 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objectives
Immunogenicity between 15-valent V114 (PCV15) and 20-valent PCV20 pneumococcal conjugate vaccines in healthy infants is compared in an indirect treatment comparison and matching-adjusted indirect comparison. Hypotheses: immunogenicity of V114 is non-inferior to PCV20 for all PCV13 serotypes, and superior to PCV20 for serotype 3 based on lower bound margins.
Methods
Two phase 3 pivotal studies on 3 + 1 pediatric vaccination schedule at age 2, 4, 6, and 12–15 months compared V114 (N = 858) to PCV13 (N = 856) and PCV20 (N = 1001) to PCV13 (N = 987). Infant’s age and race in V114 study were matched to those in PCV20 study. Primary endpoints were serotype-specific Immunoglobulin G (IgG) response rate difference (RRD) 30 days post-dose (PD)3; IgG geometric mean concentration (GMC) ratios 30 days PD3 and PD4.
Results
V114 was non-inferior (>-10%-point;
>0.5) to PCV20 (p-value <0.001) for all endpoints. V114 was superior (
>0%-point;
>1.2) to PCV20 (p-value <0.001) for serotype 3: RRD was 34.5% (95%CI 27.9%-41.1%) PD3, and IgG GMC ratios were 2.39 (95%CI 2.12–2.68) PD3 and 2.15 (95%CI 1.90–2.41) PD4.
Conclusion
Immune response to V114 administered in a 3 + 1 schedule in healthy infants was considered non-inferior to PCV20 for all 13 PCV13 serotypes and superior for serotype 3 PD3 and PD4.
Clinical trial registration
www.clinicaltrials.gov identifiers NCT03893448, NCT04382326
1. Introduction
Disease caused by Streptococcus pneumoniae (pneumococcus) can be serious and sometimes life threatening. Pneumococci are gram-positive bacteria surrounded by a complex polysaccharide capsule, which is a key factor for the bacteria’s virulence, pathogenicity, and evasion of phagocytosis by the host’s immune system [Citation1–4]. Based on the unique composition of its capsule (or lack thereof), over 100 serotypes of S. pneumoniae have been identified [Citation3,Citation4]. Pneumococcal colonization peaks in young children (~3 years of age) [Citation1] and can lead to noninvasive or invasive disease. Invasive pneumococcal disease (IPD) mainly affects young children due to an immature immune system, and people with a weakened or defective immune system (e.g. the elderly and immunocompromised people) [Citation5–7].
The introduction of pneumococcal conjugate vaccines in national immunization programs has significantly reduced IPD incidence and mortality in children [Citation8,Citation9]. In the last decade, 10-valent (PCV10, SynflorixTM) and 13-valent (PCV13, Prevnar 13TM) pneumococcal conjugate vaccines were the most frequently used in childhood vaccination programs worldwide [Citation10]. PCV10 and PCV13 both include serotypes 1, 4, 5, 6B, 7F, 9 V, 14, 18C, 19F, and 23F, with PCV13 additionally including serotypes 3, 6A, and 19A. Pneumococcal conjugate vaccines are generally effective in the prevention of IPD and other disease manifestations such as acute otitis media [Citation11,Citation12]. However, residual IPD burden remains, especially in children <2 years of age; residual disease is often due to invasive S. pneumoniae serotypes not included in PCV10 and PCV13, but they can also be caused by vaccines serotypes in the setting of breakthrough disease (receipt of incomplete vaccination series) or vaccine failures or lack of vaccination [Citation9,Citation13,Citation14]. Research is ongoing to develop new vaccines that expand protection to additional pathogenic serotypes whilst maintaining or even improving protection for current vaccine serotypes. The 15-valent pneumococcal conjugate vaccine V114 (PCV15, VaxneuvanceTM) and 20-valent pneumococcal conjugate vaccine PCV20 (Prevnar 20®) received United States Food and Drug Administration (US FDA) approval for the prevention of IPD in infants (6 weeks through 11 months of age) and children (12 months through ≥24 months of age) in 2022 and 2023, respectively [Citation15,Citation16]. V114 covers the 13 serotypes included in PCV13 plus serotypes 22F and 33F, and PCV20 covers these 15 serotypes plus serotypes 10A, 11A, 12F, and 15B. Both approvals were based on immunogenicity bridging in phase 3 studies, which compared the study vaccine (V114 or PCV20) to PCV13 in a 3 + 1 dose schedule with doses at 2, 4, 6, and 12 to 15 months of age in healthy infants. In the pivotal phase 3 study of V114 in healthy infants [Citation17], V114 met non-inferiority criteria compared to PCV13 for all serotypes for Immunoglobulin G (IgG) response rates (proportion of infants having an IgG ≥0.35 µg/mL in the enzyme-linked immunosorbent assay [ELISA] standardized by the World Health Organization [WHO]) 30 days after dose 3. Evaluation of the IgG geometric mean concentrations (GMCs) showed that V114 met non-inferiority criteria for all serotypes after the third and fourth doses, except for serotype 6A after dose 3. Additionally, the V114 IgG GMC was superior for the shared serotype 3 and the unique serotypes 22F and 33F compared to PCV13, after both the third and fourth doses. In the pivotal phase 3 study of PCV20 in healthy infants, IgG response rates met non-inferiority criteria after the third dose for types 5, 6A, 6B, 7F, 14, 18C, 19A, and 19F, but did not meet non-inferiority criteria for serotypes 1, 3, 4, 9 V, and 23F [Citation18]. IgG GMC ratios were non-inferior for all 13 vaccine types shared with PCV13 after both the third and fourth doses.
