed in outbreak-affected areas and allowed outbreak viruses to spread more broadly, and thus created further increased demand of doses from the stockpile [Citation40].
Figure 3. Dramatic shift in the total number of children targeted by outbreak response campaigns using Sabin OPV2 (mOPV2 and tOPV) to nOPV2 between 2017 and 2022 (data manually digitized from in reference [Citation65]).
![Figure 3. Dramatic shift in the total number of children targeted by outbreak response campaigns using Sabin OPV2 (mOPV2 and tOPV) to nOPV2 between 2017 and 2022 (data manually digitized from Figure 2 in reference [Citation65]).](/cms/asset/788ae109-6b7a-4cdf-81fb-0c3b47caa174/ierv_a_2263096_f0003_b.gif)
3.3. Other poliovirus vaccine stockpiles
Extended timelines for globally-coordinated cessation of OPV types 1 and 3 and use of stockpile doses for type 1 and 3 mOPVs left 300 million doses of each unused as of September 2017 [Citation68].
The increasing number of cVDPV2 outbreaks since OPV2 cessation created the need for more OPV2 vaccine doses from the stockpile and for more external financial support for outbreak response. However, the GPEI budgets did not anticipate the need for these funds. Consequently, despite plans to maintain high population immunity for types 1 and 3 after OPV2 cessation and prior to bOPV cessation [Citation69], the limited funds for SIAs led the GPEI to prioritize OPV2 oSIAs instead of bOPV pSIAs [Citation70,Citation71]. This, in conjunction with disruption caused by the COVID-19 pandemic, led to notable differences between forecast demand and actual use for bOPV () [Citation70,Citation71]. In order to mitigate some of the effects of these disruptions on manufacturers and countries, UNICEF facilitated the release of aging stock to third parties, and worked with countries to ensure acceptance of vaccine doses with shorter than ideal shelf life [Citation72]. As such, some of the economic costs of mismatch between bOPV supply and demand () shifted from the manufacturers to consumers. In addition, manufacturers adjusted production in response to ongoing collaboration and discussions with UNICEF, with awards reflecting some carry-over stocks from past years to future years. Ultimately, the implications of the reduction in bOPV pSIAs could manifest as a need for relatively larger stockpiles for OPVs for types 1 or 3, particularly for type 1 due to its greater transmissibility and neurovirulence [Citation35,Citation44].
Figure 4. UNICEF bOPV vaccine demand forecasts for SIAs (including outbreak response) and actual doses used, excluding bOPV demand for routine immunization and excluding bOPV used by self-procuring countries. Mismatches between supply and demand forecasts have resulted in a large estimated excess supply over the 2019–2022 period (solid black line) (supply and demand data manually digitized from Fig. 4 of reference [Citation70]).
![Figure 4. UNICEF bOPV vaccine demand forecasts for SIAs (including outbreak response) and actual doses used, excluding bOPV demand for routine immunization and excluding bOPV used by self-procuring countries. Mismatches between supply and demand forecasts have resulted in a large estimated excess supply over the 2019–2022 period (solid black line) (supply and demand data manually digitized from Fig. 4 of reference [Citation70]).](/cms/asset/19425f8a-1e19-4607-9543-a5dd8d46c451/ierv_a_2263096_f0004_oc.jpg)
3.4. Forecasting routine poliovirus vaccine needs
Prospective demand estimates depend on uncertain future recommendations related to the choices made by national immunization programs, the availability of vaccines, and the number of surviving infants. shows the estimated number of surviving infants for each WBIL projected for 2023–2030, which in combination with RI schedule, provide the base for estimating the vaccine needs for control programs. For polio vaccines used in RI, existing market segmentation can make some forecasting relatively easy. For example, demand appears stable for developed countries that achieved and maintain high coverage, which appear likely to continue to use IPV in IPV-containing combination vaccines in schedules that include 3 or more doses for the foreseeable future. However, for developing countries and countries facing many options, substantial uncertainty enters any forecast. In addition, the widely different views on what polio eradication means (i.e. ending all use of live poliovirus vaccines, including OPV [Citation73], or not [Citation74]) and the continued development of new poliovirus vaccines [Citation23,Citation65] make choosing the right immunization strategy and therefore forecasting future demands for all polio vaccines challenging [Citation24]. Variability in the forecasts range by hundreds of millions of doses per year, with much less IPV needed if lower-income countries continue to use OPV and minimize or stop their use of IPV or only use IPV with fractional dosing, to much more potentially needed if all countries shift to using 4 full doses of IPV per child in a combination vaccine. If OPV-using countries shift to sequential IPV/OPV schedules, then this would imply continuation of substantial quantities of both IPV and OPV. With uncertainty about whether and when polio eradication will occur, and potential success of ending all OPV use, the costs of continuing to pay to for polio vaccines annually may also lead to efforts by many countries to seek the most cost-effective polio immunization option. Given the choice of control with either IPV or with OPV, shifting back to tOPV could emerge as the option that offers both the lowest total expected cost and lowest expected total polio cases for many countries, if available [Citation75]. However, as shown in , demand for Sabin OPV can rapidly disappear with the promise of an alternative (e.g., nOPV), even in the absence of sufficient supplies of nOPVs to initially meet demand.
Figure 5. Estimated numbers of surviving infants for each World Bank income level (WBIL) projected for 2023–2035, separately showing the countries dependent on external resources for vaccine procurement (panel a, including all low-income (LI) countries) and countries that self-procure vaccines (panel b, including all high-income (HI) countries). Lower middle-income (LMI) and upper middle-income (UMI) countries split between both panels, with China, India, Indonesia, and the Russian Federation considered self-producing and thus self-procuring middle-income countries.
