ABSTRACT
Introduction
Diabetic patients are at a higher risk of getting pneumococcal disease and are therefore recommended to get vaccinated. The aim of our systematic review is the retrieval and analysis of all available evidence on the effect of pneumococcal vaccination on the risk of hospitalization and death in adult patients with diabetes.
Research design and methods
MEDLINEand EMBASE were searched from inception until January 2023. We included all studies investigating whether pneumococcal vaccination reduces the risk of dying or being hospitalized in diabetic patients. The Newcastle-Ottawa scale was used to assess risk of bias.
Results
Only two studies, encompassing a total of 68,246 subjects, were considered eligible for inclusion and of high quality. In both studies polysaccharide pneumococcal vaccination was associated with a reduction of the risk of hospitalization or death in adult diabetic patients (aHR: 0.76 in one study, aOR: 0.97 in the other one). However, in neither of the two included studies the lower risk was statistically significant.
Conclusions
Further research is needed due to the potentially major clinical implications for diabetic patients. The results of this systematic review can serve as a foundation for future studies, indicating the importance of continuing research in this area to improve patient outcomes.
1. Introduction
The increase in life expectancy has led to more than one-fifth of the population in Europe aged 65 years or over in 2021 [Citation1]. Older age, a fragility factor itself, and chronic illnesses lead to a greater susceptibility to infectious diseases with consequently higher mortality and morbidity rate [Citation2,Citation3]. Diabetes mellitus (DM) is an increasingly important public health issue worldwide, considering the number of cases more doubled in the last two decades [Citation4]: moreover, due to its impact on the immune system it causes greater susceptibility to infections and leads to a higher risk of hospitalization and death due to infectious diseases [Citation5,Citation6].
Among the pathogens that most affect diabetic patients is S. pneumoniae (commonly known as pneumococcus), a gram-positive bacterium that causes most cases of community-acquired pneumonia [Citation7]; over 90 serotypes of S. pneumoniae are known [Citation8]. Diseases caused by S. pneumoniae can vary in severity: otitis media, sinusitis, and conjunctivitis are generally milder, while pneumonia, meningitis and sepsis often lead to hospitalization and death. In particular, the most serious pneumococcal-related outcomes are invasive pneumococcal disease (IPD) and pneumococcal pneumonia (PP), recognized causes of high mortality worldwide [Citation7].
Individuals with DM are among those who present a higher risk of contracting the pneumococcal disease [Citation9]: they have up to a 1.4-fold increased risk of developing PP and a 1.4- to 4.6-fold increased risk of developing IPD as well as an increased incidence of hospitalizations due to these two conditions [Citation10]. Therefore, these subjects are highly recommended to get vaccinated against pneumococcus [Citation11]. Two types of vaccines are available against pneumococcus: polysaccharide vaccines (PPV) which contain purified capsular polysaccharides of the pathogen, and conjugate vaccines (PCV) which contain antigens derived from the most virulent subtypes conjugated to carrier proteins [Citation12]. In the adult population, PPV is licensed for individuals aged 65 years or older and immunocompromised persons or those at high risk of infection. It protects against 23 serotypes of S. pneumoniae: it is effective in preventing 50–70% of IPDs in adults and requires a booster vaccination after 5 years [Citation13,Citation14]. The PCV is also recommended for the population aged 65 years and over and for people of all ages with some health conditions who are at greater risk of complications from pneumococcal disease [Citation15]. Vaccine recommendations around the world are very heterogeneous: PCV is recommended in a number of countries (e.g. Poland, Slovakia), PPV in others (e.g. Denmark, Germany, Spain), while some countries are recommending vaccinating with PCV followed by PPV (e.g. Italy, France, United States) in order to take advantage of the benefits of both vaccines [Citation16,Citation17].
Due to the strong impact that pneumococcal pneumonia and invasive pneumococcal disease have on diabetic subjects, pneumococcal vaccination plays a key role in preventing the associated S. pneumoniae diseases.
The aim of our systematic review is the retrieval and analysis of all available evidence on the effect of pneumococcal vaccination on the risk of hospitalization and death in adult patients with diabetes.
