?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background
Because SARS-CoV-2 mutations and immunity wane over time, a third dose of heterologous COVID-19 vaccine is proposed for individuals primed with inactivated COVID-19 vaccine.
Research design and methods
We conducted a single-center, open-label trial to assess the safety, immunogenicity, and immune-persistence of a heterologous BBIBP-CorV/ZF2001 prime-boost vaccination in Chinese adults. 480 participants who had been primed with two doses of BBIBP-CorV, received a third dose of ZF2001 after an interval of 3–4, 5–6, or 7–9 months.
Results
The overall incidence of adverse reactions within 30 days after vaccination was 5.83%. No serious adverse reactions were reported. The respective geometric mean titers (GMTs) of neutralizing antibodies for 3–4, 5–6, and 7–9 months groups at baseline were 2.06, 2.02, and 2.10; which increased to 55.42, 63.45, and 62.06 on day 14; then decreased to 17.53, 23.79, and 26.73 on day 30; before finally waning to 8.29, 9.24, and 9.51 on day 180. After the booster, the three groups showed no significant differences in GMTs. GMTs were lower in older participants than younger participants.
Conclusions
A heterologous BBIBP-CorV/ZF2001 prime-boost vaccination was safe and immunogenic. Prime-boost intervals did not affect the immune response. The immune response was weaker in older adults than younger adults.
Clinical trial identifier
NCT05205083
1. Introduction
Coronavirus disease 2019 (COVID-19) caused a global pandemic and seriously threatened human health and public safety. According to public data from the World Health Organization, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused 99,315,684 COVID-19 confirmed cases with 121,742 deaths in China from 3 January 2020 to 18 October 2023. The development and deployment of COVID-19 vaccines represents a fundamental step toward ending the pandemic, protecting the health of world populations and restoring global economies.
In March 2021, China launched a national mass vaccination campaign with five COVID-19 vaccines, among which four (BBIBP-CorV, CoronaVac, Convidecia, and WIBP-CorV) were approved for conditional listing, and one vaccine (ZF2001) was authorized for emergency use by the China National Medical Products Administration. All the vaccines had shown good protection against COVID-19 infections and significant protection against severe cases of infection and deaths. In phase 3 trials, the inactivated COVID-19 vaccine BBIBP-CorV showed an overall vaccine efficacy of 78.1%, with 50.3% vaccine effectiveness against symptomatic COVID-19 cases and 100% protection against hospitalization and severe illness [Citation1]. The results of a clinical study showed the efficacy of the recombinant protein subunit vaccine ZF2001 to be 75.7% for symptomatic COVID-19 cases, 87.6% for severe-to-critical COVID-19 cases, and 86.5% for COVID-19-related deaths [Citation2].
However, the continuous emergence of SARS-CoV-2 variants of concern (VOC) with increased infectivity, transmissibility, and immune escape ability, such as the Omicron strain, challenges the immunogenicity and immunoprotection of existing vaccines. On the other hand, vaccine-induced immunity typically wanes over time, which might lead to lower vaccine protection effectiveness. There is evidence that humoral immune responses decline quickly (within six months) after a standard two-dose regimen of inactivated SARS-CoV-2 vaccination [Citation3]. Waning immune humoral response to mRNA vaccine was also reported. Levin et al. found that humoral response was substantially decreased six months after full BNT162b2 vaccination, especially among men, among persons 65 years of age or older, and among immunosuppressed persons [Citation4].
Previous studies have reported that a third homologous prime-boost regimen demonstrates satisfactory safety, and a higher immune response is elicited by a third dose of vaccine, including BNT162b2, CoronaVac, and BBIBP-CorV [Citation5–7]. Compared with the homologous prime-boost vaccination schedule, a heterologous schedule incorporating COVID-19 vaccines across different platforms may promote antibody affinity maturation and influence the breadth of vaccine-elicited neutralizing antibodies by including different antigens, types of vectors, delivery routes, doses and/or adjuvants at different times [Citation8]. Robust data on the safety and immunogenicity of heterologous schedules with different COVID-19 vaccines will help enhance deployment flexibility and improve access to vaccines [Citation9]. Recent studies have explored the effectiveness of heterologous prime-boost vaccinations with the combinations ChAdOx1 nCoV-19 and BNT162b2, Ad26.COV2-S and BNT162b2, CoronaVac and ChAdOx1 nCoV-19, and CoronaVac and Convidecia [Citation8,Citation10–13]. The results showed that heterologous prime-boost vaccination improves immunogenicity compared with homologous vaccination. However, most of the reports were limited to 28 days or 3 months after the booster dose. The long-term immunity of heterologous prime-boost regimens are rarely reported, particularly for older people.