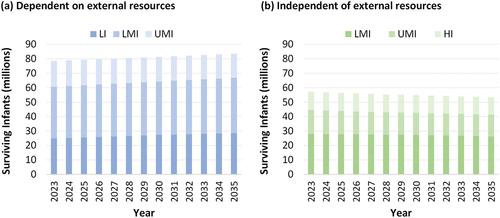
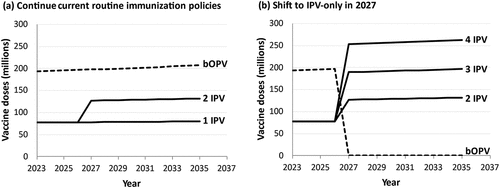
demonstrates the substantial differences between global demand for bOPV and IPV with current coverage levels (without adjustments for wastage) if OPV-using countries dependent on external resources for vaccine procurement continue to use (a) bOPV plus one or two doses of IPV in RI, or (b) shift to IPV-only with 2, 3, or 4 full doses in RI in 2027, at which point all RI demand for bOPV will disappear. We exclude self-procuring countries since they manage their own demand. Uncertainty about whether, when, and which shifts will occur pose substantial challenges for forecasting poliovirus vaccine needs. does not consider the potential shift toward 2 fractional IPV doses as the preferred schedule by more countries than those currently using it, which could reduce the demand substantially. also does not include adjustments for wastage or demand of OPV doses for SIAs (neither preventive nor outbreak response) or the possibility of reintroduction of OPV2 doses in RI with or without restarting a trivalent OPV formulation [Citation75]. Restarting OPV in RI could substantially reduce demand for IPV in RI, but substantially increase demand for OPV [Citation75], which would contrast significantly from the options shown in . Vaccine demand for SIAs will depend directly on the specific vaccine(s) used in RI (i.e., IPV and/or bOPV) and coverage, and whether the SIAs would occur preventively or reactively. Thus, from a modeling perspective, forecasting the demand of OPV for SIAs from a stockpile depends on interactions within the complex system, and the extent to which the need for doses only materializes reactively.
Figure 6. Estimated approximate numbers of bOPV and IPV doses for routine immunization (without adjustments for wastage) for surviving infants in countries dependent on external resources. A slow and steady increase in demand is predicted if countries continue current routine immunization policies using both bOPV and IPV, with a shift from a minimum of 1 IPV to 2 IPV doses in all countries (shown as a jump in 2027, although adoption likely to be gradual) (panel a). However, shifting to IPV-only due to planned bOPV cessation in 2027 will result in a significant shift in demand for both vaccines, with IPV demand depending on the minimum number of IPV doses (panel b), requiring significant planning for finances, supplies and manufacturing infrastructure.
4. Discussion
Following on the heels of successful global smallpox eradication, countries anticipated that after eradicating polio by the year 2000, the use of polio vaccines might similarly stop and yield a substantial eradication financial dividend [Citation76,Citation77]. With the promise of both a world free of poliomyelitis and substantial future savings on polio vaccines, surveillance, and management, donors chose to make substantial investments in polio eradication [Citation78]. However, the global landscape for polio vaccines continues to evolve into a highly complex [Citation24] and unpredictable future that will likely require ongoing production of various forms of IPV and OPV to respond to national immunization preferences and ongoing national and GPEI transitions in strategic objectives.
4.1. Dynamics of polio vaccine demands
As polio eradication efforts began, OPV manufacturers simultaneously encountered both increased vaccine demand for RI and SIAs used to increase coverage and the prospects of zero demand after successful eradication. Delays in global eradication combined with the risks associated with OPV also created incentives for additional IPV production and use, particularly in relatively higher-income countries that independently ended the transmission of indigenous WPVs. Specifically, due to the risks of imported polioviruses, countries that stopped poliovirus transmission need(ed) to continue to protect their populations, and the market share for more expensive IPV grew. In the meantime, OPV manufacturers continued to produce higher-risk low-margin OPVs (first tOPV, then mOPVs and bOPV and now nOPVs) to support global polio eradication efforts.
As a result of significant delays in global polio eradication, the original plans of sunsetting all polio vaccine production (first OPVs, and perhaps then IPVs), evolved with market segmentation and substantially increased production and use of polio vaccines, most notably IPV [Citation66]. With increased IPV demand, new manufacturers that entered the market responded to market incentives and chose to use Sabin OPV seed strains instead of the highly virulent WPV seed strains [Citation79,Citation80]. Notably, Sabin IPV may offer lower risks in the event of releases [Citation81] and could serve as a ‘warm base’ for OPV restart (if needed) [Citation35,Citation82]. Traditional IPV manufacturers increasingly moved toward including IPV in combination vaccines with more antigens, with hexavalent products now a standard in many higher-income countries. For OPV, manufacturers continued production substantially longer and in larger quantities than they anticipated, in some cases extending the use of outdated facilities to the extent possible or supported by demand.
Based on the experience of increasing use of mOPV2 from the stockpile as of 2019, which contrasted with initial assumptions related to die out of cVDPV2s within 5 years, GPEI accelerated the development of nOPV2 in 2018 and ordered the new production of bulk Sabin OPV2 in 2020 as a back-up plan (). The detection of a cVDPV2 outbreak in 2019 in Pakistan in the background of ongoing indigenous transmission of WPV type 1, also motivated the new production of tOPV [Citation83], which modeling identified as a better option for responding to outbreaks of cocirculating type 1 and 2 polioviruses for Pakistan and Afghanistan [Citation84]. Despite the increased demand in recent years, as of early 2023 only one manufacturer continued to produce bulk OPV for global demand [Citation47].
The 2022 GPEI stockpile strategy prioritized diversification of the OPV supply chain [Citation47]. As of 2023, India does not produce bulk OPV, although several Indian manufacturers fill OPV using bulk produced elsewhere, and they may at some point seek to produce bulk novel OPV that could reduce some of the risks of current reliance on a single supplier, albeit potentially with higher costs due to lower economies of scale for each manufacturer [Citation47,Citation85]. Recent innovations and accelerated development of nOPV2 (and other nOPVs), currently used only for outbreak response SIAs, raise further questions about the long-term use of different OPV formulations, and the possibility of other manufacturers entering the market. Countries that self-produce polio vaccine must decide whether to continue navigating global uncertainties while sustaining their own production, or seeking to procure vaccines that they need from manufacturers in other countries that could imply potentially incurring delays if or when shortages occur. Those that continue self-producing and filling OPV could potentially choose to export OPV in response to global demand.
