ABSTRACT
Background
Research on immunogenicity after 3rd SARS-CoV-2 vaccine in elder hepatocellular carcinoma (HCC) was limited. This study aimed to investigate the efficacy and influencing factors of inactivated SARS-CoV-2 vaccine in elder HCC.
Research design and methods
We assessed total antibodies, anti-RBD IgG, and neutralizing antibodies (NAb) toward SARS-CoV-2 wild type (WT) as well as BA.4/5 in 304 uninfected HCC, 147 matched healthy control (HC), and 53 SARS-CoV-2 infected HCC, all aged over 60 years. The levels of antibodies were compared in the period 7–90, 91–180, and >180 days after 2nd or 3rd vaccination, respectively.
Results
HCC had lower seropositivity than HC after 2nd dose (total antibodies, 64% vs. 92%, P < 0.0001; anti-RBD IgG, 50% vs. 77%, P < 0.0001). But 3rd dose can efficaciously close the gap (total antibodies, 96% vs. 100%, P = 0.1212; anti-RBD IgG: 87% vs. 87%, P > 0.9999). Booster effect of 3rd dose can persist >180 days in HCC (2nd vs. 3rd: total antibodies, 0.60 vs. 3.20, P < 0.0001; anti-RBD IgG, 13.86 vs. 68.85, P < 0.0001; WT NAb, 11.70 vs. 22.47, P < 0.0001). Vaccinated HCC had more evident humoral responses than unvaccinated ones after infection (total antibodies: 3.85 vs. 3.20, P < 0.0001; anti-RBD IgG: 910.92 vs. 68.85, P < 0.0001; WT NAb: 96.09 vs. 22.47, P < 0.0001; BA.4/5 NAb: 86.53 vs. 5.59, P < 0.0001).
Conclusions
Our findings highlight the booster effect and protective role of 3rd dose. Our results could provide a theoretical foundation for informing decisions regarding SARS-CoV-2 vaccination in elder HCC.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), also known as Corona Virus Disease 2019 (COVID-19), is a highly infectious virus that has spread rapidly around the world. Multiple studies have revealed that patients with cancer were at a higher risk of breakthrough infections and severe COVID-19 symptoms compared to healthy people [Citation1–5]. Besides, age was considered a risk factor for SARS-CoV-2 infection. A cohort study identified that increased age [per 10 years; partially adjusted odds ratio (OR): 1.84, 95% confidence interval (95% CI): 1.53–2.21] and active cancer (progressing vs. Remission; OR: 5.20, 95% CI: 2.77–9.77) were independent factors associated with 30-day mortality among cancer patients with SARS-CoV-2 infection [Citation4]. Additionally, age (per 10 years, OR 0.5, 95% CI 0.2–0.8, P = .008) was also identified as an independent predictor for seroconversion after COVID-19 vaccination [Citation6]. Elder cancer patients generally develop degenerative performance status and have impaired immune systems, which may be associated with low immune responses toward vaccines.
Various vaccines effectively reduced SARS-CoV-2 morbidity and the rate of severe cases. Although the safety and efficacy of the vaccines have been proven [Citation7–9], the data on cancer patients, especially those with active malignancies are limited. Most studies reported lower humoral and cellular reactions in cancer patients [Citation10–12]. Besides, the results varied in the types of cancer, particularly in solid cancer and hematological cancer [Citation13,Citation14]. Age, gender, therapies, and immunity status were also considered possible influencing factors of vaccine response [Citation11,Citation14–16]. However, whether cancer patients’ response toward SARS-CoV-2 vaccines after the third vaccination differs from healthy people remains to be clarified, and the influencing factors need further confirmation. Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third leading cause of cancer deaths worldwide [Citation17], as well as the second leading cause of cancer deaths in China [Citation18]. In China, the hepatitis B virus (HBV), the etiological agent of chronic hepatitis B (CHB), is the main cause of cirrhosis and HCC. A review on chronic liver disease (CLD) indicated that immune response to vaccination was impaired in alcohol-associated liver disease, CLD, and cirrhosis [Citation19]. Another research further found that HBV-DNA load was a risk factor for seropositivity responses toward SARS-CoV-2 in HCC patients [Citation20]. However, other research on CLD and cirrhosis did not report HBV infection as a hazardous factor [Citation21–23]. Reliable evidence is still lacking between HBV infection and SARS-CoV-2 vaccine response in HCC patients.
The durability and determinants of immunogenicity response to inactivated SARS-CoV-2 vaccine in elder HCC have yet to be determined. To fill the gap, we designed a retrospective study enrolling HCC aged over 60 years and matched healthy controls. This study aimed to investigate the immunogenicity and risk factors of inactivated SARS-COV-2 vaccines. Our study provides evidence for the effectiveness of SARS-COV-2 vaccination in elder HCC patients, which also has a certain reference value for other solid tumors.
2. Materials and methods
2.1. Patients characteristics and specimen collection
From Jan. 2022 to Jan. 2023, we collected 357 HCC samples and 147 Healthy control (HC) plasma samples. HCC patients were collected from the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital. The HC group was collected from the Peking Union Medical College Hospital. The inclusion criteria of the HCC group were as follows: (1) primary hepatocellular carcinoma patients; (2) aged over 60 years; (3) 304 HCC previously inoculated with at least one dose of inactivated whole-virion SARS-CoV-2 vaccine without previous or current infection before sampling, 53 unvaccinated HCC were collected after COVID-19 infection 1–3 months. The exclusion criteria were as follows: (i) patients with cholangiocarcinoma, hepatic metastasis, or other types of primary liver cancer like hepatic sarcoma, hepatic melanoma, and so on; (ii) patients who were vaccinated with SARS-CoV-2 vaccines other than inactivated whole-virion vaccine, such as adenovirus vector vaccine, mRNA vaccine, recombinant vaccine; (iii) patients with acquired immune deficiency syndrome (AIDS) or autoimmune diseases. The inclusion criteria of the HC group were as follows: (1) previously healthy with no malignant tumors; (2) aged over 60 years; (3) previously inoculated with at least one dose of inactivated whole-virion SARS-CoV-2 vaccine without previous or current infection before sampling; (4) gender, age, and days after vaccination were strictly matched with HCC subgroups. The exclusion criteria of the HC group were as follows: (i) patients with suspected malignant tumor, serum tumor markers detection elevated or lung computerized tomography (CT) scan showed suspected nodules; (ii) patients with diabetes, AIDS or autoimmune diseases; (iii) patients who were vaccinated with SARS-CoV-2 vaccines other than inactivated vaccine, such as adenovirus vector vaccine, mRNA vaccine, recombinant vaccine. The vaccine administered was Chinese-made inactivated whole virion vaccine (Sinopharm [Vero Cell]-Inactivated, COVID-19 Vaccine, from Beijing Institute of Biological Products Co., Ltd.; Sinovac COVID-19 Vaccine (Vero Cell), Inactivated, from Sinovac Life Sciences Co., Ltd.). All infection information was collected by epidemiological screening and confirmed via nucleic acid testing or antigen detection. After collecting, we centrifuged the blood samples at 4°C, 3000 rpm × 10 min, then separated plasma into 2 ml tubes and stored them at −80°C.
