ABSTRACT
Introduction
Hepatitis B remains a major cause of death and morbidity worldwide. Universal childhood immunization programs have been very successful, but many adults remain unprotected or are not optimally protected. PreHevbrio [Hepatitis B Vaccine (recombinant)] is a highly immunogenic 3-antigen (S/pre-S1/pre-S2) hepatitis B vaccine (3A-HBV) that recently received marketing authorization in the United States (2021), the European Union, United Kingdom (2022 – brand name PreHevbri), and Canada (2022– brand name PreHevbrio) for the prevention of infection caused by all known subtypes of the hepatitis B virus and the delta virus in adults 18 years and older.
Areas covered
This review details the development of 3A-HBV and summarizes the results of the phase 3 clinical trials that support its immunogenicity and safety in adults.
Expert Opinion
3A-HBV is highly immunogenic in adults of all ages, including older adults and subgroups that respond sub-optimally to conventional single S-antigen hepatitis B vaccines (1A-HBV), such as those with obesity, type 2 diabetes, and smokers. 3A-HBV provides higher seroprotection rates after each vaccination compared to conventional 1A-HBV vaccines, allowing for more rapid protection. The higher overall immunogenicity is also reflected in more durable seroprotection years after vaccination, as supported by a follow-up study to one of the phase 3 studies.
1. Background
Since its discovery in the 1960’s [Citation1], the hepatitis B virus (HBV) has been recognized as the cause of a spectrum of liver illness, including chronic infections that can lead to life-threatening hepatocellular carcinoma (HCC) and liver cirrhosis. Acute hepatitis is generally asymptomatic in infants and young children, but is characterized by nausea, abdominal pain, vomiting, and jaundice [Citation2] in up to 50% of the infected adults [Citation3]. In non-endemic countries, most HBV infections are transmitted in adults through sexual intercourse, injection drug use, and in the health care setting through contaminated devices or from infected personnel [Citation4].
Humoral and cellular immune responses are essential for viral clearance from HBV infection [Citation5]. If HBV infection is not cleared within 6 months, the infection is considered chronic. Chronic HBV occurs in up to 90% of the infants infected through peripartum-transmission [Citation6], with a high risk of later liver damage including cirrhosis and a subsequent 30% lifetime risk of developing HCC. While immunocompetent people infected with HBV as adults develop chronic hepatitis at a rate of 5–10% [Citation6], there is a higher risk of developing chronic HBV in immunocompromised persons [Citation3,Citation7], of up to 20% [Citation8,Citation9].
Active immunization against hepatitis B is the only way to reduce HBV-associated morbidity and mortality. Universal infant HBV campaigns using recombinant S-antigen vaccines have led to a dramatic decrease in new infections and cases of HCC and liver cirrhosis [Citation10]. However, in the last decade, the number of new HBV infections has plateaued and has even increased in some countries [Citation11,Citation12]. In the US and EU, most new infections occur in adults [Citation13,Citation14]. Almost 300 million people worldwide are chronically infected with hepatitis B, which in 2019 resulted in 820,000 HBV-related deaths [Citation15].
Conventional recombinant S-antigen vaccines have reduced immunogenicity in adults. This is particularly true in older adults and those with comorbid disease, in whom less than 75% achieve seroprotective levels of antibodies against HBV (anti-HBs ≥10 mIU/mL) after completing the 3-dose regimen [Citation16–18]. Factors associated with lower response rates to HBV vaccination include obesity [Citation19], older age, male sex [Citation20] and chronic diseases such as kidney disease and diabetes mellitus [Citation21]. Non-responders to HBV vaccination remain susceptible to infection.
In 2022, the Advisory Committee of Immunization Practices (ACIP) in the US expanded the universal age range for HBV vaccination to all adults 18–59 years old to increase the vaccine coverage among US adults [Citation22], which remains low (~30%) even among those with risk factors such as liver disease or diabetes [Citation23,Citation24]. Efforts to protect adults against HBV infection will only be maximized to the extent that highly immunogenic and fast acting vaccines are used to vaccinate this susceptible population.
PreHevbrio is a highly immunogenic hepatitis B vaccine which was approved for use in the US in November 2021 and received marketing authorization in the EU in April 2022 (brand name PreHevbri), the UK in June 2022 (brand name PreHevbri) and in Canada in December 2022 (brand name PreHevbrio) for prevention of infection caused by all known subtypes of the hepatitis B virus in adults 18 years and older. PreHevbrio is produced by expression of the Pre-S1, Pre-S2, and S protein components of the hepatitis B virus surface antigen (HBsAg) in genetically modified mammalian Chinese Hamster Ovary (CHO) cells; this contrasts with other approved HBV vaccines which contain only the small ‘S’ surface antigen and are produced in yeast [Citation25]. This 3-antigen HBV vaccine (PreHevbrio, 3A-HBV) has a similar protein composition and glycosylation pattern to native hepatitis B viral particles and expresses all antigenic epitopes and domains [Citation26].
Interest in the pre-S region of the HBV particle in vaccine development was based on early research that showed that not only did the pre-S region play an important role in virus-hepatocyte binding and penetration during infection but that the anti-pre-S immune response played an important role in viral clearance and recovery [Citation27–29]. In earlier investigations, mammalian cell-derived HBsAg envelope particles containing all three antigens (S, pre-S1, and pre-S2) were shown to elicit a broader and more robust immune response than single antigen yeast-derived vaccines in mice and were able to overcome genetic resistance to the small ‘S’ HBs [Citation27,Citation30]. This provides additional evidence to support the clinical development of the CHO-derived 3A-HBV vaccine candidate. It is noteworthy that since the original development and testing of 3A-HBV in mice, antibodies against the pre-S domain of the HBsAg have also been detected clinically with the 3A-HBV [Citation31–33], with higher anti-HBs titers noted in study participants with antibodies against pre-S1 [Citation32] or pre-S2 [Citation31] compared to subjects that were not reactive to anti-preS1 and anti-preS2, respectively.
This review describes the development of PreHevbrio and summarizes the phase 3 immunogenicity and safety results that supported its approval in the US, EU, and Canada. Clinical trial data included in this review are limited to the prophylactic use of the vaccine in the adult population. While pediatric formulations of the vaccine at 2.5 µg and 5 µg doses have been approved in some jurisdictions, they are not approved in the US, Europe, or Canada, and therefore the results of clinical trials in children are not considered in this review.
2. Pharmacology
PreHevbrio [Hepatitis B Vaccine (Recombinant)] is produced by expression of the pre-S1, pre-S2 and S protein components of HBsAg in genetically modified mammalian CHO cells. The 1.0 mL adult single-dose of 3A-HBV is formulated with 10 µg/mL of HBsAg (S [83%], pre-S1 [6%], and pre-S2 [11%]) adsorbed on aluminum hydroxide [Al(OH)3] 0.5 mg/mL alum as an adjuvant [Citation34, ] [Citation35], *The immunization schedule for 3A-HBV was set by convention as three 10 µg/mL doses administered intramuscularly (IM) at months 0, 1 and 6.
During the development of 3A-HBV three other proposed brand names, ‘Bio-Hep-B,’ ‘Hepimmune’, and ‘Sci-B-Vac’ were used for the same product. Although these names are not the brand name in the US, Europe, and Canada, they are mentioned in the literature. Additionally, only the name Sci-B-Vac remains active, as the brand name in Israel and Hong-Kong [Citation26,Citation36–38]. The vaccine was first marketed in Israel starting in 2000 and approved for five dosages, 2.5 µg and 5 µg for use in infants and children less than 10 years old and 10 µg for use in children older than 10 years and adults. Since its approval in Israel in 2000, based on global market distribution data and using the conservative assumption that all individuals received the full 3-dose regimen, it is estimated that over 750,000 infants, children, and adults have received this 3A-HB vaccine, with over 300, 000 individuals receiving the 10 µg dose.