4.2. Consequences of delays in polio eradication
The delays in achieving polio eradication led to subtle but substantial shifts of expectations for continued polio vaccination after eradication. Specifically, in contrast to early expectations by some countries of enjoying the polio eradication dividend and ending polio vaccination, the polio end game no longer promises an eradication dividend and come with substantially higher expected costs for polio vaccines for the foreseeable future [Citation86]. In 2015 the GPEI and WHO recommended a minimum of one IPV dose in RI for all countries (in addition to their OPV use for OPV-using countries) to support the globally coordinated OPV2 cessation in 2016 [Citation87]. This recommendation came with the possibility that countries might stop using IPV in RI a few years after successful eradication and global cessation of all OPV use [Citation87]. However, updated global recommendations now include a minimum or 2 or more doses of IPV in RI, in addition to multiple OPV doses in RI in OPV-using countries, potentially including some SIA OPV doses as well [Citation11]. Despite earlier emphasis on reducing IPV costs [Citation81], market segmentation currently reflects movement toward immunization schedules that use 4 full doses of IPV formulated in hexavalent combination vaccines [Citation88], which may increase coverage and substantially increase costs [Citation89]. Countries looking to manage costs may lean toward increased adoption of the use of 2 fractional doses IPV [Citation90]. In the context of considering IPV supply and potential stockpiling, the use of combination vaccine formulations in RI can create supply chain and logistical challenges for outbreak response [Citation10]. Recent outbreaks due to importations of type 2 cVDPVs in IPV-only countries (e.g. US, UK, Israel), also raised questions about the use of standalone IPV and/or OPV for response, although these countries fall outside the scope used for GPEI forecasting [Citation31,Citation47].
4.3. Implications for modeling polio vaccine needs
In the current polio vaccine landscape, the value of an OPV stockpile for emergency use could disappear or ramp up. Pre-OPV2 cessation modeling assumed the GPEI and countries would aggressively respond to any outbreak using any available vaccine from the stockpile to shut down transmission, but actual outbreak response implementation substantially differed from model recommendations [Citation34,Citation84,Citation91,Citation92]. A recent look back analysis showed that if the GPEI and countries had managed risks and implemented outbreak response as originally assumed, then the Sabin OPV2 stockpile size might have been sufficient and OPV2 cessation would have had a relatively high chance of succeeding within 5 years after OPV2 cessation [Citation93]. Conversely, nOPV2 would not have resulted in successful OPV2 cessation if the global community had responded to outbreaks as it actually did, even with ideal nOPV2 available in 2016 [Citation93].
When planning and stockpile resource development are discordant with actual practice, then mismatches between vaccine supply and demand occur. One lesson from the polio vaccine stockpile to date is the need to consider the perceived benefits, risks, and costs and other factors that influence the acceptability of different polio vaccines. Notably, the recent precipitous decline of the use of Sabin OPVs () in spite of available stockpiles () and ongoing transmission of types 1 and 2, suggest that Sabin OPVs may no longer be the vaccine of choice for polio eradication. This implies that any future stockpile decisions and vaccine use will likely need to focus on alternative vaccine choices. However, countries that do not achieve and maintain high coverage and that choose not to use available Sabin OPVs for outbreak response should expect potentially larger numbers of preventable polio cases [Citation40]. National immunization programs also need to recognize that the preferences of some communities and individuals for OPV and IPV may vary due to different perceptions associated with injections and/or other factors. The introduction of new vaccines also comes with uncertainty about risks and properties that we can only observe over time, as the experience with nOPV2 demonstrates [Citation94–96].
Moving forward, if the trends in nOPV2 production and use for emergency outbreak response () continue to imply ongoing demand averaging approximately 350 million doses per year, then this could lead to further policy shifts. At this level of expected use and given that cVDPV2 outbreaks represent an ongoing challenge, continued manufacture and use of nOPV2 in RI may emerge as the logical choice instead of relying on reactive immunization using doses from a stockpile. Experience with OPV2 cessation also raises questions about the prospects for bOPV cessation and the role of nOPVs for types 1 and 3 in different formulations, IPV, and other potential future poliovirus vaccines.
Modelers who seek to forecast poliovirus vaccine demand, including health economists and policy makers, must keep track of the evolving landscape and understand the complexity in the system. Vaccine production comes with long time delays and the doses available for delivery today entered production between 1–2 years ago, then proceeded through a series of stages, from bulk production to filling and finishing, with multiple testing and regulatory requirements completed along the way [Citation3]. In some cases, manufacturers can produce excess vaccine bulk stocks and store these, which can enable shorter times for delivery (6–12 months) for filling and finishing, or potentially as short as 5 months for semifinished mOPV2 [Citation36]. However, in general, time delays in the system imply that demand forecasts must anticipate needs 1–2 years in advance.
4.4. Implications for other stakeholders
The optimal market matches supply and demand, with manufacturers preferring excess demand, and stakeholders preferring excess supply. Getting it right depends on forecasting, truly understanding the system dynamics, and agreements among all stakeholders about strategies, even in the face of substantial challenges. Oversupply of vaccines wastes resources, because unused vaccines expire and must be destroyed. However, when manufacturers produce vaccine with agreements guaranteeing their purchase, then the costs of oversupply can shift to purchasers, or in this case, to the GPEI. In addition, market signals of lower demand than the available production capacity combined with real opportunity costs for manufacturers may motivate manufacturers to leave the market. Manufacturers already operating production facilities operating beyond their expected lifetime and for low margin products could see the complexity and uncertainty as motivation to end production. Two major Sabin OPV manufacturers ended their bulk OPV production by the early 2020s. In contrast, undersupply of vaccines results in difficult choices to prioritize the allocation of available doses to some countries and not others. Supply issues such as this have hampered nOPV2 roll out at various times [Citation97].