Patients’ data including age, gender, clinical diagnosis, Barcelona Clinic Liver Cancer (BCLC) stage, peripheral blood cell counts as well as percentage, and hepatitis B virus (HBV) or hepatitis C virus (HCV) infection indexes were collected from electronic medical records. In our study, BCLC stage A was considered as the early stage. Stages B, C, and D were considered as the middle and advanced stage. CD3+CD4+ or CD3+CD8+ T lymphocytes, B lymphocytes, and natural killer (NK) cells of 75 blood samples were detected by Flow Cytometry. This study was approved by the Medical Ethics Committee of the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital and the Peking Union Medical College Hospital with all participants’ informed consent.
2.2. Detection of total antibodies against SARS-CoV-2
We used the double antigen sandwich enzyme-linked immunosorbent assay (ELISA) kit from WANTAI BioPharm Co., Ltd to assess the level of total antibodies (including lgM and lgG) against SARS-COV-2 in serum samples. The SARS-COV-2 antigens were pre-coated on the microporous strip to bind to the antibodies in the samples. After the reaction, we used the Thermo Scientific Microplate Reader to detect the optical density (OD) of every microwell and analyzed the receiver operating characteristic (ROC) curve to count the cutoff value. Finally, 0.19 was calculated as the cutoff to measure the positive samples and the negative ones.
2.3. Detection of neutralizing antibody (NAb) toward SARS-CoV-2 wild type (WT) and omicron BA.4/5 subvariant
We used the GenScript SARS-CoV-2 Surrogate Virus Neutralization Test (sVNT) Kit to detect circulating NAb against SARS-CoV-2. This kit contains two key components: the HRP conjugated recombinant SARS-CoV-2 RBD fragment (HRP-RBD) and the humanACE2 receptor protein (hACE2). The protein-protein interaction between HRP-RBD and hACE2 can be blocked by NAb against SARS-CoV-2 RBD. Inhibition rate (%) calculation: Inhibition = (1 - OD value of sample/OD value of negative control) * 100%. 30% was selected as a threshold value according to the kit instructions.
2.4. Detection of IgG anti-SARS-CoV-2 spike receptor binding domain (RBD) antibody
Another ELISA kit that pre-coated the SARS-CoV-2 spike RBD protein was operated to detect the IgG antibody in plasma (PROPRIUM Co., Ltd.). The concentration of anti-RBD IgG was calculated by standard curve. A threshold value of 13.6 (BAU/ml) was chosen following the advice of the kit company.
2.5. Follow-up survey
From Jan. 2023 to Feb. 2023, we followed up with the HCC patients to receive their health status. The follow-up survey consisted of a series of questions about COVID-19 infection conditions. Patients who died without SARS-CoV-2 infection or couldn’t be contacted were excluded. Finally, 166 HCC patients were included for analysis.
2.6. Statistical analysis
Normality tests were played before analysis. Normally distributed data were expressed by mean±standard deviation (SD), and non-normally distributed data were described by median or percentage. For intergroup comparison, categorical variables were analyzed by the Chi-square test. Mann Whitey U test and t-test were used for non-normally distributed and normally distributed continuous variables, respectively. The Spearman r correlation method was employed for correlation analyses. To find out possible influencing factors of seroconversion rates of SARS-CoV-2 antibodies, we did univariate analyses. The Chi-square test and t-test were applied in categorical variables and continuous variables, respectively. Parameters with a P value < 0.05 by univariate analysis were included in multivariate analyses. A multivariable logistic regression model was performed to further identify potential risk factors and to explore the influence of risk factors [Citation24]. All analyses and graphs were performed by IBM SPSS Statistics (version 25.0), GraphPad Prism (version 8.0), and R (version 4.1.1). A P value <0.05 was considered statistically significant.
3. Results
3.1. Baseline demographics and clinical characteristics
In total, 304 uninfected HCC, 147 uninfected HC, and 53 infected HCC were included in the study. According to the vaccination dose and the days after vaccination, the patients were divided into nine subgroups. The demographic and clinical characteristics of the cohorts are shown in . The three cohorts’ baseline characteristics of different vaccination backgrounds were approximately matched (Table S1). Uninfected HCC and HC in different time periods after vaccination were also generally well-matched (Table S2). The baseline characteristics indicated that the HBsAg(+) and HBsAg(-) subgroups were also comparable (Table S3).
Table 1. Demographics and clinical characterization of the cohorts.