3. Clinical data
A total of 24 studies using the current and/or prior formulations of the 3A-HBV have been conducted in HBV-naïve neonates (8 studies), children (4 studies), and adults (12 studies) from 1989 until January 2023. Clinical studies in adults have been performed in both healthy adults and in older adults with controlled chronic diseases such as Type 2 diabetes and included male and female populations with different body weights, and racial and ethnic backgrounds. Studies in adults have been conducted in Israel, Singapore, Vietnam, Russia, and more recently in the US, Canada, Finland, Belgium, Germany, and England. All adult study participants received 5 µg or 10 µg HBsAg of 3A-HBV in a 3-dose regimen, with 10 µg being used in the most recent phase 3 studies that supported licensure in the US, Europe, and Canada.
The phase 3 studies conducted in North America and Europe (PROTECT and CONSTANT) were studies that assessed immunogenicity and safety of the 3A-HBV in adults and were pivotal to its approval in the US, EU, and Canada. These studies are discussed in detail in Section 3.2. Prior to the phase 3 development of this vaccine in North America and Europe, 10 studies had already been conducted in previously unvaccinated healthy adults between 1989 and 2017, using the current or prior formulations of 3A-HBV (referred to as the ‘early studies’). The results of these studies provide additional support for the immunogenicity and safety of the vaccine and provide justification for the dose and adjuvant of the final approved 3A-HBV formulation. The results of these studies are summarized in Section 3.1.
3.1. Early studies
The 10 early studies that were conducted in adults between 1989 and 2016 included three dose-ranging studies, five controlled phase 2 and 3 studies, and two single-arm open-label studies. A titer of anti-HBs of 10 mIU/mL is an established immunological correlate of protection against infection with HBV in humans [Citation39,Citation40]. This threshold was applied in all clinical studies to define seroprotection and to determine the seroprotection rate (SPR), which in all studies was defined as the percentage of subjects achieving seroprotection (anti-HBs ≥10 mIU/mL).
3.1.1. Dose-ranging studies
Three phase 1 and 2 dose-ranging studies (HB-88002 S, HB-88002 T, HBV-003-89) were conducted between 1989 and 1993 to compare the safety and immunogenicity of the 5 µg and 10 µg doses. As detailed in and reported in the literature [Citation41,Citation42], these studies were carried out both in countries with high and low HBV endemicity. The results of these studies demonstrated an SPR of 75–100% with the 5 µg dose and 96–100% with the 10 µg dose at month 6 (following two vaccinations), and 92–100% with the 5 µg dose and 100% with the 10 µg dose at month 7 (1 month after the third vaccination). Peak geometric mean titers (GMTs) at month 7 ranged from 1473 to 12157 mIU/mL for the 5 µg dose level and 2687–13483 mIU/mL for the 10 µg dose level, with higher anti-HBs titers achieved with the 10 µg dose compared to the 5 µg in each study (). Local solicited adverse events (AEs) of itching, pain, and redness at the injection site occurred with approximately equal frequency at the 5 µg and 10 µg doses in all three studies (HBV-003-89: 41% vs 58%, HB-88002-T: 65% vs 61%, HB-88002-S: 67% vs 66%), with pain at the injection site reported most frequently. Other AEs occurred with approximately equal frequency at both doses across all studies and were not found to be clinically meaningful [Citation41,Citation42].
Table 1. Dose-ranging studies in adults aged 18–45 years (5 µg and 10 µg).
In all three dose-ranging studies, the 10 µg dose of 3A-HBV consistency produced higher rates of seroprotection than the 5 µg dose. Collectively, these studies showed that the 10 µg dose induced better seroprotection at all time points, reaching rates of seroprotection of 96–100% by month 6, before the third vaccination. The 10 µg dose level was selected for further development for all studies in adults carried out after 1994, given (1) higher rates of seroprotection observed at earlier timepoints than the 5 µg formulation; (2) higher peak anti-HBs levels achieved 1 month after the third vaccination; and (3) comparable tolerability and safety profiles of the two dose strengths.
3.1.2. Comparative studies
Comparative phase 2 trials conducted early in the development of the 3A-HBV evaluated both the 5 µg and 10 µg doses, as detailed in . The first of these two studies (HBA-9006-S) compared the safety and immunogenicity of the 3A-HBV (10 µg dose) to two single-antigen hepatitis B vaccine (1A-HBV) vaccines, Engerix-B (20 µg dose) and Hepavac II (10 µg dose), in healthy adults 18–45 years. The 3A-HBV had higher SPR (93%) and GMT levels (295 mIU/mL) just prior to the third vaccination compared to Engerix-B (81%, 143 mIU/mL) and Hepavac II (83%, 93 mIU/mL) [Citation43]. SPR rates higher than 94% were comparable after the third vaccination and were maintained to the end of study [Citation43]. While injection site pain was higher in the 3A-HBV group (48.5%; 145/299) compared to the 1A-HBV Hepavac II group (36.8%; 110/299) and the Engerix-B group (27.0%; 81/300) (p < 0.001), the frequency of other solicited injection site AEs were comparable [Citation43].
Table 2. Phase 2 and 3 early comparative studies and phase 4 investigator initiated studies.
The second phase 2 comparative study (38-92-001), conducted in 1993, compared the safety and immunogenicity of two lots (‘A’ and ‘B’ manufactured in different GMP facilities) of the 3A-HBV (5 µg dose) and 1A-HBV (Engerix-B, 20 µg dose) in healthy adults 18–45 years (). Following the first and second injections, SPRs at months 1 and 6 were higher in the 3A-HBV vaccine group ‘A’ (21%, 92%) and group ‘B’ (21%, 88%), than in the 1A-HBV vaccine group (4%, 66%) (p < 0.02); however, SPR following the third injection did not differ significantly. The number of reports of local symptoms (itching, pain, and redness) as a proportion of the total number of injections showed a significant difference between the 3A-HBV groups ‘A’ (65.2%; 259/397) and ‘B’ (50.3%; 178/354) and 1A-HBV vaccine (35.1%; 53/151) (p < 0.01), mainly due to higher pain at the injection site in the 3A-HBV vaccine groups. Unsolicited AEs were reported by 44.4% subjects in the 3A-HBV vaccine ‘A’ group, 22.9% in the 3A-HBV vaccine ‘B’ group, and 33.3% subjects in the 1A-HBV vaccine group. Together, the above studies provided the initial clinical evidence that a 3-dose series of the 3A-HBV induced high rates of seroprotection in young adults aged 18–45 years faster than 1A-HBV, with higher rates of seroprotection after the first and second doses.
Between 1998 and 2015, three randomized phase 3 studies were carried out in Israel [Citation26], Vietnam [Citation38] and Russia [Citation44] to evaluate the immunogenicity and safety of the 3A-HBV compared to conventional 1A-HBV (). The Israeli study compared the safety and immunogenicity of the 3A-HBV (10 µg) to a 1A-HBV (Engerix-B, 20 µg) in adults aged 18–60 years [Citation26] (). This study evaluates a new formulation of the 3A-HBV adjuvanted with Al(OH)3 based on the results of a single-arm extension to the phase 2 study 38-92-001 (38-92-001 ext), detailed in Section 3.1.2 below. Seroprotection was achieved more rapidly in the 3A-HBV vaccine group than in the 1A-HBV vaccine group. Following the first injection at month 1, the seroprotection rate was significantly higher in the 3A-HBV vaccine group than in the 1A-HBV vaccine group (35% vs 5%, p < 0.001) [Citation26]. Both the 3A-HBV and 1A-HBV groups achieved high SPR 1 month after the third vaccination (99.2% and 92.4%, respectively); however, the peak GMT was higher in the 3A-HBV group compared to the 1A-HBV group (9254 mIU/mL vs 1812 mIU/mL) [Citation26]. The frequencies of vaccine-related AEs and incidence of local signs and symptoms were similar between vaccine groups, with pain (upon pressure or movement) being the most common local reaction [Citation26].