Here, we report the safety, immunogenicity, and immune-persistence of an initial vaccination with two doses of BBIBP-CorV followed by heterologous boosting with ZF2001 in Chinese adults aged 18 years and older.
2. Materials and methods
2.1. Study design and participants
We conducted a single-center, open-label trial to access the safety, immunogenicity, and immune-persistence of heterologous prime-boost immunization with a two-dose inactivated BBIBP-CorV vaccine and a recombinant protein subunit booster vaccine ZF2001. Participants were recruited at two study sites (Yonghe and Yiting) in community health centers in Shangyu County, Zhejiang Province, China.
Healthy participants, male or female, 18 years of age and older, who had completed two-dose priming with BBIBP-CorV in the past 3–9 months were recruited for eligibility screening. Investigators verified the vaccination records and checked the medical history of each participant. Eligible participants were allocated (1:1:1) to various prime-boost interval groups to receive a booster dose of ZF2001: a 3–4 months group (Group A), a 5–6-months group (Group B), and a 7–9 months group (Group C). For each group, participants aged 18–59 years and ≥60 years were assigned at a ratio of 3:1.
Participants with a previous COVID-19 diagnosis or SARS-CoV-2 infection or women with positive urine pregnancy test results were excluded from this study. All participants were required to have no or well-controlled comorbidities. Key exclusion criteria were congenital or acquired immunodeficiency, serious acute hypersensitive reaction to vaccines, breastfeeding, intent to conceive, and thrombocytopenia or other coagulation disorders. Details of the inclusion and exclusion criteria are presented in the Supplementary information.
Ethics approval was obtained from the Research Ethics Committee of Zhejiang Provincial Center for Disease Control and Prevention (2021-030-01) for this study. No changes to the protocol were made after the initiation of the study. Written informed consent was obtained from each participant before screening. This trail was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice, and Chinese regulatory requirements.
2.2. Study vaccine
The recombinant protein subunit COVID-19 vaccine, ZF2001, was jointly developed by the Institute of Microbiology, Chinese Academy of Sciences, and Anhui Zhifei Longcom Biopharmaceuticals and manufactured by Anhui Zhifei Longcom Biopharmaceuticals [Citation14]. The vaccine uses the tandem-repeat dimeric receptor-binding domain (RBD) of the SARS-CoV-2 spike protein (from the original Wuhan-Hu-1 strain) as the antigen. This vaccine was manufactured in the CHOZN CHO K1 cell line and produced as a liquid formulation containing 25 mg of NCP-RBD protein per 0.5 mL in a vial, with aluminum hydroxide as the adjuvant. All participants received the vaccine intramuscularly in their upper arms. In this trial, the batch number of the vaccine was A202107150.
2.3. Assessment
After the booster vaccination, all participants were observed at the community health center for 30 minutes for any immediate adverse reactions and then they were instructed on how to keep a daily record of any adverse events occurring within the next seven days on a participant diary card. Solicited injection site events included pain, redness, swelling, induration, rash and pruritus, while solicited systemic events included fever, headache, fatigue, diarrhea, nausea, vomiting, muscle ache, acute allergic reaction, and coughing. Adverse events occurring 8–30 days later were recorded on a contact card by participants. Throughout the trial, participants were requested to report all serious adverse events (SAEs) and pregnancy events. Adverse events were graded for severity according to the Guideline of the Grading Standards for Adverse Events Toxicity in Clinical Trials on Prophylactic Vaccines issued by the China National Medical Products Administration. The causal association between vaccination and adverse events was determined by trained investigators. A 5-ml blood sample was collected from each participant at baseline (day 0) before they received the booster dose and on day 14, day 30 and day 180 post-vaccination.
2.4. Live SARS-CoV-2 neutralization assay
Live viral neutralizing antibody titers against the wild-type SARS-CoV-2 was determined by using a cytopathic effect (CPE) assay with the wild-type SARS-CoV-2 virus (GDPCC-nCOV-8, CDPCC.2.00096) in Vero-E6 cells. Serum dilutions were mixed with the same volume of viral solution to achieve a final concentration of 100 TCID50 per well. The reported titer was the reciprocal of the highest sample dilution that protected at least 50% of cells from cytopathic effects. The positive cutoff for defining seropositivity for neutralizing antibodies to live SARS-CoV-2 was 1:4. Neutralizing antibody detection data below 1:4 were calculated as 1:2, and data above 1024 were calculated as 1025 in the immunology analysis.
2.5. Outcomes
Safety outcomes were the occurrence of adverse reactions within 30 days after the booster and SAEs and pregnancy within 180 days after the booster.
