ABSTRACT
Background
As pregnant women are excluded from clinical trials of inactivated SARS-CoV-2 vaccines, it is important to assess the immune response in women receiving the vaccination while unknowingly pregnant.
Methods
In a multicenter cross-sectional study, we enrolled 873 pregnant women aged 18–45 years. Serum antibody levels induced by inactivated vaccines were determined. Adverse events were collected by self-reported survey after vaccination. Logistic regression model and restricted cubic spline model were used to investigate the association of factors with antibody positivity.
Results
As the doses of the vaccine increase, neutralizing antibody (NAb) positivity was 98.3%, 39.5%, and 9.5% in pregnant women, respectively. The dose of vaccine and duration since vaccination were associated with NAb positivity. The OR of two and three doses of vaccines were 7.20 and 458.33 (P < 0.05). NAb levels and duration since vaccination showed a linear relationship in pregnant women vaccinated two doses, with a decrease to a near seropositivity threshold at 22 weeks. Adverse events were mainly mild or moderate after vaccinated during pregnancy, with no increase in incidence compared with whom vaccinated during pre-pregnancy.
Conclusions
The use of inactivated vaccines during pregnancy induced favorable immune persistence, and the incidence of adverse events did not increase.
1. Introduction
Coronavirus disease 2019 (COVID-19) remains an important public health concern worldwide, and vaccination is still the most effective measure for establishing an immune barrier and reducing the rate of severe disease in the absence of effective drug treatments. As of August 2023, more than 13 billion doses had been administered globally [Citation1]. However, during the clinical trial phase of COVID-19 vaccine development, researchers have had limited knowledge regarding its safety; therefore, special populations such as pregnant women have not yet been included in trials [Citation2,Citation3]. Moreover, data on the immunogenicity and safety of these vaccines are limited, and thus pregnant women have not been officially included in the target population of these vaccines [Citation4,Citation5].
As a special population, pregnant women are more susceptible to COVID-19 and are at an increased risk of severe disease and mortality, especially when the infection occurs in the third trimester [Citation6,Citation7] or in those with complications such as high blood pressure and diabetes [Citation8,Citation9]. At minimum, women infected with COVID-19 during pregnancy have a substantially increased risk of adverse outcomes [Citation6,Citation10,Citation11]. Although women of reproductive age are among the key populations for achieving herd immunity via COVID-19 vaccination, they carry an inherent risk of receiving the vaccination while unknowingly pregnant. Therefore, there is an urgent need to evaluate vaccine safety and immune persistence in this population.
Current research on the immunogenicity and safety of COVID-19 vaccination in pregnant women has primarily focused on mRNA vaccines, which have been reported to generate robust immune responses in pregnant women while maintaining immunogenicity and safety similar to those found in non-pregnant populations [Citation8]. However, the inactivated vaccine has been used in its place in many countries [Citation12,Citation13], owing to the higher incidence of adverse reactions and higher cold chain transportation requirements of mRNA vaccines compared to the more mature technology, guaranteed safety, and relatively stable characteristics of the inactivated vaccine. As one of the most used vaccines globally, more than 3.4 billion doses of COVID-19 vaccines having been administered by 2023 in China [Citation14], the vast majority have been inactivated vaccines. This large amount of Chinese vaccination data provides us with an opportunity to explore vaccination safety in pregnant women through observational research. Therefore, this study aimed to evaluate the serum Severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) antibody levels and immunity persistence in pregnant women who received an inactivated COVID-19 vaccine before or during pregnancy based on real-world data. We also analyzed adverse events associated with vaccination as well as factors associated with antibody positivity.
2. Patients and methods
2.1. Study participants
This multicenter study was conducted in the obstetric clinics of three hospitals in Guangdong Province, China, from December 2021 to March 2022. The inclusion criteria allowed for the participation of pregnant women who were: (1) aged 18–45 years, (2) administered inactivated vaccines obtained from Sinopharm Beijing Biotech or Sinovac Biotech, (3) administered at least one dose of the inactivated vaccine before or during pregnancy, and (4) had not previously been infected with COVID-19. The exclusion criteria eliminated pregnant women who were: (1) infected with COVID-19 after enrollment, (2) vaccinated with non-inactivated COVID-19 vaccines, (3) undergoing assisted reproductive technology.