The second phase 3 comparative study (SG-005-05, NCT04531098) was designed to demonstrate the equivalence of two production lots (‘A’ and ‘B’) of 3A-HBV (10 µg) in terms of anti-HBs response and to compare immunogenicity with 1A-HBV (Engerix-B, 20 µg) in adults aged 18–45 years in Vietnam [Citation38] (). Equivalence between 3A-HBV lots based on SPR was demonstrated at day 210 (1 month after the third dose) (‘A’ = 97.3%, ‘B’ = 100%), with similar to SPRs to that achieved by 1A-HBV at day 210 (98.3%) [Citation38]. Vaccine-related AEs occurred more frequently in 3A-HBV ‘A’ (61.9%) and ‘B’ (48.1%) groups compared to 1A-HBV (19.5%), with injection site pain being the most common vaccine-related AE in all recipients, independent of vaccine received [Citation38].
The third phase 3 comparative study (38-13-040, NCT04209400) compared the immunogenicity and safety of 3A-HBV (10 µg dose) to 1A-HBV (Engerix-B, 20 µg dose) in adults aged 18–45 years in Russia [Citation44]. () Seroprotection rates were measured at day 90 (95.9% vs 87.2%) and day 180 (100% vs 89.4%), both time points being after the second vaccination, and at day 210, being and after the third vaccination (100% vs 97.9%). The incidence rates of solicited AEs (erythema, itch, and pain) were similar between 3A-HBV and 1A-HBV across all three vaccinations [Citation44]. There were no serious AEs reported, and all AEs were of mild or moderate severity [Citation44].
3.1.3. Single-arm open-label trials
A single-arm extension study was added to the comparative trial 38-92-001 (38-92-001 ext) in 1994 to evaluate a new formulation of 3A-HBV adjuvanted with Al(OH)3 . This study established that 5 µg of 3A-HBV with the Al(OH)3 adjuvant was as immunogenic as the 5 µg formulation adjuvanted with AlPO4 used in the original study (SPR = 96% after three doses with both formulations) and resulted in a lower frequency of local symptoms at the injection site (47.0%) compared to the two lots of the 3A-HBV vaccine adjuvanted with aluminum phosphate (65.2% and 50.3% with 3A-HBV vaccines ‘A’ and ‘B’ lots) in the original comparative study 38-92-001. The Al(OH)3 adjuvant was used in all subsequent formulations of 3A-HBV.
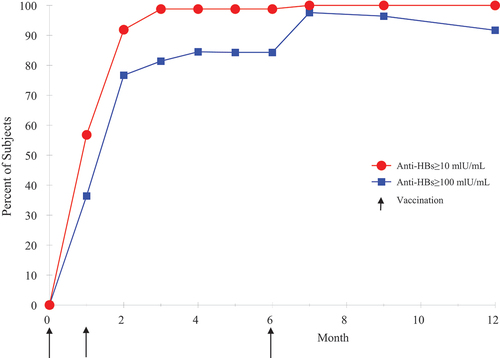
A second single-arm study was conducted in 2017 with 3A-HBV to qualify a new reference standard for batch release. SciB018 (NCT04179786) was an open-label study in 91 healthy subjects (aged 20–40 years) in Israel to evaluate the immunogenicity of a batch of 3A-HBV (10 µg), which was evaluated monthly until month 7 then at months 9 and 12. The primary endpoint was based on the time at which SPR of ≥95% was achieved following vaccination. As detailed in the literature [Citation45], the primary endpoint was met at month 3, 2 months after the second vaccination, when the SPR reached 98.8%. The SPR 1 month after the third vaccination was 100%, which was maintained to Month 12 (). Geometric mean concentration (GMC) of anti-HBs titers peaked at month 7 (6800 mIU/mL) [Citation45]. These results, combined with earlier reports [Citation36], suggest that 3A-HBV is highly immunogenic and can rapidly induce seroprotective levels of antibodies, which is an important characteristic for at-risk adults requiring urgent protection against hepatitis B.
Figure 1. Seroprotection by month (3A-HBV) in adults 18–45 years of age.
3.2. Phase 3 pivotal studies: PROTECT and CONSTANT
Two large phase 3 randomized clinical trials (PROTECT and CONSTANT) comparing 3A-HBV (PreHevbrio 10 µg) to 1A-HBV (Engerix-B 20 µg) were conducted in Europe and North America from 2017 to 2020 to further evaluate the immunogenicity, safety, and manufacturing consistency of 3A-HBV. These studies enrolled subjects from the US, Canada, Finland, Belgium, Germany, and England [Citation18,Citation46]. The PROTECT study purposefully included older adults (about 80% of the participants were aged 45 years and older) and those with controlled chronic diseases (e.g. higher body mass index (BMI), smoking status, and Type 2 diabetes), allowing for an assessment of immunogenicity in other subpopulations of interest [Citation18]. Together, PROTECT and CONSTANT were pivotal to the marketing authorization of 3A-HVB in the US, EU, and Canada. Since the PROTECT and CONSTANT studies had different study designs and different primary endpoints, the studies are discussed separately below in Section 3.2.1. However, as PROTECT and CONSTANT collected the same five safety parameters using similar methods, namely, diary recorded solicited AEs (reactogenicity) in the 7 days after vaccination, unsolicited AEs for 28 days after vaccination, serious adverse events (SAE), medically attended adverse events (MAAE), and new onset of chronic illness (NOCI) from the day of first vaccination to the end of the study [Citation28,Citation46], their safety data is pooled and is presented in Section 3.2.2. In the CONSTANT study, one in-clinic visit was replaced with a safety phone call.
3.2.1. Immunogenicity
3.2.1.1. PROTECT study
PROTECT (NCT03393754) was a double-blind, randomized, controlled study designed to compare the immunogenicity and safety of three 1 mL 10 µg doses of 3A-HBV to three 1 mL doses of 1A-HBV (Engerix-B, 20 µg) [Citation18]. Subjects 18 years of age or older in stable health or with controlled chronic conditions and no history of past HBV infection or immunization with HBV vaccines were eligible for the study. The study subjects were randomized 1:1 and stratified by age group (18–44, 45–64, 65+ years) at the study site to receive either a total of three 1 mL injections of either 3A-HBV or 1A-HBV, administered IM at 0, 1 and 6 months. Participants were followed for 24 weeks (6 months) after receiving the third vaccination [Citation18]. The study aimed to enroll approximately 80% of the subjects aged ≥45 years with an equal proportion of study subjects (i.e. 40% each) in the two older age strata (45–64 years and ≥65 years). Immunogenicity was assessed by measurement of anti-HBs levels at baseline, then 4 weeks after the first dose (day 28) and second dose (day 56), just prior to the third dose (day 168), 4 weeks after the third dose (day 196) and 24 weeks after the third dose (day 336). The co-primary immunogenicity objectives of PROTECT, tested hierarchically and assessed 4 weeks after the third dose (day 196), were to (1) demonstrate the non-inferiority of the SPR of 3A-HBV compared to 1A-HBV in adults ≥18 years old and (2) demonstrate the superiority of SPR of 3A-HBV compared to 1A-HBV in adults ≥45 years old [Citation18]. The non-inferiority of SPR of 3A-HBV in adults ≥18 years old was based on the subjects treated per protocol, with no major protocol deviations leading to exclusion (Per Protocol Set, PPS), and was established if the lower bound of the 2-sided 95% confidence interval (CI) of the difference in SPR was >-5%. Superiority of SPR of 3A-HBV in adults ≥45 years old was based on the set of subjects who received at least one vaccination and provided at least one evaluable serum immunogenicity sample both at and after baseline (Full Analysis Set, FAS) and was established if the lower bound of the 2-sided 95% CI of the difference of SPR was >0% (defined as statistical superiority) and >5% (defined as clinical superiority) [Citation18].
