ABSTRACT
Objectives
Despite their use, differences in human papillomavirus (HPV) vaccine efficacies remain uncertain. This study assesses efficacy differences among bivalent, quadrivalent, and nine-valent HPV (2vHPV, 4vHPV, and 9vHPV) vaccines.
Methods
PubMed, Web of Science, Embase, and the Cochrane Library were searched for randomized controlled trials comparing HPV vaccine efficacy against persistent infection (≥6 months) and cervical intraepithelial neoplasia grade 2 or worse (CIN2+). Network meta-analysis yielded direct and indirect comparisons. Risk ratios (RRs) and 95% confidence intervals (95% CIs) were reported, and robustness was evaluated via sensitivity analysis.
Results
In 11 randomized controlled trials with 58,881 healthy women, for persistent infection with HPV 16, 9vHPV was most effective at 97% (RR = 0.03, 95% CI: 0.01–0.08); for HPV 18, 2vHPV (Cecolin) was most effective at 98% (RR = 0.02, 95% CI: 0.00–0.29); for CIN2+ associated with HPV 16 and 18, 4vHPV was most effective at 99% (RR = 0.01, 95% CI: 0.00–0.10) and 97% (RR = 0.03, 95% CI: 0.00–0.45), respectively; for persistent infection with HPV 31, 33, 45, 52, and 58, 9vHPV was ≥ 95% effective; both 2vHPV vaccines were cross-effective against HPV 31, 33, and 45; and 4vHPV was cross-effective against HPV 31.
Conclusions
HPV vaccine efficacies differ for different HPV types. Additional data are needed to determine the cross-efficacy of 2vHPV (Cecolin).
1. Introduction
In 2020, 0.6 million cervical cancer cases and 0.34 million deaths were reported worldwide [Citation1]. Persistent infection with human papillomavirus (HPV) is a prerequisite for the development of cervical intraepithelial lesions and cervical cancer [Citation2] and associated with other cancers of the anogenital organs (vulva, vagina, anus, and penis) [Citation3,Citation4] and cancers of the head and neck [Citation5]. More than a dozen HPV types are considered to be oncogenic [Citation6]. Globally, 90% of patients with invasive cervical cancer are associated with the seven most common oncogenic HPV infections (16, 18, 31, 33, 45, 52, and 58) [Citation7]. HPV 16 and 18 are detected in 70% of the patients [Citation8]. Currently, there are four preventive HPV vaccines available worldwide: (a) a bivalent HPV (2vHPV) vaccine against HPV 16 and HPV 18 (Cervarix, GlaxoSmithKline Biologicals); (b) a newly developed 2vHPV vaccine against HPV 16 and HPV 18 (Cecolin, Xiamen Innovax Biotech Co.); (c) a quadrivalent HPV (4vHPV) vaccine against HPV 6, 11, 16, and 18 (Gardasil, Merck); and (d) a nine-valent HPV (9vHPV) vaccine against HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58 (Gardasil-9, Merck). One of the most important primary prevention measures for cervical cancer is prophylactic HPV vaccination [Citation9]. Studies have shown that HPV vaccines are safe and effective in preventing infection and precancerous lesions of the vaccine HPV types [Citation10–14]. Additionally, an increasing number of studies have reported cross-efficacy of the 2vHPV and 4vHPV vaccines against non-vaccine HPV types [Citation15,Citation16].
However, previous studies have rarely emphasized on the level of HPV vaccine efficacy. The 9vHPV vaccine contains nine HPV virus-like particles, thereby making its efficacy relatively comprehensive. However, the 9vHPV vaccine is more expensive than the other vaccines, and whether it is the most effective against the common oncogenic HPVs, especially HPV 16 and 18, has not yet been confirmed. In addition, the distribution of HPV types is heterogeneous in different regions [Citation17]. Thus, efficient and relatively inexpensive HPV vaccines must be identified for different HPV types, especially in economically poorly developed areas. To address these issues, we aimed to compare the levels of efficacy of HPV vaccines with respect to the outcomes associated with different HPV types and assess the cross-efficacy of the 2vHPV and 4vHPV vaccines through network meta-analysis. Compared to pairwise meta-analysis, network meta-analysis represents a new approach and provides the ability to indirectly compare arms from distinct randomized controlled trial. Therefore, the method can well fulfill the purpose of this study.
2. Materials and methods
We performed this network meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Extended Guidelines for NMAs [Citation18,Citation19].
2.1. Eligibility criteria and literature search
We included studies based on the following four criteria: (1) Healthy women were enrolled, regardless of nationality or ethnicity, who were HPV-DNA negative for the appropriate outcome at baseline; (2) at least one dose of the HPV vaccine, including 2vHPV vaccine (Cervarix or Cecolin), 4vHPV vaccine, or 9vHPV vaccine, was administered in the intervention group and at least one dose of the above HPV vaccine or placebo was administered in the control group; (3) vaccine efficacy outcomes included but were not limited to persistent infection with oncogenic HPV, cervical intraepithelial neoplasia, and cervical cancer; (4) randomized controlled trial was performed. Repeatedly reported studies extracted data with the most complete information. The exclusion criteria were based on the following four guidelines: (1) single-arm studies; (2) studies with ambiguous data and outcome indicators that could not be calculated; (3) studies not reported in English; and (4) duplicate studies.