3.2. SARS-CoV-2 antibodies increased evidently with the 2nd and 3rd dose of vaccination in HCC
The positive rate comparison of serum antibodies against SARS-CoV-2 showed remarkable results. Comparison between 2nd dose > 180d and 3rd dose > 180d showed that 3rd dose vaccination can intensify the humoral responses toward SARS-CoV-2 in HCC (total antibodies, 64% vs. 96%, P < 0.0001; anti-RBD IgG, 50% vs. 87%, P < 0.0001; WT NAb, 5% vs. 32%, P < 0.0001) (Figure S1A, S1B, and S1C). Numerical comparisons also showed significant gains in period comparison. Comparing the first dose with the second dose, total antibodies (0.02 vs. 1.59, P < 0.0001), anti-RBD IgG (0.19 vs. 139.19, P < 0.0001), and WT NAb against SARS-CoV-2 inhibition rate (15.58 vs. 27.48, P = 0.0045) evidently enhanced (, and Table S4). Compared to the second dose, total antibodies also raised conspicuously after the third dose in period comparison (7–90 days, 1.59 vs. 3.44, P < 0.0001; 91–180 days, 1.15 vs. 3.67, P < 0.0001; >180 days, 0.60 vs. 3.20, P < 0.0001) ( and Table S4). Similar conditions also happened in the serum level of anti-RBD IgG (91–180 days, 60.33 vs. 158.94, P < 0.0001; >180 days, 13.86 vs. 68.85, P < 0.0001) ( and Table S4) and WT NAb (7–90 days, 27.48 vs. 57.48, P = 0.0259; 91–180 days, 15.95 vs. 34.27, P < 0.0001; >180 days, 11.70 vs. 22.47, P < 0.0001) ( and Table S4). Though, the humoral response toward SARS-CoV-2 Omicron BA.4/5 was lower on the whole. And NAb toward BA.4/5 had almost no significant enhancement in period comparison (, S1D, and Table S4).
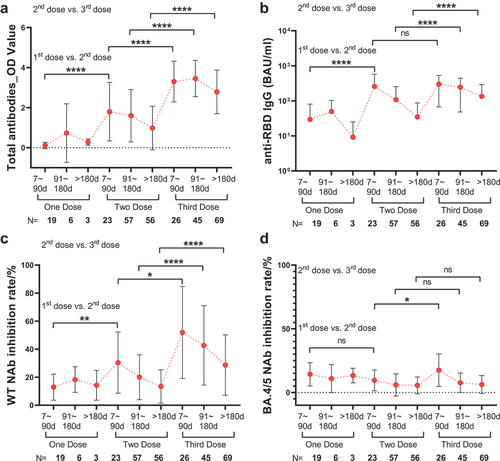
Figure 1. Time period comparison of SARS-CoV-2 antibodies between the different doses of vaccination in HCC. (a) time period comparison of total antibodies. (b) time period comparison of anti-RBD IgG. (c) time period comparison of WT NAb. (d) time period comparison of BA.4/5 NAb. Bars represent SD and spots represent the mean level. Mann-Whitney U-test. Levels of significance: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
3.3. The third dose decreased the difference between HCC and HC in total antibodies and anti-RBD IgG
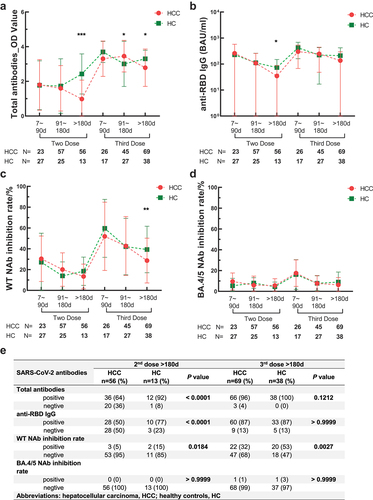
After 2nd dose > 180d, seroconversion of HCC was much lower compared to HC (HCC vs. HC: total antibodies, 64% vs. 92%, P < 0.0001; anti-RBD IgG, 50% vs. 77%, P < 0.0001). But there was no significant difference after 3rd dose (HCC vs. HC: total antibodies, 96% vs. 100%, P = 0.1212; anti-RBD IgG, 87% vs. 87%, P > 0.9999) ( and Figure S1). The results of numerical comparisons resembled. The decrement of total antibodies was apparently more rapid in HCC than in HC after the second dose (HCC vs. HC: after 2nd dose 7–90 days, 1.59 vs. 1.54, P = 0.9959; after 2nd dose >180 days, 0.60 vs. 2.68, P = 0.0005). Third dose can efficaciously wane this disparity. After 3rd dose >180 days, the difference between HCC and HC still existed but diminished compared to the second dose (HCC vs. HC: 3.200 vs. 3.417, P = 0.0174) ( and Table S4). Similarly, anti-RBD IgG between HCC and HC had a remarkable difference after 2nd dose >180 days (P = 0.0453) but was not statistically significant after the 3rd dose (P = 0.0748) ( and Table S4).
Figure 2. Time period comparison of SARS-CoV-2 antibodies between HCC and HC. (a) time period comparison of total antibodies between HCC and HC. (b) time period comparison of anti-RBD IgG between HCC and HC. (c) time period comparison of WT NAb between HCC and HC. (d) time period comparison of BA.4/5 NAb between HCC and HC. In (a), (b), (c) and (d), bars represent SD and bold lines represent the mean level. Mann-Whitney U-test. Levels of significance: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. (e) seropositive rate comparison of SARS-CoV-2 antibodies in 2nd dose and 3rd dose vaccination >180 days between HCC and HC. Chi-square test.
3.4. Third dose vaccination provided a protective effect for HCC in breakthrough infection of SARS-CoV-2 omicron BA.4/5
To evaluate the protective effect of 3rd dose vaccine on SARS-CoV-2 Omicron BA.4/5, from Jan. 2023 to Feb. 2023, we followed up with 166 HCC patients. By the time of our follow-up, 132 patients had received a 3rd dose of vaccine for more than six months and 34 patients did not get vaccinated. The infection rate of the HCC patients after 3rd vaccination was 70.45%, which was lower than those who did not get vaccinated (70.45% vs. 79.41%, P = 0.144) (). Two of the infected ones died because of SARS-CoV-2 infection. Fever and cough were the most common symptoms. Among unvaccinated HCC, 84.62% of patients had a fever, with 11.54% having severe fever (the highest temperature exceeding 39°C). Among vaccinated HCC, 66.29% had a fever, with 14.61% having severe fever (). Most patients had a fever for less than 3 days (). The symptom of fever was milder in vaccinated HCC (fever temperature: P = 0.0067; duration of fever: P = 0.0017) ().
