ABSTRACT
Introduction
Different COVID-19 vaccines are being utilized as boosters. This systematic review and meta-analysis aims to evaluate the reactogenicity of COVID-19 vaccines given as booster doses, according to vaccine type, dose, timing, participant characteristics and primary immunization regimen received.
Methods
Four databases (MEDLINE, Embase, Web of Science and CENTRAL) were searched for randomized controlled trials between 1 January 2020 and 1 January 2023 according to predetermined criteria.
Results
Twenty-eight studies describing 19 vaccines of four different types (viral vector, inactivated, mRNA and protein sub-unit) were identified. BNT162b2 vaccine (Pfizer-BioNTech) was selected as the control as it was most often compared with other vaccines. Fever, fatigue, headache, injection-site pain, redness, and swelling were the most frequently reported solicited events. mRNA vaccines were the most reactogenic, followed by viral vector vaccines and protein sub-unit vaccines, while inactivated vaccines were the least reactogenic. Full-dose vaccines were more reactogenic than half-dose vaccines. Heterologous BNT162b2 boosters were more reactogenic than boosters with the same vaccine used for primary immunization.
Conclusions
COVID-19 vaccine booster schedules have distinct reactogenicity profiles, dependent on dose and vaccine type, which may allow targeted recommendations and provide choice for specific populations. Greater standardization of adverse event reporting will aid future studies.
1. Introduction
COVID-19, caused by the coronavirus SARS-CoV2, is responsible for 6.9 million deaths and 772 million cases worldwide up to 26 November 2023 [Citation1]. The rapid development and deployment of effective vaccines is estimated to have prevented 20 million deaths in their first year [Citation2]. More than 240 COVID-19 vaccine candidates, utilizing varying technologies, are in clinical trials and 50 have now been approved for use by various regulatory authorities for primary and/or booster vaccinations [Citation3,Citation4]. As COVID-19 enters an endemic state, continued vaccination is vital, certainly for many at-risk groups, and so a better understanding of the efficacy and reactogenicity of different vaccine boosters is key to decisions about their use.
COVID-19 vaccine technologies include mRNA, inactivated, viral-vector and protein subunit and we previously showed that these different platforms have unique reactogenicity profiles when used for primary vaccination. For example, mRNA vaccines were the most reactogenic and inactivated vaccines were the least reactogenic overall [Citation5]. Booster doses are now being routinely administered to adolescents and adults. In addition to use of different vaccine platforms, different combinations of vaccines (homologous and heterologous) are being used in vaccine schedules, either because of supply issues or of perceptions of benefit.
A detailed understanding of the reactogenicity of different vaccine boosters and booster schedules is important for decisions to be made about their deployment and to build public trust. There is an urgent need of robust evidence of reactogenicity for COVID booster vaccines as this information will allow policymakers to select appropriate booster schedules. Heterologous boosters have been shown to be more immunogenic than homologous boosters but to cause greater reactogenicity [Citation6–8], which may adversely affect public acceptance of booster doses. These perceptions may however differ by risk group: for example, safety of their fetus is the most important factor influencing vaccine acceptance among pregnant women [Citation9,Citation10], or while immunocompromised populations may favor higher efficacy over safety. Vaccine schedules associated with low rates of adverse events, particularly fever, are generally prioritized for use in young children [Citation11,Citation12].
We undertook a systematic review and meta-analysis, aiming to define the reactogenicity of COVID-19 vaccines when given as booster doses, with a focus on commonly reported systemic and local adverse events.
2. Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [Citation13] and registered with the PROSPERO International Prospective Register of Systematic Reviews (registration number: CRD2022381484).
2.1. Database and search terms
Three authors (ASFR, CLS, and TBR) conducted an electronic search of the PubMed/MEDLINE, Embase, Science Citation Index (Web of Science) and Cochrane Central Register of Controlled Trials (CENTRAL) databases from 1 January 2022 to 1 January 2023. These databases were selected due to their relevance and completeness and to be consistent with our previous systematic review [Citation5] The full search strategy can be found in the supplementary material (Table S1).