Immunogenicity and immune-persistence outcomes of neutralizing antibody titers against the wild-type SARS-CoV-2 virus were reported as the geometric mean titer (GMT), geometric mean increase (GMI), seroconversion rate and seropositivity rate. The seroconversion rate was defined as a change in neutralizing titers from seronegative at baseline to seropositive, or a four-fold increase in titers for participants whose neutralizing titers were above the seropositive cutoff (1:4). The seropositivity rate was defined as the proportion of people whose neutralizing titers were 1:4 or above.
2.6. Sample size calculation
The sample size of each group was calculated according to single-arm objective performance criteria. According to the immunogenicity data of the recombinant novel coronavirus vaccine (CHO) phase II clinical trial, the GMT of subjects over 18 years old after three doses was 102.5, the expected GMT was 105.5, and the standard deviation was 10.5, with α = 0.025 (one-sided) and β = 0.10.Considering a 20% loss to follow-up, the sample size was 132 people in each group, which was adjusted to 160 people. According to the prime-boost interval, three groups were set up in this study, so the total sample size was 480 people. Among them, Z1-α and Z1-β represented the corresponding quantiles of 1-α and 1-β in the standard normal distribution, μ1 was the expected GMT, μ0 was the target GMT, and σ was the standard deviation.
2.7. Statistical analysis
Safety assessments were done on a safety set (SS) of all participants who received a booster vaccine. Immunogenicity on day 14 and day 30 and immune-persistence assessments were done on per-protocol set 1 (PPS1), per-protocol set 2 (PPS2) and immune-persistence set (IPS), respectively. PPS1 and PPS2 were composed of participants who received a booster vaccine, had no severe protocol violations, and completed the blood collections on day 14 and day 30, respectively. IPS included participants who received a booster vaccine and completed the blood collections on day 180. GMTs were calculated from the log-transformed neutralizing antibody titers. GMIs were calculated as the ratio of GMT on day 14, day 28 or day 180 after the booster to GMT at baseline.
We used the chi-squared test or Fisher’s exact test to analyze categorical data. A t-test or analysis of variance (ANOVA) was used for continuous data. A paired t test was used for paired continuous data. Comparisons of GMTs between different groups were conducted with analysis of covariance (ANCOVA). ANCOVA was performed using the following variables: the log-transformed neutralizing antibody titers on day 14 and day 30 post-booster vaccination as dependent variables, the log-transformed neutralizing antibody titers at baseline as covariates, and different prime-boost interval groups and age groups as fixed effects. Significance was set at P < 0.05 (two-sided). We used Microsoft Excel 2019, SAS 9.4 and GraphPad Prism 9 to analyze data and generate graphs.
3. Results
3.1. Participants
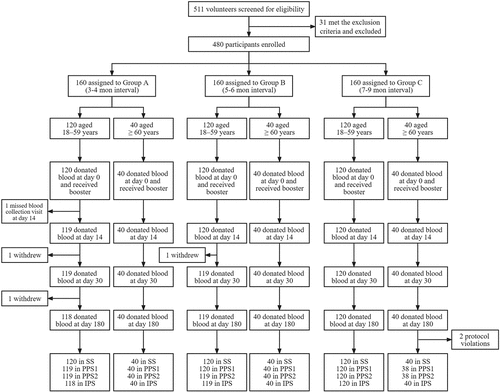
Between 10 November 2021, and 12 January 2022, we recruited 512 volunteers who had received two doses of BBIBP-CorV in the past 3–9 months for screening. A total of 480 participants were enrolled and assigned to groups A – C at a ratio of 1:1:1 according to the prime-boost intervals. Each group was composed of 120 participants aged 18–59 years and 40 participants aged 60 years or above. In total, 480 participants received a booster dose of ZF2001 and were included in the SS. Among them, two participants had protocol violations, three participants withdrew, and one participant missed the blood collection visit on day 14. Finally, 477 participants were included in PPS1, with 476 in PPS2 and 477 in IPS. Details are presented in .
Protocol violations: two participants received a fourth dose of COVID-19 vaccine on day 7 after administration of ZF2001.
The enrolled participants had a mean age of 52.7 years; the youngest participant was 18 years and the oldest was 83 years. The mean age was 48.5 years in the 18–59 years age group and 65.5 years in the ≥60 years age group. Of the 480 participants, 253 (52.7%) were female. Demographic characteristics were similar between the different prime-booster interval groups. The participants in the three groups exhibited balanced distributions in age, sex, comorbidities, medication history and vaccination within 28 days ().
Table 1. Basic characteristics of enrolled participants (SS).