Figure 3. Infection assessment and antibodies comparison between 3rd dose vaccinated HCC and unvaccinated HCC. (a) proportion of infection comparison. Chi-square test.(b) degree of fever comparison. Chi-square test. (c) duration of fever comparison. Chi-square test. (d) total antibodies comparison between infected and uninfected HCC. Mann-Whitney U-test. (e) anti-RBD IgG comparison between infected and uninfected HCC. Mann-Whitney U-test. (f) WT NAb comparison between infected and uninfected HCC. Mann-Whitney U-test. (g) BA.4/5 NAb comparison between infected and uninfected HCC. Mann-Whitney U-test. (h) seropositive rate comparison of vaccinated and unvaccinated HCC after infection. Chi-square test. Levels of significance: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
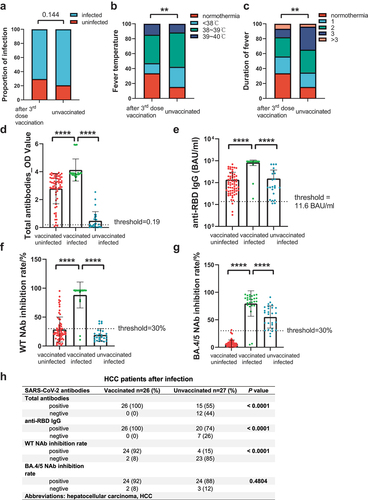
We evaluated the antibody levels of unvaccinated HCC and vaccinated HCC (after 3rd dose >180 days) after infection. Despite having been infected with SARS-Cov-2, the unvaccinated HCC still presented a lower antibody response toward SARS-CoV-2 than those who were vaccinated. (total antibodies, 55.56% vs. 100.00%, P < 0.0001; anti-RBD IgG, 74.75% vs. 100.00%, P < 0.0001; WT NAb, 13.21% vs. 86.79%, P < 0.0001) (). But the positive rate of Omicron BA.4/5 NAb was resembled (92.93% vs. 88.89%, P = 0.4593) (). Numerical comparisons showed similar results. Antibody levels of the vaccinated patients were significantly higher than unvaccinated ones (total antibodies: 0.22 vs. 3.85, P < 0.0001; anti-RBD IgGs: 58.84 vs. 910.92, P < 0.0001; WT NAb: 61.54 vs. 96.09, P < 0.0001; BA.4/5 Nab: 5.59 vs. 86.53, P < 0.0001) (, and Table S4).
3.5. Determinants associated with antibodies against SARS-CoV-2 in HCC
To find out possible factors associated with SARS-CoV-2 antibody levels, we performed Spearman’s correlation analysis of antibodies, clinical characteristics, and laboratory elements (Figure S2A). Compact relations were found between the four antibodies (Figure S2C). Besides, powerful correlations were found between antibodies and vaccine dose (Figure S3A), as well as time after vaccination (Figure S2B). Additionally, HBsAg (P = 0.0077) (Figure S3C), HBeAg (P = 0.0060) (Figure S3D), CD3+CD4+ T cell (P = 0.0439) (Figure S3E), and WBC (P = 0.0372) (Figure S3F) were also observed associated with total antibodies.
To further explore the determinants of SARS-CoV-2 antibodies, we did univariate analyses and multivariate logistic regression analysis. Univariate analysis identified age (y), HBeAg (S/CO), HBeAg-Q, CD3+CD4+ T cell counts (109/L), total T cell counts (109/L), B cell counts (109/L), and monocyte (%) were possible risk factors (Table S5). Then, forward logistic regression was selected for multivariate logistic regression analysis in 2nd dose and 3rd dose, respectively. The results showed that age (2nd: OR = 0.918, P = 0.026) and anti-HCV (3rd: OR = 0.815, P = 0.023) were significant factors impacting the seropositivity of total antibodies. Anti-HCV (2nd: OR = 0.926, P = 0.034) was also found as an independent affecting factor of anti-RBD IgG. As for WT NAb, days after vaccination (2nd: OR = 0.989, P = 0.003; 3rd: OR = 0.995, P = 0.020) and monocyte (3rd: OR = 1.313, P = 0.004) were identified ().
Table 2. Multivariate logistic regression analysis of independent risk factors affecting seropositivity of SARS-CoV-2 antibodies in HCC.
3.6. Humoral responses toward SARS-CoV-2 boosted in HCC patients undergoing hepatitis virus infection
To make certain of the effect of HBV and HCV infection on antibody responses, we compared SARS-CoV-2 antibody levels between HBsAg, HBeAg, as well as anti-HCV antibodies positive(+) and negative(-) patients. The judge criterion of the threshold was from the clinical laboratory of the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital. In the period after 3rd dose, HBsAg(+) group possessed a higher extent of seropositivity response toward total antibodies (7–90 days, 100.00% vs. 88.89%, P = 0.0007; >180 days, 98.00% vs. 88.24%, P = 0.0101) and anti-RBD IgG (7–90 days, 100.00% vs. 77.78%, P < 0.0001) (Figure S5A and S5B). The entire differential between HBsAg(+) and HBsAg(-) only showed a significant distinction in total antibodies (Figure S4). In contrast to HBeAg(-) group, HBeAg(+) group revealed a higher level of total antibodies (P = 0.0405), anti-RBD IgG (P = 0.0261), and WT NAb (P = 0.0271) (Figure S5E). Additionally, the anti-HCV antibodies (+) group displayed a weaker response toward anti-RBD IgG (P = 0.0228) and WT NAb (P = 0.0736) (Figure S5F).
4. Discussion
The SARS-CoV-2 epidemic has dramatically affected the world, which has a profound impact on cancer patients, especially the elderly. Thus, the assessment of vaccines was anxiously needed in the cancer community. Inactivated SARS-CoV-2 vaccine has shown safety and efficacy in clinical trials [Citation9,Citation25–27], but sequential immunogenicity evaluation after the vaccine in elder malignant tumor patients was not reported. Therefore, our study focused on the immunoreactivity of elder HCC patients after 3rd dose inactivated SARS-CoV-2 vaccine.