2.2. Selection of studies: inclusion and exclusion criteria
The search results were imported into Rayyan, a web application for systematic reviews [Citation14]. After removal of duplicate records, titles and abstracts were screened (ASFR, CLS, TBR, TM, DS, and NT). Each record was reviewed by two authors independently, excluding the ones that did not meet the inclusion criteria. Articles were deemed suitable for inclusion if they described a blinded, randomized clinical trial of a COVID-19 vaccine booster, the vaccine had reached at least phase III of clinical development, the trial included participants who had previously received an approved COVID-19 primary vaccine regimen, had an intervention and at least one comparator arm (either placebo or a different vaccine) and had assessed safety or reactogenicity. Abstracts were excluded if they described open label or non-randomized studies, pre-print or protocol only articles, primary COVID-19 vaccine schedules, vaccines that were not given intramuscularly, co-administration of COVID-19 vaccines with other vaccines, or trials where safety data were not reported.
Full-text articles were retrieved for every abstract included during the initial screening phase. The same six authors assessed the full-text articles and excluded the ones that did not meet the eligibility criteria. Disagreements were resolved by consensus between the two opposing authors and the senior author. Reference lists of included articles were reviewed to identify additional studies that met the eligibility criteria. The eligibility criteria and the rationale for inclusion/exclusion can be found in the supplementary material.
2.3. Risk of bias assessment
Each article was assessed independently and in duplicate for risk of bias by ASFR, CLS, TBR, TM, DS, and NT using the Revised Cochrane Risk of Bias Tool for randomized trials (RoB 2) [Citation15]. Disagreements were resolved by discussion between two authors or the rest of the team when a consensus was not reached. Studies that were deemed to have a high risk of bias were included in the qualitative synthesis but not in the meta-analysis.
2.4. Data extraction
Study characteristics were manually extracted and in duplicate for all full-text articles included after the screening phase: study year, phase, setting, participant characteristics (age and sex), intervention characteristics (vaccine name, type (technology), manufacturer, dose, adjuvant, primary COVID-19 vaccination regimen, timing of booster after primary vaccination), and control vaccine used. Data on reactogenicity were only extracted from studies which were deemed to have a ‘low’ or ‘some concerns’ risk of bias.
The number of participants who experienced fever, fatigue, headache, pain at injection site, redness, and swelling is extracted for each vaccine study arm. If the trials reported data on different doses of vaccines, only data related to the dose that was approved for clinical use or had reached Phase III development as of December 2023 were collected.
2.5. Missing data requested from authors
For articles which did not provide full reactogenicity data or where data were only presented in graphs, the authors were emailed to request the missing data. Two different denominators were provided by authors: number of participants receiving the booster vaccine included in the safety analysis or number of participants who completed a solicited e-diary entry; the former was used in our analysis for consistency. When data were only available in graph format, solicited adverse event data was extracted by means of a web-based plot digitizer tool (WebPlotDigitiser V4.6) [Citation16].
2.6. Statistical analysis
A descriptive analysis was performed to summarize study characteristics (percentage, frequency, and median with minimum-maximum ranges). The meta-analyses were performed using Review Manager (RevMan) version 5.4 (the Cochrane Collaboration, 2020). From the findings in our previous study [Citation5], high heterogeneity of reactogenicity between studies was anticipated for COVID-19 vaccine boosters, therefore the Mantel–Haenszel random-effects model was selected to estimate Risk Ratios (RRs) and 95% confidence intervals (CIs) for each symptom (fever, fatigue, headache, injection site pain, redness, and swelling) [Citation17]. The I2 values were presented in each individual forest plot to indicate the heterogeneity between studies. In order to have a consistent control group with which to estimate RRs between trials, we selected the BNT162b2 vaccine (Pfizer-BioNTech), as it is the most commonly used vaccine worldwide [Citation18] and was most consistently used in booster trials. The estimated RRs were performed separately for normal saline (and other) controlled trials. The subgroup meta-analyses were performed by vaccine type, but Individual vaccines were analyzed as independent subgroups if two or more datasets were available for the same vaccine dose. Low, moderate, and high heterogeneity were defined as I2 values of 25%, 50%, and 75%, respectively [Citation19]. Funnel plots were generated for each symptom to assess publication bias.
Sensitivity analysis was performed to evaluate whether inclusion of papers with high risk of bias (RoB) affected reactogenicity estimates. Forest plots were generated to include and exclude papers with high RoB to evaluate differences in heterogeneity and estimated RR.
We collected data on a range of clinically relevant variables that could potentially contribute to the heterogeneity of solicited symptoms, including participant age, ethnicity, the socio-economic status of the country in which the study was conducted (measured by Human Development Index [HDI]), primary COVID-19 vaccine schedule, homologous versus heterologous booster, vaccine technology, booster dose (full or half dose), number of vaccines received prior to booster and interval between primary schedule and booster dose. A multivariate meta-regression model using backward stepwise regression [Citation20] was conducted to evaluate the impact of these variables on the effect estimates for each symptom in the trials which had a booster BNT162b2 arm. This analysis was performed using STATA version 17 (StataCorp LLC, College Station, TX, USA).