A questionnaire survey was conducted among the enrolled women to collect demographic and lifestyle data such as age (stratified by the advanced maternal age defined by the World Health Organization), body mass index, gravidity and parity, smoking status, and alcohol consumption. We also collected information through the vaccination registration platform regarding adverse events of vaccination, type and doses of COVID-19 vaccine received, timing of vaccination (before or during pregnancy), and the time interval between vaccination and blood collection. The study was approved by the Medical Ethics Review Board of the School of Public Health at Guangdong Pharmaceutical University (IRB 2021–01) and complied with the guidelines of the Declaration of Helsinki ICMJE guidelines. Informed consent was provided by all participants.
2.2. Sample collection and analysis
After enrollment, 5 mL of venous blood was collected from each participant, centrifuged to separate the serum, and stored in a −80°C freezer. Neutralizing antibody (NAb) levels were then determined using a microcytopathogenic effect assay in which serum samples were inactivated at 56°C for 30 min, diluted four-fold, and incubated with an equal volume (50 μL) of live SARS-CoV-2 virus suspension at 36.5°C in 5% CO2 for 2 h. Vero cells (1.0–2.0 × 105 cells/mL) were then added to the serum-virus suspension in the microplates and incubated in duplicate at 36.5°C in 5% CO2 for 5 days. Cytopathological effects were then observed under a microscope. An NAb titer ≥ 1:8 was considered positive. The antibodies against SARS-CoV-2 Immunoglobulin G (IgG) and Immunoglobulin M (IgM) were detected using a chemiluminescence kit (Bioscience, Chongqing, China), with an antibody level of ≥ 1.00 signal to cutoff ratio (S/CO) considered positive.
2.3. Statistical analysis
The mean±standard deviation, median, and interquartile range were determined for continuous variables, whereas categorical variables were expressed as percentages. Pearson’s χ2-test or rank sum test was used to examine differences between groups in the distribution of demographic characteristics. The relationship between potential factors and antibody positivity was analyzed using logistic regression analysis and a restricted cubic spline (RCS) model with four nodes. If Poverall <0.05, there was a dose-response relationship between the time lapsed since vaccination and antibody levels. If Poverall <0.05, and Pfor nonlinearity >0.05, there was a linear dose-response relationship between the time lapsed since vaccination and antibody levels. If Poverall <0.05 and Pfor nonlinearity <0.05, there was a nonlinear dose-response relationship between the time lapsed since vaccination and antibody levels. Statistical analyses were performed using SPSS V22.0, R V4.1.0 (IBM Corp., Armonk, NY, U.S.A.). All P-values were two-sided, and differences with P-values <0.05 were considered statistically significant.
3. Results
3.1. Demographics of the study participants
The characteristics of participants are shown in and S1. A total of 873 pregnant women were recruited from the obstetrics and gynecology clinics of three hospitals. 669 of whom received two doses of inactivated COVID-19 vaccine and 120 received three doses. 87.9% were aged <35 years. The average duration since vaccination for participants was 196 days. There were significant differences in SARS-CoV-2 NAb positivity between the dose of inactivated vaccine received (P<0.001), duration since vaccination(P<0.001), and the presence and absence of adverse events (P=0.028). There were significant differences in IgG positivity between age(P=0.043), number of doses received(P<0.001), duration since vaccination(P<0.001), and the presence and absence of adverse events (P=0.026).
Table 1. Demographic characteristics of the participants.