No direct head-to-head comparison exists between V114 and PCV20. Therefore, an unadjusted indirect treatment comparison (ITC) and a matching-adjusted indirect comparison (MAIC) were conducted using data from the abovementioned two phase 3 pivotal studies to compare immunogenicity of V114 versus PCV20 in a 3 + 1 schedule in healthy infants. Only phase 3 pivotal studies were considered due to the use final formulation of the experimental vaccines and adequately powered study for hypothesis testing. It was hypothesized that both after the third and fourth doses, 1) immunogenicity of V114 would be non-inferior to PCV20 for the 13 serotypes shared with PCV13, and 2) immunogenicity of V114 would be superior to PCV20 for serotype 3.
2. Patients and methods
2.1. Data sources and data collection
This study analyzed data from two phase 3, randomized, double-blind clinical trials that evaluated the safety, tolerability, and immunogenicity of a 4-dose regimen (3 + 1 schedule) of V114 (clinicaltrials.gov registration number: NCT03893448) [Citation17,Citation19,Citation20] or PCV20 (clinicaltrials.gov registration number: NCT04382326) [Citation18,Citation21,Citation22] as compared to PCV13 in healthy infants. These studies were selected for the MAIC based on their similar designs (e.g. the same vaccination schedule, endpoints, and control arm) and good quality. Infants were randomized 1:1 to receive a 3 + 1 vaccination schedule of the studied vaccine (PCV20 or V114) or PCV13 concomitant with other routine pediatric vaccines, administered at the age of 2, 4, 6, and 12 to 15 months. The V114 study was conducted from June 2019 to May 2021, the PCV20 study from May 2020 to September 2022. Both studies recruited infants in the United States and Puerto Rico; additionally, the V114 study also recruited infants in Turkey and Thailand [Citation20,Citation21], and applied similar inclusion and exclusion criteria. Both studies evaluated the outcomes of serotype-specific IgG response rates (the proportion of infants meeting serotype-specific IgG threshold values of ≥0.35 µg/mL in the WHO reference ELISA) and serotype-specific IgG GMC ratios 30 days after dose 3 and dose 4. Both phase 3 studies were approved by the appropriate ethics committees and were performed in accordance with the Declaration of Helsinki; written informed consent was obtained from all parents (or legally acceptable representatives) before the infants participated in the studies [Citation20,Citation22].
Researchers had full access to the in-house individual level data from the V114 phase 3 study, and all relevant data were used in the ITC and MAIC analysis. For the PCV20 study, data were extracted from a publicly available data presentation for the Advisory Committee on immunization Practices (ACIP) at the Centers for Disease Control and Prevention (CDC) on 22 February 2023 [Citation18], and from data available in the EU Clinical Trials register [Citation22]. Data used for the ITC and MAIC were extracted by a statistician and validated by a second statistician. Data were extracted and compared for the 13 serotypes in PCV13 (1, 3, 4, 5, 6A, 6B, 7F, 9 V, 14, 18C, 19A, 19F, 23F). Whilst the two additional serotypes in V114 (22F, 33F) are also in the PCV20 vaccine, the immune response to these serotypes were below the lower limit of quantification for the common comparator PCV13, and therefore, were not compared.
2.2. Endpoints and hypotheses
This study had three primary endpoints: 1) serotype-specific IgG response rates ~30 days after the third dose; 2) serotype-specific IgG GMC ratios ~30 days after the third dose; and 3) serotype-specific IgG GMC ratios ~30 days after the fourth dose. In both the V114 and PCV20 phase 3 studies, antibody concentrations were bridged to the WHO reference ELISA to normalize antibody concentrations and successfully validated. Based on this bridging, thresholds for serotype-specific response rates were selected to correspond to 0.35 µg/mL in the WHO reference ELISA: in the V114 study, results confirmed that a single threshold value of 0.35 µg/mL should be applied to each serotype, whilst in the PCV20 study, 0.35 µg/mL was applied to all serotypes except for serotypes 5 (0.23 µg/mL), 6B (0.10 µg/mL), and 19A (0.12 µg/mL). Serotype-specific IgG response rates ~30 days after the fourth dose were not evaluated, due to its limited value for comparative immunogenicity evaluation in toddlers who generally have more robust immune responses to PCVs.
For all three endpoints, it was hypothesized that V114 was non-inferior to PCV20 for the 13 serotypes shared with PCV13. Non-inferiority was defined as a lower bound of the 95% confidence interval (CI) above -10% points for the serotype-specific IgG response rate differences and above 0.5 for the IgG GMC ratios. Additionally, it was hypothesized that V114 was superior to PCV20 for serotype 3 for all three endpoints. Superiority was defined as a lower bound of the 95% CI above 0% points for the response rate differences and above 1.2 for the GMC ratios. These selected non-inferiority and superiority hypotheses and margins are consistent with those specified in the V114 phase 3 study protocol [Citation17,Citation20], substituting the comparator PCV13 with PCV20 in the indirect comparison hypotheses. Furthermore, any other observed statistical differences were described as well.