All the studies were obtained from four databases (PubMed, Embase, Web of Science, and Cochrane Library) from establishment to 10 November 2022. We used the following MeSH terms or text words: ‘papillomavirus vaccines,’ ‘HPV vaccine,’ ‘papillomavirus infections,’ ‘cervical intraepithelial neoplasia,’ ‘uterine cervical neoplasms,’ ‘condyloma acuminate,’ ‘genital warts,’ ‘trial,’ and ‘Randomized Controlled Trial.’ Specific information on the search strategy is provided in the supplementary materials. In addition, we supplemented the retrieved data on randomized controlled trials by manually searching references.
2.2. Study screening and data extraction
Two researchers (R.L and X.F) independently completed the study screening process. All the studies included were first initially screened based on titles and abstracts, and then, the full texts were obtained and assessed. Data and information, including authors, year of report, study areas, number of participants, age, types of HPV vaccine, dosing schedule, number of vaccinations, duration of follow-up, vaccine manufacturer, serologic and DNA status at screening and baseline, experimental data from intervention and control groups, and outcome indicators of efficacy (persistent infection with HPV 16, 18, 31, 33, 45, 52, or 58 for at least 6 months; CIN2+ associated with HPV 16 and 18), for the selected studies were extracted independently by two researchers. Any disagreements that arose during the above process were discussed and resolved with another author (H.J).
2.3. Bias risk assessment
Two reviewers (R.L and X.F) used the Cochrane Risk of Bias Assessment Tool, ROB-II, to independently assess the risk of bias for the selected randomized controlled trials with respect to several aspects, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. Any disagreements that arose during the above process were discussed and resolved with another author (H.J).
2.4. Statistical analyses
The primary aim of this study was to compare the efficacy of four types of prophylactic HPV vaccines against persistent infection (lasting ≥6 months) and CIN2+ associated with HPV 16 and 18. The secondary aim was to compare the efficacy of prophylactic HPV vaccines against persistent infection (lasting ≥6 months) associated with HPV 31, 33, 45, 52, and 58.
First, we conducted a pairwise meta-analysis, followed by a network meta-analysis. Combined effect sizes were reported as risk ratios (RRs) and 95% confidence intervals (95% CIs). In the pairwise meta-analysis, we calculated I2 and χ2 statistics by the DerSimonian-Laird random effects meta-analysis method to test the heterogeneity of the combined data from randomized controlled trials of HPV vaccines [Citation20]. I2 values were used to quantify the percentage of variation due to heterogeneity [Citation21], and χ2 values were used to determine the statistical significance of heterogeneity. Data presenting I2 values < 50% were considered to have low heterogeneity, and those with > 50% were considered to have substantial heterogeneity. In the network meta-analysis, we plotted network diagrams to illustrate the different types of each intervention in the network and cumulative probability diagrams of outcomes associated with different HPV types to examine the level of efficacy of the HPV vaccines [Citation22]. Inconsistency analysis was conducted when a closed loop of evidence occurred in the network diagrams. We first statistically evaluated the global consistency of direct and indirect evidence. A p value lower than 0.05 for the inconsistency test indicated global inconsistency. Then, node splitting was performed by separating direct evidence from indirect evidence. The inconsistency factor close to zero indicated that the two evidence sources were consistent [Citation23]. In addition, we excluded individuals older than 25 years in the 2vHPV vaccine (Cervarix) trial in the sensitivity analysis to evaluate the robustness of the results.
Statistical analyses were performed using Stata 15.0 software. The quality and risk of bias of the randomized controlled trials were assessed using the Cochrane Risk of Bias Assessment Tool ROB-II. P < 0.05 was considered statistically significant.
3. Results
3.1. Characteristics and risk of bias of the studies included
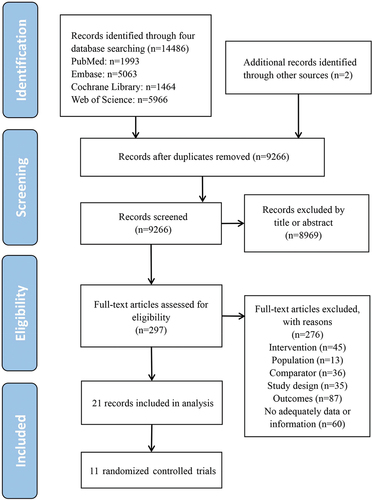
The study selection process is shown in . Eleven two-arm randomized controlled trials were included, with data extracted from 21 studies [Citation15,Citation24–43]. The main characteristics of the studies included are shown in . A total of seven of the 11 randomized controlled trials were on the efficacy of the 2vHPV vaccine (Cervarix), one on the efficacy of the 2vHPV vaccine (Cecolin), two on the efficacy of the 4vHPV vaccine, and one on the efficacy of the 9vHPV vaccine. The studies included were reported between 2000–2022 and involved 58,881 healthy women. Of the 11 studies, 10 involved persistent infection with HPV 16 and 18, nine involved CIN2+ associated with HPV 16 and 18, and nine involved persistent infection with HPV 31, 33, 45, 52, and 58. Extracted efficacy data are shown in Table S1. Characteristics of licensed prophylactic HPV vaccines are presented in Table S2.
Table 1. Characteristics of the 11 randomized controlled trials.
Of the 11 randomized controlled trials, Japan Trial 1 and Japan Trial 2 had uncertain risk because of unclear randomized sequence generation. HPV-007, Japan Trial 1, and Japan Trial 2 showed inadequate allocation concealment. The other eight randomized controlled trials did not identify clear risk of bias (Figure S1).
