ABSTRACT
Background
Pneumococcal vaccines are effective in preventing pneumococcal diseases in adults. The evaluation of the antibodies persistence to the 23-valent pneumococcal polysaccharide vaccine (PPV23) could provide evidence on PPV23 revaccination.
Research design and methods
Adults aged ≥ 60 years were selected and vaccinated with PPV23 in Shanghai, and followed up for 5 years with blood samples collection of a 1-year interval. The geometric mean concentrations (GMC) of the IgG against 23 pneumococcal serotypes covered by PPV23 were detected using enzyme-linked immunosorbent assay. The antibodies to 23 pneumococcal serotypes among different groups was analyzed using statistical analysis.
Results
Overall, 517 participants completed all six visits over a 5-year period (2013–2018). The GMC of 23 serotypes in adults aged ≥ 60 years decreased slowly after PPV23 vaccination compared to baseline pre-vaccination (P < 0.05), except serotype 3. Additionally, the multiplicative increase in the antibody concentration after PPV23 vaccination was greater, and the antibody levels of serotypes 1 and 6B were significantly higher at visit 5 than at visit 4 (P < 0.05).
Conclusions
The pneumococcal antibodies in elderly after PPV23 vaccination could sustain high levels over long-term follow-up, which suggested that the interval of revaccination with PPV23 in elderly should be at least 5 years after the first vaccination.
1. Introduction
Streptococcus pneumoniae (the pneumococcus, Spn), an encapsulated bacteria with a polysaccharide capsule, is the leading cause of community-acquired pneumonia (CAP), with more than 90 distinct pneumococcal serotypes identified worldwide [Citation1]. Young children and older adults are the most affected in developing countries, which has become a major public health problem [Citation2]. The World Health Organization (WHO) reported that pneumonia killed 740,180 children under the age of 5 years in 2019, accounting for 14% of all deaths in children under 5 years of age and 22% of all deaths in children aged 1–5 years [Citation3], mainly in developing countries in southern Asia and sub-Saharan Africa. In developed countries such as Japan, more than 95% of pneumococcal disease (PD) deaths occur in people aged ≥65 years [Citation4]. Spn infection is also an important cause of morbidity and mortality in infants, children, and older adults in China [Citation5,Citation6]. Approximately 2.5 million people suffer from PD, which causes 125,000 deaths annually, particularly in infants and children under the age of 1 year and in adults aged ≥50 years [Citation7].
Currently, antibacterial drugs are the first-line treatment for PD, and antibiotic resistance in Spn is becoming a healthcare concern [Citation8,Citation9]. According to the Asian Network for Surveillance of Resistant Pathogens report, the prevalence of multidrug resistance in Spn in China was as high as 83.3% in 2012 [Citation10]. Pneumococcal vaccination is the most effective measure to prevent CAP [Citation11,Citation12]. The WHO listed PD as a disease requiring ‘high priority’ for vaccine prevention and recommended that pneumococcal vaccines should be included in national immunization programs as a priority [Citation13]. Currently, two classes of pneumococcal vaccines are available: pneumococcal polysaccharide vaccines (PPV) based on polysaccharides, and pneumococcal conjugate vaccines (PCV) based on polysaccharides conjugated to a carrier protein [Citation14].
The 23-valent PPV (PPV23), 7-valent PCV (PCV7), and 13-valent PCV (PCV13) have been approved for marketing in China, of which PPV23 is recommended for people aged ≥60 years [Citation15]. PPV23 was developed in the United States in 1983 and is effective against 23 different pneumococcal capsular types (serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9 V, 10A, 11A, 12F, 14, 15 B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F), covering more than 85% of the types found in pneumococcal bloodstream infections [Citation16]. A previous study from Chaoyang District, Beijing, in 2010 found that the pneumonia vaccination rate of community-dwelling adults aged 60–79 years was only 2.1% [Citation17]. In September 2013, Shanghai launched a major public health service project to provide one dose of PPV23 free of charge to adults aged ≥60 years to prevent pneumonia and protect this vulnerable population. Preliminary results indicated that one dose of PPV23 in adults in Shanghai could reduce the incidence of CAP to a certain extent with good safety [Citation18,Citation19]. In general, PPV23-induced antibody levels declined over time. Currently, research on the persistence of antibodies after PPV23 vaccination in the older adult population is limited, and has not yet been conducted in China. Moreover, the interval between the first and second PPV23 vaccinations has not been confirmed because of insufficient evidence. In this study, the antibody persistence of PPV23 in an adult population aged ≥60 years was evaluated through a 5-year follow-up cohort study, which aimed to provide more evidence on the revaccination of PPV23.