Inactivated SARS-CoV-2 vaccine show an ideal protective effect after 2nd and 3rd doses in healthy people [Citation28,Citation29]. In our results, the 2nd and 3rd vaccinations can also prominently improve serum SARS-CoV-2 antibodies of HCC. Consistent with other studies on cancer patients [Citation8,Citation13,Citation26,Citation30], we found malignant tumor patients had lower serum responses toward SARS-CoV-2 than healthy controls. After 2nd dose, compared to HC, HCC had a quicker decay and shorter-lived antibodies. Nevertheless, third-dose vaccination can effectively reduce the huge disparity between HCC and HC in total antibodies and anti-RBD IgG. This suggests that HCC patients would gain more benefits from a third vaccine to further develop humoral immunity than healthy people. A current study of inactivated vaccine toward SARS-CoV-2 showed that NAb induced by the second dose peaked at month 2 [Citation30]. Comparing the three time periods after the second dose, we also found that the antibody levels peaked at the first three months and decreased over time. However, the peak time seemed to be delayed after the 3rd dose and did not show apparent descending over time. A likely explanation is that HCC patients may have slower reactions, so the peak response time was delayed. This may also indicate that 3rd dose of the vaccine resulted in a longer time persistence of SARS-CoV-2 antibodies than 2nd dose.
Recently, omicron variants rapidly became dominant in the epidemic. Given the complete dominance of Omicron BA.4/5 in China and surging prevalence globally, our data on BA.4/5 NAb activity have illuminating implications for elder patients who are at incremental risk of SARS-CoV-2 infection. In terms of the protective antibody levels, we found that the antibody response of vaccinated HCC after infection was more dramatic than those who were not vaccinated. Additionally, in the positive rate comparison, BA.4/5 NAb of unvaccinated HCC had no significant difference with vaccinated HCC, but total antibodies, anti-RBD IgG, and WT NAb were significantly lower. This indicated that compared to being infected by a single SARS-CoV-2 subvariant, vaccination can produce more varied antibodies than infection. As a result, we suggest all the HCC patients get vaccinated. The inactivated SARS-CoV-2 vaccine may not protect from infection of the BA.4/5 subvariant but can prevent HCC patients from developing severe infections. Even though most of the Omicron BA.4/5 infections have mild symptoms, whether the immunity system and performance status of cancer patients were harmed after infection remains to be evaluated. We noted with regret that two patients died of SARS-CoV-2 infection, indicating that the Omicron variant still has a threat to patients with poor general conditions. Elder patients with malignant tumors not only had a lower vaccine reaction but also possibly faced more damage from SARS-CoV-2 infection [Citation2–4]. Consequently, advanced measures should be taken to strengthen their defense. Evidence showed that a fourth dose of vaccination was efficacious for restoring the antibodies against Omicron in healthy young workers [Citation31]. And researches on the elderly aged over 60 years old also suggested the effectiveness and necessity of the fourth dose against severe disease caused by the Omicron variant [Citation32,Citation33]. A recent study suggests another strategy to increase humoral immunity to Omicron and possibly future variants is homologous mRNA and heterologous boost combinations [Citation34]. It is necessary to take the fourth dose of vaccine or combined vaccination into consideration.
In correlation analysis and univariate analysis, we found several potential risk factors that were related to serological response toward SARS-CoV-2. Multivariate logistic analyses suggested age as a hazardous factor. In accordance with recent evidence [Citation6,Citation24,Citation35,Citation36], age was considered correlated with low immune response after vaccination. It appeared that antibody profiles had an inverse interrelationship with age. Thus, it is essential to give priority to elder patients’ third shots before the new waves of the pandemic break out. Interestingly, we found monocyte (%) was a protective element for WT NAb. This result has not been described in the previous research on SARS-CoV-2 vaccines. In consideration that monocytes can induce dendritic cells (DCs) generation, we suspect this phenomenon might be related to the function of DCs, which are powerful antigen-presenting cells and essential to initiating and regulating immune responses [Citation37]. In addition, the most recent study reported that hemodialysis patients with fewer monocyte-derived DCs had a poor SARS-CoV-2 vaccine response [Citation38]. Single-cell RNA sequencing (scRNA-seq) analysis also testified to the expansions of CD16+ monocytes and plasmacytoid DCs 14 and 28 days after SARS-CoV-2 vaccination41. We also discerned CD3+CD4+ T cell counts, total T cell counts, and B cell counts as influencing factors in univariate analyses, consistent with the previous findings [Citation6,Citation24,Citation39,Citation40].
Another potential hazard factor was hepatitis virus infection. We discovered that anti-HCV antibody was a negative factor in the SARS-CoV-2 vaccine response. Interestingly, in HBsAg-positive HCC, vaccine-induced immunity was not inferior to those without infection after 3rd dose. HBeAg-positive also presented a protective effect. But limited by the sample size, we did not compare HBeAg in the different time periods. Another research on chronic hepatitis B (CHB) patients also found that HBeAg-positive patients had higher titers of SARS-CoV-2 antibodies and a slower decline in antibody titers [Citation21]. According to the present studies on the SARS-CoV-2 vaccine in HBV infection patients [Citation21–23] and our results, there was no sufficient evidence that HBV virus infection would be a hazard factor of SARS-CoV-2 humoral response. Moreover, in our univariate analyses of risk factors affecting seropositivity of SARS-CoV-2 antibodies in HCC, targeted, immunotherapy, resection, or percutaneous coronary intervention was not detrimental to the induction of humoral immune response to SARS-CoV-2 vaccination. This is reassuring and further suggests elder HCC with prior treatment can acquire acceptable vaccine responses.