3.2. Antibody responses to inactivated vaccines
The overall serum SARS-CoV-2 NAb, IgG, and IgM positivity rates were 44.7%, 46.4%, and 3.3%, respectively. Specifically, NAb, IgG, and IgM positivity rates were 39.5%, 41.5%, and 2.6% in pregnant women who received two doses of inactivated vaccine, and 98.3%, 100%, and 8.3% in those who received three doses, respectively. As shown in , in pregnant women receiving two doses of vaccine, serum IgG positivity was higher than that of the NAb within <20 weeks of duration since vaccination. But with increasing time, serum IgG positivity was similar to that of NAb, and positivity of the two antibodies was correlated (r = 0.582, P < 0.05). As shown in and S2, the Mean geometric titer (GMT) of NAb and positivity of IgG and IgM displayed a decreasing trend with increasing duration since vaccination and an increasing trend with increasing doses of vaccine in both pregnant women vaccinated during during pre-pregnancy and pregnancy. Meanwhile, the result indicated the GMT of NAb in pregnant women vaccinated during pregnancy was higher than that in pregnant women vaccinated before pregnancy at three doses (P < 0.05). By stratified analysis (Table S3), There was no significant difference in the positivity of NAb between pregnant women vaccinated during pre-pregnancy and pregnancy within different duration since vaccination. NAb positivity showed similar results in pregnant women who received two doses of the vaccine (Table S4).
Figure 1. Positive rates of antibodies at different inoculation durations in pregnant women who received two doses of inactivated COVID-19 vaccine.
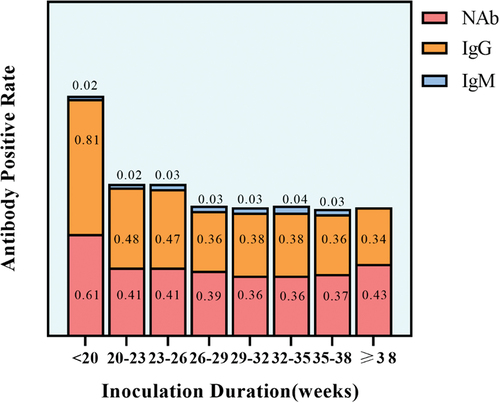
Table 2. Antibody positivity in pregnant women with different doses of vaccine.
3.2.1. Factors associated with antibody positivity
As shown in Table S5, the factors of age, dose of vaccine and duration since vaccination in pregnant women were associated with antibody positivity (P = 0.039). After adjusting for confounding factors, dose of vaccine and duration since vaccination were identified as factors associated with NAb and IgG positivity (). Positivity rates increased with increasing doses of vaccine (Pfor trend <0.001). Compared with those receiving only one dose, the odds ratio (OR) for NAb in pregnant women receiving two and three doses of vaccine were 7.20 (P < 0.001, 95% CI: 3.20–16.19) and 458.33 (P < 0.001, 95% CI: 90.55–2319.82), respectively. The OR for IgG in those receiving two doses was 9.39 (P < 0.001, 95% CI: 3.96–22.24). Compared with <20 weeks since vaccination, the positivity of NAb at all longer time points (at an increment of 4 weeks) were decreased significantly (P < 0.05). Similar results were observed for IgG.
Table 3. Multifactorial logistic regression analysis of antibody positivity.
3.2.2. RCS models for antibodies positivity
The dose-response relationship between duration since vaccination and positivity was analyzed in pregnant women receiving two doses of inactivated vaccine. Results showed that the OR of NAb positivity decreased until it leveled off as duration since vaccination increased. By week 22, duration since vaccination was not correlated with NAb positivity. Moreover, there was a linear dose-response relationship between duration since vaccination and the OR of NAb positivity (Pfor nonlinearity >0.05, ). In , the OR of IgG positivity also decreased until it leveled off, indicating a nonlinear relationship between duration since vaccination and the OR of IgG positivity (Pfor nonlinearity <0.05). By week 24, duration since vaccination was not correlated with IgG positivity.