2. Methods
2.1. Study design and recruitment
This was a prospective follow-up cohort study. Stratified random sampling was used to select the study participants. There are 16 districts in Shanghai including seven urban districts, four suburban districts near urban, and five suburban districts far from urban. Firstly, two districts were randomly selected from each type of district. Secondly, the communities in the districts were divided into three types according to the number of resident populations, including < 20,000, 20000–50,000, and > 50,000; subsequently, two communities were randomly selected from each type, resulting in a total of six communities being selected.
Adult participants were randomly selected from each community, with a total sample size of 1,200. The inclusion criteria were as follows: households registered in Shanghai; age ≥60 years on the day of vaccination; no contraindications to PPV23 vaccination; and voluntary participation with informed consent. The exclusion criteria were as follows: contraindications to PPV23 vaccination; fever, acute infection, or acute onset of chronic diseases (withholding vaccination) on the day of vaccination; and history of PPV23 vaccination within the past 5 years.
2.2. Data collection and vaccination
Baseline data were collected before vaccination using a questionnaire designed by the Shanghai Municipal Center for Disease Control and Prevention (SCDC), which included general demographic information (name, sex, age, and district of inhabitation), lifestyle information (smoking, drinking, and exercise), and disease history (chronic obstructive pulmonary diseases and chronic diseases).
PPV23 was produced by Chengdu Institute of Biological Products Co., Ltd. and consisted of 23 serotypes of pneumococcal polysaccharide antigens, including serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9 V, 10A, 11A, 12F, 14, 15 B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F. The immunization procedure and dose of PPV23 was 0.5 mL per injection containing 25 µg each of purified 23 serotypes of pneumococcal capsule polysaccharide by subcutaneous or intramuscular injection into the lateral deltoid muscle of the upper arm.
2.3. Blood sample collection
Venous blood samples were collected at the first visit (label as ‘S0’) before vaccination and at five additional visits ((label as ‘S1,’ ‘S2,’ ‘S3,’ ‘S4,’ and ‘S5’) over the next 5 years, with an interval of 1 year. Approximately 500 μL serum of each blood sample was separated by centrifuging the next day and stored at −20°C until being tested. The ID numbers of serum samples were reset in the SCDC using a single-blind method before antibody testing. The antibodies and serotypes were tested at the Chengdu Institute of Biological Products Co., Ltd. Duplicate samples were set up in the same batch for quality control of the test results.
2.4. Antibody testing
The immunoglobulin G (IgG) antibody concentration of pneumococcal capsular polysaccharides from all 23 serotypes was determined using an enzyme-linked immunosorbent assay protocol for the quantitation of human IgG antibodies specific for Streptococcus pneumoniae capsular polysaccharides (Pn PS ELISA) [Citation20]. First, the microplate was coated with polysaccharide for each serotype at 37°C for 5 h under humid conditions. The samples to be tested were absorbed by Pneumococcal Cell Wall Polysaccharide-Multi at room temperature for at least 30 min, added into the washed microplate with 50 μL/well, and incubated overnight at 2–8°C (within 18 h). After washing the plate, alkaline phosphatase labeled sheep anti-human IgG (1:2000) was added with 100 μL/well and incubated for 2 h. After washing the plate, freshly prepared substrate solution was added, and the plate was incubated in the dark for 2 h. The reaction was terminated with the termination solution, and the A405-A690 values were read. The reference serum BW09 was used as a standard. SoftMax software was used to perform four-parameter fitting to establish a standard curve and to calculate the IgG antibody level of each serum sample.