2.3. Statistical analyses
Statistical analyses were conducted using SAS software version 9.4 (https://www.sas.com/; copyright 2023; SAS Institute Inc., Cary, NC, U.S.A.). All infants who received a third and/or fourth vaccination dose and had an IgG measurement ~30 days after this dose were included in the respective analyses. Infants with missing data (age or race) needed for matching were excluded from the MAIC. All endpoints were analyzed per serotype, i.e. for each of the 13 shared serotypes individually. Baseline characteristics such as infant demographics were summarized per study and/or treatment arm using the mean and standard deviation for continuous variables; categorical variables were displayed as absolute number and percentage per category.
The network of evidence for the analysis consisted of unadjusted ITC (comparing the two study populations without matching by age and race) and MAIC (). The indirect vaccination effect (VE) of V114 versus PCV20 was calculated using the Bucher method via the following equation [Citation23]:
Figure 1. Indirect treatment comparison network.
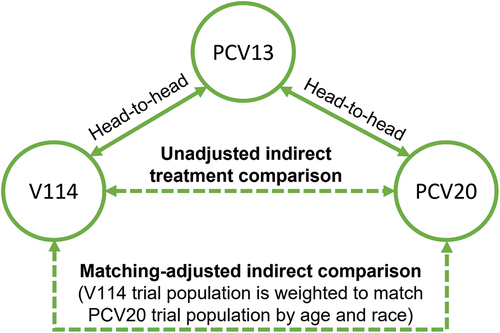
in which was the vaccination effect of V114 versus PCV13, and
was the vaccination effect of PCV20 versus PCV13.
The standard error (SE) was calculated with the standard variance formula for two independent additive normal distributions, i.e. by taking the square root of the sum of the variances:
The 95% CI was then calculated as:
in which was the top 2.5th percentile of the inverse cumulative standard normal distribution. The test of non-inferiority of vaccination effect was based on the Z-statistic for the non-inferiority margin:
Under the null hypothesis, the Z-statistic in EquationEquation 4(4)
(4) approximately follows a standard normal distribution. The one-sided p-value was calculated from the above Z-statistic test. For the analysis of the IgG GMC ratios, the calculation above was performed following a log-transformation.
MAIC was used to adjust for effect modifiers [Citation24] in combination with the Bucher method [Citation23], as in the previous comparisons of V114 and PCV20 in adults [Citation25]. Infant’s characteristics including birthdate and race were collected from the participant’s legally acceptable representative in the V114 and PCV20 studies [Citation20,Citation21]. Age (at first dose) and race were identified as potential effect modifiers based on data from previous studies, and other factors (sex and gestational age), were also discussed but excluded as they were not identified as potential effect modifiers for the immunogenicity of pneumococcal conjugate vaccines in healthy infants [Citation26–28], due to insufficient evidence and inconsistency of reporting in the literature. The decision to use age and race as potential effect modifiers in the analyses was made prior to any analysis and prior to the first announcement of the PCV20 study results [Citation18], and was documented in an ITC statistical analysis plan. To adjust for differences in age (continuous variable) and race (categorical variable) between studies, infants in the V114 study were re-weighted to match the aggregate baseline characteristics of the infant population in the PCV20 study [Citation24,Citation29,Citation30], which then equalizes the contribution of this demographic to the MAIC estimates. It was assumed that the per protocol baseline population was similar to the baseline characteristics in the analyzed populations, i.e. the infants who had IgG measurements ~30 days after dose 3 and 4. Healthy infants (i) in the V114 study were reweighted with weights (wi) estimated from a logistic regression model:
The method of moments was used to estimate the beta values in the absence of individual-level data from the PCV20 study. The effective sample size was computed as the square of the summed weights divided by the sum of the squared weights.
To compare IgG response rates between infants in the V114/PCV20 arm versus infants in the control (PCV13) arm, the unstratified weighted Miettinen and Nurminen method was used to estimate serotype-specific differences in response rates, using a natural scale [Citation31]. A weighted analysis of the serotype-specific IgG GMC ratio for V114/PCV20 versus control (PCV13) was conducted to compare IgG GMC ratios. This entailed estimating the t-distribution variance from a linear model with natural log-transformed antibody concentrations as the response and a single covariate term for vaccination group. Subsequently, the Bucher method [Citation23] was applied to indirectly compare V114 to PCV20. The Bucher approach requires standard errors for the estimated treatment effect in both studies, but the PCV20 study did not report standard errors. Therefore, asymptotic Gaussian approximation was assumed, which exploits the symmetry of confidence intervals in both studies, to calculate the standard errors.
3. Results
The V114 phase 3 study used in this analysis randomized 1720 healthy infants, of whom 1714 received a first dose of V114 (N = 858) or PCV13 (N = 856). In the PCV20 phase 3 study, 1997 healthy infants were randomized, and 1988 received a first dose of PCV20 (N = 1001) or PCV13 (N = 987). Mean age (at first dose) was 61.8 days (SD 8.5 days) in the V114 phase 3 study population and 65.8 days (SD 7.6 days) in the PCV20 phase 3 study population (). Due to the countries where infants were recruited for the two studies, the percentage of Asian infants was higher in the V114 study population compared to the PCV20 study population (26.2% versus 1.7%), whereas the proportion of White (55.5% versus 78.8%) and Black (6.1% versus 11.5%) infants were higher in the PCV20 study. Moment matching of the age and race data in the V114 phase 3 study resulted in an effective sample size of 1049.9 infants and resulted in the same mean age and race distribution of the PCV20 study population, equalizing the contribution of this demographic to the MAIC estimates. Therefore, any differences in general baseline characteristics (Supplementary Table S1) between the two studies are unlikely to matter in the MAIC analysis.