Figure 2. RCS models for antibodies in pregnant women who received two doses of inactivated COVID-19 vaccine. a: RCS model for NAb of pregnant women who received two doses of inactivated vaccine. b: RCS model for IgG antibody of pregnant women who received two doses of inactivated vaccine. c: RCS model for NAb of pregnant women who received two doses of inactivated vaccine during pregnancy. d: RCS model for IgG antibody of pregnant women who received two doses of inactivated vaccine during pregnancy. The x-axis is the duration of inoculation. The y-axis is the predicted or values of antibody positivity. The shaded area represents the 95% confidence interval. These models were adjusted for age.
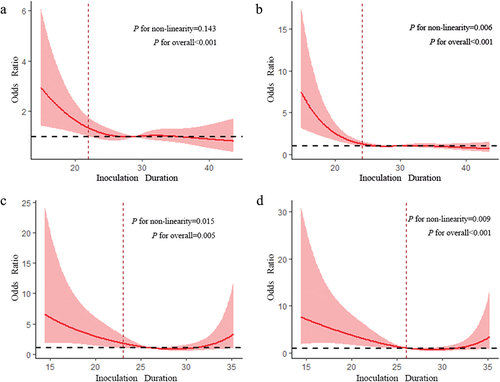
We further analyzed whether there were differences in immune responses after the second dose of inactivated vaccine before pregnancy and during pregnancy (). In pregnant women who received the second dose of inactivated vaccine during pregnancy, the OR of NAb positivity decreased slowly with increasing duration since vaccination. Duration since vaccination was not correlated with NAb positivity when it increased to 23 weeks. The OR of IgG positivity also showed similar trend. Duration since vaccination was not correlated with IgG positivity when it increased to 26 weeks. In addition, both models predicted a nonlinear dose-response relationship between duration since vaccination and antibody levels (Pfor nonlinearity <0.05). The RCS models of pregnant women receiving the second dose of vaccine before pregnancy were showed in Figure S1. A linear dose-response relationship was observed between duration since vaccination and the OR of IgG positivity (Pfor nonlinearity >0.05).
3.3. Local and systemic reactogenic events among pregnant women
No serious adverse events related to inactivated vaccines were reported in the enrolled pregnant women. The most common adverse events included pain and swelling at the injection site, fatigue, or asthenia, muscle pain, and drowsiness (Figure S2). These symptoms usually occurred on the day of or one day after vaccination, and usually disappeared within two or three days. For pregnant women who received different doses of vaccine, the incidence of adverse events in those vaccinated during pregnancy was not higher than in those vaccinated before pregnancy (Table S6). For pregnant women vaccinated before pregnancy who received one, two, and three doses of vaccine, the overall incidence of adverse events was 38.1%, 33.5%, and 52.2%, respectively, and 36.1%, 24.1%, and 47.1%, in those vaccinated during pregnancy, respectively. There was no significant difference in the incidence of adverse events between those vaccinated before and during pregnancy, except for injection-site pain when getting the second dose (P = 0.014).
4. Discussion
Our results indicated that the positivity rates of NAb and IgG in pregnant women vaccinated during pregnancy were similar to those in pregnant women vaccinated pre-pregnancy. Moreover, SARS-CoV-2 NAb and IgG remained observable in approximately half of the patients, with a median duration of 6 months following vaccination. In addition, the antibody levels of women vaccinated during pregnancy were higher than in those vaccinated pre-pregnancy vaccination in terms of vaccination duration and dose. Overall, these findings suggest that inactivated COVID-19 vaccines may provide substantial protection for pregnant women; moreover, they provide insight into the dynamics of post-vaccination immunity for the development of vaccination strategies for this population.
SARS-CoV-2 NAbs are considered protective against COVID-19 [Citation15,Citation16], and studies have shown that inactivated vaccines are 50–90% effective in protecting against infection [Citation12,Citation17]. For example, Gray et al. found that antibody levels in pregnant and lactating women administered the mRNA vaccine were similar to those in non-pregnant and non-lactating women, and were higher than those in pregnant women who were naturally infected [Citation5]. Additionally, a test-negative case-control study of routine system data in Brazil showed that the effectiveness in pregnant women with symptomatic COVID-19 was 41.0% 14 days after two doses of the inactivated vaccine were received [Citation13]. It has also been reported that the positive rate of NAb can reach 99% within 28 days of receiving two doses of the inactivated vaccine. In our study, the NAb positivity of the two doses of the vaccine was 39.5%, with an average duration of 28 weeks after vaccination. This suggests that the inactivated vaccine effectively induced humoral immune responses in pregnant women and had considerable immune persistence, which may provide protection against COVID-19 similar to that found in other adult populations. Our results also showed that IgM positivity was 3.3%, showing a markedly lower positivity, consistent with those of a previous study [Citation18].