3.2. Safety
Within 30 days after the booster vaccination, the overall incidence of adverse reactions was 5.83% (28/480), and they were predominantly solicited adverse reactions (5.63%, 27/480) (). Pain (2.29%) was the most common solicited injection-site adverse reaction, while headache (1.67%) was the most common solicited systemic reaction. Pruritus (0.83%), fever (0.42%), fatigue (0.83%), diarrhea (0.42%), nausea (0.42%), muscle pain (0.21%), acute allergic reaction (0.21%) and coughing (0.42%) also occurred after the booster vaccination. Younger participants (18–59 years of age) reported similar incidences of adverse reactions as older participants (≥60 years of age) (P > 0.05, and Table S1). The only unsolicited adverse reaction was dizziness (0.21%). All adverse reactions were grade 1 (mild) or grade 2 (moderate) in severity and typically resolved within a few days. No adverse reactions of grade 3 or above occurred within 30 days post-booster vaccination. In total, three (0.63%) participants reported four SAEs during the 180 days after the booster, but none of them were considered vaccine related. No pregnancy events were reported.
Figure 2. Adverse reactions between different age groups within 30 days after booster vaccination (SS).
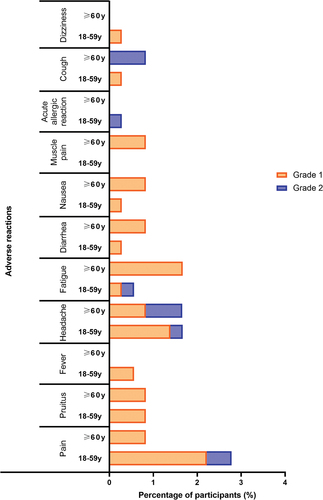
Table 2. Adverse reactions within 30 days among different interval groups after booster vaccination (SS).
3.3. Immunogenicity
At baseline, neutralizing antibody levels against wild-type SARS-CoV-2 were close to the negative detection point (titer <1:4) for most individuals. The baseline GMTs in PPS1 were 2.06, 2.02 and 2.10 for groups A – C, respectively. The respective seropositivity rates were 2.52%, 0.63% and 3.80%. The baseline GMTs showed no difference among the three groups (P > 0.05).
After the booster dose vaccination with ZF2001, significant increases in neutralizing antibody levels were observed in all groups (all P < 0.0001) (). For participants in groups A – C, the respective GMTs of neutralizing antibody increased considerably to 55.42, 63.45 and 62.06 on day 14, then decreased to 17.53, 23.79 and 26.73 on day 30 (). The respective seroconversion rates for groups A – C were 91.82%, 93.75%, and 92.41% on day 14, and 80.50%, 85.53%, and 87.34% on day 30. Additionally, the GMIs of participants in groups A – C were 26.88, 31.45, and 29.57, respectively, on day 14, and 8.50, 11.79, and 12.74, respectively, on day 30. Although the neutralizing antibody titers decreased from day 14 to day 30, they were still higher on day 30 than on day 0 (P < 0.0001). No statistically significant differences were observed in the levels of neutralizing antibodies to live SARS-CoV-2 among groups A – C on day 14 or day 30 after controlling for the influence of neutralizing antibody titers at baseline (P = 0.7461, P = 0.0553).
Figure 3. Neutralizing antibodies against wild-type SARS-CoV-2 before and after booster vaccination among different interval groups.
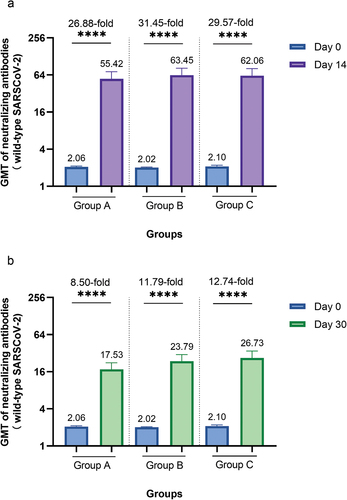
Table 3. Live-virus neutralizing antibody response results among different interval groups.
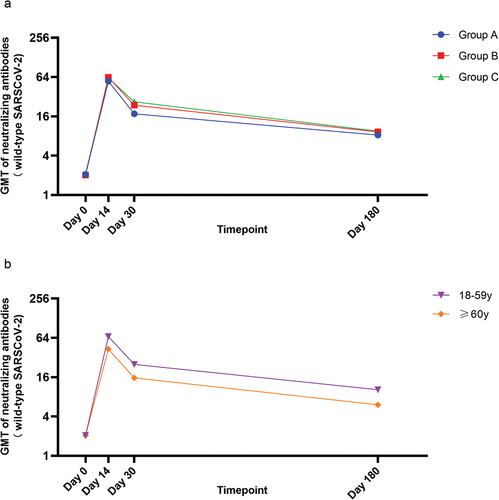
We also analyzed the immunogenicity results for participants aged 18–59 years and ≥60 years. At baseline, the GMTs were 2.06 and 2.05 for participants aged 18–59 years and ≥60 years, with seropositivity rates of 2.23% and 2.54% (P > 0.05), respectively (Table S2). After the booster dose, a similar pattern was observed in participants aged 18–59 years and ≥60 years, in which neutralizing antibody responses peaked on day 14 and then declined from 67.17 and 43.18 on day 14 to 25.12 and 15.63, respectively, on day 30; however, they were still higher on day 30 than at baseline (P < 0.0001) (). Compared with older participants, younger participants had greater GMT on both day 14 and day 30 after controlling for the influence of neutralizing antibody titers at baseline (P = 0.0145, P = 0.0055).