Table 1. Summary description of demographic variables inserted as potential effect modifiers in the MAIC, before and after matching.
Serotype-specific IgG concentrations of samples taken approximately 30 days after the third dose were analyzed in 702 and 665 infants in the V114 and PCV13 arms of the V114 study, respectively, and 833 and 803 infants in the PCV20 and PCV13 arms of the PCV20 study, respectively (Supplementary Table S2). IgG response rates were non-inferior for all serotypes in infants having received V114 compared to those having received PCV20, both in the ITC () and MAIC ( and ) analyses (p < 0.001 for all outcomes). The IgG response rate for serotype 3 was superior in infants receiving V114: the response rate difference was 34.5% (95% CI 27.9% to 41.1%, one-sided p < 0.0001) after matching. Other response rates that differed between V114 and PCV20 in the MAIC were serotypes 4 (risk difference 5.5%, 95% CI 1.2% to 9.7%), 9 V (risk difference 7.1%, 95% CI 3.0% to 11.2%), and 23F (risk difference 6.9%, 95% CI 1.4% to 12.3%) in favor of V114, and serotype 6A (3.5%, 95% CI 6.8% to 0.2%) in favor of PCV20.
Figure 2. Matching-adjusted indirect comparison analysis of the IgG response rate difference for V114 vs PCV20 in healthy infants, ~30 days after completing the third dose (administered at ~6 months of age).
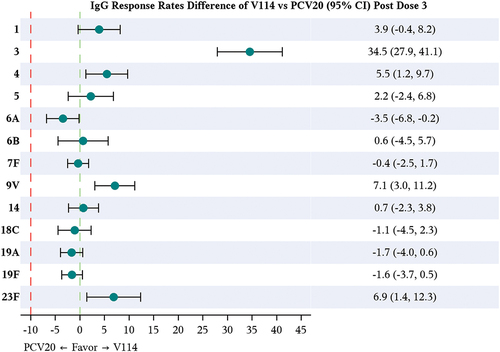
Table 2. Analysis of percentage of infants with a serological response (IgG ≥0.35 µg/mL) 30 days after the third dose (administered at ~6 months of age) – ITC and MAIC of V114 to PCV20.
As with the IgG response rates, IgG GMC ratios were non-inferior ~30 days after the third dose for all serotypes in the analyzed V114 vaccinated infants compared to PCV20 vaccinated infants and superior for serotype 3, both before () and after ( and ) matching of the V114 study population (p < 0.001 for all measurements). The IgG GMC ratio in the MAIC was 2.39 (95% CI 2.12 to 2.68) for serotype 3; no other IgG GMC ratio had the lower bound of the 95% CI above the predefined superiority margin of 1.2. In the MAIC, the lower bound of the 95% CI of the IgG GMC ratio was above 1.00 for four additional serotypes: serotype 4 (1.36, 95% CI 1.19 to 1.55), 6B (1.38, 95% CI 1.13 to 1.70), 9 V (1.35, 95% CI 1.18 to 1.54), and 23F (1.36, 95% CI 1.16 to 1.61); and the upper bound of the 95% CI of the IgG GMC ratio was below 1.00 for serotype 6A (0.72, 95% CI 0.63 to 0.83).
Figure 3. Matching-adjusted indirect comparison analysis of the IgG GMC ratio for V114 vs PCV20 in healthy infants, ~30 days after completing the third (figure A) and fourth (figure B) dose.
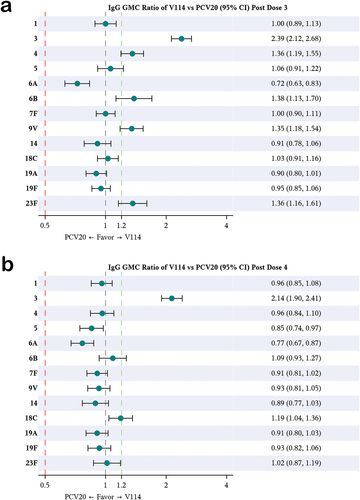
Table 3. Analysis of IgG GMC ratio at 30 days after the third dose (administered at ~6 months of age) – ITC and MAIC of V114 vs PCV20.
Approximately 30 days after the fourth dose, 716 and 686 infants in the V114 and PCV13 arms of the V114 study, respectively, and 755 and 745 infants in the PCV20 and PCV13 arms of the PCV20 study, respectively, had serotype-specific IgG concentrations analyzed (Supplementary Table S2). Consistent with the findings after the third dose, ~30 days after the fourth dose, the lower bound of the 95% CI of all 13 IgG GMC ratios was above the prespecified non-inferiority margin of 0.5, both in the ITC () and MAIC ( and ) analyses between V114 and PCV20. Hence, V114 met the non-inferiority criteria for all 13 serotypes at this time point too. Additionally, the IgG GMC ratio in the MAIC was 2.14 (95% CI 1.90 to 2.41, one-sided p < 0.0001) for serotype 3, indicating superiority of serotype 3 immunogenicity in V114 compared to PCV20. Besides serotype 3, the lower bound of the 95% CI of the IgG GMC ratios was above 1.00 for serotype 18C (1.19, 95% CI 1.04 to 1.36); and the upper bound of the 95% CI was below 1.00 for serotypes 5 (0.85, 95% CI 0.74 to 0.97) and 6A (0.77, 95% CI 0.67 to 0.87) in the MAIC.