3.2. Pairwise meta-analysis and heterogeneity tests
Figure S2 shows the results of paired meta-analysis forest plots. HPV vaccines were significantly effective against all vaccine HPV types, except for 2vHPV vaccine (Cecolin) for CIN2+ associated with HPV18. The 9vHPV vaccine was superior to the 4vHPV vaccine in preventing persistent infection of all seven HPV types. In addition, the 2vHPV vaccine (Cervarix) was significantly effective against persistent infection with non-vaccine HPV types 31, 33, and 45, although its efficacy declined in studies with longer follow-ups. The 4vHPV vaccine was significantly effective against persistent infection with non-vaccine HPV type 31. In the heterogeneity test, there was low heterogeneity in the 2vHPV vaccine (Cervarix) for persistent infection with HPV 33, 45, and 52, with I2 values of 11.0%, 32.7%, and 17.1%, respectively. Two studies on the 4vHPV vaccine, Future I and Future II, consistently had combined results; therefore, we were unable to assess the heterogeneity. The 2vHPV vaccine (Cecolin) and 9vHPV vaccine each had one randomized controlled trial with no results of heterogeneity assessment.
3.3. Network meta-analysis and sensitivity analysis
In , none of the network diagrams form a closed loop of evidence. Therefore, this network meta-analysis does not require an inconsistency test. For persistent infection with HPV 16 and 18, we included 10 randomized controlled trials (). In terms of persistent infection with HPV 16, the efficacy of the 2vHPV vaccine (Cervarix) was 93% (RR = 0.07, 95% CI: 0.05–0.10), that of the 2vHPV vaccine (Cecolin) was 96% (RR = 0.04, 95% CI: 0.01–0.18), that of the 4vHPV vaccine was 95% (RR = 0.05, 95% CI: 0.02–0.12), and that of the 9vHPV vaccine was 97% (RR = 0.03, 95% CI: 0.01–0.08). In terms of persistent infection with HPV 18, the efficacy of the 2vHPV vaccine (Cervarix) was 93% (RR = 0.07, 95% CI: 0.04–0.12), that of the 2vHPV vaccine (Cecolin) was 98% (RR = 0.02, 95% CI: 0.00–0.29), that of the 4vHPV vaccine was 96% (RR = 0.04, 95% CI: 0.01–0.17), and that of the 9vHPV vaccine was 96% (RR = 0.04, 95% CI: 0.01–0.20). Comparisons of efficacy between the four HPV vaccines were not significantly different (p > 0.05), but the 9vHPV vaccine and 2vHPV vaccine (Cecolin) appear to be more effective against persistent infection with HPV 16 or 18 than the other vaccines ().
Figure 2. Network plots. Network plots for persistent infection with HPV 16 (a), Persistent infection with HPV 18 (b), Cervical intraepithelial neoplasia grade 2 or worse associated with HPV 16 (c), Cervical intraepithelial neoplasia grade 2 or worse associated with HPV 18 (d), Persistent infection with HPV 31 (e), Persistent infection with HPV 33 (f), Persistent infection with HPV 45 (g), Persistent infection with HPV 52 (h), and persistent infection with HPV 58 (i). The area of the circle represents the number of participants. The width of the line represents the number of trials of the two treatments. vHPV, valent human papillomavirus.
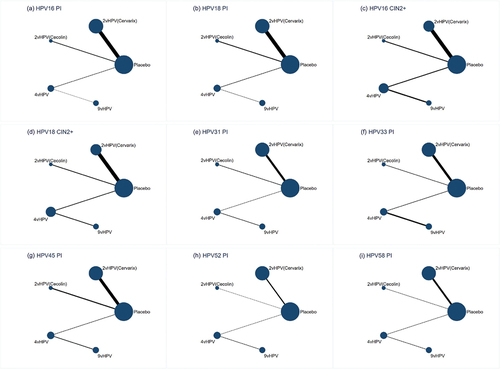
Figure 3. Network meta-analysis. Network meta-analysis for persistent infection with HPV 16 (a), Persistent infection with HPV 18 (b), Cervical intraepithelial neoplasia grade 2 or worse associated with HPV 16 (c), Cervical intraepithelial neoplasia grade 2 or worse associated with HPV 18 (d), Persistent infection with HPV 31 (e), Persistent infection with HPV 33 (f), Persistent infection with HPV 45 (g), Persistent infection with HPV 52 (h), and persistent infection with HPV 58 (i). Results are column-defined RRs in treatment compared with row-defined RRs in treatment. RR, risk ratio; vHPV, valent human papillomavirus.
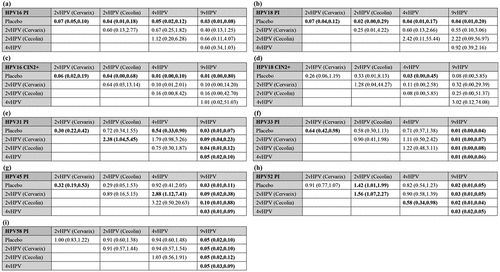
For CIN2+ associated with HPV 16 and 18, we included nine randomized controlled trials (). In terms of CIN2+ associated with HPV 16, the efficacy of the 2vHPV vaccine (Cervarix) was 94% (RR = 0.06, 95% CI: 0.02–0.19), that of the 2vHPV vaccine (Cecolin) was 96% (RR = 0.04, 95% CI: 0.00–0.68), that of the 4vHPV vaccine was 99% (RR = 0.01, 95% CI: 0.00–0.10), and that of the 9vHPV vaccine was 99% (RR = 0.01, 95% CI: 0.00–0.80). In terms of CIN2+ associated with HPV 18, both 2vHPV and 9vHPV vaccines were effective, although the results were not statistically significant, and the 4vHPV vaccine was 97% effective (RR = 0.03, 95% CI: 0.00–0.45). Comparisons of efficacy among the four HPV vaccines indicated that the values were not significantly different (p > 0.05). However, the 4vHPV vaccine appeared more effective against CIN2+ associated with HPV 16 and 18 than the other vaccines ().