2. Materials and methods
2.1. Protocol and registration
The methods and inclusion criteria of this systematic review were described according to PRISMA statement (Supplementary File 1) and registered in the PROSPERO International prospective register of systematic reviews (CRD42021248179) [Citation18].
2.2. Search strategy
The literature search was conducted on MEDLINE and EMBASE, up to 31 January 2023, on diabetic subjects exposed to pneumococcal vaccination. The search was performed by using a string built on the following terms: ‘diabetic patients’ (and variations such as ‘diabetes’), ‘pneumococcal vaccination’ (and variations such as PCV or PPV). No time, geographical, or language restriction was applied as long as an English abstract was available to assess the eligibility of each record.
2.3. Study selection
After removing duplicates, the articles were screened based on their title and abstract, and those considered potentially eligible for inclusion were read in full. The literature search and article selection were conducted independently by two researchers, and any disagreements were resolved through consensus or by seeking advice from a third, more experienced researcher. Additionally, further eligible articles were searched for through backward citation chaining and by examining the reference lists of previously published reviews and meta-analyses, if available.
To be eligible for inclusion in this systematic review, a study had to be an original study evaluating and reporting (by means of Risk Ratios (RR), Odds Ratios (OR), Hazard Ratios (HR) and corresponding 95% confidence intervals (CI) or another measure of statistical uncertainty such as standard errors, variance, or exact p-values) the effectiveness of pneumococcal vaccination (either PCV or PPV, or both) in adult diabetic patients (type 1 and/or type 2). Studies based on the pediatric population were not considered eligible for inclusion. The main endpoints were hospitalization and death in vaccinated diabetic patients. The non-exposed or control group were unvaccinated diabetic patients. Only observational studies were included.
2.4. Data retrieval
A dedicated extraction spreadsheet was developed to collect data. The following parameters were extracted from each eligible article: (1) Study identification characteristics; title, first author, year of publication; (2) study design; (3) duration of follow-up; (4) country in which the study was conducted; (5) number, age and sex of enrolled subjects; (6) type of vaccine received; (7) type of outcome measure; (8) details on statistical analysis methods and variables used for estimates adjustment. Measures of risk were extracted directly or calculated from the publications.
2.5. Statistical analyses and quality assessment
We had planned to use study-specific measures of risk to calculate a pooled OR (with the Mantel-Haenszel formula) with 95% CI and quantify heterogeneity across studies by using the I2 statistics [Citation19]. Moreover, subgroup analysis and leave-one-out sensitivity analysis to identify sources of the observed heterogeneity (in case I2 exceeded 50%) were planned. In case of a limited number of eligible studies, we would not perform a formal meta-analysis and study specific results would therefore be reported in tables and commented on in the text. Finally, the study quality and susceptibility to bias was independently evaluated by two authors by using the Newcastle-Ottawa Scale [Citation20].
3. Results
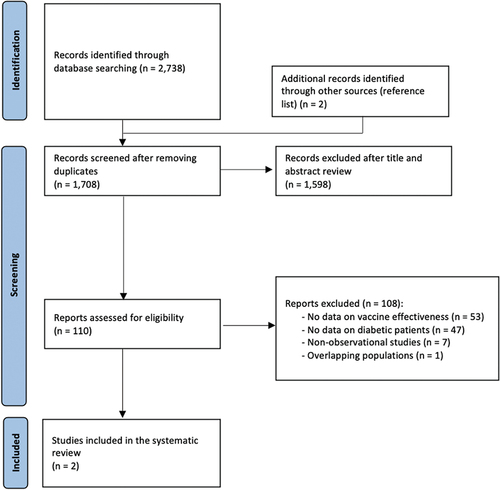
The literature search returned 2,738 records, and two additional studies were identified by checking reference lists ().
