ABSTRACT
Background
There is a lack of synthesis of literature to determine hepatitis B vaccine (HepB) strategies for hepatitis B virus (HBV) supported by quality evidence. We aimed to explore the efficacy and safety of HepB strategies among people with different characteristics.
Research design and methods
PubMed, Cochrane Library, Embase, and Web of Science were searched for meta-analyses comparing the efficacy and safety of HepB up to July 2023.
Results
Twenty-one meta-analyses comparing 83 associations were included, with 16 high quality, 4 moderate, and 1 low quality assessed by AMSTAR 2. Highly suggestive evidence supports HepB booster and HepB with 1018 adjuvant (HBsAg-1018) for improved seroprotection, and targeted and universal HepB vaccination reduced HBV infection Suggestive evidence indicated that targeted vaccination decreased the rate of hepatitis B surface antibody positivity and booster doses increased seroprotection in people aged 10–20. Weak evidence suggests potential local/systemic reaction risk with nucleotide analogs or HBsAg-1018. Convincing evidence shows HLA-DPB1*04:01 and DPB1*04:02 increased, while DPB1*05:01 decreased, hepatitis B antibody response. Obesity may reduce HepB seroprotection, as highly suggested.
Conclusion
Targeted vaccination could effectively reduce HBV infection, and adjuvant and booster vaccinations enhance seroprotection without significant reaction. Factors such as obesity and genetic polymorphisms may affect the efficacy.
1. Introduction
Hepatitis B virus (HBV) infection is a major public health problem, which has caused a high direct and indirect disease burden worldwide [Citation1]. The global burden of HBV infection is unevenly distributed, with particularly high prevalence in some countries in Africa and Southeast Asia [Citation2]. However, persistent HBV infection can lead to varying degrees of liver damage, leading to various complications such as hepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) [Citation3], causing nearly 900,000 deaths every year. The anti-HBV vaccine has been available to people since 1982 [Citation4], study estimated that a timely scale-up of the birth dose vaccine to 90% by 2030 would immediately reduce the incidence of chronic HBV, and the number of deaths in the global population born between 2020 and 2030 will be reduced by 710,000 [Citation5]. Studies have shown that hepatitis B vaccine (HepB) is an economically attractive option compared to other interventions [Citation6,Citation7], but it has not reached universal coverage worldwide, with global coverage remaining at only 42% and 17% in the WHO African Region by 2021. Between 1990 and 2020, 310 million people worldwide have benefited from hepatitis B vaccination and were protected from hepatitis B virus infect [Citation2,Citation5,Citation6].
With medical progress, HepB and the corresponding vaccination program are constantly updated. As the specific situation varies from one country region to another, the World Health Organization (WHO) encouraged vaccination campaigns based on the specific prevalence of hepatitis B antigen carriers in the geographic area where vaccination is implemented [Citation4]. Therefore, several vaccination strategies, such as vaccination protocols and different doses, have been proposed. For instance, the use of adjuvants and a booster dose has demonstrated the ability to enhance the immunological protection properties of the HepB vaccine according to studies [Citation8,Citation9]. The anti-HBs titers of two accelerated vaccination schedules for high-risk healthy adults [Citation10], 0-7-21 days and 0-1-2-12 months, were confirmed to have the capacity of reaching seroprotective levels more rapidly than the standard group [Citation11,Citation12]. Also, individual compliance with a full course of vaccine should also be considered when assessing the efficacy of the vaccine program. However, the effectiveness and the safety of the hepatitis B vaccine varied among different populations.
In addition, it has been suggested that even if the same HepB strategy is used, the effectiveness of immunization is affected by different individual characteristics. Genome-wide association studies have shown that some DRB1 and DQB1 genes and associated single nucleotide polymorphisms (SNPs) play a key role in HepB response in different populations [Citation13,Citation14], studies shown that HLA–DPB1 alleles * 02:01, * 02:02, * 03:01, * 04:01, and * 14:01 were associated with vaccine response, while in contrast, * 05:01 was associated with non-response to HepB. It has also been suggested that HBV gene expression adopts a similar pattern to that of the major metabolic genes in the liver [Citation15], so the impact that different metabolic states (e.g. diabetes, obesity, etc.) may have disorders of innate immunity that can negatively affect vaccine response [Citation16–18]. In northern China, after the implementation of universal hepatitis B vaccination for infants starting in 1992, the prevalence of hepatitis B infection in children aged 1–14 years fell from 46% in 1992 to 4% in 2006 [Citation19]. Age at acquisition of HBV infection is a major determinant of the clinical presentation of acute disease and the development of chronic infection, and perinatal and early postnatal transmission is the main cause of chronic hepatitis infection.
There is a growing number of meta-analyses summarizing the impact of current vaccination strategies and characteristics of the inoculated population for HepB. Due to the heterogeneity of study methods and findings, as well as other limitations, a single meta-analysis may not provide a comprehensive picture of its safety and efficacy. Just as systematic reviews synthesize data from existing individual studies [Citation20], umbrella reviews summarized evidence from existing systematic reviews. Umbrella reviews can provide summaries of evidence from multiple systematic reviews covering different populations, research questions, or time periods [Citation21]. It is important at this time to systematically assess the efficacy of HepB in vaccinated individuals, focusing on the overall picture of the higher level of evidence for all its effects.
Thus, the present umbrella review provides a comprehensive review of existing meta-analyses related to HepB, with the aim of summarizing the evidence on its efficacy and safety, including vaccine strategies, immune response, and vaccinated populations with different characteristics. In addition, it highlights areas of contradiction and consistency in the evidence base for HBV vaccination, as well as research gaps. This overview can be used as a guide for finding a high-quality systematic review of specific outcomes, patient populations, or vaccine types, with a view to bringing better available evidence to healthcare decision-makers [Citation22].
2. Methodology
This review was registered and conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the Grading of Recommendations, Assessment, Development and Evaluation (GRADE). Our study protocol is registered with PROSPERO (registration number: CRD42023413135).
2.1. Literature search strategy and eligibility criteria
An umbrella review was conducted on all available systematic reviews and meta-analyses centering on the topic of the efficacy and safety of HepB associated with different vaccine factors or populations. PubMed, Cochrane Library, Embase, and Web of Science were searched for papers published between the database inception and 14 July 2023, and no language restriction was applied. A manual search on Google Scholar was also conducted to include articles covering the topics of interest. The literature search strategy wasattached in the Supplement. Two independent reviewers (JQ and SZ) undertook screening, data extraction, and quality assessment according to the PRISMA guidelines and disagreements were judged by a third reviewer (FZ) to reach a consensus after discussions.