Manufacturers navigating the complexity and uncertainty will likely look for multi-year projections and advanced market commitments (i.e. guarantees for future purchases [Citation98]) when they consider any new investments. New manufacturers must offset all of the substantial upfront investment costs for (1) developing products, (2) building, maintaining, and operating production capacity, and (3) licensing and stewardship of products with funds upfront and/or an expected multi-year stream of income. This means that new manufacturers will likely need to anticipate a decade (or more) of sufficient demand to support substantial investments, and self-producing countries need to anticipate substantial national costs of major changes in global strategy. Notably, at a time by which polio vaccine production might have stopped if polio eradication had achieved milestones and successfully stopped OPV2 preventive use, new polio manufacturers continue to enter the market, and demand projections extend through 2027 [Citation64].
As immunization recommendations change, polio eradication delays continue, and polio end game strategies evolve, national leaders must finance (or find external support) for continued polio immunization and eradication activities. In addition to immunization, polio endgame activities also include compliance with global requirements for the containment of live polioviruses and ongoing (and increasingly costly) surveillance programs, which also imply ongoing costs. Containment requirements add costs associated with the identification and destruction of non-essential samples or laboratory stocks of live poliovirus vaccines, and the establishment of national advisory committees to oversee compliance for facilities that continue to hold, store, or use live polioviruses [Citation99], which can increase costs for vaccine manufacturers and the prices of vaccines as well as national diagnostic facilities. In addition, areas around vaccine manufacturers need to maintain high levels of immunization and surveillance to protect the population in the event of a containment breach [Citation100,Citation101].
5. Conclusions
Now 23 years after the original target for eradicating polio, the number of doses and the costs per dose of polio vaccines continue to increase [Citation86]. The increasing costs imply no expected eradication dividend for polio eradication. The increasing complexity of poliovirus vaccine options and uncertainties about mid- to long-term strategies and timelines for the polio endgame limit the ability of models to provide reliable forecasts of vaccines needs [Citation24]. Perhaps the largest hurdle to forecasting vaccine needs for poliovirus stockpiles relates to uncertainty about whether countries will use the resources as planned and developed, or if they will pursue their own preferences and accept mismatches between vaccine supply and demand along with the associated health and financial consequences.
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors substantially contributed to the conception and design of the review article, interpreting the relevant literature, and writing and revising it for intellectual content.
Supplemental Material
Download MS Word (40.1 KB)Acknowledgments
The authors thank Ann Ottosen, Ian Lewis, Vachagan Harutyunyan, David Woods, and Eric Wiesen for helpful comments and discussions.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2023.2263096
Additional information
Funding
References
- Fine PE, Sutter RW, Orenstein WA. Stopping a polio outbreak in the post-eradication era. Dev Biol (Basel). 2001;105:129–150.
- Esbitt D. The strategic national stockpile: roles and responsibilities of health care professionals for receiving the stockpile assets. Disaster Manag Response. 2003 Jul;1(3):68–70. doi: 10.1016/S1540-2487(03)00044-0
- Duintjer Tebbens RJ, Pallansch MA, Alexander JP, et al. Optimal vaccine stockpile design for an eradicated disease: application to polio. Vaccine. [2010 Jun 11];28(26):4312–4327. doi: 10.1016/j.vaccine.2010.04.001
- Jarrett S, Pagliusi S, Park R, et al. The importance of vaccine stockpiling to respond to epidemics and remediate global supply shortages affecting immunization: strategic challenges and risks identified by manufacturers. Vaccine: X. 2021 Dec;9:100119.
- Thompson KM, Duintjer Tebbens RJ. Framework for optimal global vaccine stockpile design for vaccine-preventable diseases: application to measles and cholera vaccines as contrasting examples. Risk Anal. 2016 Jul;36(7):1487–1509. doi: 10.1111/risa.12265
- Yen C, Hyde TB, Costa AJ, et al. The development of global vaccine stockpiles. Lancet Infect Dis. 2015 Mar;15(3):340–347. doi: 10.1016/S1473-3099(14)70999-5
- Prevots DR, Burr RK, Sutter RW, et al. Poliomyelitis prevention in the United States. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. [2000 May 19];49(RR–5):1–22.
- Alexander L, Birkhead G, Guerra F, et al. Ensuring preparedness for potential poliomyelitis outbreaks: recommendations for the US poliovirus vaccine stockpile from the National Vaccine Advisory Committee (NVAC) and the Advisory Committee on Immunization Practices (ACIP). Archives Of Pediatrics & Adolescent Medicine. 2004 Dec;158(12):1106–1112.
- Jenkins PC, Modlin JF. Decision analysis in planning for a polio outbreak in the United States. Pediatrics. 2006 Aug;118(2):611–618. doi: 10.1542/peds.2005-2358
- Thompson KM, Wallace GS, Tebbens RJ, et al. Trends in the risk of U.S. polio outbreaks and poliovirus vaccine availability for response. Public Health Rep. 2012 Jan;127(1):23–37. doi: 10.1177/003335491212700104
- WHO. Polio vaccines: WHO position paper - June 2022. Weekly Epidemiol Rec. 2022;97(25):277–300.
- Duintjer Tebbens RJ, Pallansch MA, Chumakov KM, et al. Expert review on poliovirus immunity and transmission. Risk Anal. 2013 Apr;33(4):544–605. doi: 10.1111/j.1539-6924.2012.01864.x
- Duintjer Tebbens RJ, Pallansch MA, Kew OM, et al. Risks of paralytic disease due to wild or vaccine-derived poliovirus after eradication. Risk Anal. 2006 Dec;26(6):1471–1505. doi: 10.1111/j.1539-6924.2006.00827.x
- Platt LR, Estivariz CF, Sutter RW. Vaccine-associated paralytic poliomyelitis: a review of the epidemiology and estimation of the global burden. J Infect Dis. [2014 Nov 1];210(suppl 1):S380–9. doi: 10.1093/infdis/jiu184
- Duintjer Tebbens RJ, Pallansch MA, Kim JH, et al. Oral poliovirus vaccine evolution and insights relevant to modeling the risks of circulating vaccine-derived polioviruses (cVdpvs). Risk Anal. 2013 Apr;33(4):680–702. doi: 10.1111/risa.12022
- Konopka-Anstadt JL, Campagnoli R, Vincent A, et al. Development of a new oral poliovirus vaccine for the eradication end game using codon deoptimization. NPJ Vaccines. 2020;5(1):26. doi: 10.1038/s41541-020-0176-7
- GPEI. GPEI statement on cVDPV2 detections in Burundi and Democratic Republic of the Congo. Geneva (Switzerland): World Health Organization; 2023 [2023 Apr 25]. Available from: https://polioeradication.org/news-post/gpei-statement-on-cvdpv2-detections-in-burundi-and-democratic-republic-of-the-congo/.