For persistent infection with HPV 31, 33, 45, 52, and 58, we included nine randomized controlled trials (). Relative to placebo, the efficacy of the 2vHPV vaccine (Cervarix) against persistent infection with HPV 31, 33, and 45 was 70% (RR = 0.30, 95% CI: 0.22–0.42), 36% (RR = 0.64, 95% CI: 0.42–0.98), and 68% (RR = 0.32, 95% CI: 0.19–0.53), respectively. The 2vHPV vaccine (Cecolin) was effective against persistent infection with HPV 31, 33, and 45, but the results were insignificant. The 4vHPV vaccine was effective against persistent infection with HPV 31 (RR = 0.54, 95% CI: 0.33–0.90) and appeared to be effective against persistent infection with HPV 33. The 9vHPV vaccine was ≥ 95% effective against persistent infection with HPV 31, 33, 45, 52, and 58. In inter-vaccine comparisons, the 9vHPV vaccine was always the most effective. In addition, both 2vHPV vaccines had better cross-protective efficacy than the other vaccines ().
The sensitivity analysis results are shown in Figure S3. The findings were not inconsistent after excluding the VIVIANE (Cervarix) trial, in which the study participants were older than 25 years.
3.4. Ranking
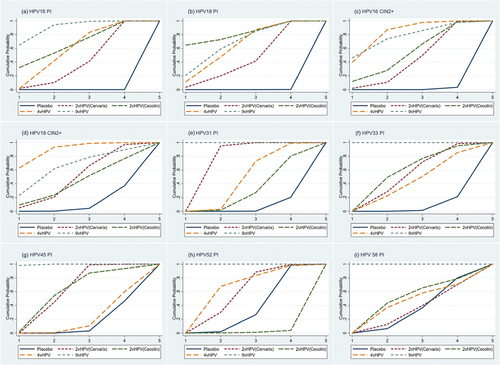
The results of the vaccine efficacy ranking are shown in . For persistent infection with HPV 16, the ranking order is 9vHPV, 2vHPV (Cecolin), 4vHPV, and 2vHPV (Cervarix). For persistent infection with HPV 18, the ranking order is 2vHPV (Cecolin), 9vHPV, 4vHPV, and 2vHPV (Cervarix). For CIN2+ associated with HPV 16, the ranking order is 4vHPV, 9vHPV, 2vHPV (Cecolin), and 2vHPV (Cervarix). For CIN2+ associated with HPV 18, the ranking order is 4vHPV, 9vHPV, 2vHPV (Cervarix), and 2vHPV (Cecolin). For persistent infection with HPV 31, the rank order is 9vHPV, 2vHPV (Cervarix), 4vHPV, and 2vHPV (Cecolin). For persistent infection with HPV 33, the rank order is 9vHPV, 2vHPV (Cecolin), 2vHPV (Cervarix), and 4vHPV. For persistent infection with HPV 45, the ranking order is 9vHPV, 2vHPV (Cervarix), and 2vHPV (Cecolin). Neither of the 2vHPV vaccine was cross-protective against persistent infection with HPV 52 and 58. The 4vHPV vaccine was not cross-protective against persistent infection with HPV 45, 52, and 58. Therefore, they were not involved in the ranking.
Figure 4. Probability of efficacy rankings for each vaccine. Probability of vaccines’ efficacy for persistent infection with HPV 16 (a), Persistent infection with HPV 18 (b), Cervical intraepithelial neoplasia grade 2 or worse associated with HPV 16 (c), Cervical intraepithelial neoplasia grade 2 or worse associated with HPV 18 (d), Persistent infection with HPV 31 (e), Persistent infection with HPV 33 (f), Persistent infection with HPV 45 (g), Persistent infection with HPV 52 (h), and persistent infection with HPV 58 (i). The ranking for each vaccine is shown on a cumulative probability plot, with the area under the curve representing the magnitude of the probability. Higher rankings indicate greater efficacy. vHPV, valent human papillomavirus.
4. Discussion
4.1. Summary of findings
The network meta-analysis results showed that the HPV vaccines were significantly effective against vaccine HPV types (except for CIN2+ associated with HPV 18). The 9vHPV vaccine and 2vHPV vaccine (Cecolin) were the most effective against persistent infection with HPV 16 and 18. The 4vHPV vaccine was the most effective against CIN2+ associated with HPV 16 and 18. Both 2vHPV vaccines and the 4vHPV vaccine provided cross-efficacy against some uninfected non-vaccine HPV types. In our analysis, both 2vHPV vaccines were effective against persistent infection with HPV 31, 33, and 45, although the 2vHPV vaccine (Cecolin) was not significantly effective. The 4vHPV vaccine was effective against persistent infection with HPV 31 and not significantly effective against persistent infection with HPV 33. There appeared to be a difference in cross-efficacy between the 2vHPV and 4vHPV vaccines, with both 2vHPV vaccines appearing to be more effective than the 4vHPV vaccine for persistent infections with HPV 31, 33, and 45. Few studies have shown cross-efficacy of both 2vHPV and 4vHPV vaccines against HPV 52 and 58. In studies with longer follow-up, the 2vHPV vaccine (Cervarix) appeared to have reduced efficacy against persistent infection with HPV 31, 33, and 45 (Figures S2E – S2G), suggesting diminished cross-efficacy.