After removing duplicates, a total of 1,598 articles were excluded based on their title or abstract and 110 were read in full text. Of these, 108 were not eligible because they did not present data on vaccine effectiveness (n = 53), were not focused or did not present data on the diabetic population (n = 47), were editorials, letters without data or systematic reviews with a different research question (n = 7). Among the studies that focused on diabetic patients but were found not to be eligible, the studies by Butler et al. and Sotiropoulos et al. were excluded [Citation21,Citation22]. Butler et al. did not focus on the adult population only, while Sotiropoulos et al. did not mention whether both children and adults were included [Citation21,Citation22]. The same was true for the abstract by Blinova et al. [Citation23]. Lastly, Gilbertson et al. analyzed the association of pneumococcal vaccination with hospitalization and mortality in hemodialysis patients (40% of whom were diabetic) but did not report measures of association regarding the effectiveness of pneumococcal vaccination in the specific subgroup of diabetic patients [Citation24]. Only two articles published between 2016 and 2017 were found eligible for inclusion in this systematic review [Citation25,Citation26]. Overall, the two studies had good methodological quality, with the risk of bias judged to be low (overall score 9 out of 9 for both studies) (Supplementary File 2). The two studies encompassed a total of 68,246 subjects, of which most were contributed by Kuo et al., and were conducted in the Asia-Pacific WHO region. The main characteristics of these studies are summarized in .
Table 1. Characteristics of included studies on pneumococcal vaccine effectiveness in diabetic patients.
The manuscript by Davis and colleagues reports a longitudinal prospective cohort study that aimed to assess the importance of pneumococcal vaccination in preventing hospitalizations or death due to pneumonia in type 2 diabetic adult patients [Citation25]. Of the 1,465 included participants, 624 (42.6%) recalled pneumococcal vaccination in the previous 5 years. Over the course of the follow-up period, 72 patients were hospitalized and 9 died due to pneumonia. Although the results of the adjusted analysis suggested that pneumococcal vaccination may have a potential impact in preventing poor outcomes (aHR: 0.76), the vaccination status did not emerge as a significant predictor of pneumonia-related hospitalization or mortality (95%CI: 0.47–1.26) ().
Table 2. Results of studies on the association between pneumococcal vaccination and the risk of hospitalization or death in diabetic adult patients.
4. Discussion
The present systematic review aimed to assess the impact of pneumococcal vaccination on the risk of hospitalization and death in adult patients with diabetes. Only two studies were included in the present work as a result of the selection process described in the methods section [Citation25,Citation26]. Both studies found that pneumococcal vaccination had a positive impact in reducing hospitalizations or deaths, as indicated by measures of association below one. Both direct (e.g. reduction of pneumococcal infections) and indirect mechanisms could play a relevant role; it has been demonstrated, for example, that pneumococcal infections can worsen glycemic control in patients with diabetes and increase blood glucose in non-diabetic patients [Citation27]. Pneumococcal infections can also be associated with a higher number of cardiovascular events, even if no higher risk of hospitalization or death have been observed in the two included studies [Citation25,Citation26,Citation28].
There are several studies performed in adult population aimed at assessing the effectiveness of pneumococcal vaccination. Huss and colleagues conducted a meta-analysis on 22 trials showing trivial protective effects of vaccine in trials with a higher methodological quality [Citation29]. Similar results were found by a Cochrane review in which PPV seems to be associated with a lower risk of IPD, but not mortality [Citation30]. Despite these potential favorable effects, there is very limited evidence focusing on the effectiveness of conjugated and polysaccharide pneumococcal vaccines on the diabetic adult population. This lack of evidence is particularly problematic due to the importance of protecting frail population, affected by chronic disease, such as diabetes, potentially affecting the immune response [Citation5]. Vaccination has been considered one of the most effective methods for preventing pneumococcal infections, which are associated with relevant morbidity and mortality, particularly among immunocompromised individuals [Citation11,Citation31]. Gilbertson and colleagues reported a small but significant association between pneumococcal vaccination and lower mortality and hospitalization risk in patients with end-stage renal disease [Citation24]. Similarly, Blinova and colleagues showed that pneumococcal vaccination in patients with chronic obstructive pulmonary disease (COPD) and DM can improve the course of the COPD by reducing the number of exacerbations [Citation23].