3. Results
3.1. Literature search and risk of bias (RoB) assessment
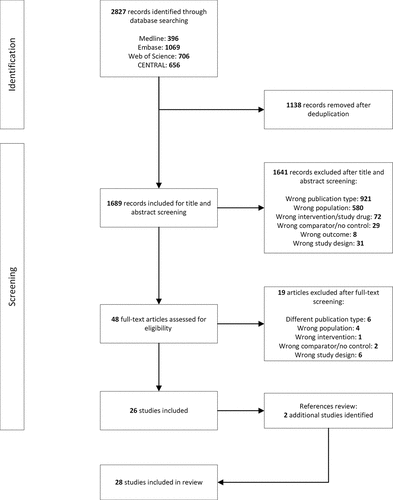
The four databases were searched from 1 January 2022 to 1 January 2023 and yielded 2827 results. After duplicates were removed, 1689 articles were screened. The PRISMA flow diagram is presented in . A total of 28 studies describing 19 vaccines met the inclusion criteria for the systematic review and underwent RoB assessment. Most studies were deemed to have a low RoB while eight had some concerns [Citation21–28] and three had a high RoB [Citation29–31]. RoB assessments for individual papers can be found in the supplement (Table S2).
3.2. Systematic review
Nineteen vaccines were included in this systematic review: three were viral vector, five inactivated, two mRNA and nine protein subunit vaccines (). BNT162b2 (Pfizer-BioNTech) was the most frequently evaluated vaccine (used in 14 studies), followed by mRNA-1273 (Moderna, 10 studies), CoronaVac (Sinovac, 6 studies), ChAdOx-1 (Oxford-AstraZeneca, 4 studies) and Ad26.COV2.S (Janssen, 4 studies). Regarding control arms, 12 studies incorporated a BNT162b2 booster arm: two studies compared a full dose (30ug) to a half dose (15 µg) [Citation30,Citation32]; BNT162b2 was compared with one other COVID-19 vaccine in five studies [Citation29,Citation31,Citation33–35], with two other COVID-19 vaccines in one study [Citation36] and with three or more vaccines in four studies [Citation26,Citation37–39]. Seven trials used 0.9% normal saline [Citation22–25,Citation27,Citation40,Citation41], one used an aluminum adjuvant + fusion protein without spike protein component [Citation28], one used an inactivated influenza vaccine [Citation42] and one used a Meningitis ACWY conjugate vaccine [Citation37] as their controls.
Table 1. Study characteristics.
All trials evaluated a first booster dose after the primary schedule except one, which studied a second booster dose [Citation34]. The time interval between primary vaccination and booster ranged from 1 to 9 months. Primary COVID-19 vaccination schedules varied among the different trials, the majority had received a primary series of an mRNA vaccine (BNT162b2 or mNRA-1273) or inactivated (CoronaVac). Most trials recruited adults over 18 years of age, one trial enrolled participants aged 16 or older [Citation25], another 21 years or older [Citation35] while two others recruited 30 year-olds and older [Citation34,Citation37]. Overall, there was balanced proportion of male to female participants among trials, although four trials had less than 30% female participants [Citation21,Citation23,Citation28,Citation31]. Trials took place across all WHO regions (), although the African region was under-represented. Ethnicity data were only reported in 10 (35.7%; 10/28) of the trials. Four trials recruited immunocompromised patients: three enrolled solid organ transplant patients [Citation22,Citation24,Citation26] and one recruited patients treated with Rituximab [Citation36].
There was considerable variability in the number of solicited adverse events reported across studies: a median of four (range 2–6) local and a median of eight (range 2–11) systemic. For this reason, analyses of solicited events focused on the six main symptoms most consistently reported: three systemic (fever, fatigue, and headache) and three local (pain at injection site, redness, and swelling).
3.3. Meta-analysis - reactogenicity of COVID-19 booster vaccines in comparison with BNT162b2 30ug booster
Five studies were excluded from the meta-analysis: three due to high RoB [Citation29–31], one due to repetition of safety data [Citation24] and another due to missing data which was not available from the authors [Citation21]. Of the 23 studies included in the meta-analysis, 15 [Citation26,Citation32–39,Citation43–48] included two or more booster vaccine arms or stratified patients according to primary COVID-19 received, therefore a total of 52 datasets were available for meta-analysis.