4.2. Interpretation of the findings
For persistent infection with HPV 16 and 18, the 2vHPV vaccine (Cecolin) produced by Xiamen Innovative Biotechnology appears to have greater efficacy than the 4vHPV vaccine and 2vHPV vaccine (Cervarix), which may be real differences in vaccine efficacy or differences in other factors between trials. Therefore, further studies on the efficacy of the 2vHPV vaccine (Cecolin) are needed. In addition, the 4vHPV vaccine appears to be more effective than the 2vHPV vaccine (Cervarix), in contrast to the results of a previous immunogenicity study, which suggested that the number of anti-HPV 16 and 18 antibodies produced after 1 month of use of the 2vHPV vaccine (Cervarix) was several times higher than that after the use of the 4vHPV vaccine [Citation44]. This may be because in the included studies, the follow-up time of the 4vHPV vaccine trials (mean 3.6 years) was shorter than that of the 2vHPV vaccine (Cervarix) trials. Vaccine effectiveness decreases with longer follow-up. However, when we compared only the 4vHPV vaccine trials FUTURE I/II with the 2vHPV vaccine (Cervarix) trial PATRICIA (Figures S2A and S2B) with similar follow-up times, we noted that the 4vHPV vaccine was more effective than the 2vHPV vaccine (Cervarix). For CIN2+ associated with HPV 16 and 18, the 4vHPV vaccine appears to be the most effective. However, previous studies have shown that the 2vHPV vaccine (Cervarix) appears to be more effective than the 4vHPV vaccine [Citation45]. Notably, our study controlled for the baseline status of individuals who received three doses of the HPV vaccine and had a negative DNA status for the corresponding HPV, thus making the findings plausible. In addition, studies have suggested that the prophylactic 4vHPV vaccine has a therapeutic effect on cervical intraepithelial neoplasia and reduces the detection rate of CIN2+ [Citation46,Citation47]. The combination of the HPV vaccine with radiotherapy, chemotherapy, or immune checkpoint inhibitors may represent the potential basis for a new therapeutic regimen for gynecologic diseases [Citation48]. For CIN2+ associated with HPV 18, the 2vHPV and 9vHPV vaccines were not significantly effective, mainly due to the low prevalence of CIN2+ associated with HPV 18 among the population, resulting in too few cases in the trials.
The cross-protection provided by the 2vHPV and 4vHPV vaccines has been proven to be biologically plausible [Citation49]. Cross-immunity may result from phylogenetic similarities between vaccine and non-vaccine L1 genes. HPV 16 is similar to HPV 31, 33, 52, and 58 (A9 species); HPV 18 is similar to HPV 45 (A7 species) [Citation50]. Furthermore, the cross-protection between 2vHPV and 4vHPV vaccines may also be due to different adjuvant systems. We noted significant cross-protective efficacy of the 2vHPV vaccine (Cervarix) against persistent infection with HPV 31, 33, and 45. The 2vHPV vaccine (Cecolin) appears to be cross-protective but not significantly effective against persistent infection with HPV 31, 33, and 45. The cumulative probability plot showed that the 2vHPV vaccine (Cecolin) was noninferior to the 2vHPV vaccine (Cervarix). Therefore, the cross-protective effect of the 2vHPV vaccine (Cecolin) requires further investigation. The 4vHPV vaccine was significantly effective against persistent infection with HPV 31 and somewhat effective against persistent infection with HPV 33. This is in line with the results of a previous review study [Citation15]. In addition, the efficacy of the 2vHPV vaccine (Cervarix) against persistent infection with HPV31, 33, and 45 decreased in studies with longer follow-up, while the efficacy against HPV16 and 18-related outcomes remained stable (Figure S2). This was also demonstrated in immunogenicity trial studies. Antibody titer levels for HPV 31, 33, and 45 were significantly lower over 2 years with extended follow-up, while antibody titers for HPV 16 and 18 were generally high [Citation51]. This suggests that the cross-efficacy of the vaccine may diminish over time. However, these results require careful interpretation, as there may be differences in other characteristics of the experimental subgroups being compared not just the duration of follow-up.
4.3. Strengths and limitations
The outcome indicator that best reflects the efficacy of HPV vaccines remains unclear. Persistent infection outcomes are the most useful and least controversial indicators because HPV persistence is strongly associated with cervical precancer and cervical cancer [Citation52,Citation53]. In addition, a recent study showed that persistent infection with HPV (<12 months) is one of the most important predictors of CIN2+ recurrence [Citation54]. Moreover, the outcome of persistent infection is not biased by misclassification due to co-infection with other HPV types [Citation55]. However, the results of persistent infections may underestimate vaccine efficacy because baseline infections are not detected. In addition, we only analyzed CIN2+ associated with HPV 16 and 18 and not CIN2+ associated with other HPV types because this outcome is vulnerable to various biases. Co-infection of HPV is more common in most CIN2+ cases. We were unable to determine which HPV type was dominant in the lesions. We included the population that was negative for the corresponding outcome HPV-DNA at baseline and serologically negative at screening, thereby minimizing the bias associated with baseline infection. The analyzed subgroup received three doses of vaccine according to the immunization schedule (97.2% and 92% for the three doses in the FUTURE I/II [Gardasil] and PATRICIA [Cervarix] vaccine trials, respectively), thus minimizing the bias caused by differences in vaccination rates across vaccines.