Table 4. Analysis of IgG GMC ratio at 30 days after the fourth dose (administered at ~ 12 to 15 months of age) – ITC and MAIC of V114 vs PCV20.
4. Discussion
In this study, ITC and MAIC were conducted to compare the 15-valent V114 to the 20-valent PCV20 in healthy infants. Based on available phase 3 data, it was hypothesized that immunogenicity of V114 would be non-inferior to PCV20 for all serotypes compared and would be superior for serotype 3. Analyzing immunogenicity data both after 3 and 4 doses, these hypotheses were confirmed. Compared to PCV20, V114 immunogenicity was lowest for serotype 6A for all outcomes but remained above prespecified non-inferiority margins. When considering the complete immunogenicity profile of V114 for serotype 6A, the totality of the data suggest immunity is conferred after the infant series and after the toddler dose, including robust functional activity as measured by OPA. In the V114 pivotal study, approximately 94% of participants in the V114 group met the IgG threshold value of 0.35 g/mL for serotype 6A at 30 days after the third dose; similar results were seen in the PCV13 group [Citation17]. Serotype 6A IgG response rates and IgG GMCs were also comparable between the V114 and PCV13 intervention groups at 30 days after fourth dose; the prespecified noninferiority criterion for IgG GMCs was met at the completion of a full PCV regimen [Citation17]. Importantly, serotypes are not equivalent in terms of the antibody level needed for protection: some serotypes require more than the 0.35
g/mL threshold, while others require less [Citation32]. The estimated antibody concentration needed for protection against IPD with serotype 6A in the literature is approximately 0.16
g/mL (95% CI 0.08 to 1.05) [Citation32]. Superiority criteria were met for serotype 3 in V114 as compared to PCV20 in all analyses. Beyond these prespecified hypotheses, several other statistical differences in immunogenicity were observed between V114 and PCV20; for example, following three doses, response rates and antibody levels were higher for serotypes 4, 9 V and 23F, and lower for serotype 6A, with V114 than PCV20.
In the PCV20 phase 3 study, immunogenicity of PCV20 for serotype 3 was inferior to PCV13, with response rates being 52.1% and 67.6%, respectively (response rate difference − 15.5%, 95% CI − 20.1% to 10.8%) [Citation18]. In contrast, the immunogenicity of V114 for serotype 3 was superior to PCV13 in the V114 phase 3 study, with response rates being 94.7% and 79.2%, respectively (response rate difference 15.6%, 95% CI 12.1% to 19.2%). The hypothesized superiority of V114 for serotype 3 compared to PCV20 was confirmed in the current MAIC analysis; the sensitivity analysis using ITC with unweighted V114 data showed similar results. Considering that serotype 3 is one of the most prevalent invasive pneumococcal serotypes [Citation14], further studies are needed to determine how the superior immunogenicity for serotype 3 observed with V114 affects vaccine effectiveness against this serotype. Additionally, studies are needed to evaluate vaccine effectiveness as measured by the incidence of IPD in infants after completion of the V114 and PCV20 vaccination series in general, as no such data are available yet. Previous research has shown that immunogenicity, including the minimum protective concentration of 0.35 µg/mL as recommended by the WHO in their guidance for manufacturers and licensing authorities [Citation33,Citation34], may serve as an acceptable surrogate endpoint to evaluate protection against IPD of pneumococcal vaccines; research is ongoing to provide a definitive answer on the correlation between serotype-specific IgG concentrations and long-term protection in infants [Citation32,Citation35,Citation36].
The major strength of this analysis of V114 and PCV20 in healthy infants is that the two studies used for this analysis were large, well-designed, and well-executed phase 3 studies with comparable designs. Although a peer reviewed publication for PCV20 phase 3 study for 3 + 1 vaccination schedule was not available at the time of writing this manuscript, the two data sources used [Citation18,Citation22], reported reliable and sufficient information to allow the ITC and MAIC analyses to be conducted. The same 3 + 1 vaccination schedule was used in the same age groups, both studies recruited most infants in the United States and Puerto Rico, and the same outcomes were measured at the same time points (~30 days after dose 3 and 4). Both studies had good compliance rates: in the V114 study, 89% (1531/1720) of the randomized infants completed the four doses [Citation17]; in the PCV20 study, at least 85% (1694/1988) of the randomized infants completed the series [Citation22]. Whilst the V114 study enrolled relatively more Asian infants than the PCV20 study, population adjustment methodology equalized the contribution of this demographic to the MAIC estimates. However, infants with missing race data (4.5% [n = 90] in the PCV20 study) had to be excluded in the MAIC weights calculation. The weights and therefore the MAIC analyses between V114 and PCV20 effectively represent a comparison in a population as in the PCV20 study rather than another general population of interest. Furthermore, whereas serotype-specific and dose-specific population adjustment – i.e. matching the baseline characteristics of exactly those subjects measured at a given time point for a given serotype – would have been ideal, this information was not published for the PCV20 phase 3 study. Our analysis cannot eliminate potential bias due to the impact of unknown effect modifiers. Whilst this possibility cannot be excluded and is not testable per se, the MAIC analysis removed bias due to known potential effect modifiers. As the studies used different assays, measured IgG concentrations may have been different because of the assay used. The use of the 0.35
g/mL threshold value measured by the WHO reference enzyme-linked immunosorbent assay (ELISA) has been recommended by a WHO expert panel as an acceptable threshold value for evaluating the clinical performance of PCVs following a routine childhood vaccination regimen [Citation37]. This pooled correlate of protection against IPD of
0.35
g/mL was derived from a meta-analysis of three efficacy trials based on the ranking of serotypes by their epidemiologic and clinical significance [35]. The concentration of circulating serum antibody sufficient to protect infants against pneumococcal disease during the postvaccination period is not well defined but is generally higher for mucosal endpoints (non-IPD) than IPD and varies between serotypes [Citation32,Citation38–40].