- WHO. Report of the second joint meeting (hybrid) of the WHO global advisory committee on vaccine safety and the WHO advisory committee on safety of medicinal products. Wkly Epidemiol Rec. [2022 Dec 14–16] Mar 3 2023;98(9):83–92.
- Haldar P, Agrawal P, Bhatnagar P, et al. Fractional-dose inactivated poliovirus vaccine, India. Bull World Health Organ. 2019 May 1;97(5):328–334. doi: 10.2471/BLT.18.218370
- Gamage D, Ginige S, Palihawadana P. National introduction of fractional-dose inactivated polio vaccine in Sri Lanka following the global “switch”. WHO South East Asia J Public Health. 2018 Sep;7(2):79–83. doi: 10.4103/2224-3151.239418
- Bashorun AO, Badjie Hydara M, Adigweme I, et al. Intradermal administration of fractional doses of the inactivated poliovirus vaccine in a campaign: a pragmatic, open-label, non-inferiority trial in the Gambia. Lancet Glob Health. 2022 Feb;10(2):e257–e268. doi: 10.1016/S2214-109X(21)00497-6
- Okayasu H, Sein C, Chang Blanc D, et al. Intradermal administration of fractional doses of inactivated poliovirus vaccine: a dose-sparing option for polio immunization. J Infect Dis. [2017 Jul 1];216(suppl_1):S161–s167. doi: 10.1093/infdis/jix038
- Macklin GR, Peak C, Eisenhawer M, et al. Enabling accelerated vaccine roll-out for Public Health Emergencies of International Concern (PHEICs): novel oral polio vaccine type 2 (nOPV2) experience. Vaccine. 2023 Apr 6;41(Suppl 1):A122–A127. doi: 10.1016/j.vaccine.2022.02.050
- Kalkowska DA, Wassilak SGF, Wiesen E, et al. Complexity of options related to restarting Oral Poliovirus Vaccine (OPV) in national immunization programs after OPV cessation. Gates Open Res. 2023;7:55. doi: 10.12688/gatesopenres.14511.1
- Yeh MT, Smith M, Carlyle S, et al. Genetic stabilization of attenuated oral vaccines against poliovirus types 1 and 3. Nature. 2023 [2023 Jul 1];619(7968):135–142. doi: 10.1038/s41586-023-06212-3
- Bahar MW, Porta C, Fox H, et al. Mammalian expression of virus-like particles as a proof of principle for next generation polio vaccines. NPJ Vaccines. [2021 Jan 8];6(1):5. doi: 10.1038/s41541-020-00267-3
- Xu Y, Ma S, Huang Y, et al. Virus-like particle vaccines for poliovirus types 1, 2, and 3 with enhanced thermostability expressed in insect cells. Vaccine. [2019 Apr 17];37(17):2340–2347. doi: 10.1016/j.vaccine.2019.03.031
- WHO. Poliomyelitis: mechanism for management of potetnial risks to eradication. Geneva (Switzerland): World Health Organization; 2008 [cited May 9, 2023]. Available from: https://polioeradication.org/wp-content/uploads/2016/07/WHA61_Resolution_English.pdf.
- WHO. Poliomyelitis. Geneva (Switzerland): World Health Organization; 2015 [cited May 9, 2023]. Available from: https://polioeradication.org/wp-content/uploads/2016/07/A68_R3-en.pdf.
- Duintjer Tebbens RJ, Sangrujee N, Thompson KM. The costs of polio risk management policies after eradication. Risk Analysis. 2006 Dec;26(6):1507–1531. doi: 10.1111/j.1539-6924.2006.00842.x
- Harutyunyan V, Quddus A, Pallansch M, et al. Global oral poliovirus vaccine stockpile management as an essential preparedness and response mechanism for type 2 poliovirus outbreaks following global oral poliovirus vaccine type 2 withdrawal. Vaccine. [2023 Apr 6];41 (Suppl 1):A70–A78. doi: 10.1016/j.vaccine.2022.02.058
- GPEI. Responding to a poliovirus outbreak - part 2: protocol for poliovirus type 2. Geneva (Switzerland): World Health Organization; 2017 [cited 2023 May 10]. Available from: https://polioeradication.org/wp-content/uploads/2018/01/pol-sop-responding-polio-event-outbreak-part2-20180117.pdf.
- Duintjer Tebbens RJ, Thompson KM. Managing the risk of circulating vaccine-derived poliovirus during the endgame: oral poliovirus vaccine needs. BMC Infect Dis. [2015 Sep 24];15(1):390. doi: 10.1186/s12879-015-1114-6
- Duintjer Tebbens RJ, Pallansch MA, Wassilak SGF, et al. Characterization of outbreak response strategies and potential vaccine stockpile needs for the polio endgame. BMC Infecti Dis. 2016;16(1):137. doi: 10.1186/s12879-016-1465-7
- Duintjer Tebbens RJ, Thompson KM. Poliovirus vaccination during the endgame: insights from integrated modeling. Expert Rev Vaccines. 2017;16(6):577–586. doi: 10.1080/14760584.2017.1322514
- Duintjer Tebbens RJ, Thompson KM. Polio endgame risks and the possibility of restarting the use of oral poliovirus vaccine. Expert Rev Vaccines. 2018 [2018 Aug 3];17(8):739–751. doi: 10.1080/14760584.2018.1506333.