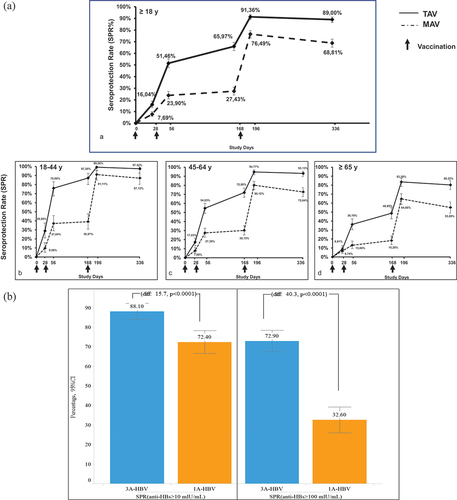
A total of 1607 subjects were randomized to the 3A-HBV (n = 796) or 1A-HBV (n = 811) groups. At baseline, 61.5% (989/1607) of the subjects were female and 89.9% (1445/1607) were white. As planned, more than 80% of the subjects enrolled in the study were ≥45 years of age, with the mean age of the study subjects being 56.6 years and the maximum age of the study subjects being 91 years. In total, 36.7% (589/1607) of subjects had BMI > 30 kg/m2, 13.5% (217/1607) were current smokers and 7.8% (125/1607) had Type 2 diabetes mellitus. Baseline characteristics were balanced between the two vaccine groups [Citation18]. The study met both co-primary immunogenicity endpoints. At day 196, the SPR in subjects ≥18 years of age was 91.4% in the 3A-HBV group compared to 76.5% in the 1A-HBV group, a difference of 14.9% (95% CI: 11.2, 18.6), achieving non-inferiority. Sensitivity analysis of the FAS was consistent with the PPS analysis used for the co-primary endpoint. Additionally, at day 196, the SPR in subjects ≥45 years of age was 89.4% in the 3A-HBV group, compared to 73.1% in the 1A-HBV group, a difference of 16.4% (95% CI: 12.2%, 20.7), achieving both statistical superiority and clinical superiority as defined in the protocol [Citation18]. Sensitivity analysis in the intent to treat (ITT) population was consistent with the FAS analysis used for the co-primary endpoint. Results were consistent across key subgroups based on age, gender, diabetes status, BMI, daily alcohol consumption, and smoking status, with all lower bounds of 2-sided 95% CIs of the difference in SPR being above the pre-set margins (). Moreover, the SPR in the 3A-HBV group was at least twice that of the SPR in the 1A-HBV group at all timepoints before receiving the third vaccination: 16.0% vs. 7.7% at day 28, 51.5% vs. 23.9% at day 56, and 66.0% vs. 27.4% at day 168. The higher SPR of the 3A-HBV vaccine compared to the 1A-HBV vaccine in the 6 months after the first vaccination was even more pronounced in younger adults aged 18–44 years: 28.8% vs. 9.6% at day 28, 76.0% vs. 37.0% at day 56, and 87.2% vs. 39.0% at day 168 (). Anti-HBs titers peaked at day 196 in both vaccine groups, at which timepoint the mean adjusted GMC of anti-HBs for the 3A-HBV group was sixfold greater than the 1A-HBV group in subjects treated per protocol [Citation18].
Figure 2. Seroprotection by subgroup (PROTECT).
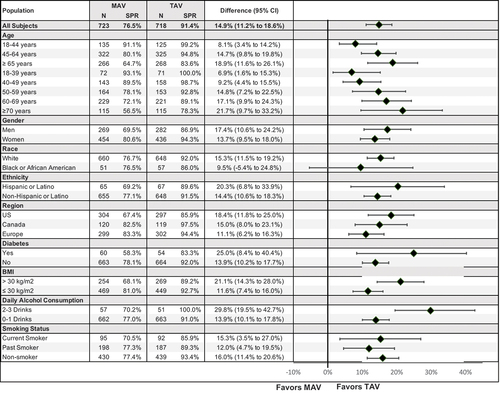
Figure 3. A. Seroprotection rate over 12 months and by age group. B. Retention of seroprotection after 2–3 years of follow-up (PROTECT).
3.2.1.2. CONSTANT study
CONSTANT (NCT03408730) was a double-blind, 4-arm, randomized study designed to demonstrate the manufacturing equivalence in terms of immunogenicity of 3 lots (A, B, and C) of 3A-HBV (10 µg), assessed at 4 weeks after the third vaccination (day 196) and to compare the immunogenicity and safety of three 1 mL 10 µg doses of 3A-HBV (PreHevbrio) to three 1 mL 20 µg doses of 1A-HBV (Engerix-B), administered at 0, 1 and 6 months [Citation46]. Eligible subjects were healthy adults aged 18–45 years with no history of past HBV infection or immunization with HBV vaccines. The subjects were stratified by a study center and randomized 1:1:1:1 to receive three doses of one of the 3 lots of 3A-HBV (A, B, or C) or to 1A-HBV. The primary immunogenicity endpoint of lot-to-lot consistency was assessed by measuring GMC of anti-HBs across the 3 lots of 3A-HBV at study day 196, 4 weeks after the third vaccination, and was established if the 2-sided 95% CI of the GMC ratios of all three pairwise comparisons between lots in the PPS was between (0.67, 1.5).
A total of 2838 subjects were randomized to 3A-HBV Lot A (n = 711), 3A-HBV Lot B (n = 709), 3A-HBV Lot C (n = 706), or 1A-HBV (n = 712). The two subjects randomized to 3A-HBV (Lot B and Lot C) did not get vaccinated due to noncompliance (n = 1) and withdrawal of consent (n = 1). 57.8% (1638/2836) of subjects were female and 91.5% (2595/2836) of subjects were white. Median ages across the vaccine groups ranged from 34 to 36 years. The median BMI of subjects in the safety set was 25.4 kg/m2. Demographic and baseline characteristics were well balanced between the study groups [Citation46].
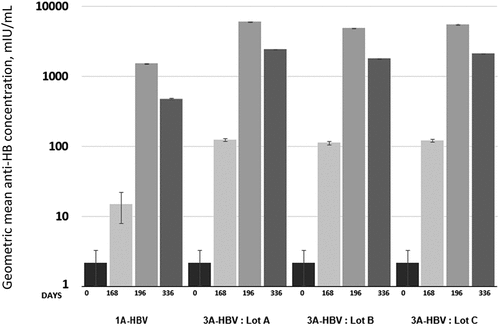
Lot-to-lot manufacturing consistency of 3A-HBV was demonstrated in the primary analysis population. The 2-sided 95% CIs of the adjusted GMC of anti-HBs ratios between lots were Lot A/Lot B = 0.82 [0.67, 1.00], Lot A/Lot C = 0.95 [0.78, 1.15], and Lot B/Lot C = 1.16 [0.95, 1.41]. The results of the sensitivity analysis of the FAS were consistent with the PPS analysis used for the primary endpoint. The mean GMCs of anti-HBs concentrations were higher across all three 3A-HBV lots compared to 1A-HBV at day 168 (after two vaccinations) – Lot A 124.1 mIU/mL, Lot B 112.5 mIU/mL, Lot C 120.5 mIU/mL, and 1A-HBV 14.9 mIU/mL. While both vaccines demonstrated a marked increase in anti-HBs between the second and third vaccinations as shown in , the mean adjusted GMC in the pooled 3A-HBV (5442.4 mIU/mL; 95% CI, 4967.0–5963.0 mIU/mL) was 3.5 times higher than 1A-HBV (1567.2 mIU/mL; 95% CI, 1338.0–1834.0 mIU/mL) at day 196 [Citation46], 4.4 times higher at day 336 (473.0 mIU/mL vs 2093.8 mIU/mL), 6 months after the third vaccination with 3A-HBV (). The pooled SPR at day 196 in subjects in the per protocol set who received the 3A-HBV was 99.3% compared to 94.8% for those who received the 1A-HBV, a difference of 4.50% (95% CI: 2.9, 6.6), establishing the non-inferiority of the 3A-HBV vaccine compared to 1A-HBV [Citation46].