We included studies of HepB if they met the following inclusion criteria: (i) Systematic reviews and meta-analyses that focused on the safety or efficacy of HepB among various populations; (ii) Meta-analyses conducted based on original studies providing details about the efficacy (e.g. anti-HBs IgG titers; the response rate, etc.) or safety (e.g. risk for cardiovascular-related mortality) with different HepB strategies or among populations with different characteristics; and (iii) Studies reported at least one of these outcomes as pooled odds ratios (ORs), relative risks (RRs), hazard ratios (HRs), or health-related diameters (e.g. standardized mean difference (SMD), weighted mean difference or mean difference [MD]) concerning health outcomes in association with HepB. When two or more systematic reviews existed for the same intervention and comparison, only the most recent systematic review with the largest number of individual studies providing study-level estimates would be included to avoid duplication of samples. No language or date restrictions were applied. Studies published in full peer-reviewed literature. Articles without study-level effect sizes and 95% confidence intervals (CIs) for systematic reviews, or those were conducted on animal models were excluded. We also exclude the conference abstracts, editorials, narrative reviews, or systematic review protocols.
We divided the included studies into three major categories, the effects of different vaccine strategies (e.g. vaccine composition, route of administration, vaccination procedure, dose, etc.), various characteristics of included populations (e.g. gender, race, genetics, disease status, etc.) on the effectiveness of HepB, and side effects (e.g. adverse effects, mortality, etc.) due to different vaccine strategies.
2.2. Data extraction and quality assessment
We retrieved the first author, the year of publication, population, reported HepB-related interventions and comparisons, outcomes, number of included studies, study design, and an aggregated meta-analysis estimate from each included systematic review. The following information was extracted from each individual study: publication year, study design (i.e. cohort design, case-control design, or clinical trial design), population (e.g. adolescents, the general population, chronic kidney disease population, etc.), sample size, reported HepB-related information (e.g. type, route, protocol, dosage of vaccination, etc.), and corresponding evaluation criteria for control information, and maximum adjusted study specific estimates [i.e. mean deviation (MD), normalized mean deviation (SMD); including Hedges’ g and Cohen’s d), OR, or RR], 95% confidence interval. We extracted any estimates reported by subgroup analysis as well. The protection threshold for anti-HBs concentration was set at 10 mIU/mL based on vaccine efficacy study [Citation23].
AMSTAR (A Measurement Tool to Assessment Systems Reviews) 2 was used to evaluate the overall confidence in the results of all the included reviews, which was divided into four levels: high, moderate, low, and critically low to evaluate review design, literature screening, data extraction, and individual study quality assessment [Citation24]. The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) was also used to assess the certainty of the evidence. GRADE would be categorized as high quality, moderate quality, low quality, or very low quality, then evidence can be downgraded or upgraded by the following criteria [Citation25,Citation26].
2.3. Dataanalysis
Based on the re-analyses of the findings of the reviews, data were presented along with summary tables on the characteristics and findings of the reviews.
A unit of analysis was formed by a systematic review and meta-analysis that met the inclusion criteria. We used random-effects models (the DerSimonian and Laird) to re-estimate the pooled effect sizes, 95% CIs, and P-values for each meta-analysis by referring to the analysis of previous umbrella reviews [Citation27–29]. P Value thresholds for statistical significance for the pooled effect estimate are set at <0.05, and P-value thresholds were set at <10−3 and <10−6 to assess the credibility of evidence [Citation30,Citation31]. For inter-group heterogeneity, we conducted Cochran’s Q (P < 0.10 indicated the existence of heterogeneity) testing and we calculated the I2statistic (I2 ≥50% represented high inconsistency) [Citation32,Citation33]. In addition, to better compare the effects of different interventions on HepB safety and efficacy, we used Cohen’s method MDs were normalized to SMDs and further converted to ORs using Hasselblad and Hedges’ method. We also estimated 95% prediction intervals in order to investigate whether the effect on the unified study topic could persist in the future, and when the interval excluded null values (i.e. RRs or ORs equal to 1), the effect was inferred to occur in the new study [Citation34,Citation35]. To identify potential publication bias, we used Egger’s regression asymmetry test and presented it as a funnel plot [Citation36]. Small-study effect was established with Egger’s P < 0.10, with the estimation for the largest component study (the study on the lowest SE) being more conservative than the summary estimate based on the random-effects models [Citation27–29].
We assessed the excess significance for any observed number of studies (O) with nominally meaningful results from every association (P < 0.05) in order to see whether or not it is greater than their expected number (E) [Citation37]. In each association, an expected amount of significant studies was estimated by summing up the estimated statistical power of each individual study using an algorithm from the non-central t distribution, in which the largest effect in each association is the plausible power of the tested association [Citation38]. Each association was compared with the results of the studies, and the excess significance thresholds for the association were established at P < 0.10. At P < 0.10, excess significance was established for a single association. All analyses were performed by Stata version 17.7. The P-values of the test results were two-tailed.
2.4. Determining the credibility of evidence
Based on previous umbrella reviews [Citation27–29,Citation39], we used the following criteria to determine the level of evidence: (1) P < 10−6based on random-effects meta-analysis; (2) >1000 participants; (3) P < 0.05 of the largest study; (4) between-study heterogeneity with I2 <50%; (5) no evidence of small-study effects; (6) 95% prediction interval that excluded the null value; and (7) no excess significance bias.
According to the results of statistical analysis, we sorted out each evidence’scredibility as .
Table 1. Rating overall evidence’s credibility of the review.
3. Results
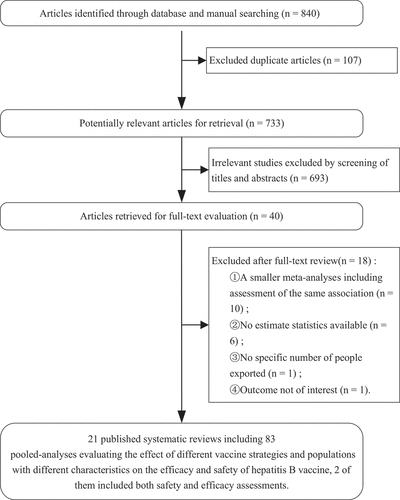
By systematic database searching, we identified 840 records. Forty full-text articles were screened for eligibility after being cleared of duplicates and examined for titles and abstracts. Ultimately, 21 meta analyses comprising 89 associations were included for re-analysis [Citation6,Citation24,Citation40–58] (). Supplemental results provide a detailed explanation for the exclusion reviews and the corresponding reasons. Of the 21 included metaanalyses, 15 were rated as high quality according to the AMSTAR 2 scoring system [Citation41–49,Citation52,Citation53,Citation57], 4 were rated as moderate quality (40, 49, 51, 54), and 2 were rated as low quality [Citation50,Citation58] (, sTables 2–4). Supplemental file 7 shows the poolGRADE evidence of association of different factors and HepB.
Figure 2. Pool GRADE of evidence of association of different factors and HepB.