- GPEI. Polio eradication & endgame strategic Plan 2013-2018. Geneva (Switzerland): World Health Organization; 2013 [cited 2023 May 9]. Available from: http://polioeradication.org/wp-content/uploads/2016/07/PEESP_EN_A4.pdf.
- Thompson KM, Kalkowska DA. Review of poliovirus modeling performed from 2000-2019 to support global polio eradication. Expert Rev Vaccines. 2020;19(7):661–686. doi: 10.1080/14760584.2020.1791093
- Kalkowska DA, Voorman A, Pallansch MA, et al. The impact of disruptions caused by the COVID-19 pandemic on global polio eradication. Vaccine. [2023 Apr 6];41 (Suppl 1):A12–A18. doi: 10.1016/j.vaccine.2021.04.026
- Kalkowska DA, Pallansch MA, Wassilak SGF, et al. Serotype 2 oral poliovirus vaccine (OPV2) choices and the consequences of delaying outbreak response. Vaccine. [2023 Apr 6];41(Suppl 1):A136–A141. doi: 10.1016/j.vaccine.2021.04.061
- Kalkowska DA, Wassilak SGF, Pallansch MA, et al. Outbreak response strategies with type 2-containing oral poliovirus vaccines. Vaccine. [2023 Apr 6];41 (Suppl 1):A142–A152. doi: 10.1016/j.vaccine.2022.10.060
- Kalkowska DA, Pallansch MA, Wilkinson A, et al. Updated characterization of poliovirus outbreak response strategies for 2019-2029: impacts of the use of novel OPV2 strains. Risk Anal. 2021;41(2):329–348. doi: 10.1111/risa.13622
- Kalkowska DA, Thompson KM. Expected implications of globally-coordinated cessation of serotype 3 oral poliovirus vaccine (OPV) before serotype 1 OPV. Risk Anal. 2021;41(2):312–319. doi: 10.1111/risa.13590
- Kalkowska DA, Wassilak SGF, Wiesen E, et al. Coordinated global cessation of oral poliovirus vaccine use: options and potential consequences. Risk Anal. 2023 Jun 21. Online ahead of print. doi: 10.1111/risa.14158
- Kalkowska DA, Wiesen E, Wassilak SGF, et al. Worst-case scenarios: modeling uncontrolled type 2 polio transmission. Risk Anal. 2023 Jun 21. Online ahead of print. doi: 10.1111/risa.14159
- UNICEF Supply Division. Consultation between GPEI and poliovirus vaccine manufacturers, national authorities for containment and national regulatory authorities, Virtual meeting, 11 October 2022. 2023 [cited 2023 Feb 27]]. Available from: https://polioeradication.org/wp-content/uploads/2023/01/Annual-GPEI-Consultation-with-polio-vaccine-manufacturers-NACs-NRAsv-prepublication-version.pdf.
- World Health Organization. Global OPV stockpile strategy 2022-2026, November 2022. Geneva (Switzerland); 2023 [cited 2023 Aug 10]. https://polioeradication.org/wp-content/uploads/2023/06/Global-OPV-Stockpile-Strategy-31052023.pdf
- GPEI. Polio endgame strategy 2019-2023: eradication, integration, certification and containmen. Geneva (Switzerland): World Health Organization; 2019 [cited 2020 Jun 18]. Available from: https://apps.who.int/iris/bitstream/handle/10665/329948/WHO-Polio-19.04-eng.pdf.
- GPEI. Strategy for response to type 2 circulating vaccine-derived poliovirus 2020-2021: An addendum to the polio endgame strategy 2019-2023. Geneva (Switzerland): World Health Organization; 2020 [cited 2020 Mar 10]. Available from: http://polioeradication.org/wpcontent/uploads/2020/04/Strategy-for-the-response-to-type-2-circulating-Vaccine-DerivedPoliovirus-20200406.pdf.
- GPEI. Delivering on a promise. Polio eradication strategy 2022–2026. Geneva (Switzerland): World Health Organization; 2021 [cited 2021 Jun 18]. Available from: https://polioeradication.org/wp-content/uploads/2021/06/polio-eradication-strategy-2022-2026-pre-publication-version-20210609.pdf.
- GPEI. Library - policy and reports. Geneva (Switzerland): World Health Organization; 2023 [cited 2023 Mar 1]. Available from: https://polioeradication.org/library/.
- UNICEF. Search. New york (NY): United Nations Children’s Fund; 2023 [cited 2023 Mar 1]. Available from: https://www.unicef.org/search.
- United Nations. World population prospects 2019. (NY): United Nations, Department of Economic and Social Affairs, Population Division; 2019 [cited 2023 May 9]. Available from: https://www.un.org/development/desa/pd/news/world-population-prospects-2019-0.
- World Bank. World Bank country and lending groups. Washington DC: The World Bank; 2023 [cited 2023 May 9]. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- Sriudomporn S, Watts E, Yoon Sim S, et al. Achieving immunization agenda 2030 coverage targets for 14 pathogens: projected product and immunization delivery costs for 194 countries, 2021–2030. Vaccine: X. 2023 [2023 Apr 1];13:100256.
- Smith G, Michelson J, Singh R, et al. Is there enough vaccine to eradicate measles? An integrated analysis of measles-containing vaccine supply and demand. J Infect Dis. 2011;204(suppl_1):S62–S70. doi: 10.1093/infdis/jir130
- Kalkowska DA, Badizadegan K, Thompson KM. Outbreak management strategies for cocirculation of multiple poliovirus types. Vaccine. [2023 Jun 7];41(25):3718–3727. doi: 10.1016/j.vaccine.2023.04.037
- Thompson KM, Duintjer Tebbens RJ. The case for cooperation in managing and maintaining the end of poliomyelitis: stockpile needs and coordinated OPV cessation. Medscape J Med. 2008;10(8):190.