Several limitations of the present systematic review should be recognized: the first is the limited number of included studies, which prevented formal meta-analysis and did not allow to draw any reliable conclusions on the effectiveness of pneumococcal vaccination in reducing the risk of hospitalization and death in adult diabetic patients. The paucity of studies has an impact as well on the geographical representativeness of the conclusions that might be taken, considering that it is not possible to assess any differences between countries, where there are different recommendations or vaccination coverage rates. The review process and research questions used in this study had been chosen to be appropriate to achieve reliable conclusions; however, this approach could have limited the inclusion of other studies, such as those conducted on mixed populations (both adults and children).
Despite these limitations, it must be acknowledged that the two included studies represent a good starting point for future primary studies or systematic reviews on this topic, considering that both are good quality studies: as an example, despite conducting a retrospective study (thus possibly limiting the reliability of the used classifications, such as the chosen outcomes), Kuo and colleagues used ‘hospitalization’ as outcome rather than ‘pneumonia hospitalization,’ therefore reducing the chances of misclassification, as they included all hospitalized patients, which is an objective measure (Supplementary File 2).
To obtain a more comprehensive understanding of the topic, further studies are needed, both primary studies and secondary studies that could focus on a broader range of endpoints, including pneumonia, bacteremia, and other relevant health outcomes. Furthermore, future research should delve into various aspects in depth to unearth potential nuances that may have eluded prior studies: this includes investigating the effects of the recommended combination of pneumococcal and influenza vaccinations in these subjects, as well as examining the implications of different vaccination schedules. In fact, no studies fulfilling our inclusion criteria have explored the effects of conjugated pneumococcal vaccines on health outcomes [Citation32].
5. Conclusions
This study attempted at evaluating the effectiveness of pneumococcal vaccination on the risk of all-cause mortality and hospitalization in adult diabetic population. This attempt is particularly important due to the high risk of adverse events in case of pneumococcal pneumonia in patients with diabetes. This subgroup of patients, in fact, should be considered at high risk because of the impaired immune response. This may have a relevant impact on public health policies and ultimately on the burden of the diabetic disease, considering that 537 million adults (20–79 years) worldwide suffer from diabetes [Citation33]; moreover, considering the aging population and the increase in chronic diseases, it is conceivable that vaccination should be implemented in the near future. Moreover, the onset of the COVID-19 pandemic has rapidly changed lifestyle and disease management of elderly patients with diabetes and has negatively impacted routine immunization [Citation34,Citation35].
Despite the aforementioned limitations, the results of this systematic review should be viewed as a starting point for future studies aimed at clarifying the effectiveness of pneumococcal vaccination in specific immunocompromised populations. This can help optimize vaccine policies and improve overall health outcomes.
Article highlights
Diabetic patients are at a greater risk of getting pneumococcal disease and suffer unfavorable outcomes from it;
National and international public health organizations recommend diabetic patients to vaccinate against pneumococcus;
Our study evaluated the effectiveness of pneumococcal vaccination in reducing the risk of hospitalization and death in diabetic patients;
Only two studies met the inclusion criteria. Both reported that pneumococcal vaccination was associated with a reduction of the risk of hospitalization or death in adult diabetic patients, but in neither the lower risk was statistically significant;
More studies aimed at assessing the effectiveness of pneumococcal vaccination in specific risk groups are needed.
Author contributions
Conceptualization: A.B., M.D.R., S.B., P.B., B.B., B.Z., M.M.; methodology: all authors; formal analysis: A.B., M.D.R., C.C., G.V., S.B.; investigation, M.D.R., C.C., G.V., C.S.,L.R., D.G., M.A.B., A.B., S.B.; data curation: A.B., M.D.R., S.B.; writing – original draft preparation, M.D.R., C.C., G.V.; writing – review and editing: A.B., S.B., P.B., L.R., D.G., M.A.B, C.S., M.M.; visualization: M.D.R., C.C. G.V.; supervision, A.B., S.B., P.B.; project administration, A.B., S.B., P.B. All authors should have contributed to the conception and design of the review article and interpreting the relevant literature; all authors have been involved in writing the review article or revised it for intellectual content. All authors have read and agreed to the published version of the manuscript.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
A peer reviewer on this manuscript received honoraria for their review work. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki. Ethical approval was waived for this study, due to the deidentified nature of the data presented.