3.1. Effect of different vaccine strategies on the efficacy of HepB
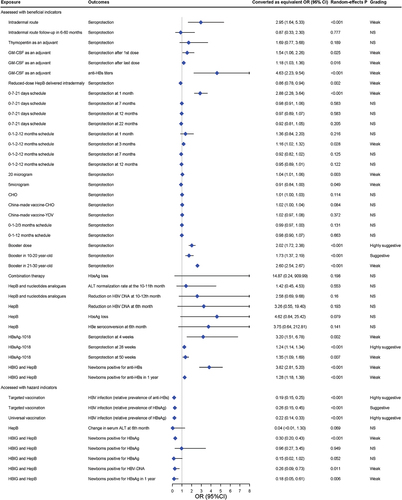
Intotal, 45 associations in 11 articles evaluated the effectiveness of various HepB strategies, including different vaccination protocols, doses, routes, and origins of HepB, and whether universal vaccinated. Of these, only 24 re-analyses reported nominally statistically significant pooled effects by the random-effects estimate (P < 0.05), and only 6 had 95% of the prediction intervals excluding null values (sTable 5). In these comparisons, we observed 13 associations with significant heterogeneity (I2 >50%). Twenty-eight analyses consisted of fewer than five separate studies, in which case the power of the test was diminished.
A total of 45 GRADE evidence quality classifications were conducted to evaluate the impact of different vaccine strategies on hepatitis B efficacy (Supplementary file 7). Among these classifications, 15 were rated as very low quality (15/45, 33.3%), 7 were rated as low quality (7/45, 15.6%), 11 were rated as moderate quality (11/45, 24.4%), and 12 were rated as high quality (12/45, 26.7%) (.
Figure 5. Forest plot of different characteristics and the efficacy of HepB.
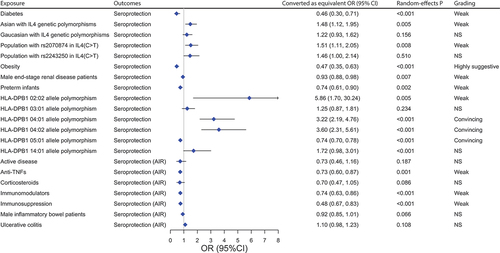
Out of 45 associations, 4 showed highly suggestive evidence, 2 suggestive evidence, and 20 weak evidence, according to the quantitative umbrella review criteria (, sTable 5). Highly suggestive evidence showed that vaccination of HepB booster (pooled RR: 2.023; P-random effects: <0.001) and HepB with 1018 as adjuvant (HBsAg-1018, pooled RR: 1.235; P-random effects: <0.001) could improve the seroprotection rate of vaccine, targeted vaccination could effectively reduce HBV infection (with the positive expression of hepatitis B surface antigen, HBsAg as an indicator) compared to non-vaccinated populations (pooled OR: 0.194; P-random effects: <0.001). Similarly, universal vaccination could also effectively have the same effect on HBV infection (with the positive of hepatitis B surface) (pooled OR: 0.217; P-random effects: <0.001), just as the suggestive evidence suggested that targeted vaccination decreased HBsAg positivity in the population (pooled OR: 0.261; P-random effects: <0.001). Suggestive evidence showed that HepB booster could improve the seroprotection of 10–20 years old people (pooled RR: 1.729; P-random effects: <0.001). Limited evidence suggests that HepB vaccination effectively boosts immune response, especially when combined with hepatitis B immune globulin (HBIG) in newborns of HBsAg-positive mothers, reducing HBsAg and HBV-DNA positivity rates. After a year, this combo continues to increase anti-HBs rates. Using granulocyte macrophage colony-stimulating factor (GM-CSF) as adjuvant therapy, both first and last doses enhance anti-HBs titers. Intradermal vaccination improves seroprotection more than intramuscular, but the trend reverses with reduced doses. 0-7-21 days and 0-1-2-12 months schedules enhance seroprotection. The 20 μg vaccine dose is effective, while 5 μg has the opposite effect. When 1018 was used as an adjuvant (HBsAg-1018), seroprotection could also be improved, extending to boosters in 21–30-year-olds.
3.2. Effect of different vaccine strategies on the safety of HepB
A total of 19 associations assessing the vaccine strategies on vaccine safety were reassessed, and 11 associations reported statistically significant results (p < 0.05) using random-effects model analysis, with null values excluded from the 95% prediction interval in 2 of these analyses. Excess of significance bias was detected in three comparisons and five comparisons consisted of less than 5 individual studies (sTable 6).
In the 17 GRADE evidence quality classifications of effect of different vaccine strategies on the safety of HepB, the quality of evidence was very low in 9 items (9/17, 52.9%), moderatein 3 items (3/17, 42.9%), and high in 5 items (5/17, 29.4%), with no items graded as low (Supplemental file 7).
None of the 19 associations had a convincing, highly suggestive or suggestive strength of evidence according to the quantitative omnibus evaluation criteria. In addition, nine associations had weak strength of evidence (, sTable 6). For example, weak evidence suggested that vaccine combined with nucleotide analogues compared to nucleotide analogues (NAs) increased the risk of injection site pain, fatigue, myalgia, headache, and nausea.
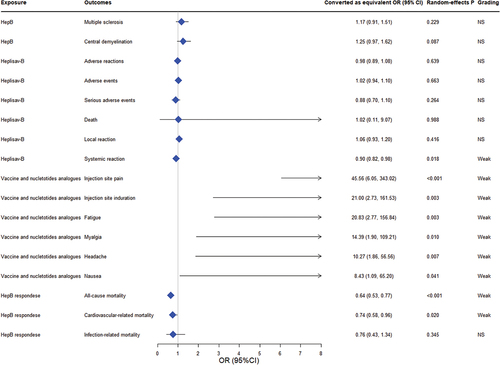
3.3. Effect of populations with different characteristics on the efficacy of HepB
Twenty-two associations assessing the impact of populations with different characteristics (e.g. gender, disease or health status, genetics, etc.) on vaccine efficacy were reassessed, and 14 associations reported statistically significant results by using random-effects model analysis (p < 0.05), 6 of these analyses showed null values excluded from the 95% prediction interval. Heterogeneity (I2 >50%) was observed in people who were obese, with HLA-DPB1*02:02 allelepolymorphisms, using immunosuppression (anti-TNFs, immunomodulators) (sTable7). We found a risk of small-study effect bias in one pooled analysis, excess of significance bias was detected in three comparisons and 5 comparisons consisted of less than 5 individual studies ().