Figure 4. Neutralizing antibodies against wild-type SARS-CoV-2 before and after booster vaccination between different age groups.
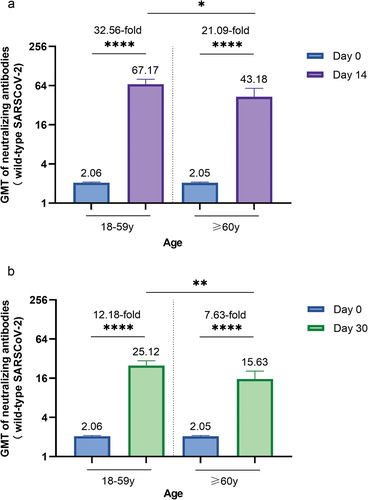
GMTs of neutralizing antibodies to wild-type SARS-CoV-2 of each age group on day 0 and day 14 (a) or day 30 (b). A paired t test was used to compare the GMTs before and after booster vaccination. Comparisons of GMTs between different age groups on day 14 and day 30 were conducted with ANCOVA. Error bars are 95% CI. *P < 0.05, **P < 0.01, ****P < 0.0001.
3.4. Immune-persistence
By 180 days after the booster dose, the GMT of neutralizing antibody was 9.00, and the seropositivity rate remained at 66.25%. For participants in groups A – C, the respective GMTs were 8.29, 9.24, and 9.51 on day 180, with seropositivity rates of 64.56%, 72.33%, and 61.88%. There was no difference in the levels of neutralizing antibodies among groups A – C. The GMTs of neutralizing antibodies for younger participants were 10.28 on day 180, while those of older participants were lower, at 6.06, and the differences were statistically significant (P = 0.0006). The dynamic change indicated the occurrence of a decreasing trend in neutralizing antibody titers from day 14 after the booster dose ().
4. Discussion
A primary series of BBIBP-CorV vaccination has been used on a large scale in populations worldwide. Additionally, compared to inactivated vaccines, ZF2001 can induce humoral immunity that exhibits better tolerance to current VOCs, which suggests it is an ideal heterologous booster [Citation19]. Therefore, we evaluated the safety, immunogenicity, and immune-persistence of a heterologous BBIBP-CorV/ZF2001 prime-boost vaccination. Our study indicated that a heterologous prime-boost regimen of one dose of ZF2001 administered at an interval of 3–9 months after two doses of BBIBP-CorV induced sufficient immunogenicity and had a satisfactory safety profile in healthy adults aged 18 years or older.
In our study, all of the observed adverse reactions were mild to moderate, with the most common symptom being injection site pain, which is in accordance with the priming vaccinations described in the published literature [Citation2,Citation20]. There were no severe adverse reactions (grade 3) within 30 days of the booster. The frequency of adverse reactions between the younger participants and older participants was similar in our study.
The neutralizing antibody titers were close to negative for 97.69% of the participants at baseline. This shows that antibody levels were attenuated within the 3–9 months after the two-dose regimen of BBIBP-CorV, suggesting the need for booster vaccinations. After the use of ZF2001 as a booster dose, a 29.24-fold increase in neutralizing antibody GMT and a 92.66% seroconversion rate were obtained on day 14. Previous studies have evaluated the short-term immunogenicity of different vaccines as a homologous or heterologous booster dose (). A phase 2 clinical trial showed that neutralizing antibody GMTs had increased 2.58- to 7.36-fold on day 14 after a homologous booster of BBIBP-CorV, while 13.95- to 35.86-fold after a heterologous booster of NVSI-06-07 [Citation15]. Gilboa et al. reported a neutralizing antibody GMR of 7.06 between those received a third dose of BNT162b2 and second dose [Citation16]. Jin et al. reported that neutralizing antibody GMRs of Convidecia/ZF2001/ZF2001 (D0–D28–M5) group and Convidecia/ZF2001/ZF2001 (D0–D56–M6) group at 14 days post first booster were 2.0 and 3.0, respectively, compared to placebo control group [Citation9]. Combined with our results, it demonstrates that the two-dose inactivated prime vaccine elicits durable humoral immunity that is successfully recalled by a third booster dose of the recombinant protein subunit vaccine (ZF2001) to provide protection against SARS-CoV-2. Furthermore, a heterologous prime boost may lead to a higher fold increase in neutralizing GMTs than a homologous boost.