This study has certain limitations. First, although we selected comparable subgroups, differences in subgroup characteristics and trial protocols could not be fully explained. Second, owing to the small number of randomized controlled trials of the 9vHPV vaccine and 2vHPV vaccine (Cecolin), there are some instances of large CIs in the comparison of vaccine efficacy, thereby resulting in insignificant differences in the comparison results. Therefore, further studies are needed to provide evidence. In addition, we only compared the efficacy of vaccines against HPV monotypic persistent infection and CIN2+. Owing to data limitations, we were unable to compare the overall efficacy of the vaccine in preventing oncogenic HPV-related outcomes. Finally, while persistent infection with HPV is strongly associated with cervical precancer and cervical cancer, the extent to which reported differences and rankings might translate into a difference in the risk of CIN2+ and subsequent invasive cancer is unknown.
4.4. Implications for policy and research
Our findings are important for medical professionals and policymakers who can understand the differences between vaccine efficacy and make recommendations for HPV vaccine selection. The 9vHPV vaccine has broad and robust protection but is not widely available in many developing countries owing to its high price [Citation56]. Therefore, the relatively inexpensive 2vHPV and 4vHPV vaccines still have an indispensable role. In particular, the 2vHPV vaccine (Cecolin) produced by Xiamen Innovax Biotech is the most cost-effective [Citation57,Citation58]. Among patients with HPV infection, 4% are infected with HPV 31 and 33, and 6% with HPV 45 [Citation7]. Both 2vHPV vaccines have cross-protective efficacy, which can reduce the incidence of associated precancerous lesions and cancers. However, the choice of the 2vHPV vaccine with better cross-protection must be weighed against the 4vHPV vaccine with genital wart protection and better efficacy for CIN2+ associated with HPV 16 and 18. As HPV vaccines are mainly administered to prepubertal girls, the impact on the population is not significant if the cross-protection period is short.
5. Conclusions
Four HPV vaccines show significant efficacy against vaccine HPV types. The 9vHPV vaccine and 2vHPV vaccine (Cecolin) are the most effective against persistent infection with HPV 16 and 18. The 4vHPV vaccine is the most effective against CIN2+ associated with HPV 16 and 18. Both 2vHPV vaccines are better than the 4vHPV vaccine for cross-protection. This study provides evidence-based support for selecting the most appropriate HPV vaccine. However, further studies are needed to confirm the non-significant findings of differences in vaccine efficacy.
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
A peer reviewer on this manuscript received honoraria for their review work. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
R Lin conceptualized and designed the study, performed data extraction and analysis, drafted the initial manuscript, and revised the manuscript. X Fu performed data extraction and analysis, reviewed and revised the manuscript. H Jin conceptualized and designed the study and critically revised and reviewed the manuscript. All authors approved the final manuscript as submitted.
Ethical approval
Patients or the public were not involved in setting the research question or the outcome measures, nor were they involved in the design or conduct of the study. No participants were asked to advise on interpreting or writing the manuscript. There are no plans to involve patients in the dissemination of the study findings.
Supplemental Material
Download Zip (8.1 MB)Acknowledgments
We are grateful for the financial support from the Chinese National Natural Fund, Science Technology Demonstration Project for Emerging Infectious Diseases Control and Prevention, Jiangsu Provincial Six Talent Peak, Southeast University Novel Coronavirus Research, and Jiangsu Provincial Key Medical Discipline.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2023.2287135.
Additional information
Funding
References
- Singh D, Vignat J, Lorenzoni V, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO global cervical cancer elimination initiative. Lancet Glob Health. 2023 Feb;11(2):e197–e206. doi: 10.1016/S2214-109X(22)00501-0
- Alfaro K, Maza M, Cremer M, et al. Removing global barriers to cervical cancer prevention and moving towards elimination. Nat Rev Cancer. 2021 Oct;21(10):607–608. doi: 10.1038/s41568-021-00396-4
- Senkomago V, Henley SJ, Thomas CC, et al. Human papillomavirus-attributable cancers - United States, 2012-2016. MMWR Morb Mortal Wkly Rep. 2019 Aug 23;68(33):724–728. doi: 10.15585/mmwr.mm6833a3
- Melkonian SC, Henley SJ, Senkomago V, et al. Cancers associated with human papillomavirus in American Indian and Alaska native populations - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2020 Sep 18;69(37):1283–1287. doi: 10.15585/mmwr.mm6937a2
- Sabatini ME, Chiocca S. Human papillomavirus as a driver of head and neck cancers. Br J Cancer. 2020 Feb;122(3):306–314. doi: 10.1038/s41416-019-0602-7
- Perkins RB, Wentzensen N, Guido RS, et al. Cervical cancer screening: a review. JAMA. 2023 Aug 8;330(6):547–558. doi: 10.1001/jama.2023.13174