Figure 4. Geometric mean concentration anti-HBs by timepoint and type of vaccine and lot administered in the phase 3 CONSTANT study.
3.2.1.3. Integrated efficacy analysis in adults ≤45 years
In an integrated post-hoc analysis of subjects ≤ 18–45 years from PROTECT and CONSTANT-treated per-protocol (per protocol set) the SPR of 3A-HBV (90.2%) after two doses (Day 168) was non-inferior to the SPR of 1A-HBV (93.9%) after three doses (Day 196), given that the lower limit of the 95% CI for the between-group difference (−3.76% [95%CI:-5.85, -1.44%]) was greater than −10%, a commonly used margin of non-inferiority for vaccines [Citation47]. Sensitivity analysis in the integrated ITT population yielded similar results.
3.2.2. Antibody persistence
Available evidence suggests that the high peak anti-HBs titers achieved with 3A-HBV afford durable seroprotection [Citation48]. A follow-up study in 465 participants from the PROTECT study, enrolled in Finland, found that 2–3 years after vaccination, 88.1% of subjects that received 3A-HBV had retained seroprotective levels of anti-HBs (i.e. ≥10 mIU/mL) compared with 72.4% of participants that received 1A-HBV. Similarly, 72.9% of the subjects in the 3A-HBV group and only 32.3% of the subjects in the 1A-HBV group retained ≥ 100 mIU/mL. The mean concentration of anti-HBs in the 3A-HBV group was also five times greater in follow-up than in the 1A-HBV group (1382.9 mIU/mL vs 252.6 mIU/mL). The results of this follow-up study highlight the importance of a robust immune response to vaccination for durable seroprotection against HBV. The role of 3A-HBV in protecting all adults against HBV infection, independent of age, BMI, sex, or smoking status, was evidenced by the higher rates of seroprotection compared to 1A-HBV observed across all subgroups in the follow-up study [Citation48].
3.2.3. Investigator initiated studies in immunocompromised patients
Several investigator-initiated studies with 3A-HBV have been conducted in adults with underlying health conditions (), including in adults with HIV [Citation49], inflammatory bowel disease [Citation50], end-stage renal disease [Citation51], and in patients on dialysis [Citation52]. Depending on the degree of immunosuppression and/or age, the non-response rate to 3A-HBV was observed in 14–32% compared to the 1A-HBV non-response rate of 19–44%. In these published reports in adults no ≥Grade 3 AE related to administration of 3A-HBV have been reported.
3.2.4. Safety
The pooled safety data from PROTECT and CONSTANT, which compared 3A-HBV (10 µg) with the control 1A-HBV (Engerix-B, 20 µg), include 4,443 unique subjects, 2,920 of whom received 3A-HBV.
In both studies, subjects recorded solicited adverse events (reactogenicity), including local reactions at the injection site (erythema, pain, tenderness, edema, and pruritus) and systemic reactions (nausea/vomiting, diarrhea, headache, fatigue, and myalgia) and body temperature (oral) daily in a diary card, from the day of vaccination and during the subsequent 6 days. In addition, the subject also recorded any unsolicited adverse events on the day of vaccination and during the subsequent 27 days on the 28-day diary card, including any changes in concomitant medication. Serious adverse events such as MAAE and NOCI were also recorded by the study staff at each study visit for the study duration. Adverse events were graded according to the FDA Guidance for Industry Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials [Citation53].
3.2.4.1. Safety set information
Of the randomized subjects, 99.96% received their assigned treatment. Across both pivotal studies, most subjects in both vaccine groups completed study treatment as planned, including 2,725 subjects (93.3%) in the 3A-HBV group and 1,456 subjects (95.6%) in the 1A-HBV group. Treatment discontinuation due to non-serious AEs was uncommon, reported in 0.4% and 0.3% of the subjects in the 3A-HBV and 1A-HBV groups, respectively. Similarly, treatment discontinuations due to SAEs were rare, reported in 0.1% and 0.2% of the subjects in the 3A-HBV and 1A-HBV groups, respectively.
3.2.4.2. Solicited adverse events
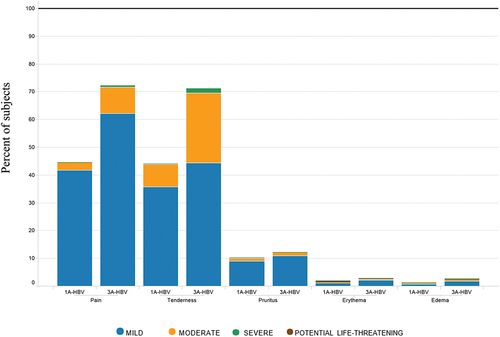
Subjects in the 3A-HBV group compared to the 1A-HBV group had a higher rate of solicited local AEs (81.4% vs. 55.7%), however most local solicited AEs reported the maximum severity as Grade 1 (50.2% and 44.2%) or Grade 2 (28.3% and 10.0%) for 3A-HBV and 1A-HBV, respectively. Grade 3 and Grade 4 local solicited AEs were uncommon; Grade 3: 2.4% and 0.9% and Grade 4: 0.5% and 0.7% for subjects receiving 3A-HBV and 1A-HBV, respectively. Importantly, the Grade 4 events resolved with no sequelae within 1–7 days of vaccination, with all subjects completing their 3-dose regimen and no recurrence of Grade 4 events. The higher percentage of subjects with locally solicited AEs in the 3A-HBV group compared to the 1A-HBV group was largely attributable to the higher frequency of injection site pain (72.2% vs 44.5%) and tenderness (71.2% vs 44.2%), respectively, which was of Grade 1 and 2 severity (). The overall incidence of solicited local AEs, including pain and tenderness, decreased with age in both vaccine groups and was of short duration.
Figure 5a. Severity of local reactions by type of vaccine administered in the pivotal phase 3 studies CONSTANT and PROTECT.
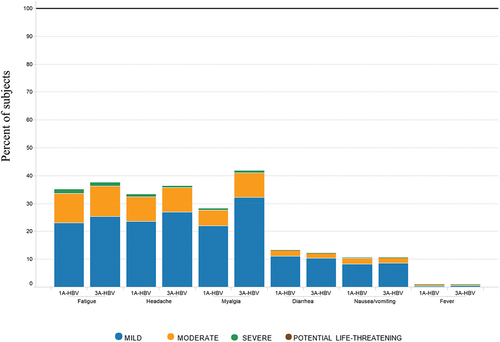
Subjects in the 3A-HBV compared to the 1A-HBV group also had a higher rate of solicited systemic AEs (64.7% vs. 54.1%), however most subjects who experienced solicited systemic AEs reported the maximum severity as mostly Grade 1 (40.7% and 32.6%) or Grade 2 (21.1% and 18.9%) for 3A-HBV and 1A-HBV, respectively. Grade 3 and Grade 4 systemic solicited AEs were uncommon; Grade 3: 2.8% and 2.6%, with Grade 4: 0.1% and 0% for subjects receiving 3A-HBV and 1A-HBV, respectively. Rates of most systemic solicited AEs were comparable between vaccine groups except for myalgia, which was more common in 3A-HBV than 1A-HBV (41.7% and 28.1%, respectively) but was mostly of grade 1 and 2 severities. Fever following vaccination was uncommon in both 3A-HBV and 1A-HBV (). The overall incidence of solicited systemic AEs, including myalgia, decreased with age in both vaccine groups and was of short duration.