There were a total of 21 results of GRADE evidence quality classification on populations with different characteristics and the efficacy of HepB outcomes. Of these outcomes, the quality of evidence was very low in 9 (9/21, 12.3%), low in 19 (10/21, 47.6%), moderate in 2 (2/21, 9.5%), and there were no items with a high grade (Supplemental file 7). Of the 23 associations, we found three convincing evidence, one highly suggestive evidence, and nine evidence of weak strength (, sTable 7). Convincing evidence suggested that alleles HLA-DPB1 *04:01 (pooled OR: 3.224; P-random effects: <0.001), DPB1*04:02 (pooled OR: 3.601; P-random effects: <0.001) were found to be associated with a significant increase in the antibody response to HepB, whereas DPB1*05:01 (pooled OR: 0.736; P-random effects: <0.001) showed the opposite association. Highly suggestive evidence indicated obesity decreased HepB seroprotection (pooled OR: 0.468; P-random effects: <0.001). Moreover, weak evidences suggested that Asian with IL4 genetic polymorphisms and population with the T allele of rs2070874 or HLA-DPB1* 02:02 allele polymorphism increased HepB protection. Diabetes, preterm infants, males end-stage renal disease patients were associated with the reduction of the seroprotection, and those using immunosuppression, including anti-TNFs andimmunomodulators also have the same effect.
4. Discussion
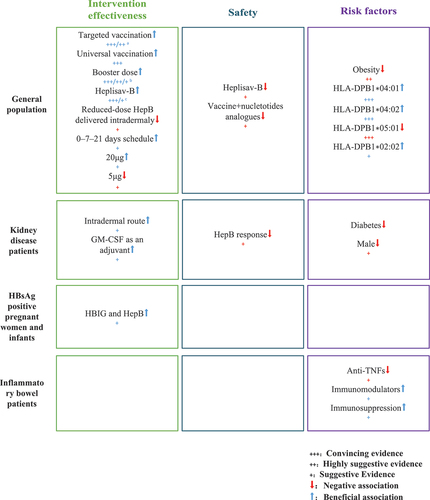
This umbrella review includes 83 associations on the residual safety of the effectiveness of HepB, and it presents compelling evidence suggesting HepB boosters, HBsAg-1018, targeted and universal vaccination were effective in increasing the immune response and thus protection against HBVinfection without major side effects. The finding also shows that people with different alleles have different immune responses to hepatitis B vaccines, while obesity may reduce the immune protection.
The findings of this umbrella review show the effectiveness of booster doses in augmenting seroprotection rates across diverse age groups, with a notable impact observed in individuals aged 10–20 years. Previously, various attempts have also been made to improve the protective effect of the HepB, including higher antigen concentration, vaccine boosters, the intradermal vaccine route, and new adjuvant systems. The concentration of protective antibody decreases over time, with less than 70% of people having more than 10mIU/ml of seroprotection present 10 years after vaccination. The term ’booster’ in the context of immunization refers to a subsequent vaccination administered after a primary vaccination series [Citation59]. Based on current scientific evidence, primary vaccination is required for all people [Citation60,Citation61]. However, in immunocompromised patients, or for major breakthrough infections, serologic monitoring of this population is necessary, and if their anti-HBs level is below 10 mIU/mL, they need to receive a booster vaccination to provide rapid protective immunity, while booster vaccination of immunocompetent children and adults is not recommended [Citation62].
Additionally, we demonstrated the efficacy of hepatitis B vaccine in combination with 1018 adjuvant to significantly increase hepatitis B vaccine response rates. HBsAg-1018 is a HepB that uses cytidine-phosphate-guanosine oligodeoxynucleotide (CpGODN), 1018, as an adjuvant. This adjuvant stimulates hepatitis B surface antigen (HBsAg) directly, producing a targeted immune response, compared to a multi-pathway, broad immune stimulation response [Citation63]. In two previous phase 3 trials, HBsAg-1018 has been shown to induce higher and earlier seroprotection rate in healthy adults [Citation64,Citation65]. It has been noted that the seroprotection rate in the HBsAg-1018 group was 95% or higher in all pre-specified populations except for diabetics or those aged 60–70 years [Citation65]. Accordingly, this greater immunogenicity may not be due to the induction of higher overall antibody levels in the vaccinated population, but to the induction of higher antibody levels in a broad of the recipient population, and the induction of more uniform and effective levels of protective antibodies.
Similarly, universal and targeted vaccination strategies have proven effective in reducing HBV infection in our study. Universal infant and birth dose immunization against hepatitis B is key in global efforts to eliminate HBV infection. WHO estimated a significant reduction in HBsAg prevalence among children under 5, from 4.7% to 1.3% following the implementation of universal vaccination. This highlights the vital role of vaccination programs in curbing HBV transmission and moving closer to global eradication goals [Citation66]. Certain countries have implemented targeted vaccination programs, including catch-up vaccination initiatives, for children born shortly before the introduction of universal infant immunization [Citation57]. These targeted efforts have successfully narrowed national coverage disparities and achieved HepB coverage of ≥80% in all districts [Citation67].
The assessment related to safety to HepB in this study was generally limited, and safety assessments indicate evidence supporting a slight increase in local and systemic reactions such as injection-site pain, fatigue, and myalgia when HepB is administered concurrently with nucleotide analogues or HBsAg-1018. According to the Centers for Disease Control and Prevention (CDC) [Citation68], administration of the HepB is just like taking any other medications, and there may be side effects, but the HepB is so safe that most people do not experience side effects, and if they do occur, they are relatively mild [Citation69]. Our study concluded that all-cause mortality and cardiovascular disease mortality were significantly lower among dialysis patients who were HBV vaccine responders than among non-responders [Citation56], suggesting that immunization against HBV in dialysis patients is essential for the protection of dialysis patients.
Some of the factors associated with vaccine non-responsiveness include obesity [Citation17], age at first vaccination, gender, immune status, and genetic factors [Citation70]. In the present study, the DPGly84-related alleles DPB1*04:01 and DPB1*04:02 were associated with high HepB response, whereas DPB1*05:01 was related to lower HepB response [Citation71]. The reason for this may be due to the fact that DPGly84-related alleles can use class I and class II antigen processing pathways to present endogenous and exogenous peptides, while DP84Asp (including DPB1*05:01) does not have this function of endogenous antigen presentation.
Obesity status also affects the immune response to HepB. Hepatitis B virus is considered to be a metabolic virus and obesity is known to be a risk factor for many metabolic diseases [Citation15], with one study finding diabetes to be an independent risk factor for liver cirrhosis [Citation72]. There may be an impaired immune response in obese individuals due to leptin-induced systemic and B-cell intrinsic inflammation [Citation73], and there is also a hypothesis pointed that obesity or obesity-related disease influences HepB escape mutations [Citation74], and even more researches have concluded that individuals with a BMI of 25–30 and greater than 30 were less likely to be seroprotected after hepatitis B vaccination than those with a BMI less than 25 [Citation17]. The short-term response of HepB to obesity can be enhanced by improved immunisation methods, but the impaired vaccine response due to obesity still needs to be addressed by more robust mechanistic studies.