The relationship between vaccination and the risk of severe disease and death due to COVID-19 became clear with the progression of the pandemic. Research suggests that pregnant women at any stage of pregnancy should be vaccinated to reduce their risk of contracting COVID-19 during pregnancy [Citation19]. However, clinical trials of vaccines have COVID-19 have not yet provided premarketing data on immunity in vaccinated pregnant women.
Early studies focused on mRNA vaccines and found that women had a good immune response regardless of pregnancy status. In our study, multivariate analysis revealed that vaccine dose was associated with antibody positivity. The result that pregnant women who received two and three doses of the vaccine had higher antibody positivity than those who received only one partially explains why completing a full course of vaccination provides protection against the incidence of death or severe disease caused by COVID-19 [Citation20,Citation21]. A previous multicenter retrospective cohort study reported that individuals who completed the full course of vaccination had fewer symptoms than unvaccinated individuals in cases of breakthrough infection with the Delta variant [Citation22]. Another also noted that the protection rate against various SARS-CoV-2 strains including the delta variant was significantly higher with two doses of vaccine than with one, regardless of vaccine type (mRNA, ChAdOx1 nCoV-19 vaccine, or inactivated vaccine) [Citation23]. Our results demonstrate that the vaccination strategy of inactivated COVID-19 vaccines conforms to the working mode of immune memory.
Additionally, the RCS model used in this study revealed that SARS-CoV-2 NAb and IgG levels gradually decreased in pregnant women after two doses of inactivated vaccine, approaching the seropositivity threshold around the 24th week. It is well known that antibody levels gradually decrease over time following vaccination; previous studies have reported a decrease in serum antibody levels after vaccination [Citation24,Citation25]. Moreover, a phase II clinical trial of the inactivated CoronaVac COVID-19 vaccine showed that the antibody levels were close to the seropositivity threshold 6 months after the second dose of the vaccine [Citation26].
This study also further analyzed potential differences in immune responses after the second dose of the inactivated vaccine before and during pregnancy using the RCS model. The results showed that the time required for IgG levels to decrease to nearly the seropositivity threshold was approximately 24 weeks after the second dose of the vaccine in pregnant women who received it before pregnancy. Although no statistical significance was observed for Nab, the OR for Nab-positivity decreased with time lapsed since vaccination. Additionally, the duration for which IgG levels decreased to nearly the seropositivity threshold was approximately 26 weeks in pregnant women who received the second dose during pregnancy, whereas it was approximately 23 weeks for NAb. Thus, pregnant women who are vaccinated during pregnancy may have a longer duration of immunity. A previous retrospective cohort study of pregnant women who received three doses of COVID-19 vaccines (mRNA or adenovirus vector vaccine) found that IgG levels were significantly higher in pregnant women receiving the third dose of the vaccine during pregnancy, especially in the second and third trimesters, than in those who received it before pregnancy [Citation27]. This may be related to changes in the immune system and a series of complex physiological changes that occur during pregnancy [Citation28].
Our results also showed that the inactivated SARS-CoV-2 vaccine had an acceptable safety profile in the enrolled pregnant women, with adverse events that were mostly mild and short-lived. Additionally, there was no difference in the incidence of adverse events between women vaccinated before and during pregnancy; at some doses, lower rates of significant adverse events after vaccination were found during pregnancy, consistent with the findings of previous studies [Citation29–31]. Pregnant women who received the booster dose were also included and reported fewer types of adverse events with no systematic differences in reactogenicity compared to those vaccinated before pregnancy. In general, our real-world data suggest that inactivated vaccines are safe during pregnancy.