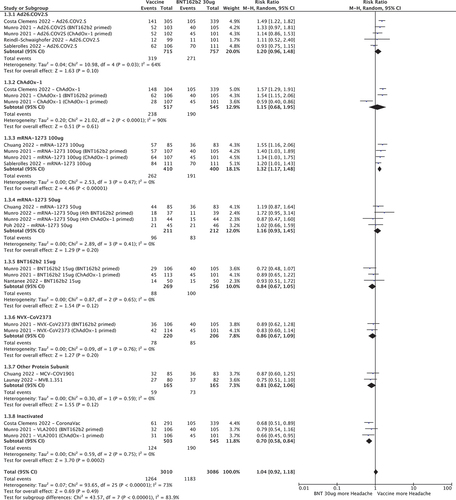
3.3.1. Fever
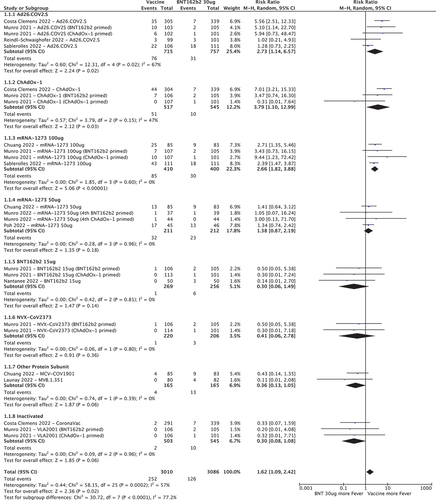
shows the RR for developing fever after a COVID-19 vaccine booster, with BNT162b2 30ug as the control group. Compared to BNT162b2 30ug, Ad26.COV2.S (RR 2.73 [95%CI 1.14–6.57]), ChAdOx-1 (RR 3.79 [95%CI 1.10–12.99]) and mRNA-1273 100ug (RR 2.66 [95%CI 1.82–3.88] had increased risk of fever whereas half-dose (15ug) BNT162b2, half-dose (50ug) mRNA-1273, NVX-CoV2373 and other protein sub-unit and inactivated vaccines had similar or lower rates.
3.3.2. Fatigue
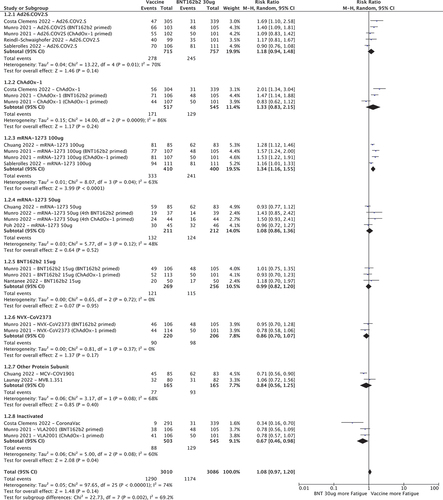
shows that mRNA-1273 100 µg (1.34 [95%CI 1.16–1.55]) had a higher RR of fatigue compared to BNT162b2 30ug. Ad26.COV2.S, ChAdOx-1, BNT162b2 15ug, mRNA-1273 50ug, NVX-CoV2373 and other protein sub-unit vaccines had similar RRs while inactivated vaccines had lower RR for fatigue (0.67 [95%CI 0.46–0.98]).
3.3.3. Headache
mRNA-1273 100ug was the only vaccine associated with increased risk of headache when compared to BNT162b2 30ug (RR 1.32 [95%CI 1.17–1.48]. Ad26.COV2.S, ChAdOx-1, BNT162b2 15ug, mRNA-1273 50ug, NVX-CoV2373 and other protein sub-unit vaccines had comparable RRs (). Inactivated vaccines were less likely to cause headache (RR 0.70 [95%CI 0.58–0.84]).