- Duintjer Tebbens RJ, Pallansch MA, Cochi SL, et al. An economic analysis of poliovirus risk management policy options for 2013–2052. BMC Infect Dis. 2015 [2015 Sep 24];15(1):389. doi: 10.1186/s12879-015-1112-8
- Kalkowska DA, Pallansch MA, Wassilak SGF, et al. Global transmission of live polioviruses: updated dynamic modeling of the polio endgame. Risk Anal. 2021 Feb;41(2):248–265. doi: 10.1111/risa.13447
- UNICEF. Request for proposals - monovalent (Sabin) oral polio vaccine type 2 in bulk form, potential conversion into finished product and delivery, including the services for storage, management, and maintenance in a global stockpile. UNICEF Supply Division; 2020 [cited 2023 May 10]. Available from: https://www.ungm.org/Public/Notice/118260.
- WHO. Circulating vaccine-derived poliovirus. 2023. Available from: https://polioeradication.org/polio-today/polio-now/this-week/circulating-vaccine-derived-poliovirus/.
- WHO. Statement of the thirty-sixth meeting of the polio IHR emergency committee. Geneva (Switzerland): World Health Organization; 2023 [cited 2023 Sep 1]. Available from: https://www.who.int/news/item/25-08-2023-statement-of-the-thirty-sixth-meeting-of-the-polio-ihr-emergency-committee.
- UNICEF. Vaccine industry consultation 2022: product updates for the portfolio of polio vaccines. UNICEF Supply Division; 2022 [cited 2023 Apr 21]. Available from: https://www.unicef.org/supply/media/14136/file/Portfolio-Polio-products-Ian-Lewis-Jason-Thompson-2022.pdf.
- GPEI. Delivering on the promise of a polio free world for every child - vaccine industry consultation, September 2022. Geneva (Switzerland): Global Polio Eradication Initiative; 2022 [cited 2023 Apr 21]. Available from: https://www.unicef.org/supply/media/14131/file/Programme-Market-Update-Polio-Steven-Lauwerier-2022.pdf.
- Lewis I, Ottosen A, Rubin J, et al. A supply and demand management perspective on the accelerated global introductions of inactivated poliovirus vaccine in a constrained supply market. J Infect Dis. [2017 Jul 1];216(suppl_1):S33–s39. doi: 10.1093/infdis/jiw550
- Rubin J, Ottosen A, Ghazieh A, et al. Managing the planned cessation of a global supply market: lessons learned from the global cessation of the trivalent oral poliovirus vaccine market. J Infect Dis. [2017 Jul 1];216(suppl_1):S40–s45. doi: 10.1093/infdis/jiw571
- WHO. 14th meeting of the SAGE polio Working group - Conclusions and recommendations. Geneva (Switzerland): World Health Organization; 2017 [cited 2023 May 10]. Available from: https://terrance.who.int/mediacentre/data/sage/SAGE_Docs_Ppt_Oct2017/5_session_polio/Oct2019_session5_14th_SAGE_PolioWG.pdf.
- Duintjer Tebbens RJ, Hampton LM, Wassilak SGF, et al. Maintenance and intensification of bivalent oral poliovirus vaccine use prior to its coordinated global cessation. J Vaccines Vaccin. 2016 Oct;7(5). doi: 10.4172/2157-7560.1000340
- GPEI. Consultation between the GPEI and polio vaccine manufacturers, national authorities for containment and national regulatory authorities. Geneva (Switzerland): Global Polio Eradication Initiative; 2022 [cited 2023 SepSep 1]. Available from: https://polioeradication.org/wp-content/uploads/2023/04/Annual-GPEI-Consultation-with-polio-vaccine-manufacturers-NACs-NRAs-V1.2-20220331.pdf
- UNICEF. Market supply and update: oral polio vaccine, bivalent (bOPV). UNICEF; 2022 [cited 2023 Apr 21]. Available from: https://www.unicef.org/supply/media/14396/file/bOPV-Poster-2022.pdf.
- GPEI. Consultation between the global polio eradication Initiative and poliovirus vaccine manufacturers, national authorities for containment and national regulatory authorities. Geneva (Switzerland): Global Polio Eradication Initiative; 2021 [cited 2023 Apr 21]. Available from: https://polioeradication.org/wp-content/uploads/2022/01/GPEI-Consultation-with-Polio-Vaccine-Manufacturers-NACs-NRAs-2021-v1.0.pdf.
- Aylward RB, Sutter RW, Heymann DL. OPV cessation–the final step to a “polio-free” world. Science. 2005 Oct 28;310(5748):625–626.
- Chumakov K, Ehrenfeld E, VI A, et al. Polio eradication at the crossroads. Lancet Glob Health. 2021;9(8):E1172–5. doi: 10.1016/S2214-109X(21)00205-9
- Thompson KM, Kalkowska DA, Badizadegan K. Health economic analysis of vaccine options for the polio eradication endgame: 2022-2036. Expert Rev Vaccines. 2022 Oct;5(11):1–8. doi: 10.1080/14760584.2022.2128108
- Bart KJ, Foulds J, Patriarca P. Global eradication of poliomyelitis: benefit-cost analysis. Bull World Health Organ. 1996;74(1):35–45.