2.5. Statistical analysis
Baseline data were analyzed using Microsoft Excel 2019, and statistical analyses were performed using SPSS software (version 26.0). The geometric mean concentrations (GMC) values of each IgG antibody serotype were log-transformed and compared between the two groups using Student’s t test, analysis of variance was used to compare multiple groups. The Least Significant Difference multiple comparisons method was used for two-by-two comparisons, and the Chi-square test was used for rate comparisons. Two-sided tests were used for all analyses. The maps were created using ArcGIS 10.1 (Environmental Systems Research Institute, Inc., Redlands, CA, U.S.A.). Statistical significance was set at P < 0.05.
2.6. Ethics statement
This study was approved by the Institutional Review Board of SCDC. Informed consent was obtained from each participant before enrollment in the study.
3. Results
3.1. Study site
Shanghai, one of the four municipalities, is the largest city and global financial hub in the country and is located on China’s central coast. Shanghai has a land area of 6,340.5 km2 and is divided into 16 county-level districts (). Among them, seven (Huangpu, Xuhui, Jingan, Changning, Putuo, Hong Kou, and Yangpu) were urban districts, four (Minghang, Baoshan, Jiading, and Pudong New Districts) were suburban districts near urban, and five (Fengxian, Songjiang, Qingpu, Jinshan, and Chongming) were suburban districts far from urban.
Six communities from six districts were selected, with one community from each district, including Xiaodongmen in Huangpu District, Daqiao in Yangpu District, Gucun in Baoshan District, Huating Town in Jiading District, Xinbang Town in Songjiang District, and Baihe Town in Qingpu District ().
3.2. Sample collection and demographic characteristics
The number of the first visit (S0) before vaccination was 1044 at the baseline and a total of 517 completed the following additional visits (S1, S2, S3, S4, and S5) over the next 5 years. Therefore, all participants were divided into two groups: one included participant who completed the entire follow-up, and the other was the group that did not complete the entire follow-up (517 and 527 participants, respectively).
Among the 517 participants enrolled in the study, the majority were aged 60–69 years (70.79%, 366/517). A proportion of 47.00% (243/517) was male, and 52.41% were from suburban districts. Among the remaining 527 participants, the proportion of those aged 60–69 years was as high as 70.02%, with 43.83% (231/527) men and 44.78% (236/527) women from suburban areas, far from urban areas. There were no significant differences between the two groups of participants in age or sex (P > 0.05); however, there were significant differences in district of inhabitation, smoking, drinking, exercise, chronic obstructive pulmonary diseases, and chronic diseases (i.e., hypertension, diabetes, coronary heart disease, chronic kidney disease, and chronic liver disease) (P < 0.05) ().
Table 1. Demographic characteristics of participants in the study.
3.3. Antibody testing
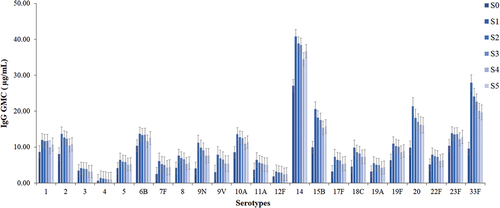
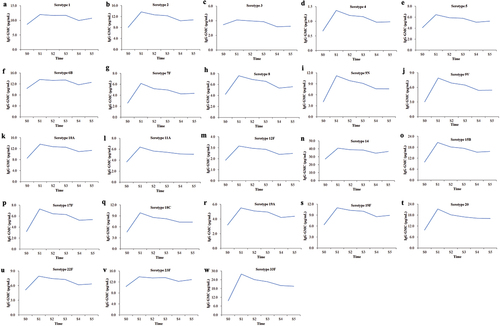
The IgG concentrations of 23 serotypes except 9N and 20, indicated significant difference between those who completed all visits and partial visits (P < 0.05) (). The persistence of antibody evaluation was focused on samples from the 517 participants who completed all visits. The antibody levels of the 23 serotypes after PPV23 vaccination in the adult population aged ≥60 years slowly decreased over time (P < 0.05). Additionally, the antibody levels of 22 serotypes from S1 to S5 were higher than those at S0, except for serotype 3, which was slightly lower from S4 to S5 than at S0 ().