- de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010 Nov;11(11):1048–1056. doi: 10.1016/S1470-2045(10)70230-8
- Castellsagué X, Díaz M, de Sanjosé S, et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst. 2006 Mar 1;98(5):303–315. doi: 10.1093/jnci/djj067
- Harper DM, Jimbo M. Elimination of cervical cancer depends on HPV vaccination and primary HPV screening. Lancet Infect Dis. 2021 Oct;21(10):1342–1344. doi: 10.1016/S1473-3099(20)30992-0
- Kamolratanakul S, Pitisuttithum P. Human papillomavirus vaccine efficacy and effectiveness against cancer. Vaccines (Basel). 2021 Nov 30;9(12):1413. doi: 10.3390/vaccines9121413
- Huang R, Gan R, Zhang D, et al. The comparative safety of human papillomavirus vaccines: a bayesian network meta-analysis. J Med Virol. 2022 Feb;94(2):729–736. doi: 10.1002/jmv.27304
- Goldstone SE, Giuliano AR, Palefsky JM, et al. Efficacy, immunogenicity, and safety of a quadrivalent HPV vaccine in men: results of an open-label, long-term extension of a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2022 Mar;22(3):413–425. doi: 10.1016/S1473-3099(21)00327-3
- Joura EA, Ulied A, Vandermeulen C, et al. Immunogenicity and safety of a nine-valent human papillomavirus vaccine in women 27-45 years of age compared to women 16-26 years of age: an open-label phase 3 study. Vaccine. 2021 May 12;39(20):2800–2809. doi: 10.1016/j.vaccine.2021.01.074
- Li M, Zhao C, Zhao Y, et al. Immunogenicity, efficacy, and safety of human papillomavirus vaccine: data from China. Front Immunol. 2023;14:1112750. doi: 10.3389/fimmu.2023.1112750
- Malagón T, Drolet M, Boily MC, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012 Oct;12(10):781–789. doi: 10.1016/S1473-3099(12)70187-1
- Mariz FC, Gray P, Bender N, et al. Sustainability of neutralising antibodies induced by bivalent or quadrivalent HPV vaccines and correlation with efficacy: a combined follow-up analysis of data from two randomised, double-blind, multicentre, phase 3 trials. Lancet Infect Dis. 2021 Oct;21(10):1458–1468. doi: 10.1016/S1473-3099(20)30873-2
- Clifford GM, Gallus S, Herrero R, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on cancer HPV prevalence surveys: a pooled analysis. Lancet. 2005 Sep 17-23;366(9490):991–998. doi: 10.1016/S0140-6736(05)67069-9
- Cornell JE. The PRISMA extension for network meta-analysis: bringing clarity and guidance to the reporting of systematic reviews incorporating network meta-analyses. Ann Intern Med. 2015 Jun 2;162(11):797–798. doi: 10.7326/M15-0930
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021 Mar 29;10(1):89. doi: 10.1186/s13643-021-01626-4
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986 Sep;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003 Sep 6;327(7414):557–560. doi: 10.1136/bmj.327.7414.557
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011 Feb;64(2):163–171. doi: 10.1016/j.jclinepi.2010.03.016
- White IR, Barrett JK, Jackson D, et al. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. 2012 Jun;3(2):111–125. doi: 10.1002/jrsm.1045
- Huh WK, Joura EA, Giuliano AR, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16-26 years: a randomised, double-blind trial. Lancet. 2017 Nov 11;390(10108):2143–2159. doi: 10.1016/S0140-6736(17)31821-4
- Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015 Feb 19;372(8):711–723. doi: 10.1056/NEJMoa1405044
- Muñoz N, Kjaer SK, Sigurdsson K, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010 Mar 3;102(5):325–339. doi:10.1093/jnci/djp534
- Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16-26 years. J Infect Dis. 2009 Apr 1;199(7):926–935. doi: 10.1086/597307
- Zhao FH, Wu T, Hu YM, et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced human papillomavirus (16 and 18) L1 virus-like-particle vaccine: end-of-study analysis of a phase 3, double-blind, randomised, controlled trial. Lancet Infect Dis. 2022 Dec;22(12):1756–1768. doi: 10.1016/S1473-3099(22)00435-2
- Qiao YL, Wu T, Li RC, et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced Bivalent human papillomavirus vaccine: an interim analysis of a randomized clinical trial. J Natl Cancer Inst. 2020 Feb 1;112(2):145–153. doi: 10.1093/jnci/djz074
- Zhu FC, Chen W, Hu YM, et al. Efficacy, immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Chinese women aged 18-25 years: results from a randomized controlled trial. Int J Cancer. 2014 Dec 1;135(11):2612–2622. doi: 10.1002/ijc.28897
- Zhu FC, Hu SY, Hong Y, et al. Efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine in Chinese women aged 18-25 years: event-triggered analysis of a randomized controlled trial. Cancer Med. 2017 Jan;6(1):12–25. doi: 10.1002/cam4.869
- Zhu FC, Hu SY, Hong Y, et al. Efficacy, immunogenicity and safety of the AS04-HPV-16/18 vaccine in Chinese women aged 18-25 years: end-of-study results from a phase II/III, randomised, controlled trial. Cancer Med. 2019 Oct;8(14):6195–6211. doi: 10.1002/cam4.2399
- Hildesheim A, Wacholder S, Catteau G, et al. Efficacy of the HPV-16/18 vaccine: final according to protocol results from the blinded phase of the randomized Costa Rica HPV-16/18 vaccine trial. Vaccine. 2014 Sep 3;32(39):5087–5097. doi: 10.1016/j.vaccine.2014.06.038
- Herrero R, Wacholder S, Rodríguez AC, et al. Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: a community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer Discov. 2011 Oct;1(5):408–419. doi: 10.1158/2159-8290.CD-11-0131
- Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006 Apr 15;367(9518):1247–1255. doi: 10.1016/S0140-6736(06)68439-0
- Romanowski B, de Borba PC, Naud PS, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009 Dec 12;374(9706):1975–1985. doi: 10.1016/S0140-6736(09)61567-1
- Konno R, Tamura S, Dobbelaere K, et al. Efficacy of human papillomavirus 16/18 AS04-adjuvanted vaccine in Japanese women aged 20 to 25 years: interim analysis of a phase 2 double-blind, randomized, controlled trial. Int J Gynecol Cancer. 2010 Apr;20(3):404–410. doi: 10.1111/IGC.0b013e3181d373a5
- Konno R, Yoshikawa H, Okutani M, et al. Efficacy of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical intraepithelial neoplasia and cervical infection in young Japanese women. Hum Vaccin Immunother. 2014;10(7):1781–1794. doi: 10.4161/hv.28712
- Wheeler CM, Castellsagué X, Garland SM, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012 Jan;13(1):100–110. doi: 10.1016/S1470-2045(11)70287-X
- Apter D, Wheeler CM, Paavonen J, et al. Efficacy of human papillomavirus 16 and 18 (HPV-16/18) AS04-adjuvanted vaccine against cervical infection and precancer in young women: final event-driven analysis of the randomized, double-blind PATRICIA trial. Clin Vaccine Immunol. 2015 Apr;22(4):361–373. doi: 10.1128/CVI.00591-14
- Lehtinen M, Paavonen J, Wheeler CM, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012 Jan;13(1):89–99. doi: 10.1016/S1470-2045(11)70286-8
- Skinner SR, Szarewski A, Romanowski B, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 4-year interim follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet. 2014 Dec 20;384(9961):2213–2227. doi: 10.1016/S0140-6736(14)60920-X
- Wheeler CM, Skinner SR, Del Rosario-Raymundo MR, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infect Dis. 2016 Oct;16(10):1154–1168. doi: 10.1016/S1473-3099(16)30120-7
- Einstein MH, Baron M, Levin MJ, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 vaccine and HPV-6/11/16/18 vaccine: follow-up from months 12-24 in a phase III randomized study of healthy women aged 18-45 years. Hum Vaccin. 2011 Dec;7(12):1343–1358. doi: 10.4161/hv.7.12.18281
- Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018 May 9;5(5):Cd009069. doi: 10.1002/14651858.CD009069.pub3
- Karimi-Zarchi M, Allahqoli L, Nehmati A, et al. Can the prophylactic quadrivalent HPV vaccine be used as a therapeutic agent in women with CIN? A randomized trial. BMC Public Health. 2020 Feb 27;20(1):274. doi: 10.1186/s12889-020-8371-z
- Kang WD, Choi HS, Kim SM. Is vaccination with quadrivalent HPV vaccine after loop electrosurgical excision procedure effective in preventing recurrence in patients with high-grade cervical intraepithelial neoplasia (CIN2-3)? Gynecol Oncol. 2013 Aug;130(2):264–268. doi: 10.1016/j.ygyno.2013.04.050
- Di Tucci C, Schiavi MC, Faiano P, et al. Therapeutic vaccines and immune checkpoints inhibition options for gynecological cancers. Crit Rev Oncol Hematol. 2018 Aug;128:30–42. doi: 10.1016/j.critrevonc.2018.05.011
- Mariani L, Venuti A. HPV vaccine: an overview of immune response, clinical protection, and new approaches for the future. J Transl Med. 2010 Oct 27;8(1):105. doi: 10.1186/1479-5876-8-105
- de Villiers, EM, Fauquet C, Broker TR, et al. Classification of papillomaviruses. Virology. 2004 Jun 20;324(1):17–27. doi: 10.1016/j.virol.2004.03.033
- Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity of the human papillomavirus (HPV)-16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18-45 years. Hum Vaccin. 2011 Dec;7(12):1359–1373. doi: 10.4161/hv.7.12.18282
- Bowden SJ, Doulgeraki T, Bouras E, et al. Risk factors for human papillomavirus infection, cervical intraepithelial neoplasia and cervical cancer: an umbrella review and follow-up Mendelian randomisation studies. BMC Med. 2023 Jul 27;21(1):274. doi: 10.1186/s12916-023-02965-w
- Yuan Y, Cai X, Shen F, et al. HPV post-infection microenvironment and cervical cancer. Cancer Lett. 2021 Jan 28;497:243–254. doi: 10.1016/j.canlet.2020.10.034
- Bogani G, Sopracordevole F, Ciavattini A, et al. Duration of human papillomavirus persistence and its relationship with recurrent cervical dysplasia. Eur J Cancer Prev. 2023 Nov 1;32(6):525–532. doi: 10.1097/CEJ.0000000000000822
- Jenkins D. A review of cross-protection against oncogenic HPV by an HPV-16/18 AS04-adjuvanted cervical cancer vaccine: importance of virological and clinical endpoints and implications for mass vaccination in cervical cancer prevention. Gynecol Oncol. 2008 Sep;110(3 Suppl 1):S18–25. doi: 10.1016/j.ygyno.2008.06.027
- Akhatova A, Azizan A, Atageldiyeva K, et al. Prophylactic human papillomavirus vaccination: from the origin to the Current state. Vaccines (Basel). 2022 Nov 11;10(11):1912. doi: 10.3390/vaccines10111912
- Llave CL, MEV U, Lam HY, et al. The cost-effectiveness of human papillomavirus vaccination in the Philippines. Vaccine. 2022 Jun 15;40(27):3802–3811. doi:10.1016/j.vaccine.2022.05.025
- Zou Z, Fairley CK, Ong JJ, et al. Domestic HPV vaccine price and economic returns for cervical cancer prevention in China: a cost-effectiveness analysis. Lancet Glob Health. 2020 Oct;8(10):e1335–e1344. doi: 10.1016/S2214-109X(20)30277-1