Figure 5b. Severity of systemic reactions and fever by type of vaccine administered in the pivotal phase 3 studies CONSTANT and PROTECT.
3.2.4.3. Unsolicited adverse events
Treatment-emergent AEs (TEAEs) that occurred within 28 days of any vaccination were reported with approximately equal frequency in 3A-HBV (48.3%) and 1A-HBV (48.4%) groups. The most frequently reported events for 3A-HBV vs 1A-HBV, respectively, were headache (11.0% vs. 10.0%), upper respiratory tract infection (8.4% vs. 7.6%), nasopharyngitis (4.6% vs. 4.8%), fatigue (3.9% vs. 3.7%), dysmenorrhea (3.2% vs. 2.4%), back pain (3.1% vs. 2.6%), myalgia (2.9% vs. 3.4%), oropharyngeal pain (2.7% vs. 2.6%), and injection site pain (2.4% vs. 1.6%) with most events being of Grade 1 or Grade 2 in severity. In the first 28 days after vaccination, the rates of Grade 3 and Grade 4 combined in 3A-HBV and 1A-HBV groups were low and reported at similar frequencies (6.3% vs 6.4%, respectively).
3.2.4.4. Serious adverse events, medically attended events, new onset of chronic illnesses
Serious adverse events were infrequent in both vaccine groups, reported by 2.5% in the 3A-HBV group and 1.6% n the 1A-HBV group in the pooled dataset. Within 28 days of any vaccination, the percentage of subjects with SAEs were similar (0.9% and 0.6% for 3A-HBV and 1A-HBV, respectively). One SAE of viral gastroenteritis was assessed by the investigator as probably related to 3A-HBV. There was 1 sudden cardiac death that was unrelated to vaccination with 3A-HBV in a subject whose medical history included open-heart surgery and biventricular hypertrophy.
Medically attended adverse events were reported in 22.7% of 3A-HBV subjects and 23.4% 1A-HBV subjects, with the majority of MAAEs assessed as unlikely to be related or unrelated to the study of vaccines. The most common MAAEs were upper respiratory tract infection (1.5% vs. 1.3%), sinusitis (1.3% vs 1.2%), and urinary tract infection (1.2% vs 1.6%), in the 3A-HBV and 1A-HBV groups, respectively. Adverse events that represent NOCI were reported in 2.0% of 3A-HBV and 2.5% of 1A-HBV subjects. Hypertension was the most reported NOCI, occurring in 9 (0.3%) 3A-HBV subjects and 7 (0.5%) 1A-HBV subjects. The majority of NOCIs were Grade 1 or 2 in severity.
In summary, whilst the solicited AEs representing local and systemic reactogenicity were higher in the 3A-HBV group than the 1A-HBV group, the rates of unsolicited AEs, AEs leading to discontinuation, MAAE, NOCI, and SAE were similar in both groups. There were no unexpected safety signals associated with 3A-HBV and no findings of clinical concern. The increased level of mild and moderate solicited events in the first 7 days was consistent with the higher reactogenicity of 3A-HBV, and these events were mostly self-limited, of mild-to-moderate intensity and short duration and did not require medical intervention.
3.3. Pharmacovigilance
The safety of 3A-HBV in vaccine recipients has been continuously monitored since 2000, when it was first authorized for use in Israel. Over 750,000 infants, children, and adults have been exposed to PreHevbrio (Sci-B-Vac, Bio-Hep-B). Post-marketing exposure and overall safety based on a review of pharmacovigilance data, which included spontaneous reports, literature reviews, and a review of safety results in investigator-initiated trials using 3A-HBV, have been summarized in Periodic Benefit Risk Evaluation Reports (PBRERs) in accordance with regulatory requirements. To date, the safety profile in marketed use is not meaningfully different from clinical trial safety data.
3.4. Long-term antibody persistence
A follow-up study in a subset of participants from PROTECT enrolled in Finland found that 2–3 years after vaccination, 88.1% of the subjects that received 3A-HBV had retained seroprotective levels of anti-HBs (i.e. ≥10 mIU/mL), compared with only 72.4% of the participants that received 1A-HBV. The mean concentration of anti-HBs in the 3A-HBV group was also five times greater in follow-up than in the 1A-HBV group (1382.9 mIU/mL vs 252.6 mIU/mL). The results of this follow-up study highlight the importance of a robust immune response to vaccination for durable seroprotection against HBV [Citation54].
4. Expert opinion
Most new infections of HBV in North America and Europe occur in adults [Citation13,Citation14]. Increased hepatitis B vaccination in adults, as supported by the recent adoption of a universal hepatitis B vaccination recommendation by the Advisory Committee on Immunization Practices (ACIP) in the US for adults aged 19–59 years [Citation22], is critical given current US adult vaccination rates remain low at only 30% [Citation48]. However, new tools may be needed to ensure the protection of all adults. PreHevbrio is a highly immunogenic 3-antigen hepatitis B vaccine recently approved for adults ≥18 years in the US, Europe, and Canada, and may be a beneficial new tool for health-care providers in the fight against the spread of hepatitis B.
In adults, response rates to conventional single S-antigen, alum-adjuvanted vaccines may be suboptimal because of secondary immune deficiency and genetic resistance [Citation55,Citation56]. Seroprotection is often delayed until after the third vaccination, with limited durability of antibody titers, leaving many adults at risk, particularly those with underlying diseases. 3A-HBV induces higher rates of seroprotection after the first and second vaccinations. The rapid seroprotection of 3A-HBV in younger adults (18–45 years) is relevant to at-risk adults such as health-care workers, travelers to countries where hepatitis B is endemic, or those whose lifestyle behaviors increase their risk.
Heplisav-B, which is a single-antigen vaccine adjuvanted with CpG 1018, was approved for adults in the US in 2017 and the EU in 2021. It is approved as a two-dose schedule. PreHevbrio and Heplisav have not been directly compared in a clinical trial. However, the SPR rates of PreHevbrio discussed above are comparable to the SPR rates of Heplisav-B after the last vaccine dose [Citation57].
The phase 3 clinical trial data from the PROTECT and CONSTANT studies detailed herein demonstrate the consistency of immunogenicity of 3A-HBV in adults of all ages, including in those over 45 and in other subgroups known to have reduced responses to single S-antigen vaccines, including obesity, male gender, diabetes, or smoking status. Therefore, a highly immunogenic hepatitis B vaccine with a good safety profile, such as 3A-HBV, may be a good vaccine option for all adults and may be preferred for use in adults over the age of 45 and those with risk factors that reduce their immune response to conventional single antigen hepatitis B vaccines.
Article highlights
Almost 300 million people worldwide are chronically infected with hepatitis B.
Approximately 90% of the individuals with chronic HBV infection are not aware of their status, allowing for ongoing transmission to susceptible populations.
In North America and Europe, most new infections occur in adults.
Conventional single S-antigen HBV vaccines (1A-HBV) adjuvanted with alum have reduced immunogenicity in adults.
Effective and fast-acting adult HBV vaccines are required to maximize the benefit of expanded adult vaccination rates, as supported by the recent adoption of a universal hepatitis B vaccination recommendation by the Advisory Committee on Immunization Practices (ACIP) in the United States for adults 19–59 years.