Table 2. The baseline antibody levels between participants who completed all visits and partial visits.
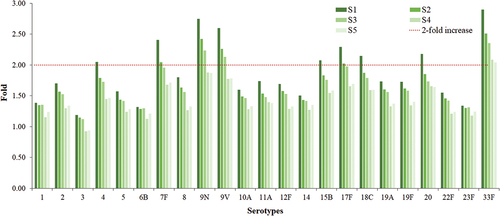
After PPV23 vaccination in the adult population aged ≥60 years, the increase in the antibody level of each serotype varied greatly compared to that before vaccination (S0). The IgG concentrations of serotypes 1, 2, 3, 5, 6B, 8, 10A, 11A, 12F, 14, 19A, 19F, 22F, and 23F did not increase by ≥ 2-fold at S1, whereas those of serotypes 4, 7F, 9N, 9 V, 15B, 17F, 18C, 20, and 33F showed ≥ 2-fold increase at S1. In addition, serotype 7F, 9N, 9 V, 17F, and 33F antibody concentrations showed ≥ 2-fold rise at S1 and S2; serotypes 9N, 9 V, and 33F antibody concentrations showed ≥ 2-fold increase at S1, S2, and S3; and serotype 33F antibody concentrations showed ≥ 2-fold increase at all five visits from S1 to S5 ().
Figure 3. Fold increase in GMC of 23 serotypes from 1 to 5 years after PPV23 vaccination comparing with that before vaccination.
In addition, the antibody level at S0, S1, S2, S3, S4, and S5 in different two age group of 60–69 years old (N = 366, 70.79%) and ≥70 years old (N = 151, 29.21%) was analyzed. All serotypes except 1,3, and 9 V showed no significant difference between these two groups (Table S1). The IgG concentration of serotype 1 was lower in the group of 60–69 years than that in ≥70 age group at S1, S2, S3, and S4 (P < 0.05). The antibody level of serotype 3 was lower at S4 in 60–69 age group than that in ≥70 age group (P < 0.05). Similarly, the IgG concentration of serotype 9 V was lower at S0, S1, and S2 in 60–69 age group than that in ≥70 age group (P < 0.05).
According to the dynamic change in antibodies in the 5-year cohort study, the antibody concentrations of all 23 serotypes, except serotypes 1 and 6B, decreased after PPV23 vaccination from S1 to S5. Interestingly, the antibody concentrations of serotypes 1 and 6B showed an inverse pattern, with the concentrations at S5 being significantly higher than that at S4 (P = 0.046 and 0.045, respectively) (, Table S2).
4. Discussion
PD is a major disease that endangers the physical and mental health of older adults and remains an important public health problem in China [Citation21]. Since the first use of PPV23 in 1983, it has been proved to be safe and effective in preventing PD in older adults [Citation22,Citation23]. At present, PPV23 is recommended for adults aged ≥60 years and for high-risk populations with underlying diseases [Citation24,Citation25]. A high vaccination rate is a prerequisite for improved vaccination efficacy. However, the vaccination rate of PPV23 was low compared to that of the national immunization program vaccine, especially in developed countries. In recent years, PPV23 has been adopted in the provincial immunization program in China [Citation22,Citation26,Citation27]; however, it was not mandatory and required self-payment. Nevertheless, Shanghai launched a major public health service project in September 2013, in which residents aged ≥60 years received free PPV23 vaccination.