Supplemental Material
Download Zip (38 KB)Acknowledgments
We would like to acknowledge Marco Menicacci, who contributed to this work before his untimely passing. With his ideas, Marco participated in designing this study. We are grateful for his dedication, and he will be greatly missed.
Data availability statement
Data supporting reported results are available upon request to the corresponding author.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2023.2286374
Additional information
Funding
References
- Eurostat [Internet]. Population structure and ageing. [cited 2023 Feb 1]. Available from: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Population_structure_and_ageing.
- Gavazzi G, Krause KH. Ageing and infection. Lancet Infect Dis. 2002 Nov;2(11):659–666. doi: 10.1016/s1473-3099(02)00437-1
- Yoshikawa TT. Epidemiology and unique aspects of aging and infectious diseases. Clin Infect Dis. 2000 Jun;30(6):931–933. doi: 10.1086/313792
- Dos Santos G, Tahrat H, Bekkat-Berkani R. Immunogenicity, safety, and effectiveness of seasonal influenza vaccination in patients with diabetes mellitus: a systematic review. Hum Vaccin Immunother. 2018;14(8):1853–1866. doi: 10.1080/21645515.2018.1446719
- Berbudi A, Rahmadika N, Tjahjadi AI, et al. Type 2 diabetes and its impact on the immune System. Curr Diabetes Rev. 2020;16(5):442–449. doi: 10.2174/1573399815666191024085838
- Macounová P, Ar RM. Vaccines recommended for diabetic patients. Vnitr Lek. 2020;66(5):301–307. Spring English. doi: 10.36290/vnl.2020.085
- Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012 Jan;67(1):71–79. Epub 2010 Aug 20. doi: 10.1136/thx.2009.129502
- Henrichsen J. Six newly recognized types of streptococcus pneumoniae. J Clin Microbiol. 1995 Oct;33(10):2759–2762. doi: 10.1128/jcm.33.10.2759-2762.1995
- Wyplosz B, Fernandes J, Goussiaume G, et al. Adults at risk of pneumococcal disease in France. Infect Dis Now. 2021 Nov;51(8):661–666. Epub 2021 Jul 31. doi: 10.1016/j.idnow.2021.07.006
- Torres A, Blasi F, Dartois N, et al. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax. 2015 Oct;70(10):984–989. Epub 2015 Jul 28. doi: 10.1136/thoraxjnl-2015-206780
- Centers for Disease Control and Prevention [Internet]. About pneumococcal disease. Prevention. [2022 Jan 27; cited 2023 Feb 20]. Available from: https://www.cdc.gov/pneumococcal/about/prevention.html#:~:text=Vaccines%20are%20the%20best%20way%20to%20prevent%20pneumococcal%20disease
- Two New Pneumococcal Vaccines-Prevnar 20 and Vaxneuvance. JAMA. 2021 Dec 28;326(24):2521–2522. doi: 10.1001/jama.2021.22119
- Briles DE, Paton JC, Mukerji R, et al. Pneumococcal vaccines. Microbiol Spectr. 2019 Nov;7(6). doi: 10.1128/microbiolspec.GPP3-0028-2018
- U.S. Food and Drug Administration [Internet]. PNEUMOVAX 23 – Pneumococcal Vaccine, Polyvalent. [2021 Oct 28; cited 2023 Mar 1]. Available from: https://www.fda.gov/vaccines-blood-biologics/vaccines/pneumovax-23-pneumococcal-vaccine-polyvalent
- Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol. 2009 Mar;9(3):213–220. doi: 10.1038/nri2494
- Weinberger B. Vaccination of older adults: influenza, pneumococcal disease, herpes zoster, COVID-19 and beyond. Immun Ageing. 2021 Oct 9;18(1):38. doi: 10.1186/s12979-021-00249-6
- European Centers for Disease Control and Prevention (ECDC). Pneumococcal Disease: Recommended vaccinations. [cited 2023 Feb 1]. Available from: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=25&SelectedCountryIdByDisease=-1
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 Mar 29;372:n71. doi: 10.1136/bmj.n71
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002 Jun 15;21(11):1539–1558. doi: 10.1002/sim.1186
- Wells G, Shea B, O’Connel B, et al. The Newcastle Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 2013 [cited 2023 Feb 1]. Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Butler JC, Breiman RF, Campbell JF, et al. Pneumococcal polysaccharide vaccine efficacy. An evaluation of current recommendations. JAMA. 1993 Oct 20;270(15):1826–1831. doi: 10.1001/jama.1993.03510150060030
- Sotiropoulos A, Koutsovasilis AG, Vergidou P, et al. Influenza and pneumococcal vaccination rates among Greek diabetes patients between 2003-2013 and its influence on their morbidity and hospitalization. Diabetes. 2015;64(Suppl.1):A441.