In the V114 study, the pneumococcal electrochemiluminescence (PnECL) assay was formally bridged to the WHO reference ELISA [Citation37] to assess the threshold values that correspond to 0.35 μg/mL measured via the WHO reference ELISA for each of the 15 serotypes in V114 individually. (The study included 116 pediatric infant serum samples selected with antibody concentrations that spanned the entire range of response, with a concerted effort to secure samples with serotype-specific IgG concentrations near the WHO ELISA threshold value for all 15 serotypes.) The results confirmed that a single threshold value of 0.35 μg/mL should be applied to each of the 15 serotypes when comparing the serotype-specific response rates between V114 and licensed PCVs in children.
On the other hand, in the PCV20 study, the serotype-specific multiplex direct-binding Luminex immunoassay (dLIA) to measure IgG has been bridged to the WHO reference ELISA and resulted in the 0.35 g/mL that was applied to all 20 serotypes within PCV20 except for serotypes 5, 6B, and 19A. The assay bridging between old and newer methodologies allowed the PCV20 study to report alternative dLIA IgG thresholds for serotypes 5, 6B and 19A, which were 0.23
g/mL, 0.10
g/mL, and 0.12
g/mL respectively to be equivalent to the WHO reference ELISA threshold of 0.35
g/mL [Citation41]. However, such measurement differences could not be adjusted using standard statistical methods without further information on the specific differences between the methods of measurements in the two studies. The use of the common comparator PCV13 and the choice of endpoints have mitigated these differences, assuming that any difference in the assays is geometrically the same across the assay range in both studies. Apart from the two additional serotypes in V114 (22F, 33F) that were excluded from the comparison due to antibody response level below lower limit of quantification for PCV13, similar antibody response levels were observed across all shared PCV13 serotypes in both studies, and further support the assumption. Finally, a comparison between safety and tolerability endpoints was not conducted due to methodological differences in data collection and analysis for these outcomes between the two studies.
5. Conclusions
In conclusion, consistent with the MAIC between V114 and PCV20 in adults [Citation25], the immunogenicity of V114 in healthy infants was non-inferior to PCV20 in healthy infants for all 13 serotypes included in the PCV13 vaccine, and superior for serotype 3. As there is no direct head-to-head comparison between the two vaccines, the findings from this analysis provide early insights for policymakers to evaluate potential economic impact and to estimate the potential effectiveness of the vaccines to prevent IPD based on differences in immunogenicity responses between V114 and PCV20. Furthermore, the results may provide useful information for decision making on childhood vaccination programs and similar public health initiatives. Clinical trials directly comparing V114 and PCV20 may be needed to confirm our findings and to evaluate the impact of differences in immunogenicity for the two vaccines on the incidence of and mortality from IPD in healthy infants.
Declaration of interest
All authors are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, U.S.A., and may hold stock in Merck & Co., Inc., Rahway, NJ, U.S.A.. All authors declare no non-financial competing interests. The funder had a role in the conceptualization, design, data collection, analysis, decision to publish, and preparation of the manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors (1) substantially contributed to the conception and design of the review of the article and interpreting the relevant literature and (2) been involved in the writing the article or revised it for intellectual content.
All authors critically reviewed the manuscript and approved the final version of the manuscript.
Ethical approval
No ethical approval was required since the research presented in this manuscript was based on secondary post-hoc analyses, and individual-level data on V114 used are internal data to the Company and therefore do not require further permission. The data on PCV20 are aggregate data freely available in public domain, and therefore do not require permission to use.
Code availability
The programming codes for the analysis in this study are not publicly available but parts that do not contain proprietary information may be made available to qualified researchers on reasonable request from the corresponding author.
Supplemental Material
Download MS Word (59.7 KB)Acknowledgments
This research was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and conducted by its employees. Therefore, the funder had a role in the conceptualization, study design, data collection, analysis and interpretation of data, decision to publish, and preparation of this manuscript. The phase 3 studies of which data were used for the analyses were conducted with financial support from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (the V114 phase 3 study) for V114 and Pfizer, Inc., New York, NY, USA (the PCV20 phase 3 study) for PCV20. The authors would like to thank Merck & Co., Inc., Rahway, NJ, USA for providing the V114 study data, and Pfizer for making their PCV20 phase 3 study data used in our analyses publicly available. The authors would like to thank the following people at Merck & Co., Inc., Rahway, NJ, USA for providing input into the work presented in this manuscript: Robert Lupinacci for his help with the study design and statistical analyses, Dr. Shrita Patel for providing technical input into the study design and conducting the clinical feasibility assessment of the safety analysis, and Michael Allie for technical support of the analysis production with his statistical programming expertise. Professional medical writing support was provided by Dr. Michel D. Wissing and Holly R. Tomlin, Certara Synchrogenix.