Table 4. Immunogenicity of homologous and heterologous prime-boost vaccination of other original studies using CPE assay.
The phenomenon of higher immunogenicity after a longer prime-boost interval is well established for some viral and bacterial vaccines, as the affinity maturation of memory B cells induced by vaccination can take months [Citation15,Citation21,Citation22]. However, our study found that neutralizing antibody titers did not increase with longer prime-boost intervals. The results were consistent with another heterologous CoronaVac/ZF2001 booster study conducted by our team [Citation23]. In addition, a study of heterologous vaccination with ZF2001 after priming with two doses of inactivated vaccines conducted in Huashan Hospital found no significant difference in the levels of antibody responses at 4- to 8-month intervals [Citation24].
In this study, we demonstrated that ZF2001 given as a booster vaccine induced a significantly lower degree of humoral immunogenicity in older participants than younger participants. After administration of ZF2001, GMTs in those 60 years and above were lower than those of 18–59 years. Similar observations were also reported for those given a booster shot of ChAdOx1 nCoV-19 because of the age-associated decrease in immune function [Citation25].
Immune-persistence following heterologous vaccination with ZF2001 primed with inactivated vaccines has not been evaluated before the current study. In our study, the dynamic changes in the GMTs of neutralizing antibodies showed that antibodies were elicited quickly by a third booster ZF2001 vaccine following the two-dose inactivated vaccine BBIBP-CorV; the GMTs reached a peak on day 14, then gradually waned until day 30, and finally declined to a relatively low level on day 180. The rapid decay of neutralizing antibody titers seen on day 30 May be related to the deviations from protocol, in that the blood collections scheduled for day 30 were substantially delayed. A total of 301 participants had an 18- to 68-day delay in their day 30 blood collection due to an outbreak of COVID-19 in the Shangyu area in December 2021. Up to now, limited studies have evaluated the immune-persistence of heterologous vaccination (). A COV-BOOST trial compared the long-term immunogenicity to third booster doses of vaccines in seven study arms. It found that in participants receiving an initial schedule of ChAdOx1 nCov-19, the neutralizing antibody GMTs post third dose of BNT162b2, mRNA-1273, Ad26.COV2-S, NVXCoV2373 were 5134, 5535, 1068, 575 on Day 28, 637, 436, 281, 194 on Day 242, and the D242 to D28 ratios were 0.13, 0.07, 0.26, 0.34, respectively [Citation17]. Another study reported the antibody persistence after heterologous boosting with orally aerosolised Convidecia in individuals primed with two-dose CoronaVac. When compared with the peaking levels of neutralizing antibody GMTs on day 28 after the booster dose, GMTs decreased by 72.26% and 72.01% on day 90, 83.62% and 84.64% on day 180, 89.30% and 89.52% on day 360 in the low-dose and high-dose aerosolised Convidecia groups, respectively [Citation18]. Combined with our study, the results indicate a waning of neutralizing antibody titers over time after the booster immunization with different kinds of COVID-19 vaccines, however, the waning was slower after the third booster dose compared with the second dose and the decay rates could be varied [Citation16–18,Citation26].
As cellular immunity contributed to protection, particularly when neutralizing antibody titers declined, these reduced titers may not correspond to a less efficacious vaccine [Citation27]. Miao Xu et al., demonstrated that a heterologous booster with a recombinant subunit vaccine after two doses of inactivated vaccine induced stronger viral CD4+ T cell responses and could provide broader protection than a homologous booster [Citation26]. Our study found immune-persistence after the booster vaccination was weaker in older adults than younger adults. This is consistent with the current vaccination strategy that the government of China recommends for older people and immunocompromised individuals, i.e. the administration of a second booster dose of COVID-19 vaccines at least six months after the first booster dose. Further investigations of immune-persistence are required to determine whether a second booster is needed for a wider range of people.
In our study, no breakthrough infections were reported during the follow-up. This was probably due to the epidemic prevention and control policy in China. Since the outbreak of COVID-19 on December 2019 in Wuhan, China has coordinated a national campaign to contain outbreaks [Citation28]. The Zhejiang government initiated the level I response to major public health emergencies on 23 January 2020 and used a ‘five-colour epidemic chart’ to establish an accurate and smart control mechanism for epidemic prevention and control and resumption of work thereafter [Citation29]. Later on in August 2021, the government implemented the ‘dynamic zero tolerance’ policy, and it was replaced by the ‘Ten New Covid Rules’ On 7 December 2022 [Citation28,Citation30]. Thus, up until 7 December 2022, only 354,017 COVID-19 confirmed cases were reported in mainland China despite its huge population base [Citation31].