The guidelines for routine pneumococcal vaccination in different European countries are not similar, especially those for healthy older adults [Citation28]. A hospital-based case-control study found that protection against PPV23 was best within 3 years after vaccination and least effective after 5 years [Citation29]. Moreover, the prevalence of Spn infection was increasing with age, indicating that PPV23 revaccination was required [Citation30]. However, revaccination with PPV23 did not boost the immune response, which was similar to the basal response. The vaccine response was low, and the possibility of adverse events increased when revaccination was conducted at an interval of 1–2 years, whereas low responses were not observed when revaccination was conducted at an interval of at least 5 years [Citation31]. A post hoc analysis conducted in Japan found that a second dose of PPV23 administered at least 5 years after the first dose was immunogenic and well tolerated in people aged ≥70 years [Citation32].
Results of previous studies on the persistence of antibodies against PPV23 have been inconsistent. One study showed a significant decrease in anti-capsular antibody production in older adults the year after the first dose of PPV23 [Citation33], whereas two studies found relatively stable vaccine efficacy within 6 and 9 years after the first dose of PPV23 [Citation34,Citation35].
This study found that the IgG GMC of 23 serotypes displayed a slow declining trend during the 5 years after the first dose of PPV23 in adults aged ≥60 years. Among them, the IgG GMC of serotype 3 was the first to decrease compared with that before vaccination, and the IgG GMC of the other 22 serotypes remained higher than that in the fifth year after vaccination. This was consistent with the results of a multicenter study that compared the tolerability and immunogenicity of PPV23 (Pneumovax 23; Merck & Co.) between the first vaccination and revaccination 3–5 years later. Immune responses to eight PPV23 serotypes were compared, and antibody concentrations remained higher than those at the baseline for serotypes 4, 6B, 8, 9 V, 12F, 14, and 23F for up to 5 years after primary vaccination. Surprisingly, one study found that the antibody concentration of serotype 3 decreased to baseline levels 1 year after vaccination. The clinical significance and causes of the faster decline in antibody concentrations of serotype 3 compared with other serotypes were unclear and might require further study [Citation36].
In this cohort study, a certain of participants were lost to follow-up or withdrew, nearly all of 23 serotypes except 2 indicated no significant difference of the baseline IgG concentrations between those who completed all visits and partial visits (), which demonstrated the missing data would not affect the conclusion based on the data from participants who completed all visits. Generally, younger adults could produce stronger immune response than older adults. Interestingly, the IgG concentration of serotypes 1,3, and 9 V in younger elderly of 60–69 years was lower than that in older group of ≥70 years old (P < 0.05), the cause for this converse results was not clear, which required further study.
A previous study indicated that PPV23 could induce a strong immune response to pneumococcal serotypes 10A, 11A, 15 B, and 17F in adults 1 month after vaccination [Citation37], whereas some studies confirmed that these serotypes were associated with increased mortality or incidence of meningitis [Citation38]. Similarly, it was observed that the increase in antibody concentration varied between serotypes after PPV23 inoculation in this study, with 9 of the 23 serotypes showing ≥ 2-fold rise in antibody concentration in S1, with five in S1–S2, three in S1–S3, and one in S1–S5. Even though nearly half of serotypes showed ≥ 2-fold rise after vaccination, the immune response remained relatively lower tested by ELISA compared with the opsonophagocytic assay (OPA) [Citation39]. The causes might be the ability of OPA having a greater differential response and the reduced efficacy of PPV23 among older adults compared with young adults [Citation40,Citation41].
In addition, the concentrations of antibodies against PPV23 serotypes 1 and 6B were significantly higher in S5 than in S4, and the factors correlated with the level of IgG antibody produced by older adults after the first dose of PPV23 were not identified yet, contrary to the results showing that adult women develop stronger IgG antibody responses than men after PPV23 vaccination. This sex difference was more pronounced in those aged ≥50 years [Citation42,Citation43].
This study has two limitations. First, approximately half of the participants missed visits or withdrew from the study because of going abroad or moving their residence. Second, Spn antibodies were detected by ELISA using two absorbents as CPS-Multi, rather than CPS and purified 22F capsular polysaccharide. Although this was a deviation from the WHO protocol, CPS-Multi could greatly improve the detection efficiency and specificity of all 23 serotypes [Citation44]. Meanwhile the functional activities of antibodies, which could reflect the protective effect of antibodies produced after vaccination, was not tested [Citation45].