The strength of our study is a cohort of both infected and uninfected elder HCC with comparison across the humoral response of four antibodies, which has so far been lacking in studies of third SARS-CoV-2 vaccines. We also assessed correlations between immunity status, hepatitis virus infection, and vaccine-induced humoral response. Nonetheless, there were limitations in our studies. Firstly, this cohort was a retrospective cohort instead of a prospectively recruited cohort, which was unable to access dynamic samples to monitor the sequential immune response after each dose of vaccination. Secondly, though we assessed the relation between immunity status and humoral response, specific cellular immunity testing, which also showed great importance in SARS-CoV-2 protection [Citation41], is lacking. Hence, validation of findings in larger prospective cohorts to investigate humoral and cellular responses of SARS-CoV-2 vaccines is essential. And thirdly, we only investigated the immune response of inactivated whole-virion SARS-CoV-2 vaccine, limited by the availability of samples. As mRNA vaccines are generally more immunogenic than inactivated vaccines, further studies on various types of vaccines including mRNA vaccines in cancer patients should be explored.
5. Conclusion
In conclusion, our findings demonstrated enhanced humoral responses following the administration of booster dose inactivated whole-virion SARS-CoV-2 vaccine in patients with HCC. The booster effect of 3rd dose apparently reduced the distinction between HCC patients and healthy individuals. Moreover, it provided robust protective effects against breakthrough infections. Considering additional vaccine boosting for the elderly before a new pandemic breaks out is imperative. Age, anti-HCV, and monocyte (%) were identified as independent factors associated with seropositivity of SARS-CoV-2 antibodies in elder HCC. Interestingly, HBV infection presented a protective effect in antibody response after 3rd dose. Our results validated the efficacy of inactivated whole-virion SARS-CoV-2 vaccine in elder HCC, particularly highlighting the beneficial impact of the 3rd dose.
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
XHH conceived and designed the research. RYG, MWY, CLZ, LYD, CC, JRY, ZSZ, and LT performed the experiments. RYG extracted data, performed software analyses, and visualized graphs and tables. RYG and MWY wrote the paper. CLZ and YKS provided the clinical samples and data of participants. All authors are responsible for all aspects of the study and attest to the accuracy and completeness of the results. All authors have read and approved the final manuscript as submitted.
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital (22/363–3565) and the Medical Ethics Committee of Peking Union Medical College Hospital (I-22PJ354). Informed consent was obtained from individual or guardian participants.
Supplemental Material
Download ()Acknowledgments
We would like to thank all patients, their families, and study investigators.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2023.2274484
Additional information
Funding
References
- Ribas A, Sengupta R, Locke T, et al. Priority COVID-19 vaccination for patients with Cancer while vaccine supply is limited. Cancer Discov. 2021 Feb;11(2):233–236. PubMed PMID: 33355178; PubMed Central PMCID: PMCPMC8053003. doi: 10.1158/2159-8290.CD-20-1817
- Becerril-Gaitan A, Vaca-Cartagena BF, Ferrigno AS, et al. Immunogenicity and risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection after coronavirus disease 2019 (COVID-19) vaccination in patients with cancer: a systematic review and meta-analysis. Eur J Cancer. 2022 Jan;160:243–260. PubMed PMID: 34794855; PubMed Central PMCID: PMCPMC8548030. doi: 10.1016/j.ejca.2021.10.014.
- Au L, Boos LA, Swerdlow A, et al. Cancer, COVID-19, and antiviral immunity: the CAPTURE study. Cell. 2020 Oct 1;183(1):4–10. PubMed PMID: 32979319; PubMed Central PMCID: PMCPMC7470737 Diagnostics. L.A., L.B., F.B., S.S., A.F., A.S. have no conflicts of interest to declare. doi: 10.1016/j.cell.2020.09.005
- Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020 Jun 20;395(10241):1907–1918. PubMed PMID: 32473681; PubMed Central PMCID: PMCPMC7255743. doi: 10.1016/S0140-6736(20)31187-9.
- Desai A, Sachdeva S, Parekh T et al. COVID-19 and Cancer: lessons from a Pooled meta-analysis. JCO glob oncol. 2020 Apr;6:557–559. doi: 10.1200/GO.20.00097. PubMed PMID: 32250659; PubMed Central PMCID: PMCPMC7193801 manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to. Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments). No potential conflicts of interest were reported. www.asco.org/rwcorascopubs.org/go/site/misc/authors.html
- Liebers N, Speer C, Benning L, et al. Humoral and cellular responses after COVID-19 vaccination in anti-CD20-treated lymphoma patients. Blood. 2022 Jan 6;139(1):142–147. PubMed PMID: 34669919; PubMed Central PMCID: PMCPMC8530768. doi: 10.1182/blood.2021013445
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. [2020 Dec 31];383(27):2603–2615. PubMed PMID: 33301246; PubMed Central PMCID: PMCPMC7745181. doi: 10.1056/NEJMoa2034577
- Walsh EE, Frenck RW Jr., Falsey AR, et al. Safety and immunogenicity of two RNA-Based covid-19 vaccine candidates. N Engl J Med. [2020 Dec 17];383(25):2439–2450. PubMed PMID: 33053279; PubMed Central PMCID: PMCPMC7583697. doi: 10.1056/NEJMoa2027906
- Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021 Jan;21(1):39–51. PubMed PMID: 33069281; PubMed Central PMCID: PMCPMC7561304. doi: 10.1016/S1473-3099(20)30831-8
- Monin L, Laing AG, Munoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021 Jun;22(6):765–778. PubMed PMID: 33930323; PubMed Central PMCID: PMCPMC8078907. doi: 10.1016/S1470-2045(21)00213-8
- Herzog Tzarfati K, Gutwein O, Apel A, et al. BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies. Am J Hematol. [2021 Oct 1];96(10):1195–1203. PubMed PMID: 34185336; PubMed Central PMCID: PMCPMC8420332 interests. doi: 10.1002/ajh.26284
- Van Oekelen O, Gleason CR, Agte S, et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell. [2021 Aug 9];39(8):1028–1030. PubMed PMID: 34242572; PubMed Central PMCID: PMCPMC8238657. doi: 10.1016/j.ccell.2021.06.014
- Fendler A, Shepherd STC, Au L, et al. Adaptive immunity and neutralizing antibodies against SARS-CoV-2 variants of concern following vaccination in patients with cancer: the CAPTURE study. Nat Cancer. 2021 Dec;2(12):1321–1337. PubMed PMID: 34950880; PubMed Central PMCID: PMCPMC7612125. doi: 10.1038/s43018-021-00274-w
- Grinshpun A, Rottenberg Y, Ben-Dov IZ, et al. Serologic response to COVID-19 infection and/or vaccine in cancer patients on active treatment. ESMO Open. 2021 Dec;6(6):100283. PubMed PMID: 34634634; PubMed Central PMCID: PMCPMC8469519. doi: 10.1016/j.esmoop.2021.100283
- Buttiron Webber T, Provinciali N, Musso M, et al. Predictors of poor seroconversion and adverse events to SARS-CoV-2 mRNA BNT162b2 vaccine in cancer patients on active treatment. Eur J Cancer. 2021 Dec;159:105–112. PubMed PMID: 34742157; PubMed Central PMCID: PMCPMC8502731. doi: 10.1016/j.ejca.2021.09.030
- Chung DJ, Shah GL, Devlin SM, et al. Disease- and therapy-specific impact on humoral immune responses to COVID-19 vaccination in hematologic malignancies. Blood Cancer Discov. 2021 Nov;2(6):568–576. PubMed PMID: 34778797; PubMed Central PMCID: PMCPMC8580617. doi: 10.1158/2643-3230.BCD-21-0139
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021 May;71(3):209–249. PubMed PMID: 33538338. doi: 10.3322/caac.21660
- Zheng RS, Zhang SW, Sun KX, et al. Cancer statistics in China, 2016. Zhonghua Zhong Liu Za Zhi. 2023 Mar 23;45(3): 212–220. PubMed PMID: 36944542. 10.3760/cma.j.cn112152-20220922-00647.