PreHevbrio is a highly immunogenic 3-antigen (S/pre-S1/pre-S2) HBV vaccine (3A-HBV) approved in the United States (2021), Europe (2022), and Canada (2022) for the prevention of all known subtypes of HBV.
In phase 3 clinical trials, 3A-HBV demonstrated improved seroprotection in adults ≥45 years old and higher rates of seroprotection in adults ≥18 years old after each vaccination compared to 1A-HBV (Engerix-B).
3A-HBV was initially approved in Israel in 2000 (Brand name: Sci-B-Vac) and has a 20-year post-marketing safety history, with estimated 750,000 individuals vaccinated.
As the only approved 3-antigen HBV vaccine, PreHevbrio/PreHevbri has the potential to be a meaningful new tool in the fight to reduce the risk of HBV infection in adults, toward achieving hepatitis B elimination by 2030.
Declaration of interest
T Vesikari has received funding for PROTECT and CONSTANT studies from VBI Vaccines, Inc., through the Nordic Research Network Oy company of which he owns stock. J Langley received funding from VBI Vaccines for PROTECT and CONSTANT studies. V Popovic is an officer and owns shares of VBI Vaccines. F Diaz-Mitoma is an officer and owns shares of VBI Vaccines. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Blumberg BS, Gerstley BJ, Hungerford DA, et al. A serum antigen (Australia antigen) in down’s syndrome, leukemia, and hepatitis. Ann Intern Med. 1967;66(5):924–931. doi: 10.7326/0003-4819-66-5-924
- Jindal A, Kumar M, Sarin SK. Management of acute hepatitis B and reactivation of hepatitis B. Liver Int. 2013;33(1):164–175. doi: 10.1111/liv.12081
- Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on immunization Practices (ACIP) part II: immunization of adults. MMWR Recomm Rep. 2006;55(RR–16):1–33.
- Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on immunization Practices. MMWR Recomm Rep. 2018;67(1):1–31. doi: 10.15585/mmwr.rr6701a1
- Chisari FV, Masanori I, Wieland SF. Pathogenesis of hepatitis B virus infection. Pathol Biol. 2010;58(4):258–266. doi: 10.1016/j.patbio.2009.11.001
- Hyams KC. Risks of chronicity following acute hepatitis B virus infection: a review. Clin Infect Dis. 1995;20(4):992–1000. doi: 10.1093/clinids/20.4.992
- McKeating C, Cadden I, McDougall N, et al. Progression from acute to chronic hepatitis B is more common in older adults. Ulster Med J. 2018 Oct;87(3):177–180.
- Haber P, Schillie S, Hepatitis B. Pinkbook: HepatitisB – CDC. Available online at https://www.cdc.gov/vaccines/pubs/pinkbook/hepb.html (accessed on Nov 3, 2022).
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0
- Flores JE, Thompson AJ, Ryan M, et al. The global impact of hepatitis B vaccination on hepatocellular carcinoma. Vaccines (Basel). 2022;10(5):793. doi: 10.3390/vaccines10050793
- Kim BH, Kim WR. Epidemiology of hepatitis B virus infection in the United States. Clin Liver Dis. 2018;12(1):1–4. doi: 10.1002/cld.732
- CDC 2021 Viral Hepatitis. 2019 Viral hepatitis surveillance report. Atlanta GA. US Department of Health and Human Services, CDC, https://www.cdc.gov/hepatitis/statistics/serveillanceRpts.htm
- Department of Health and Human Services 2022. Viral hepatitis in the United States: data and trends. Available at: http://www.hhs.gov/hepatitis/learn-about-viral-hepatitis/data-and-trends/index.html#:~:text=After%20decades%20of%20declines%20in,%2C%20Tennessee%20and%20West%20Virginia). Accessed on Nov 3, 2022.
- European Centre for Disease Prevention and Control. Hepatitis B. In: ECDC. Annual epidemiological report for 2020. Stockholm: ECDC; 2022.
- WHO 2022. Hepatitis B. Available at https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. Accessed Nov 3, 2022.
- Heyward WL, Kyle M, Blumenau J, et al. Immunogenicity and safety of an investigational hepatitis B vaccine with a toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared to a licensed hepatitis B vaccine in healthy adults 40-70 years of age. Vaccine. 2013;31(46):5300–5305. doi: 10.1016/j.vaccine.2013.05.068
- Sablan BP, Kim DJ, Barzaga NG, et al. Demonstration of safety and enhanced seroprotection against hepatitis B with investigational HBsAg-1018 ISS vaccine compared to a licensed hepatitis B vaccine. Vaccine. 2012;30(16):2689–2696. doi: 10.1016/j.vaccine.2012.02.001
- Vesikari T, Langley JM, Segall N, et al. PROTECT study group immunogenicity and safety of a tri-antigenic versus a mono-antigenic hepatitis B vaccine in adults (PROTECT): a randomised, double-blind, phase 3 trial. The Lancet Infectious Diseases. 2021;21(9):1271–1281. doi: 10.1016/S1473-3099(20)30780-5
- Fan W, Chen XF, Shen C, et al. Hepatitis B vaccine response in obesity: a meta-analysis. Vaccine. 2016;34(40):4835–4841. doi: 10.1016/j.vaccine.2016.08.027
- Yang S, Tian G, Cui Y, et al. Factors influencing immunologic response to hepatitis B vaccine in adults. Sci Rep. 2016;6(1):27251. doi: 10.1038/srep27251
- Schillie SF, Spradling PR, Murphy TV. Immune response of hepatitis B vaccine among persons with diabetes. Diabetes Care. 2012;35(12):2690–2697. doi: 10.2337/dc12-0312
- Weng MK, Doshani M, Khan MA, et al. Universal hepatitis B vaccination in adults aged 18-59 years: updated recommendations of the Advisory Committee on immunization Practices – United States, 2022. MMWR. 2022;71(13):477–483. doi: 10.15585/mmwr.mm7113a1
- Yue X, Black CL, O’Halloran A, et al. Hepatitis a and hepatitis B vaccination coverage among adults with chronic liver disease. Vaccine. 2018;36(9):1183–1189. doi: 10.1016/j.vaccine.2018.01.033
- Lu P-J, Hung M-C, Srivastav A, et al. Surveillance of vaccination coverage among adult populations – United States, 2018. MMWR. 2021;70(3):1–25. doi: 10.15585/mmwr.ss7003a1
- Diminsky D, Schirmbeck R, Reimann J, et al. Comparison between hepatitis B surface antigen (HBsAg) particles derived from mammalian cells (CHO) and yeast cells (hansenula polymorpha): composition, structure and immunogenicity. Vaccine. 1997;15(6–7):637–647. doi: 10.1016/S0264-410X(96)00239-3
- Raz R, Koren R, Bass D. Safety and immunogenicity of a new mammalian cell-derived recombinant hepatitis B vaccine containing pre-S1 and pre-S2 antigens in adults. Isr Med Assoc J. 2001;3(5):328–332.
- Milich DR, Thornton GB, Neurath AR, et al. Enhanced immunogenicity of the pre-S region of hepatitis B surface antigen. Science. 1985;228(4704):1195–1199. doi: 10.1126/science.2408336
- Budkowska A, Dubreuil P, Capel F, et al. Hepatitis B virus pre-S gene-encoded antigenic specificity and anti-pre-S antibody: relationship between anti-pre-S response and recovery. Hepatology. 1986;6(3):360–368. doi: 10.1002/hep.1840060305
- Yerushalmi B, Raz R, Blondheim O, et al. Safety and immunogenicity of a novel mammalian cell-derived recombinant hepatitis B vaccine containing pre-S1 and pre-S2 antigens in neonates. Pediatr Infect Dis J. 1997;16(6):587–592. doi: 10.1097/00006454-199706000-00009
- Shouval D, Ilan Y, Adler R, et al. Improved immunogenicity in mice of a mammalian cell-derived recombinant hepatitis B vaccine containing pre-S1 and pre-S2 antigens as compared with conventional yeast-derived vaccines. Vaccine. 1994;12(15):1453–1459. doi: 10.1016/0264-410X(94)90155-4
- Madalinski K, Sylvan SPE, Hellström U, et al. Presence of anti-preS1, anti-preS2, and anti-HBs antibodies in newborns immunized with Bio-Hep-B™ vaccine. Med Sci Monit. 2004;10(s1):I10–17.