The main limitation of this study is that, similar to other umbrella reviews [Citation75], our study only reported published systematic reviews or meta-analyses, and even if a factor has a strong effect but is not systematically reviewed or meta-analyzed, it may be excluded from the umbrella evaluation, and if it is little studied, it may also be classified as weak evidence only because it involves <1000 patients. For example, the assessment related to adverse reactions to HepB in this study was generally limited, with only weak evidence showing an increased risk of fatigue, nausea, and pain with vaccine plus nucleotide analogues (NAs) compared to NAs, and for those HepB responders of dialysis patients, they would have a higher all-cause mortality rate as well as cardiovascular-related mortality rate. Possible explanations for the above results can be attributed to the selection criteria of the original study design, which are often not appropriate for very rare outcomes, such as rarely reported adverse reactions. Therefore, we may have overlooked other influences that have not been studied by meta-analysis. Secondly, different meta-analyses may differ in their selection criteria and analysis methods, which may bias the assessment of the evidence. For review included preliminary studies differing significantly in basic characteristics, the heterogeneity should be considered. Therefore, this study used a criteria of I2 <50% between studies for type I evidence for studies. However, clinical heterogeneity could exist even in the absence of statistical heterogeneity [Citation76]. Moreover, some of the biases contained in the primary studies could not be fully excluded by the comprehensive review criteria of this study, and it is beyond the scope of this study to assess the quality of primary studies, which should be conducted by the original meta-analysis [Citation29]. The entire analysis was conducted using published data and further analysis to estimate dose–response relationships was not possible due to the variability of data reported across studies. Thirdly, as many of the systematic reviews had similar objectives and were conducted in a relatively limited period of time, the results are likely to have been derived from overlapping initial studies.
5. Limitation
Our study has several limitations. Firstly, as with other umbrella reviews [Citation75], our study is based solely on published systematic reviews or meta-analyses. This approach may exclude factors with strong effects that have not been systematically reviewed or meta-analyzed. For instance, factors involving fewer than 1000 patients are often classified as weak evidence, not necessarily reflecting their actual impact. For instance, the higher mortality rates in HepB responders among dialysis patients could not be thoroughly explored due to the selection criteria of the original study designs not being suited for rare outcomes. Secondly, different meta-analyses may have varying selection criteria and analysis methods, potentially biasing the evidence assessment. While we used a criterion of I2 < 50% to identify type I evidence, clinical heterogeneity can still exist without statistical heterogeneity [Citation76], which could affect the reliability of our findings. Thirdly, the biases in primary studies included in the meta-analyses are not fully addressed in our review, which should be conducted by the original meta-analysis [Citation29]. Fourthly, our analysis was limited to published data, preventing further estimation of dose–response relationships due to data variability. Fifthly, it is crucial to mention that our included meta-analyses did not limit anti-HBs measurements, potentially leading to some discrepancies. While the predominant method was radioimmunoassay (Ausab, Abbott Laboratories, Chicago, IL, U.S.A.), certain studies still employed alternative measurement methods. Finally, many of the systematic reviews we included had similar objectives and were conducted in alimited timeframe, likely leading to overlapping initial studies. This overlap might have influenced our results.
6. Conclusions
In conclusion, our study demonstrates that the efficacy of the hepatitis B vaccine is enhanced with booster shots and the addition of adjuvant 1018. We also found that both targeted and universal vaccination strategies significantly reduce hepatitis B incidence, thereby improving population health. Notably, individuals with HLA-DPB1*04:01 and DPB1*04:02 genotype exhibit a higher immune response to the vaccine, in contrast to those with HLA-DPB1*05:01 and obese individuals. Our analysis on the safety and efficacy of HBV vaccine provides valuable insights for future research, highlighting the need for varied study designs and outcome measures. This comprehensive overview contributes to refining hepatitis B vaccination strategies and supports the development of effective booster programs. These findings are crucial for public health authorities in optimizing hepatitis B prevention and control practices.
Article highlights
Targeted hepatitis B vaccination is an effective way in reducing hepatitis B infection
Booster doses increase hepatitis B immune response
Population with specific factors such as obesity may reduce the efficacy of the vaccine
Hepatitis B vaccine strategies have no significant side effects
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or mending, or royalties.
Reviewer disclosures
A peer reviewer on this manuscript has received an honorarium for their review work. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Author contributions statement
Jiamin Qiu did the literature search and screening and extracted the data; conducted the data analyses; made the figures and tables; and drafted manuscript.
Shiwen Zhang did the literature search and screening and extracted the data; conducted the data analyses.
Yonghui Feng conducted the data analyses, participated in the interpretation of results, and critically revised the manuscript.
Xin Su and Jun Cai conducted the data analyses.
Shiyun Chen, Jiazi Liu, Sui Zhu, Shiqi Huang, Haokun Huang, Huiyan Wen, Jiaxin Li, Haoyu Yan, and Zhiquan Diao participated in the interpretation of results and critically revised the manuscript.
Xiaofeng Liang was the co-corresponding author who took responsibility for the integrity of the data and the accuracy of the data analysis.
Fangfang Zeng designed the study and also was the co-corresponding author who took responsibility for the integrity of the data and the accuracy of the data analysis.
All authors participated in the interpretation of results, critically revised the manuscript, and approved the final version for submission.
Supplemental Material
Download Zip (290.2 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2023.2289566
Additional information
Funding
References
- Pattyn J, Hendrickx G, Vorsters A, Van DP. Hepatitis B Vaccines. J Infect Dis. 2021;2241:S343–S351. doi: 10.1093/infdis/jiaa668. PubMed PMID: 34590138.
- Cooke GS, Andrieux-Meyer I, Applegate TL, et al. Accelerating the elimination of viral hepatitis: a lancet gastroenterology & hepatology commission. Lancet Gastroenterol Hepatol. 2019 Feb;4(2):135–184. PubMed PMID: 30647010. doi: 10.1016/S2468-1253(18)30270-X
- Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23–61. PubMed PMID: 18039107. doi: 10.1146/annurev.pathol.1.110304.100230
- Health Organization W, World Health O. Hepatitis B vaccines: WHO position paper, July 2017 – recommendations. Vaccine. 2019 Jan 7;37(2):223–225. PubMed PMID: 28743487. 10.1016/j.vaccine.2017.07.046
- de Villiers MJ, Nayagam S, Hallett TB. The impact of the timely birth dose vaccine on the global elimination of hepatitis B. Nat Commun. 2021 Oct 28;12(1):6223. doi: 10.1038/s41467-021-26475-6. PubMed PMID: 34711822; PMCID: PMCPMC8553835.