Our study had several limitations. First, we did not apply the homologous BBIBP-CorV booster at same time interval as the booster for the control group, thus the effects of homologous vaccination and heterologous vaccination could not be compared in this study, although we compared these with other studies. Second, the sample size was limited. More data are required to generalize the findings to larger populations and to optimize the timeframe for the third booster dose. Furthermore, cellular responses and neutralization testing against emerging VOC, such as the predominant Omicron strain, was not performed.
One strength of our study is that it is the first to report immune-persistence after a ZF2001 booster shot in China, especially in elderly people aged 60 years and above.Our data provide an important body of evidence for the government to decide if they should promote a fourth COVID-19 vaccine boost strategy. Additionally, this study has provided information on pure vaccine-induced immunity that is unaffected by exogenous boosting by natural infection.
5. Conclusion
We found that a heterologous booster vaccine with ZF2001 was safe and immunogenic in adults who have previously received two doses of BBIBP-CorV as the primary series vaccination. The prime-boost intervals did not affect the immune response. The immune response was weaker in older than younger adults.
Declaration of interest
L Gong, L Yuan and G Chen are employees of Anhui Zhifei Longcom Biopharmaceutical Co., Ltd who provided the ZF2001 vaccines. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Yingping Chen conceptualized and designed the study, data collection, analysis and interpreted the data, drafted the manuscript. Xinpei Zhang recruited participants and conducted the study, reviewed the manuscript. Lihui Gong participated in the conceptualization and design of the study, reviewed the manuscript. Zhenzhen Liang, Xiaosong Hu, Bo Xing and Yuting Liao participated in data collection, analysis, and interpretation, reviewed the manuscript. Lingfeng Yuan and Gang Chen participated in data interpretation, reviewed the manuscript. Huakun LV conceptualized and designed the study, coordinated, and supervised data collection, critically reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Supplemental Material
Download ()Acknowledgments
We thank Anhui Zhifei Longcom Biopharmaceuticals for providing the ZF2001 vaccines and Guangdong Provincial Institute of Public Health for conducting the live virus experiments. We thank all the investigators from Shangyu District Center for Disease Control and Prevention, Yonghe community health center and Yiting community health center.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2023.2274491
Additional information
Funding
References
- Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021 Jul 6; 326(1):35–45. doi: 10.1001/jama.2021.8565
- Dai L, Gao L, Tao L, et al. Efficacy and safety of the RBD-Dimer-based Covid-19 vaccine ZF2001 in adults. N Engl J Med. 2022 Jun 2;386(22):2097–2111. doi: 10.1056/NEJMoa2202261
- Liu Y, Zeng Q, Deng C, et al. Robust induction of B cell and T cell responses by a third dose of inactivated SARS-CoV-2 vaccine. Cell Discov. 2022 Feb 1; 8(1):10. doi: 10.1038/s41421-022-00373-7
- Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 covid-19 vaccine over 6 months. N Engl J Med. 2021 Dec 9; 385(24):e84. doi: 10.1056/NEJMoa2114583
- Falsey AR, Frenck RW Jr., Walsh EE, et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med. 2021 Oct 21; 385(17):1627–1629. doi: 10.1056/NEJMc2113468
- Guo W, Duan K, Zhang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18 years or older: a randomized, double-blind, placebo-controlled, phase 1/2 trial. EClinicalMedicine. 2021 Aug;38:101010. doi: 10.1016/j.eclinm.2021.101010
- Zeng G, Wu Q, Pan H, et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect Dis. 2022 Apr;22(4):483–495. doi: 10.1016/S1473-3099(21)00681-2
- Li J, Hou L, Guo X, et al. Heterologous AD5-nCOV plus CoronaVac versus homologous CoronaVac vaccination: a randomized phase 4 trial. Nat Med. 2022 Feb;28(2):401–409. doi: 10.1038/s41591-021-01677-z
- Jin P, Guo X, Chen W, et al. Safety and immunogenicity of heterologous boost immunization with an adenovirus type-5-vectored and protein-subunit-based COVID-19 vaccine (Convidecia/ZF2001): a randomized, observer-blinded, placebo-controlled trial. PLOS Med. 2022 [cited 2022 May 26;19(5):e1003953. doi: 10.1371/journal.pmed.1003953