- Cornberg M, Buti M, Eberhardt CS, et al. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021 Apr;74(4):944–951. PubMed PMID: 33563499; PubMed Central PMCID: PMCPMC7867401. doi: 10.1016/j.jhep.2021.01.032
- Liu F, Feng X, Du J, et al. Serologic status and safety of inactivated covid-19 vaccine for hepatocellular carcinoma patients with cirrhosis after curative liver resection. Cancer Commun (Lond). 2023 Mar;43(3):409–412. PubMed PMID: 36566347; PubMed Central PMCID: PMCPMC9880698. doi: 10.1002/cac2.12398
- He T, Zhou Y, Xu P, et al. Safety and antibody response to inactivated COVID-19 vaccine in patients with chronic hepatitis B virus infection. Liver Int. 2022;Jun:421:1287–1296. PubMed PMID: 35107848. doi: 10.1111/liv.15173
- Wang J, Zhang Q, Ai J, et al. Safety and immunogenicity of SARS-CoV-2 vaccines in Chinese patients with cirrhosis: a prospective multicenter study. Hepatol Int. 2022 Jun;16(3):691–701. PubMed PMID: 35403977; PubMed Central PMCID: PMCPMC8995697. doi: 10.1007/s12072-022-10332-9
- Ai J, Wang J, Liu D, et al. Safety and immunogenicity of SARS-CoV-2 vaccines in patients with chronic liver diseases (CHESS-NMCID 2101): a multicenter study. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1516–1524 e2. PubMed PMID: 34942370; PubMed Central PMCID: PMCPMC8686447. doi: 10.1016/j.cgh.2021.12.022
- Zhan H, Gao H, Liu Y, et al. Booster shot of inactivated SARS-CoV-2 vaccine induces potent immune responses in people living with HIV. J Med Virol. 2023 Jan;95(1):e28428. PubMed PMID: 36571267; PubMed Central PMCID: PMCPMC9880704. doi: 10.1002/jmv.28428
- Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: a randomised, double-blind, controlled, phase 1/2 trial. Lancet Infect Dis. 2022 Feb;22(2):196–208. PubMed PMID: 34536349; PubMed Central PMCID: PMCPMC8443232 SHZ, JJC, QQL, HF, YX, and XTZ are employees of Beijing Institute of Biological Products, which developed the vaccine and funded the trial. All other authors declare no competing interests. doi: 10.1016/S1473-3099(21)00462-X
- Ariamanesh M, Porouhan P, PeyroShabany B, et al. Immunogenicity and Safety of the inactivated SARS-CoV-2 vaccine (BBIBP-CorV) in patients with malignancy. Cancer Invest. 2022 Jan;40(1):26–34. PubMed PMID: 34634986; PubMed Central PMCID: PMCPMC8567287. doi: 10.1080/07357907.2021.1992420
- Huang R, Liu X, Xie F, et al. Safety and immunogenicity of inactivated SARS-CoV-2 vaccine (BBIBP-CorV) in hypertensive and/or diabetic people aged over 60 years: a prospective Open-label study. Diabetes Ther. 2023 Jan;14(1):139–151. PubMed PMID: 36437418; PubMed Central PMCID: PMCPMC9702925. doi: 10.1007/s13300-022-01343-8
- Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized Clinical trial. JAMA. 2021 Jul 6;326(1): 35–45. PubMed PMID: 34037666; PubMed Central PMCID: PMCPMC8156175 Ms Y. Yang, Ms Y. Zhao, Ms H.Wang, and Mr Li reported receiving grants from the Ministry of Science and Technology of the People’s Republic of China (2020YFC0842100) during the conduct of the study. Dr Yuntao Zhang, Ms Y Yang, Ms Xuqin Yang, Mr Lai, Ms Q. Wang, Mr T. Yang, Mr Liu, and Dr Xiaoming Yang are employees of the China National Biotec Group Company Limited; Mr Li, Mr Chen and Drs Huang and Duan are employees of the Wuhan Institute of Biological Products Co, Ltd; and Mr W. Wang, Mr Ma, Ms Y. Zhao, and Ms H. Wang are employees of the Beijing Institute of Biological Products Co, Ltd-all companies that developed the vaccine and sponsored the trial. Drs Jiang and G. Zhao are employees of the Beijing Key-Tech Statistical Consulting Co, Ltd, and received fees from the China National Biotec Group Company Limited to conduct the data analysis. Drs Al Kaabi, Abdalla, Hussein, Al Mazrouei, Al Karam, Hussain, and Khan and Mr Fasihuddin reported being an employee of Abu Dhabi Health Services Company. Drs Mahmoud and Zaher, Mr ElTantawy, Mr Xiao, and Mr Koshy reported being an employee of the G42 Healthcare. Ms H. Wang reported receiving grants from the Beijing Municipal Science & Technology Commission during the conduct of the study and having a patent for 202010575098.9 pending, a patent for 202010537733.4 pending, a patent for 202010537730.0 pending, a patent for 202110052921.2 pending, and a patent for 202110052933.5 pending. Drs Xiaoming Yang and Duan and Mr Li have a patent for 202010559132.3 pending. No other disclosures were reported. doi: 10.1001/jama.2021.8565