- Hellström UB, Madalinski K, Sylvan SPE. PreS1 epitope recognition in newborns after vaccination with the third-generation Sci-B-Vac™ vaccine and their relation to the antibody response to hepatitis B surface antigen. J Virol. 2009;6(1):7. doi: 10.1186/1743-422X-6-7
- Sylvan SPE, Madalinski K, Hellström UB. Anti-preS responses influence the anti-HBs response in newborns after vaccination with the third generation Sci-B-Vac™ vaccine. Vaccine. 2010;28(2):446–451. doi: 10.1016/j.vaccine.2009.10.023
- PreHevbrio. Prescribing information. https://www.prehevbrio.com/wp-content/uploads/2021/11/PreHevbrio-Full-Prescribing-Information.pdf. Accessed on Nov 2, 2022.
- CDC. Thimerosal in vaccines: a joint statement of the American academy of pediatrics and the public health Services. MMWR Morb Mortal Wkly Rep. 1999;48(26):563–565.
- Shapira MY, Zeira E, Adler R, et al. Rapid seroprotection against hepatitis B following the first dose of a pre-S1/Pre-S2/S vaccine. J Hepatol. 2001;34(1):123–127. doi: 10.1016/S0168-8278(00)00082-9
- Schumann A, Fiedler M, Dahmen U, et al. Cellular and humoral immune response to a third generation hepatitis B vaccine. J Viral Hepat. 2007;14(8):592–598. doi: 10.1111/j.1365-2893.2007.00848.x
- Diaz-Mitoma F, Popovic V, Spaans JN. Assessment of immunogenicity and safety across two manufacturing lots of a 3-antigen hepatitis B vaccine, Sci-B-Vac®, compared with Engerix-B® in healthy Asian adults: a phase 3 randomized clinical trial. Vaccine. 2021;39(29):3892–3899. doi: 10.1016/j.vaccine.2021.05.067
- Jack AD, Hall AJ, Maine N, et al. What level of hepatitis B antibody is protective? J Infect Dis. 1999;179(2):489–492. doi: 10.1086/314578
- Francis DP, Hadler SC, Thompson SE, et al. The prevention of hepatitis B with vaccine: report of the centers of disease Control multicenter efficacy trial among homosexual men. Ann Of Intern Med. 1982;97(3):362–366. doi: 10.7326/0003-4819-97-3-362
- Yap I, Guan R, Chan SH. Recombinant DNA hepatitis B vaccine containing pre-S components of the HBV coat protein – a preliminary study on immunogenicity. Vaccine. 1992;10(7):439–442. doi: 10.1016/0264-410X(92)90391-V
- Hourvitz A, Mosseri R, Solomon A, et al. Reactogenicity and immunogenicity of a new recombinant hepatitis B vaccine containing pre S antigens: a preliminary report. J Viral Hepatitis. 1996;3(1):37–42. doi: 10.1111/j.1365-2893.1996.tb00079.x
- Yap I, Guan R, Chan SH. Study on the Comparative immunogenicity of a recombinant DNA hepatitis B vaccine containing pre-S components of the HBV coat protein with non pre-S containing vaccines. J Gastroenterol Hepatol. 1995;10(1):51–55. doi: 10.1111/j.1440-1746.1995.tb01047.x
- Esaulenko EV, Yakovlev AA, Volkov GA, et al. Efficacy and safety of a 3-antigen (pre-S1/Pre-S2/S) hepatitis B vaccine: results of a phase 3 randomized clinical trial in the Russian Federation. Clin Infect Dis. 2021;73(9):e3333–e3339. doi: 10.1093/cid/ciaa1649
- Atsmon J, Machluf N, Yayon-Gur V, et al. Rapid and high seroprotection rates achieved with a tri-antigenic hepatitis B vaccine in healthy young adults: results from a phase IV study. Vaccine. 2021;39(8):1328–1332. doi: 10.1016/j.vaccine.2020.12.050
- Vesikari T, Finn A, Vac Damme P, et al. Immunogenicity and safety of a 3-antigen hepatitis b vaccine vs a single-antigen hepatitis B vaccine – a phase 3 randomized clinical trial. JAMA Netw Open. 2021;4(10):e2128652. doi: 10.1001/jamanetworkopen.2021.28652
- Donken R, de Melker HE, Rots NY, et al. Comparing vaccines: a systematic review of the use of the non-inferiority margin in vaccine trials. Vaccine. 2015;33(12):1426–1432. doi: 10.1016/j.vaccine.2015.01.072
- Centers for Disease Control and Prevention. Healthy people 2030 objectives–vaccination 2021 [1 Jul 2021]. Available from: https://health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination
- Alon D, Stein GY, Hadas-Golan V, et al. Immunogenicity of Sci-B-Vac (a third-generation hepatitis B vaccine) in HIV-Positive adults. Isr Med Assoc J. 2017;19(3):143–146.
- Etzion O, Novack V, Perl Y, et al. Sci-B-Vac vs ENGERIX- B vaccines for hepatitis B virus in patients with inflammatory bowel diseases: a randomised controlled trial. J Crohns Colitis. 2016;10(8):905–912. doi: 10.1093/ecco-jcc/jjw046
- Weinstein T, Chagnac A, Boaz M, et al. Improved immunogenicity of a novel third-generation recombinant hepatitis B vaccine in patients with end-stage renal disease. Nephron Clin Pract. 2004;97(2):c67-c–72. doi: 10.1159/000078403
- Elhanan E, Boaz M, Schwartz I, et al. A randomized, controlled clinical trial to evaluate the immunogenicity of a PreS/S hepatitis B vaccine Sci-B- Vac™, as compared to Engerix B®, among vaccine naïve and vaccine non-responder dialysis patients. Clin Exp Nephrol. 2017;22(1):151–158. doi: 10.1007/s10157-017-1416-7
- U.S. Food and Drug Administration. Guidance for industry. Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. In Us department of health and human services, food and drug administration, center for drug evaluation and research, center for biologics evaluation and research: 10. (available at: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials | FDA; Accessed Nov 3, 2022; Rockville MD: Jeffrey Shuren; 2007.
- Vesikari T, Langley JM, Spaans J, et al. The persistence of seroprotective levels of antibodies after vaccination with PreHevbrio, a 3-antigen HBV vaccine. Vaccine. 2023;41(24):3584–3588. doi: 10.1016/j.vaccine.2023.05.010
- Godkin A, Davenport M, Hill AV. Molecular analysis of HLA class II associations with hepatitis B virus clearance and vaccine nonresponsiveness. Hepatology. 2005;41(6):1383–1390. doi: 10.1002/hep.20716
- Walayat S, Ahmed Z, Martin D, et al. Recent advances in vaccination of non-responders to standard dose hepatitis B virus vaccine. World J Hepatol. 2015;7(24):2503–2509. doi: 10.4254/wjh.v7.i24.2503
- Janssen JM, Jackson S, Heyward WL, et al. Immunogenicity of an investigational hepatitis B vaccine with a toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared with a licensed hepatitis B vaccine in subpopulations of healthy adults 18–70 years of age. Vaccine. 2015;33(31):3514–3618. doi: 10.1016/j.vaccine.2015.05.070