- Zhang LL, Guo J, Duan K. Comparative analysis of the safety and efficacy of HBsAg-1018 versus HBsAg-eng: a meta-analysis. Cent Eur J Immunol. 2019;44(4):455–462. PubMed PMID: 32140059; PubMed Central PMCID: PMCPMC7050062. doi: 10.5114/ceji.2019.92808
- Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis. 2011 Jul 1;53(1):68–75. PubMed PMID: 21653306. 10.1093/cid/cir270
- Di Lello FA, Martinez AP, Flichman DM. Insights into induction of the immune response by the hepatitis B vaccine. World J Gastroenterol. 2022 Aug 21;28(31):4249–4262. PubMed PMID: 36159002; PubMed Central PMCID: PMCPMC9453777. 10.3748/wjg.v28.i31.4249
- Poorolajal J, Mahmoodi M, Majdzadeh R, et al. Long-term protection provided by hepatitis B vaccine and need for booster dose: a meta-analysis. Vaccine. 2010 Jan 8;28(3):623–631. PubMed PMID: 19887132. doi: 10.1016/j.vaccine.2009.10.068
- Chen W, Gluud C. Vaccines for preventing hepatitis B in health-care workers. Cochrane Database Syst Rev. 2005 Oct;19(4):Cd000100. doi: 10.1002/14651858.CD000100.pub3. PubMed PMID: 16235273; eng.
- Hess G, Hingst V, Cseke J, et al. Influence of vaccination schedules and host factors on antibody response following hepatitis B vaccination. Eur J Clin Microbiol Infect Dis. 1992 Apr;11(4):334–340. PubMed PMID: 1396753. doi: 10.1007/BF01962073
- Ricciardi G, Graziano G. [Safety and immunogenicity of an anti-hepatitis-B vaccine obtained using the recombinant DNA technique: results of a longitudinal study in hospital personnel]. Boll Ist Sieroter Milan. 1990 Jun;69(2):385–90. PubMed PMID: 2152297.
- Chung S, Roh EY, Park B, et al. GWAS identifying HLA-DPB1 gene variants associated with responsiveness to hepatitis B virus vaccination in Koreans: Independent association of HLA-DPB1*04: 02 possessing rs1042169 G - rs9277355 C - rs9277356 a. J Viral Hepat. 2019 Nov;26(11):1318–1329. PubMed PMID: 31243853. doi: 10.1111/jvh.13168
- Akcay IM, Katrinli S, Ozdil K, et al. Host genetic factors affecting hepatitis B infection outcomes: insights from genome-wide association studies. World J Gastroenterol. 2018 Aug 14;24(30): 3347–3360. PubMed PMID: 30122875; PubMed Central PMCID: PMCPMC6092584. 10.3748/wjg.v24.i30.3347
- Shlomai A, Shaul Y. The “metabolovirus” model of hepatitis B virus suggests nutritional therapy as an effective anti-viral weapon. Med Hypotheses. 2008;71(1):53–57. PubMed PMID: 18334285 doi: 10.1016/j.mehy.2007.08.032
- Hyer RN, Janssen RS. Immunogenicity and safety of a 2-dose hepatitis B vaccine, HBsAg/CpG 1018, in persons with diabetes mellitus aged 60–70 years. Vaccine. 2019 Sep 16;37(39):5854–5861. PubMed PMID: 31431412. 10.1016/j.vaccine.2019.08.005
- Liu F, Guo Z, Dong C. Influences of obesity on the immunogenicity of hepatitis B vaccine. Hum Vaccin Immunother. 2017 May 4;13(5):1014–1017. PubMed PMID: 28059607; PubMed Central PMCID: PMCPMC5443393. 10.1080/21645515.2016.1274475
- Verstraeten T, Fletcher MA, Suaya JA, et al. Diabetes mellitus as a vaccine-effect modifier: a review. Expert Rev Vaccines. 2020 May;19(5):445–453. PubMed PMID: 32516066. doi: 10.1080/14760584.2020.1760098
- Zhang L, Xu A, Yan B, et al. A significant reduction in hepatitis B virus infection among the children of Shandong Province, China: the effect of 15 years of universal infant hepatitis B vaccination. Int J Infect Dis. 2010 Jun;14(6):e483–8. PubMed PMID: 19939719. doi: 10.1016/j.ijid.2009.08.005
- Siddaway AP, Wood AM, Hedges LV. How to do a systematic review: a best practice guide for conducting and reporting narrative reviews, meta-analyses, and meta-syntheses. Annu Rev Psychol. 2019 Jan 4;70(1):747–770. PubMed PMID: 30089228. 10.1146/annurev-psych-010418-102803
- Aromataris E, Fernandez R, Godfrey CM, et al. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015 Sep;13(3):132–40. PubMed PMID: 26360830. doi: 10.1097/XEB.0000000000000055
- Smith V, Devane D, Begley CM, et al. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011 Feb 3;11(1):15. PubMed PMID: 21291558; PubMed Central PMCID: PMCPMC3039637. doi: 10.1186/1471-2288-11-15
- Jack AD, Hall AJ, Maine N, et al. What level of hepatitis B antibody is protective? J Infect Dis. 1999 Feb;179(2):489–492. PubMed PMID: 9878036. doi: 10.1086/314578
- Mouchet J, Salvo F, Raschi E, et al. Hepatitis B vaccination and the putative risk of central demyelinating diseases - a systematic review and meta-analysis. Vaccine. 2018 Mar 14;36(12): 1548–1555. PubMed PMID: 29454521. 10.1016/j.vaccine.2018.02.036
- Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011 Apr;64(4):383–94. PubMed PMID: 21195583. DOI:10.1016/j.jclinepi.2010.04.026
- Granholm A, Alhazzani W, Moller MH. Use of the GRADE approach in systematic reviews and guidelines. Br J Anaesth. 2019 Nov;123(5):554–559. PubMed PMID: 31558313. doi: 10.1016/j.bja.2019.08.015
- Rojas-Rueda D, Morales-Zamora E, Alsufyani WA, et al. Environmental risk factors and Health: an umbrella review of meta-analyses. Int J Environ Res Public Health. 2021 Jan 15;18(2). PubMed PMID: 33467516; PubMed Central PMCID: PMCPMC7830944 10.3390/ijerph18020704
- Gao X, Su X, Han X, et al. Unsaturated fatty acids in mental disorders: an umbrella review of meta-analyses. Adv Nutr. 2022 Dec 22;13(6):2217–2236. PubMed PMID: 36041185; PubMed Central PMCID: PMCPMC9776730. 10.1093/advances/nmac084
- Piovani D, Danese S, Peyrin-Biroulet L, et al. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology. 2019 Sep;157(3):647–659.e4. PubMed PMID: 31014995. doi:10.1053/j.gastro.2019.04.016
- Ioannidis JP, Tarone R, McLaughlin JK. The false-positive to false-negative ratio in epidemiologic studies. Epidemiology. 2011 Jul;22(4):450–6. doi: 10.1097/EDE.0b013e31821b506e. PubMed PMID: 21490505.