Data availability statement
Summary/aggregate data from the PCV20 study were used for this analysis and are publicly available online [18, 21-22]; the authors of this study do not own these data. Data from the V114 phase 3 study used in this analysis may be made available to qualified researchers upon reasonable request from the corresponding author and/or Merck & Co., Inc., Rahway, NJ, U.S.A. (Data Access mailbox).
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2023.2270039.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004 Mar;4(3):144–154. doi: 10.1016/S1473-3099(04)00938-7
- Nelson AL, Roche AM, Gould JM, et al. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect Immun. 2007 Jan;75(1):83–90. doi: 10.1128/IAI.01475-06
- Geno KA, Gilbert GL, Song JY, et al. Pneumococcal capsules and their types: past, present, and future. Clin Microbiol Rev. 2015 Jul;28(3):871–899. doi: 10.1128/CMR.00024-15
- Ganaie F, Saad JS, McGee L, et al. A New pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral Streptococcus. MBio. 2020 May 19;11(3). doi: 10.1128/mBio.00937-20
- Westerink MA, Schroeder HW Jr., Nahm MH. Immune responses to pneumococcal vaccines in children and adults: rationale for age-specific vaccination. Aging Dis. 2012 Feb;3(1):51–67.
- Berical AC, Harris D, Dela Cruz CS, et al. Pneumococcal vaccination strategies. An update and perspective. Ann Am Thorac Soc. 2016 Jun;13(6):933–944. doi: 10.1513/AnnalsATS.201511-778FR
- Active Bacterial Core Surveillance (ABCs) report, emerging infections program network, Streptococcus pneumoniae, 2019. Atlanta (GA): Centers for Disease Control and Prevention; 2019 [cited 2023 May 16]. Available from: https://www.cdc.gov/abcs/downloads/SPN_Surveillance_Report_2019.pdf
- Simonsen L, Taylor RJ, Young-Xu Y, et al. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. MBio. 2011 Jan 25;2(1):e00309–10. doi: 10.1128/mBio.00309-10
- Hu T, Song Y, Done N, et al. Incidence of invasive pneumococcal disease in children with commercial insurance or Medicaid coverage in the United States before and after the introduction of 7- and 13-valent pneumococcal conjugate vaccines during 1998-2018. BMC Public Health. 2022 Sep 5;22(1):1677. doi: 10.1186/s12889-022-14051-6
- Noharet-Koenig R, Lasota K, Faivre P, et al. Evolution of pneumococcal vaccine recommendations and criteria for decision making in 5 Western European Countries and the United States. MDM Policy Pract. 2023 Jan;8(1):23814683231174432. doi: 10.1177/23814683231174432
- Mungall BA, Hoet B, Nieto Guevara J, et al. A systematic review of invasive pneumococcal disease vaccine failures and breakthrough with higher-valency pneumococcal conjugate vaccines in children. Expert Rev Vaccines. 2022 Feb;21(2):201–214. doi: 10.1080/14760584.2022.2012455
- Pettigrew MM, Alderson MR, Bakaletz LO, et al. Panel 6: vaccines. Otolaryngol Head Neck Surg. 2017 Apr;156(4_suppl):S76–s87. doi: 10.1177/0194599816632178
- McLaughlin JM, Utt EA, Hill NM, et al. A current and historical perspective on disparities in US childhood pneumococcal conjugate vaccine adherence and in rates of invasive pneumococcal disease: considerations for the routinely-recommended, pediatric PCV dosing schedule in the United States. Hum Vaccin Immunother. 2016;12(1):206–212. doi: 10.1080/21645515.2015.1069452
- Adam HJ, Karlowsky JA, Baxter MR, et al. Analysis of MDR in the predominant Streptococcus pneumoniae serotypes in Canada: the SAVE study, 2011-2020. J Antimicrob Chemother. 2023 May 3;78(Supplement_1):i17–i25. doi: 10.1093/jac/dkad066
- VAXNEUVANCE Prescribing Information. United States Food and Drug Administration; 2022 [cited 2023 May 16]. Available from: https://www.fda.gov/media/150819/download
- PREVNAR. 20 prescribing information. United States Food and Drug Administration 2023. Available from: https://www.fda.gov/media/149987/download
- Lupinacci R, Rupp R, Wittawatmongkol O, et al. A phase 3, multicenter, randomized, double-blind, active-comparator-controlled study to evaluate the safety, tolerability, and immunogenicity of a 4-dose regimen of V114, a 15-valent pneumococcal conjugate vaccine, in healthy infants (PNEU-PED). Vaccine. 2023 Jan 27;41(5):1142–1152. doi: 10.1016/j.vaccine.2022.12.054
- Watson W 20-valent pneumococcal conjugate vaccine (PCV20) phase 3 in pediatrics. ACIP. 2023. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-02/slides-02-22/Pneumococcal-04-Watson-508.pdf
- Clinical trial results: a phase 3, multicenter, randomized, Double-blind, active-comparator-controlled study to evaluate the safety, tolerability, and immunogenicity of a 4-dose regimen of V114 in healthy infants (PNEU-PED). EU Clinical Trials Register. 2022 [cited 2023 May 15]. Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2018-004109-21/results