5. Conclusion
This study demonstrated that PPV23 could provide long-term protection in adults aged ≥60 years, which indicated that the interval of revaccination with PPV23 in elderly should be at least 5 years after the first vaccination. Nevertheless, a cohort study with short-interval surveillance and long duration is needed to understand the long-term antibody persistence of PPV23 in older adults after the first dose to provide a reference for adjusting the immunization strategy and prevention and control measures of PPV23 in different regions.
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
A reviewer on this manuscript is currently employed by Merck & Co working in pneumococcal vaccines. Other reviewers on this manuscript received honoraria for their review work. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Author contributions
XS and XG conceived and designed the study; JL, JQ, JR and FH carried out the data analysis. RZ, FL and QC conducted the laboratory work. XL contributed to the samples collection. FH and QL drafted the initial manuscript. ZH, ZL, XG, and XS contributed to data review and manuscript revision. All authors have read and provided final approval of the submitted version of the manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article.
Supplemental Material
Download MS Word (27.8 KB)Supplemental Material
Download MS Word (32.2 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2023.2296934.
Data availability statement
The data generated and/or analyzed during this study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Park IH, Pritchard DG, Cartee R, et al. Discovery of a new capsular serotype (6C) within serogroup 6 of streptococcus pneumoniae. J Clin Microbiol. 2007;45(4):1225–1233. doi: 10.1128/JCM.02199-06
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0
- WHO. Pneumonia in children. 2022 Nov11. [cited 2023 Jul 7]. Available from: https://www.who.int/news-room/fact-sheets/detail/pneumonia
- Namkoong H, Ishii M, Funatsu Y, et al. Theory and strategy for pneumococcal vaccines in the elderly. Hum Vaccin Immunother. 2016;12(2):336–343. doi: 10.1080/21645515.2015.1075678
- O’Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6
- Sun JF, Yao HY, Yu SC, et al. Disease burden of three kinds of bacterial meningitis in China,1990 and 2010. Disease Sur. 2015;30(12):1008–1013.
- Zhang Y, Shu JD. Pneumococcal pneumonia and its vaccines. Chin J Epidemiol. 2002;23(1):78.
- Lyu S, Yao KH, Dong F, et al. Vaccine serotypes of streptococcus pneumoniae with high-level antibiotic resistance isolated more frequently seven years after the licensure of PCV7 in Beijing. Pediatr Infect Dis J. 2016;35(3):316–321. doi: 10.1097/INF.0000000000001000
- Song JH. Advances in pneumococcal antibiotic resistance. Expert Rev Respir Med. 2013;7(5):491–498. doi: 10.1586/17476348.2013.816572
- Kim SH, Song JH, Chung DR, et al. Changing trends in antimicrobial resistance and serotypes of streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother. 2012;56(3):1418–1426. doi: 10.1128/AAC.05658-11
- Beard JR, Bloom DE. Towards a comprehensive public health response to population ageing. Lancet. 2015;385(9968):658–661. doi: 10.1016/S0140-6736(14)61461-6
- Diao WQ, Shen N, Yu PX, et al. Efficacy of 23-valent pneumococcal polysaccharide vaccine in preventing community-acquired pneumonia among immunocompetent adults: a systematic review and meta-analysis of randomized trials. Vaccine. 2016;34(13):1496–1503. doi: 10.1016/j.vaccine.2016.02.023
- World Health Organization. Meeting of the immunization strategic advisory group of experts, November 2007–conclusions and recommendations. Wkly Epidemiol Rec. 2008;83(1):1–15. PMID: 18175408.