- Sterne JA, Davey Smith G. Sifting the evidence-what’s wrong with significance tests? BMJ. 2001 Jan 27;322(7280):226–31. PubMed PMID: 11159626; PubMed Central PMCID: PMCPMC1119478. 10.1136/bmj.322.7280.226
- Cochran W. The combination of estimates from different experiments. Biometrics. 1954;10(1):101. doi: 10.2307/3001666
- Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007 Nov 3;335(7626):914–6. PubMed PMID: 17974687; PubMed Central PMCID: PMCPMC2048840. 10.1136/bmj.39343.408449.80
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011 Feb 10;342: d549. PubMed PMID: 21310794 10.1136/bmj.d549
- Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009 Jan;172(1):137–159. doi: 10.1111/j.1467-985X.2008.00552.x. PubMed PMID: 19381330; PubMed Central PMCID: PMCPMC2667312.
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997 Sep 13;315(7109):629–634. PubMed PMID: 9310563; PubMed Central PMCID: PMCPMC2127453. doi: 10.1136/bmj.315.7109.629
- Ioannidis JP, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials. 2007;4(3):245–253. PubMed PMID: 17715249 doi: 10.1177/1740774507079441
- Lubin JH, Gail MH. On power and sample size for studying features of the relative odds of disease. Am J Epidemiol. 1990 Mar;131(3):552–566. PubMed PMID: 2301364; eng. doi: 10.1093/oxfordjournals.aje.a115530
- Kim JH, Kim JY, Lee J, et al. Environmental risk factors, protective factors, and peripheral biomarkers for ADHD: an umbrella review. Lancet Psychiatry. 2020 Nov;7(11):955–970. PubMed PMID: 33069318. doi: 10.1016/S2215-0366(20)30312-6
- Alavian SM, Tabatabaei SV. The effect of diabetes mellitus on immunological response to hepatitis B virus vaccine in individuals with chronic kidney disease: a meta-analysis of current literature. Vaccine. 2010 May 14;28(22):3773–3777. PubMed PMID: 20371390. doi: 10.1016/j.vaccine.2010.03.038
- Chen Z, Zeng M, Liu D, et al. Antenatal administration of hepatitis B immunoglobulin and hepatitis B vaccine to prevent mother to child transmission in hepatitis B virus surface antigen positive pregnant women: a systematic review and meta-analysis. Medicine (Baltimore). 2020 Apr;99(16):e19886. PubMed PMID: 32312015; PubMed Central PMCID: PMCPMC7220666. doi: 10.1097/MD.0000000000019886
- Cruciani M, Mengoli C, Serpelloni G, et al. Granulocyte macrophage colony-stimulating factor as an adjuvant for hepatitis B vaccination: a meta-analysis. Vaccine. 2007 Jan 8;25(4):709–718. PubMed PMID: 16963165. doi: 10.1016/j.vaccine.2006.08.015
- Cui W, Sun CM, Deng BC, et al. Association of polymorphisms in the interleukin-4 gene with response to hepatitis B vaccine and susceptibility to hepatitis B virus infection: a meta-analysis. Gene. 2013 Aug 1;525(1):35–40. PubMed PMID: 23651591. doi: 10.1016/j.gene.2013.04.065
- Fabrizi F, Dixit V, Magnini M, et al. Meta-analysis: intradermal vs. intramuscular vaccination against hepatitis B virus in patients with chronic kidney disease. Aliment Pharmacol Ther. 2006 Aug 1;24(3):497–506. PubMed PMID: 16886915. doi: 10.1111/j.1365-2036.2006.03002.x
- Fabrizi F, Dixit V, Martin P. Meta-analysis: the adjuvant role of thymopentin on immunological response to hepatitis B virus vaccine in end-stage renal disease. Aliment Pharmacol Ther. 2006 Jun 1;23(11):1559–66. PubMed PMID: 16696803. 10.1111/j.1365-2036.2006.02923.x
- Fan W, Zhang M, Zhu YM, et al. Immunogenicity of hepatitis B vaccine in preterm or low birth weight infants: a meta-analysis. Am J Prev Med. 2020 Aug;59(2):278–287. PubMed PMID: 32564973. doi: 10.1016/j.amepre.2020.03.009
- Fan W, Chen XF, Shen C, et al. Hepatitis B vaccine response in obesity: a meta-analysis. Vaccine. 2016 Sep 14;34(40):4835–4841. PubMed PMID: 27546877. doi: 10.1016/j.vaccine.2016.08.027
- Ghozy S, Nam NH, Radwan I, et al. Therapeutic efficacy of hepatitis B virus vaccine in treatment of chronic HBV infections: a systematic review and meta-analysis. Rev Med Virol. 2020 May;30(3):e2089. PubMed PMID: 31811678. doi: 10.1002/rmv.2089
- Jin H, Tan Z, Zhang X, et al. Comparison of accelerated and standard hepatitis B vaccination schedules in high-risk healthy adults: a meta-analysis of randomized controlled trials. PLoS One. 2015;10(7):e0133464. PubMed PMID: 26196903; PubMed Central PMCID: PMCPMC4510064. doi: 10.1371/journal.pone.0133464
- Khedmat H, Aghaei A, Ghamar-Chehreh ME, et al. Sex bias in response to hepatitis B vaccination in end-stage renal disease patients: meta-analysis. World J Nephrol. 2016 Jan 6;5(1): 115–24. PubMed PMID: 26788471; PubMed Central PMCID: PMCPMC4707164. 10.5527/wjn.v5.i1.115
- Mahmood S, Shah KU, Khan TM. Immune persistence after infant hepatitis-B vaccination: a systematic review and meta-analysis. Sci Rep. 2018 Aug 22;8(1):12550. PubMed PMID: 30135554; PubMed Central PMCID: PMCPMC6105718. doi: 10.1038/s41598-018-30512-8
- Opri R, Veneri D, Mengoli C, et al. Immune response to hepatitis B vaccine in patients with celiac disease: a systematic review and meta-analysis. Hum Vaccin Immunother. 2015;11(12):2800–2805. PubMed PMID: 26378476; PubMed Central PMCID: PMCPMC5054794. doi: 10.1080/21645515.2015.1069448
- Ou G, Liu X, Jiang Y. HLA-DPB1 alleles in hepatitis B vaccine response: a meta-analysis. Medicine (Baltimore). 2021 Apr 9;100(14):e24904. PubMed PMID: 33832070; PubMed Central PMCID: PMCPMC8036076. doi: 10.1097/MD.0000000000024904
- Sangare L, Manhart L, Zehrung D, et al. Intradermal hepatitis B vaccination: a systematic review and meta-analysis. Vaccine. 2009 Mar 13;27(12):1777–1786. PubMed PMID: 19200451. doi: 10.1016/j.vaccine.2009.01.043