- Merck Sharp & Dohme LLC. NCT03893448: safety, tolerability, and immunogenicity of V114 in healthy infants (V114-029) (PNEU-PED). ClinicalTrials.Gov 2019 [cited 2023 May 15]. Available from: https://clinicaltrials.gov/ct2/show/NCT03893448
- Pfizer. NCT04382326: 20-valent pneumococcal conjugate vaccine safety and immunogenicity study of a 4-dose series in healthy infants. ClinicalTrials.Gov 2020 [cited 2023 May 15]. Available from: https://clinicaltrials.gov/ct2/show/NCT04382326
- Clinical trial results: a phase 3, randomized, Double-blind trial to evaluate the safety and immunogenicity of a 20-valent pneumococcal conjugate vaccine in healthy infants. EU Clinical Trials Register 2023 [cited 2023 May 15]. Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2019-003305-10/results
- Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997 Jun;50(6):683–691. doi: 10.1016/S0895-4356(97)00049-8
- Phillippo DM, Ades AE, Dias S, et al. Methods for population-adjusted indirect comparisons in Health technology appraisal. Med Decis Making. 2018 Feb;38(2):200–211. doi: 10.1177/0272989X17725740
- Mt-Isa S, Abderhalden LA, Musey L, et al. Matching-adjusted indirect comparison of pneumococcal vaccines V114 and PCV20. Expert Rev Vaccines. 2022 Jan;21(1):115–123. doi: 10.1080/14760584.2021.1994858
- Park DE, Johnson TS, Nonyane BA, et al. The differential impact of coadministered vaccines, geographic region, vaccine product and other covariates on pneumococcal conjugate vaccine immunogenicity. Pediatr Infect Dis J. 2014 Jan;33(Suppl 2):S130–9. Suppl 2 Optimum Dosing of Pneumococcal Conjugate Vaccine For Infants 0 A Landscape Analysis of Evidence Supportin g Different Schedules. doi: 10.1097/INF.0000000000000081
- O’Brien KL, Moisi J, Moulton LH, et al. Predictors of pneumococcal conjugate vaccine immunogenicity among infants and toddlers in an American Indian PnCRM7 efficacy trial. J Infect Dis. 2007 Jul 1;196(1):104–114. doi: 10.1086/518438
- Nieminen H, Rinta-Kokko H, Jokinen J, et al. Effectiveness of the 10-valent pneumococcal conjugate vaccine among girls, boys, preterm and low-birth-weight infants - results from a randomized, double-blind vaccine trial. Vaccine. 2019 Jun 19;37(28):3715–3721. doi: 10.1016/j.vaccine.2019.05.033
- Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012 Sep;15(6):940–947. doi: 10.1016/j.jval.2012.05.004
- Signorovitch JE, Wu EQ, Yu AP, et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. PharmacoEconomics. 2010;28(10):935–945. doi: 10.2165/11538370-000000000-00000
- Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985 Apr;4(2):213–226. doi: 10.1002/sim.4780040211
- Andrews NJ, Waight PA, Burbidge P, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014 Sep;14(9):839–846. doi: 10.1016/S1473-3099(14)70822-9
- WHO. Annex 3 recommendations to assure the quality, safety and efficacy of pneumococcal conjugate vaccines. 2013 [cited 2023 Jul 19]. Available from: https://cdn.who.int/media/docs/default-source/biologicals/vaccine-standardization/pneumococcus/trs_977_annex_3.pdf
- Jódar L, Butler J, Carlone G, et al. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine. 2003 Jul 4;21(23): 3265–72.
- Siber GR, Chang I, Baker S, et al. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine. 2007 May 10;25(19): 3816–26.
- Ryman J, Weaver J, Hu T, et al. Predicting vaccine effectiveness against invasive pneumococcal disease in children using immunogenicity data. NPJ Vaccines. 2022 Nov 7;7(1): 140.
- World Health Organization. Annex 3: recommendations to assure the quality, safety and efficacy of pneumococcal conjugate vaccines. WHO expert committee on biological standardization, sixtieth report. Geneva (Switzerland): World Health Organization (WHO); 2013. p. 91–151
- Dagan R, Givon-Lavi N, Fraser D, et al. Serum serotype-specific pneumococcal anticapsular immunoglobulin g concentrations after immunization with a 9-valent conjugate pneumococcal vaccine correlate with nasopharyngeal acquisition of pneumococcus. J Infect Dis. 2005 Aug 1;192(3): 367–76.
- Dagan R, Juergens C, Trammel J, et al. Modeling pneumococcal nasopharyngeal acquisition as a function of anticapsular serum antibody concentrations after pneumococcal conjugate vaccine administration. Vaccine. 2016 Aug 5;34(36): 4313–20.
- Voysey M, Fanshawe TR, Kelly DF, et al. Serotype-specific correlates of protection for pneumococcal carriage: an analysis of immunity in 19 countries. Clin Infect Dis. 2018 Mar 5;66(6):913–920.
- Tan CY, Immermann FW, Sebastian S, et al. Evaluation of a validated Luminex-based multiplex immunoassay for measuring immunoglobulin g antibodies in serum to pneumococcal capsular polysaccharides. mSphere. 2018 Aug 8;3(4):10–128.