This is the first study to analyze the immune response and safety of inactivated SARS-CoV-2 vaccines in pregnant women based on real-world data. Its findings provide a basis for further investigations into the protective efficacy of inactivated vaccines in pregnant women and contribute significantly to mass immunization with inactivated vaccines to prevent further outbreaks of COVID-19. Moreover, as this was a multicenter study, its conclusions may have broader significance and greater credibility. However, this study has some limitations. First, as a real-world study that could not be designed as precisely as a clinical trial, the data quality may vary widely. Nevertheless, as an open and non-interventional study, it has high representativeness and external authenticity, which greatly reduces selection bias. Lastly, because the adverse events in pregnant women were self-reported in the questionnaire, there may have been some recall bias. Future research should investigate the mother-to-child transfer of antibodies in pregnant women who receive inactivated vaccines.
5. Conclusions
In this study, pregnant women who received more than two doses of the inactivated COVID-19 vaccine had higher serum NAb levels. Antibody levels in pregnant women approached the seropositivity threshold around 22–24 weeks after receiving two doses of the COVID-19 vaccine. Furthermore, compared with women vaccinated before pregnancy, the incidence of adverse events among women vaccinated during pregnancy did not increase. In conclusion, our study suggests that it is safe for pregnant women to receive inactivated vaccines, which can generate effective antibodies against COVID-19 that may decrease after approximately 6 months. The results of this study may provide a scientific basis for governmental immunization strategies for pregnant women and improve their willingness to be vaccinated.
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors contributed to the study conception, design, data acquisition, analysis, interpretation, and drafting and revising the manuscript.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Availability of data and materials
De-identified data collected for the study (with data dictionary) might be made available upon approval by the study investigators, with relevant agreements (eg, data sharing agreement) and approvals (eg, relevant ethics approvals). Requests should be directed to the corresponding author in the first instance.
Ethics approval and consent to participate
The study was approved by the Medical Ethics Review Board of the School of Public Health, Guangdong Pharmaceutical University (IRB 2021–01), and complied with the Declaration of Helsinki guidelines. All participants signed consent forms.
Supplemental Material
Download MS Word (122.3 KB)Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2023.2272655.
Additional information
Funding
References
- WHO COVID-19 Dashboard. Geneva: World Health Organization. 2020 [cited 2023 Aug]; Available online https://covid19.who.int/
- Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021 Feb;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577
- Collier AY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021;325(23):2370–2380. doi: 10.1001/jama.2021.7563
- Gray KJ, Bordt EA, Atyeo C, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021 Sep;225(3):.e303.1–.e303.17. doi: 10.1016/j.ajog.2021.03.023
- Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States. MMWR Morb Mortal Wkly Rep. 2020 Nov 6;69(44):1641–1647. January 22-October 3, 2020. doi: 10.15585/mmwr.mm6944e3
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020 Sep 1;370:m3320. doi: 10.1136/bmj.m3320
- Panagiotakopoulos L, Myers TR, Gee J, et al. SARS‐CoV‐2 infection among hospitalized pregnant women: reasons for admission and pregnancy characteristics—eight U.S. Health care centers. MMWR Morb Mortal Wkly Rep. 2020;69:1355–1359. doi: 10.15585/mmwr.mm6938e2
- Ellington S, Strid P, Tong VT, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020 Jun 26;69(25):769–775. doi: 10.15585/mmwr.mm6925a1
- Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021 Aug 1;175(8):817–826. doi: 10.1001/jamapediatrics.2021.1050
- Tabacco S, Giannini A, Garufi C, et al. Complementemia in pregnancies with antiphospholipid syndrome. Lupus. 2019 Nov;28(13):1503–1509. doi: 10.1177/0961203319882507
- Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385(10):875–884. doi: 10.1056/NEJMoa2107715
- Paixao ES, Wong KLM, Alves FJO, et al. CoronaVac vaccine is effective in preventing symptomatic and severe COVID-19 in pregnant women in Brazil: a test-negative case-control study. BMC Med. 2022 Apr 5;20(1):146. doi: 10.1186/s12916-022-02353-w
- National Health Commission of the people’s Republic of China.COVID-19 vaccination status. http://www.nhc.gov.cn/xcs/yqjzqk/202212/a87d93dbfbd14fc1b7837f1a20092cbe.shtml.
- Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010 Jul;17(7):1055–1065. doi: 10.1128/CVI.00131-10
- Corbett KS, Flynn B, Foulds KE, et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med. 2020 Oct 15;383(16):1544–1555. doi: 10.1056/NEJMoa2024671
- Tanriover MD, Doğanay HL, Akova M, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021 Jul 17;398(10296):213–222. doi: 10.1016/S0140-6736(21)01429-X
- Xu QY, Xue JH, Xiao Y, et al. Response and duration of serum anti-SARS-CoV-2 antibodies after inactivated vaccination within 160 days. Front Immunol. 2021 Dec 23;12:786554. doi: 10.3389/fimmu.2021.786554
- American College of Obstetricians and Gynecologists. COVID-19 vaccination considerations for obstetric–gynecologic care.Accessed Nov 22, 2021.https://www.acog.org/clinical/clinical-guidance/practice-dvisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care.
- Moreira ED Jr, Kitchin N, Xu X, et al. Safety and efficacy of a third dose of BNT162b2 covid-19 vaccine. N Engl J Med. 2022 May 19;386(20):1910–1921. doi: 10.1056/NEJMoa2200674
- Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022 May 12;386(19):1804–1816. doi: 10.1056/NEJMoa2200797
- Chia PY, Ong SWX, Chiew CJ, et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine breakthrough infections: a multicentre cohort study. Clin Microbiol Infect. 2022 Apr;28(4):.e612.1–.e612.7. doi: 10.1016/j.cmi.2021.11.010
- Bian L, Gao Q, Gao F, et al. Impact of the delta variant on vaccine efficacy and response strategies. Expert Rev Vaccines. 2021 Oct;20(10):1201–1209. doi: 10.1080/14760584.2021.1976153
- Muecksch F, Wise H, Batchelor B, et al. Longitudinal Serological analysis and Neutralizing antibody levels in Coronavirus disease 2019 convalescent patients. J Infect Dis. 2021 Feb 13;223(3):389–398. doi: 10.1093/infdis/jiaa659
- Wang K, Long QX, Deng HJ, et al. Longitudinal dynamics of the Neutralizing antibody response to severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection. Clin Infect Dis. 2021 Aug 2;73(3):e531–e539. doi: 10.1093/cid/ciaa1143
- Zeng G, Wu Q, Pan H, et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect Dis. 2022 Apr;22(4):483–495. doi: 10.1016/S1473-3099(21)00681-2
- Yang YJ, Murphy EA, Singh S, et al. Association of gestational age at Coronavirus disease 2019 (COVID-19) vaccination, history of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection, and a vaccine booster dose with Maternal and umbilical cord antibody levels at delivery. Obstet Gynecol. 2022 Mar 1;139(3):373–380. doi: 10.1097/AOG.0000000000004693
- Abu-Raya B, Michalski C, Sadarangani M, et al. Maternal immunological adaptation during normal pregnancy. Front Immunol. 2020 Oct 7;11:575197. doi: 10.3389/fimmu.2020.575197
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021 Jun 17;384(24):2273–2282. doi: 10.1056/NEJMoa2104983
- Bookstein Peretz S, Regev N, Novick L, et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet Gynecol. 2021 Sep;58(3):450–456. doi: 10.1002/uog.23729
- Sadarangani M, Soe P, Shulha HP, et al. Safety of COVID-19 vaccines in pregnancy: a Canadian national vaccine safety (CANVAS) network cohort study. Lancet Infect Dis. 2022 Aug 11;22(11):S1473-3099(22)00426–1. doi: 10.1016/S1473-3099(22)00426-1