- Singh AK, Jena A, Mahajan G, et al. Meta-analysis: hepatitis B vaccination in inflammatory bowel disease. Aliment Pharmacol Ther. 2022 Apr;55(8):908–920. PubMed PMID: 35261057. doi: 10.1111/apt.16880
- Udomkarnjananun S, Takkavatakarn K, Praditpornsilpa K, et al. Hepatitis B virus vaccine immune response and mortality in dialysis patients: a meta-analysis. J Nephrol. 2020 Apr;33(2):343–354. PubMed PMID: 31701375. doi: 10.1007/s40620-019-00668-1
- Whitford K, Liu B, Micallef J, et al. Long-term impact of infant immunization on hepatitis B prevalence: a systematic review and meta-analysis. Bull World Health Organ. 2018 Jul 1;96(7): 484–497. PubMed PMID: 29962551; PubMed Central PMCID: PMCPMC6022616. 10.2471/BLT.17.205153
- Wu Z, Bao H, Yao J, et al. Suitable hepatitis B vaccine for adult immunization in China: a systematic review and meta-analysis. Hum Vaccin Immunother. 2019;15(1):220–227. PubMed PMID: 30089437; PubMed Central PMCID: PMCPMC6363055. doi: 10.1080/21645515.2018.1509172
- Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on immunization practices. MMWR Recomm Rep. 2018 Jan 12;67(1):1–31. PubMed PMID: 29939980;PMCID: PMC5837403. doi: 10.15585/mmwr.rr6701a1
- Watson B, West DJ, Chilkatowsky A, et al. Persistence of immunologic memory for 13 years in recipients of a recombinant hepatitis B vaccine. Vaccine. 2001 Apr 30;19(23–24):3164–3168. PubMed PMID: 11312012. doi: 10.1016/s0264-410x(01)00019-6
- McMahon BJ, Bruden DL, Petersen KM, et al. Antibody levels and protection after hepatitis B vaccination: results of a 15-year follow-up. Ann Intern Med. 2005 Mar 1;142(5):333–341. PubMed PMID: 15738452. doi: 10.7326/0003-4819-142-5-200503010-00008
- Zanetti AR, Mariano A, Romano L, et al. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet. 2005 Oct 15-21;366(9494): 1379–1384. PubMed PMID: 16226616. 10.1016/S0140-6736(05)67568-X
- De Gregorio E, Caproni E, Ulmer JB. Vaccine adjuvants: mode of action. Front Immunol. 2013;4:214. PubMed PMID: 23914187; PubMed Central PMCID: PMCPMC3728558. doi: 10.3389/fimmu.2013.00214
- Halperin SA, Ward B, Cooper C, et al. Comparison of safety and immunogenicity of two doses of investigational hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide and three doses of a licensed hepatitis B vaccine in healthy adults 18-55 years of age. Vaccine. 2012 Mar 28;30(15):2556–2563. PubMed PMID: 22326642. doi: 10.1016/j.vaccine.2012.01.087
- Heyward WL, Kyle M, Blumenau J, et al. Immunogenicity and safety of an investigational hepatitis B vaccine with a Toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared to a licensed hepatitis B vaccine in healthy adults 40–70 years of age. Vaccine. 2013 Nov 4;31(46): 5300–5. PubMed PMID: 23727002. 10.1016/j.vaccine.2013.05.068
- Indolfi G, Easterbrook P, Dusheiko G, et al. Hepatitis B virus infection in children and adolescents. Lancet Gastroenterol Hepatol. 2019 Jun;4(6):466–476. PubMed PMID: 30982722. doi: 10.1016/S2468-1253(19)30042-1
- Sandhu HS, Roesel S, Sharifuzzaman M, et al. Progress toward hepatitis B control — South-East Asia Region, 2016–2019. MMWR Morb Mortal Wkly Rep. 2020 Jul 31;69(30): 988–992. PubMed PMID: 32730237 PubMed Central PMCID: PMCPMC7392392 Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed. 10.15585/mmwr.mm6930a2
- Miller KD, Gibbs RD, Mulligan MM, et al. Intradermal hepatitis B virus vaccine: immunogenicity and side-effects in adults. Lancet. 1983 Dec 24-31;2(8365–66): 1454–6. PubMed PMID: 6140546. 10.1016/s0140-6736(83)90800-0
- Allen MI, Deslauriers M, Andrews CW, et al. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine. Lamivudine clinical investigation group. Hepatology. 1998 Jun;27(6):1670–1677. PubMed PMID: 9620341. doi: 10.1002/hep.510270628
- Yue T, Zhang Q, Cai T, et al. Trends in the disease burden of HBV and HCV infection in China from 1990-2019. Int J Infect Dis. 2022 Sep;122:476–485. PubMed PMID: 35724827. doi: 10.1016/j.ijid.2022.06.017
- Nishida N, Sugiyama M, Sawai H, et al. Key HLA-DRB1-DQB1 haplotypes and role of the BTNL2 gene for response to a hepatitis B vaccine. Hepatology. 2018 Sep;68(3):848–858. PubMed PMID: 29534301; PubMed Central PMCID: PMCPMC6175380. doi: 10.1002/hep.29876
- Huo TI, Wu JC, Lee PC, et al. Diabetes mellitus as a risk factor of liver cirrhosis in patients with chronic hepatitis B virus infection. J Clin Gastroenterol. 2000 Apr;30(3):250–254. PubMed PMID: 10777182. doi: 10.1097/00004836-200004000-00009
- Frasca D, Diaz A, Romero M, et al. Young and elderly patients with type 2 diabetes have optimal B cell responses to the seasonal influenza vaccine. Vaccine. 2013 Aug 2;31(35):3603–3610. PubMed PMID: 23711934; PubMed Central PMCID: PMCPMC3760593. doi: 10.1016/j.vaccine.2013.05.003
- Zhu H, Liu X, Ding Y, et al. Relationships between low serum vitamin D levels and HBV “a” determinant mutations in chronic hepatitis B patients. J Infect Dev Ctries. 2016 Sep 30;10(9):1025–1030. PubMed PMID: 27694737. doi: 10.3855/jidc.7459
- Fusar-Poli P, Radua J. Ten simple rules for conducting umbrella reviews. Evid Based Ment Health. 2018 Aug;21(3):95–100. PubMed PMID: 30006442; PubMed Central PMCID: PMCPMC10270421. doi: 10.1136/ebmental-2018-300014
- Ioannidis JP, Patsopoulos NA, Rothstein HR. Reasons or excuses for avoiding meta-analysis in forest plots. BMJ. 2008 Jun 21;336(7658):1413–5. PubMed PMID: 18566080; PubMed Central PMCID: PMCPMC2432114. 10.1136/bmj.a117