- Yue L, Xie T, Yang T, et al. A third booster dose may be necessary to mitigate neutralizing antibody fading after inoculation with two doses of an inactivated SARS-CoV-2 vaccine. J Med Virol. 2022 Jan;94(1):35–38. PubMed PMID: 34516026; PubMed Central PMCID: PMCPMC8661707. doi: 10.1002/jmv.27334
- Cheng ZJ, Huang H, Zheng P, et al. Humoral immune response of BBIBP COVID-19 vaccination before and after the booster immunization. Allergy. 2022 Aug;77(8):2404–2414. PubMed PMID: 35255171; PubMed Central PMCID: PMCPMC9111230. doi: 10.1111/all.15271
- Regev-Yochay G, Gonen T, Gilboa M, et al. Efficacy of a fourth dose of Covid-19 mRNA vaccine against Omicron. N Engl J Med. [2022 Apr 7];386(14):1377–1380. PubMed PMID: 35297591; PubMed Central PMCID: PMCPMC9006792. doi: 10.1056/NEJMc2202542
- Bar-On YM, Goldberg Y, Mandel M, et al. Protection by a fourth dose of BNT162b2 against Omicron in Israel. N Engl J Med. [2022 May 5];386(18):1712–1720. PubMed PMID: 35381126; PubMed Central PMCID: PMCPMC9006780. doi: 10.1056/NEJMoa2201570
- Goh YS, Rouers A, Fong SW, et al. Waning of specific antibodies against Delta and Omicron variants five months after a third dose of BNT162b2 SARS-CoV-2 vaccine in elderly individuals. Front Immunol PubMed PMID: 36451833; PubMed Central PMCID: PMCPMC9704817. 2022;13:1031852. doi: 10.3389/fimmu.2022.1031852
- Lyke KE, Atmar RL, Islas CD, et al. Rapid decline in vaccine-boosted neutralizing antibodies against SARS-CoV-2 Omicron variant. Cell Rep Med. 2022 Jul 19;3(7): 100679. PubMed PMID: 35798000; PubMed Central PMCID: PMCPMC9212999. 10.1016/j.xcrm.2022.100679.
- Collier DA, Ferreira I, Kotagiri P, et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021 Aug;596(7872):417–422. PubMed PMID: 34192737; PubMed Central PMCID: PMCPMC8373615. doi: 10.1038/s41586-021-03739-1
- Tober-Lau P, Schwarz T, Vanshylla K, et al. Long-term immunogenicity of BNT162b2 vaccination in older people and younger health-care workers. Lancet Respir Med. 2021 Nov;9(11):e104–e105. PubMed PMID: 34687656; PubMed Central PMCID: PMCPMC8528470 regarding the diagnosis of SARS-CoV-2 by antibody testing (application number EP20158626.0). HG and FKl are named on a patent application regarding neutralising antibodies against SARS-related coronaviruses (application number EP20177354). All other authors declare no competing interests. We thank all study participants at Charite - Universitatsmedizin Berlin for their participation. We also thank the entire staff of the Department for Occupational Medicine, the Charite Clinical Study Center at Charite - Universitatsmedizin Berlin, and the Berlin Institute of Health (BIH) for their support during the study. SARS-CoV-2 RBD variant antigens were kindly provided by InVivo BioTech Services (Hennigsdorf, Germany) to the Seramun Diagnostica (Heidesee, Germany). Parts of this work were supported by grants from the BIH and Berlin University Alliance. This study was further supported by the German Ministry of Education and Research through Forschungsnetzwerk der Universitatsmedizin zu COVID-19, (COVIM, FKZ: 01KX2021) to LES, FKu, FKI, CD, and VMC; through VARIPath projects (01KI2021) to VMC; and through Deutsche Forschungsgemeinschaft (SFB-TR84) to NS and LES. The study was supported by a donation from Zalando to Charite - Universitatsmedizin Berlin. doi: 10.1016/S2213-2600(21)00456-2
- Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18(1):767–811. PubMed PMID: 10837075. doi: 10.1146/annurev.immunol.18.1.767
- Valentini N, Marchitto L, Raymond M, et al. Innate immunity and SARS-CoV-2 vaccine response in hemodialysis patients. Kidney360. [2022 Oct 27];3(10):1763–1768. PubMed PMID: 36514720; PubMed Central PMCID: PMCPMC9717667. doi: 10.34067/KID.0002542022
- Lv Z, Li Q, Feng Z, et al. Inactivated SARS-CoV-2 vaccines elicit immunogenicity and T-cell responses in people living with HIV. Int Immunopharmacol. 2022 Jan;102:108383. PubMed PMID: 34824035; PubMed Central PMCID: PMCPMC8599017. doi: 10.1016/j.intimp.2021.108383
- Feng Y, Zhang Y, He Z, et al. Immunogenicity of an inactivated SARS-CoV-2 vaccine in people living with HIV-1: a non-randomized cohort study. EClinicalMedicine. 2022 Jan;43:101226. PubMed PMID: 34901799; PubMed Central PMCID: PMCPMC8642727. doi: 10.1016/j.eclinm.2021.101226
- Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature. 2020 Oct;586(7830):594–599. PubMed PMID: 32998157. doi: 10.1038/s41586-020-2814-7