- El-Beyrouty C, Buckler R, Mitchell M, et al. Pneumococcal vaccination-a literature review and practice guideline update. Pharmacotherapy. 2022;42(9):724–740. doi: 10.1002/phar.2723
- Chen Q, Wang L, Xie M, et al. Recommendations for influenza and streptococcus pneumoniae vaccination in elderly people in China. Aging med (Milton (NSW)). 2020;3(1):1–11. doi: 10.1002/agm2.12102
- Nieminen T, Käyhty H, Virolainen A, et al. Circulating antibody secreting cell response to parenteral pneumococcal vaccines as an indicator of a salivary IgA antibody response. Vaccine. 1998;16(2–3):313–319. doi: 10.1016/S0264-410X(97)00162-X
- Zhang GH, Zheng DQ, Shi NM, et al. Pneumonia vaccination coverage and influencing factors among part of community elderly in Chaoyang District, Beijing city, China. Chin J Biol. 2013;26(1):3.
- Guo X, Qiu J, Ren J, et al. Efficacy evaluation after 5 years of inoculation of 23 valent pneumococcal polysaccharide vaccine for the elderly aged 60 years old and above in Shanghai during 2013–2018. Chi J Prev Med. 2020;54(9):923–928. doi: 10.3389/fpubh.2021.647725
- Guo X, Qiu J, Ren J, et al. Safety evaluation of mass inoculation of 23 valent pneumococcal polysaccharide vaccine among elderly people aged 60 and above in Shanghai from 2013 to 2017. Chi J Prev Med. 2020;54(9):929–933. doi: 10.3760/cma.j.cn112150-20191011-00779
- Bacterial Respiratory Pathogen Reference Laboratory. Training manual for enzyme linked immunosorbent assay for the quantitation of streptococcus pneumonia serotype specific IgG (pn PS ELISA). [cited 2023 Jul 7]. (007sp Version). Available from: https://www.vaccine.uab.edu/uploads/mdocs/ELISAProtocol(007sp).pdf
- Brian W, O’Brien KL, Adena G, et al. Burden of streptococcus pneumoniae and haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health. 2018;6(7):e744–e757. doi: 10.1016/S2214-109X(18)30247-X
- Chinese Preventive Medicine Association, vaccine and Immunology Branch of the Chinese Preventive Medicine Association. Expert consensus on immunoprophylaxis of pneumococcal disease (2020 version). Chin J Epidemiol. 2020;41(12):35.
- Falkenhorst G, Remschmidt C, Harder T, et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine (PPV23) against pneumococcal disease in the elderly: systematic review and meta-analysis. PloS one. 2017;12(1):e0169368. doi: 10.1371/journal.pone.0169368
- Qiu YP, Zhao K, Li X, et al. Health economic evaluation of a 23 value pneumococcal polysaccharide vaccination pilot programme among elderly chronic obstructive pulmonary disease patients in China. Chi J Prev Med. 2016;50(12):1074–1078. doi: 10.3760/cma.j.issn.0253-9624.2016.12.010
- Remschmidt C, Harder T, Wichmann O, et al. Effectiveness, immunogenicity and safety of 23-valent pneumococcal polysaccharide vaccine revaccinations in the elderly: a systematic review. BMC Infect Dis. 2016;16(1):711. doi: 10.1186/s12879-016-2040-y
- Nuorti JP, Whitney CG. Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR–11):1–18.