- Wright PF, Kim-Farley RJ, de Quadros CA, et al. Strategies for the global eradication of poliomyelitis by the year 2000. N Engl J Med. [1991 Dec 19];325(25):1774–1779. doi: 10.1056/NEJM199112193252504
- Thompson KM, Kalkowska DA. An updated economic analysis of the global polio eradication Initiative. Risk Anal. 2021 Feb;41(2):393–406. doi: 10.1111/risa.13665
- Okayasu H, Sein C, Hamidi A, et al. Development of inactivated poliovirus vaccine from Sabin strains: a progress report. Biologicals. 2016 Nov;44(6):581–587. doi: 10.1016/j.biologicals.2016.08.005
- Capeding MR, Gomez-Go GD, Oberdorfer P, et al. Safety and immunogenicity of a new inactivated polio vaccine made from Sabin strains: a randomized, double-blind, active-controlled, phase 2/3 seamless study. J Infect Dis. 2020;226(2):308–318. doi: 10.1093/infdis/jiaa770
- Okayasu H, Sutter RW, Jafari HS, et al. Affordable inactivated poliovirus vaccine: strategies and progress. J Infect Dis. [2014 Nov 1];210(Suppl 1):S459–64. doi: 10.1093/infdis/jiu128
- Simizu B, Abe S, Yamamoto H, et al. Development of inactivated poliovirus vaccine derived from Sabin strains. Biologicals. 2006 Jun;34(2):151–154. doi: 10.1016/j.biologicals.2006.02.010
- GPEI. Consultation with OPV/IPV manufacturers, national authorities for containment and national regulatory authorities. Geneva (Switzerland): Global Polio Eradication Initiative; 2020 [cited 2023 Apr 21]. Available from: https://polioeradication.org/wp-content/uploads/2022/01/2020-GPEI-Consultation-with-OPV-IPV-Manufacturers-NACs-NRAs.pdf.
- Duintjer Tebbens RJ, Pallansch MA, Cochi SL, et al. Modeling poliovirus transmission in Pakistan and Afghanistan to inform vaccination strategies in undervaccinated subpopulations. Risk Anal. 2018 Aug;38(8):1701–1717. doi: 10.1111/risa.12962
- Sharma P. Biological E, panacea to make polio vaccine. 2023 [cited 2023 May 11]. Available from: https://www.livemint.com/news/india/india-prepares-to-produce-and-stockpile-new-polio-vaccine-to-fight-vaccine-derived-polio-virus-outbreaks-globally-11683826896263.html.
- Kalkowska DA, Thompson KM. Health and economic outcomes associated with polio vaccine policy options: 2019-2029. Risk Anal. 2021 Feb;41(2):364–375. doi: 10.1111/risa.13664
- Meeting of the strategic advisory group of experts on immunization, April 2017 – conclusions and recommendations. Wkly Epidemiol Rec. [2017 Jun 2];92(22):301–320.
- Mahmood K, Pelkowski S, Atherly D, et al. Hexavalent IPV-based combination vaccines for public-sector markets of low-resource countries. Hum Vaccin Immunother. 2013 Sep;9(9):1894–1902. doi: 10.4161/hv.25407
- Olivera I, Grau C, Dibarboure H, et al. Valuing the cost of improving Chilean primary vaccination: a cost minimization analysis of a hexavalent vaccine. BMC Health Serv Res. 2020 [2020 Apr 9];20(1):295. doi: 10.1186/s12913-020-05115-7
- Trueba G, Jeyaseelan V, Lopez L, et al. Achieving high immunogenicity against poliovirus with fractional doses of inactivated poliovirus vaccine in ecuador-results from a cross-sectional serological survey. The Lancet Regional Health – Americas. 2022;11:11. doi: 10.1016/j.lana.2022.100235
- Thompson KM, Kalkowska DA. Reflections on modeling poliovirus transmission and the polio eradication endgame. Risk Anal. 2021 Feb;41(2):229–247. doi: 10.1111/risa.13484
- Darwar R, Biya O, Greene SA, et al. Assessing country compliance with circulating vaccine-derived poliovirus type 2 outbreak response standard operating procedures: April 2016 to December 2020. Vaccine. [2023 Apr 6];41 (Suppl 1):A25–a34. doi: 10.1016/j.vaccine.2023.02.060
- Thompson KM, Kalkowska DA, Badizadegan K. Looking back at prospective modeling of outbreak response strategies for managing global type 2 oral poliovirus vaccine (OPV2) cessation [original research]. Front Public Health. 2023 [2023 Mar 24];11:11. doi: 10.3389/fpubh.2023.1098419
- Kennedy SB, Macklin GR, Mason Ross G, et al. Poliovirus antibodies following two rounds of campaigns with a type 2 novel oral poliovirus vaccine in Liberia: a clustered, population-based seroprevalence survey. Lancet Glob Health. 2023 Jun;11(6):e917–e923. doi: 10.1016/S2214-109X(23)00116-X
- Thompson KM. Polio endgame complexity: updating expectations for nOPV2. Lancet Infect Dis. [2023 May 10];23(9):992–994. doi: 10.1016/S1473-3099(23)00133-0
- Wilkinson AL, Zaman K, Hoque M, et al. Immunogenicity of novel oral poliovirus vaccine type 2 administered concomitantly with bivalent oral poliovirus vaccine: an open-label, non-inferiority, randomised, controlled trial. Lancet Infect Dis. [2023 May 10];23(9):1062–1071. doi: 10.1016/S1473-3099(23)00139-1
- WHO. Poliomyelitis eradication: report by the Director-General. Geneva (Switzerland): World Health Organization; 2022 [cited 2023 Mar 1]. https://apps.who.int/gb/ebwha/pdf_files/EB152/B152_18-en.pdf
- Batson A, Glassman A, Federgruen A, et al. The world needs to prepare now to prevent polio resurgence post eradication. BMJ Glob Health. 2022 Dec;7(12):e011485. doi: 10.1136/bmjgh-2022-011485
- WHO. Global Action Plan for poliovirus containment, fourth edition (unedited version). Geneva (Switzerland): World Health Organization; 2022 [cited 2023 Feb 10]. Available from: https://polioeradication.org/wp-content/uploads/2022/07/WHO-Global-Action-Plan-for-Poliovirus-Containment-GAPIV.pdf.
- Duintjer Tebbens RJ, Kalkowska DA, Thompson KM. Poliovirus containment risks and their management. Future Virol. 2018;13(9):617–628. doi: 10.2217/fvl-2018-0079
- Duizer E, Ruijs WL, Putri Hintaran A, et al. Wild poliovirus type 3 (WPV3)-shedding event following detection in environmental surveillance of poliovirus essential facilities, the Netherlands, November 2022 to January 2023. Eurosurveillance. 2023;28(5):2300049. doi: 10.2807/1560-7917.ES.2023.28.5.2300049