- Blinova E, Ignatova G, Antonov V. Effect of vaccination of pneumococcal infection on the course of chronic obstructive pulmonary disease in combination with diabetes mellitus. Eur Respir J. 2019 Sep;54(suppl 63):A4318. doi: 10.1183/13993003.congress-2019.PA4318
- Gilbertson DT, Guo H, Arneson TJ, et al. The association of pneumococcal vaccination with hospitalization and mortality in hemodialysis patients. Nephrol Dial Transplant. 2011 Sep;26(9):2934–2939. Epub 2011 Feb 11. doi: 10.1093/ndt/gfq853
- Davis TME, Kauhanen J, Davis WA. Pneumococcal vaccination and incident hospitalisation for pneumonia in type 2 diabetes: the Fremantle Diabetes Study Phase II. Intern Med J. 2017 Oct;47(10):1206–1210. doi: 10.1111/imj.13569
- Kuo CS, Lu CW, Chang YK, et al. Effectiveness of 23-valent pneumococcal polysaccharide vaccine on diabetic elderly. Medicine (Baltimore). 2016 Jun;95(26):e4064. doi: 10.1097/MD.0000000000004064
- Jan IS, Tsai TH, Chen JM, et al. Hypoglycemia associated with bacteremic pneumococcal infections. Int J Infect Dis. 2009 Sep;13(5):570–576. Epub 2008 Dec 13. doi: 10.1016/j.ijid.2008.08.026
- Africano HF, Serrano-Mayorga CC, Ramirez-Valbuena PC, et al. Major Adverse Cardiovascular Events During Invasive Pneumococcal Disease Are Serotype Dependent. Clin Infect Dis. 2021 Jun 1;72(11):e711–e719. doi: 10.1093/cid/ciaa1427
- Huss A, Scott P, Stuck AE, et al. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009 Jan 6;180(1):48–58. Erratum in: CMAJ. 2009 May 12;180(10):1038. doi: 10.1503/cmaj.080734
- Moberley S, Holden J, Tatham DP, et al. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013 Jan 31;2013(1):CD000422. doi: 10.1002/14651858.CD000422.pub3
- van Aalst M, Lötsch F, Spijker R, et al. Incidence of invasive pneumococcal disease in immunocompromised patients: a systematic review and meta-analysis. Travel Med Infect Dis. 2018 Jul;24:89–100. doi: 10.1016/j.tmaid.2018.05.016
- Bechini A, Ninci A, Del Riccio M, et al. Impact of influenza vaccination on all-cause mortality and hospitalization for pneumonia in adults and the elderly with diabetes: a meta-analysis of observational studies. Vaccines (Basel). 2020 May 30;8(2):263. doi: 10.3390/vaccines8020263
- International Diabetes Federation (IDF). IDF Atlas [Internet]. IDF diabetes atlas 2022 reports. [cited 2023 Feb 1]. Available from: https://diabetesatlas.org/2022-reports/
- Vigezzi GP, Bertuccio P, Bossi CB, et al. COVID-19 pandemic impact on people with diabetes: results from a large representative sample of Italian older adults. Prim Care Diabetes. 2022 Oct;16(5):650–657. doi: 10.1016/j.pcd.2022.06.001
- Ota MOC, Badur S, Romano-Mazzotti L, et al. Impact of COVID-19 pandemic on routine immunization. Ann Med. 2021 Dec;53(1):2286–2297. doi: 10.1080/07853890.2021.2009128