- Borobia AM, Carcas AJ, Perez-Olmeda M, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacs): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021 Jul 10;398(10295):121–130. doi: 10.1016/S0140-6736(21)01420-3
- Hillus D, Schwarz T, Tober-Lau P, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir Med. 2021 Nov;9(11):1255–1265. doi: 10.1016/S2213-2600(21)00357-X
- Liu X, Shaw RH, Stuart ASV, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021 Sep 4;398(10303):856–869. doi: 10.1016/S0140-6736(21)01694-9
- Munro APS, Janani L, Cornelius V, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021 Dec 18;398(10318):2258–2276. doi: 10.1016/S0140-6736(21)02717-3
- Dai L, Zheng T, Xu K, et al. A universal design of betacoronavirus vaccines against COVID-19, MERS, and SARS. Cell. 2020 Aug 6;182(3):722–733 e11. doi: 10.1016/j.cell.2020.06.035
- Kaabi NA, Yang YK, Zhang J, et al. Immunogenicity and safety of NVSI-06-07 as a heterologous booster after priming with BBIBP-CorV: a phase 2 trial. Signal Transduct Target Ther. 2022 Jun 6; 7(1):172. doi: 10.1038/s41392-022-00984-2
- Gilboa M, Regev-Yochay G, Mandelboim M, et al. Durability of immune response after COVID-19 booster vaccination and Association with COVID-19 Omicron infection. JAMA Netw Open. 2022 Sep 1; 5(9):e2231778. doi: 10.1001/jamanetworkopen.2022.31778
- Liu X, Munro APS, Wright A, et al. Persistence of immune responses after heterologous and homologous third COVID-19 vaccine dose schedules in the UK: eight-month analyses of the COV-BOOST trial. J Infect. 2023 Jul;87(1):18–26. doi: 10.1016/j.jinf.2023.04.012
- Jin L, Tang R, Wu S, et al. Antibody persistence and safety after heterologous boosting with orally aerosolised Ad5-nCov in individuals primed with two-dose CoronaVac previously: 12-month analyses of a randomized controlled trial. Emerg Microbes Infect. 2023 Dec;12(1):2155251. doi: 10.1080/22221751.2022.2155251
- Cao Y, Yisimayi A, Bai Y, et al. Humoral immune response to circulating SARS-CoV-2 variants elicited by inactivated and RBD-subunit vaccines. Cell Res. 2021 Jul;31(7):732–741. doi: 10.1038/s41422-021-00514-9
- Yang S, Li Y, Dai L, et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021 Aug;21(8):1107–1119. doi: 10.1016/S1473-3099(21)00127-4
- Juno JA, Wheatley AK. Boosting immunity to COVID-19 vaccines. Nat Med. 2021 Nov;27(11):1874–1875. doi: 10.1038/s41591-021-01560-x
- Sallusto F, Lanzavecchia A, Araki K, et al. From vaccines to memory and back. Immunity. 2010 Oct 29; 33(4):451–463. doi: 10.1016/j.immuni.2010.10.008
- Liao Y, Chen Y, Chen B, et al. Safety and immunogenicity of heterologous recombinant protein subunit vaccine (ZF2001) booster against COVID-19 at 3–9-month intervals following two-dose inactivated vaccine (CoronaVac). Front Immunol. 2022;13:1017590. doi: 10.3389/fimmu.2022.1017590
- Ai J, Zhang H, Zhang Q, et al. Recombinant protein subunit vaccine booster following two-dose inactivated vaccines dramatically enhanced anti-RBD responses and neutralizing titers against SARS-CoV-2 and variants of concern. Cell Res. 2022 Jan;32(1):103–106. doi: 10.1038/s41422-021-00590-x
- Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021 Dec 19;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1
- Wang Z, Zhao Z, Cui T, et al. Heterologous boosting with third dose of coronavirus disease recombinant subunit vaccine increases neutralizing antibodies and T cell immunity against different severe acute respiratory syndrome coronavirus 2 variants. Emerg Microbes Infect. 2022 Dec;11(1):829–840. doi: 10.1080/22221751.2022.2048969
- McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021 Feb;590(7847):630–634. doi: 10.1038/s41586-020-03041-6
- Luo M, Liu Q, Wang J, et al. From SARS to the Omicron variant of COVID-19: China’s policy adjustments and changes to prevent and control infectious diseases. Biosci Trends. 2022 Jan 23; 15(6):418–423. doi: 10.5582/bst.2021.01535
- He F, Shang X, Ling F, et al. A practice of using five-colour chart to guide the control of COVID-19 and resumption of work in Zhejiang Province, China. Sci Rep. 2021 May 31; 11(1):11317. doi: 10.1038/s41598-021-90808-0
- Zhang M, Wang Y, Zhang T, et al. Status of and perspectives on COVID-19 vaccination after lifting of the dynamic zero-COVID policy in China. Glob Health Med. 2023 Apr 30;5(2):112–117. doi: 10.35772/ghm.2022.01063
- Epidemic Notification from National Health Commission of the People’s Republic of China. [cited 2023 Oct 13]. Available from: http://www.nhc.gov.cn/xcs/yqtb/202212/cf3fb59d2e394ce2b89b5e18c60f5143.shtml