- Kohlhammer Y, Schnoor M, Schwartz M, et al. Determinants of influenza and pneumococcal vaccination in elderly people: a systematic review. Public Health. 2007;121(10):742–751. doi: 10.1016/j.puhe.2007.02.011
- Castiglia P. Recommendations for pneumococcal immunization outside routine childhood immunization programs in Western Europe. Adv Ther. 2014;31(10):1011–1044. doi: 10.1007/s12325-014-0157-1
- Shapiro ED, Berg AT, Austrian R, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325(21):1453–1460. doi: 10.1056/NEJM199111213252101
- Manoff SB, Liss C, Caulfield MJ, et al. Revaccination with a 23-valent pneumococcal polysaccharide vaccine induces elevated and persistent functional antibody responses in adults aged 65 > or = years. J Infect Dis. 2010;201(4):525–533. doi: 10.1086/651131
- Steens A, Vestrheim DF, Aaberge IS, et al. A review of the evidence to inform pneumococcal vaccine recommendations for risk groups aged 2 years and older. Epidemiol Infect. 2014;142(12):2471–2482. doi: 10.1017/S0950268814001514
- Kawakami K, Kishino H, Kanazu S, et al. Time interval of revaccination with 23-valent pneumococcal polysaccharide vaccine more than 5 years does not affect the immunogenicity and safety in the Japanese elderly. Hum Vaccin Immunother. 2018;14(8):1931–1938. doi: 10.1080/21645515.2018.1456611
- de Roux A, Schmöle-Thoma B, Siber GR, et al. Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin Infect Dis. 2008;46(7):1015–1023. doi: 10.1086/529142
- Butler JC, Breiman RF, Campbell JF, et al. Pneumococcal polysaccharide vaccine efficacy. an evaluation of current recommendations. JAMA. 1993 Oct 20;270(15):1826–1831. doi: 10.1001/jama.1993.03510150060030
- MacIntyre CR, Ridda I, Trent MJ, et al. Persistence of immunity to conjugate and polysaccharide pneumococcal vaccines in frail, hospitalised older adults in long-term follow up. Vaccine. 2019;37(35):5016–5024. doi: 10.1016/j.vaccine.2019.07.005
- Musher DM, Manof SB, Liss C, et al. Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Infect Dis. 2010;201(4):516–524. doi: 10.1086/649839
- Ciprero KL, Marchese RD, Richard P, et al. Vaccination of adults with 23-valent pneumococcal polysaccharide vaccine induces robust antibody responses against pneumococcal serotypes associated with serious clinical outcomes. Hum Vaccin Immunother. 2016;12(8):2135–2141. doi: 10.1080/21645515.2016.1156270
- Grabenstein JD, Musey LK. Differences in serious clinical outcomes of infection caused by specific pneumococcal serotypes among adults. Vaccine. 2014;32(21):2399–2405. doi: 10.1016/j.vaccine.2014.02.096
- Vernacchio L, Romero-Steiner S, Martinez JE, et al. Comparison of an opsonophagocytic assay and IgG ELISA to assess responses to pneumococcal polysaccharide and pneumococcal conjugate vaccines in children and young adults with sickle cell disease. J Infect Dis. 2000;181(3):1162–1166. doi: 10.1086/315307
- Ahn JG, Kim HW, Choi HJ, et al. Functional immune responses to twelve serotypes after immunization with a 23-valent pneumococcal polysaccharide vaccine in older adults. Vaccine. 2015;33(38):4770–4775. doi: 10.1016/j.vaccine.2015.08.002
- Park S, Nahm MH, Weiser JN. Older adults have a low capacity to opsonize pneumococci due to low IgM antibody response to pneumococcal vaccinations. Infect Immun. 2011;79(1):314–320. doi: 10.1128/IAI.00768-10
- Chiarella SE, Jenkins SM, Park MA, et al. Sex differences in antibody responses to the 23-valent pneumococcal polysaccharide vaccination. Ann Allergy Asthma Immunol. 2021;127(4):509–510. doi: 10.1016/j.anai.2021.07.013
- Parker AR, Skold M, Harding S, et al. Pneumococcal vaccination responses in adults with subnormal IgG subclass concentrations. BMC Immunol. 2019;20(1):29. doi: 10.1186/s12865-019-0310-3
- Goldblatt D, McKeen A, Burbidge P, et al. Assignment of weight-based antibody units for four additional serotypes to a human antipneumococcal standard reference serum, 007sp. Clin Vaccine Immunol. 2017 Sep 5;24(9):e00194–17. doi: 10.1128/CVI.00194-17
- LaFon DC, Nahm MH. Measuring immune responses to pneumococcal vaccines. J Immunol Methods. 2018;461:37–43. doi: 10.1016/j.jim.2018.08.002