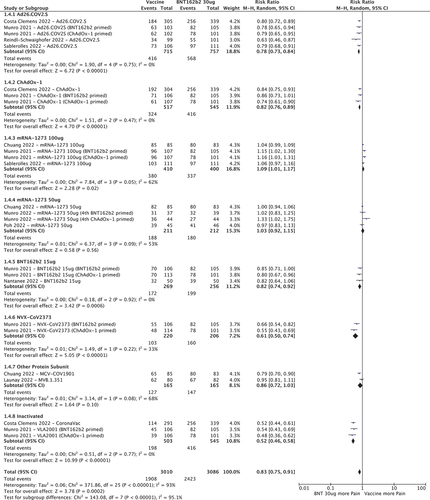
3.3.4. Pain at the injection site
Several vaccines had lower RR for pain at the injection site when compared to BNT162b2 30ug (): Ad26.COV2.S (0.78 [95%CI 0.73–0.84]), ChAdOx-1 (0.82 [95%CI 0.76–0.89]), BNT162b2 15 µg (0.82 [95%CI 0.74–0.92]), NVX-CoV2373 (0.61 [95%CI 0.50–0.74]) and inactivated vaccines (0.52 [95%CI 0.46–0.58]). mRNA-1273 100ug had an increased risk of local pain (RR 1.03 [95%CI 1.01–1.17]) while mRNA-1273 50ug and other protein subunit vaccines had similar RR compared to BNT162b2 30ug.
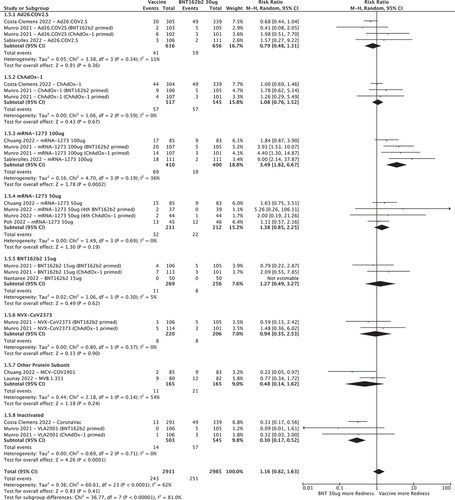
3.3.5. Redness at the injection site
mRNA-1273 100ug was the only vaccine associated with increased risk of injection site redness when compared to BNT162b2 30ug (RR 3.49 [95%CI 1.82–6.67]. Ad26.COV2.S, ChAdOx-1, BNT162b2 15ug, mRNA-1273 50ug, NVX-CoV2373 and other protein sub-unit vaccines had comparable RRs (). Inactivated vaccines were less likely to cause redness (RR 0.30 [95%CI 0.17–0.52]).
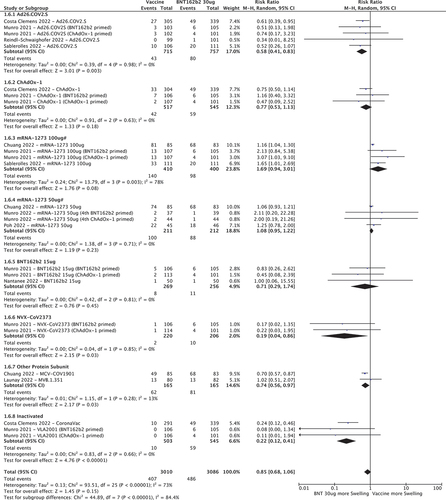
3.3.6. Swelling at the injection site
Ad26.COV2.S, NVX-CoV2373, other protein sub-unit vaccines and inactivated vaccines had lower RRs for injection site swelling (0.58 [95%CI 0.41–0.83], 0.19 [95%CI 0.04–0.86], 0.74 [95%CI 0.56–0.97] and 0.22 [95%CI 0.12–0.41] respectively) compared to BNT162b2 30ug (). ChAdOx-1, 50 and 100 µg doses of mRNA-1273 and 15ug of BNT162b2 had similar RR. Of note, although swelling was graded by most trials based on measurement of the affected area, one study [Citation39] graded according to interference with daily activities, resulting in higher swelling rates for both vaccine and control arms compared to other trials.
3.4. Meta-analysis - reactogenicity of COVID-19 vaccines against placebo, adjuvant, or other COVID-19 vaccines
Analyses of individual adverse events (fever, fatigue, headache, pain, swelling, redness) with normal saline placebo, adjuvant, or other vaccines as control groups are shown in the supplementary material (Tables S3-S8).
When compared with saline placebo, BNT162b2 30 µg and NVX-CoV2373 both had significantly raised RR for all symptoms. RRs were comparable for both vaccines, being highest for fever (34.33 [95%CI 16.21–72.71] and 34.01 [95%CI 2.07–558.16] respectively). Of note, the trial comparing BNT162b2 30ug against saline placebo [Citation25] reported unsolicited adverse event data only, based on retrospective telephone calls while other studies collected solicited adverse events prospectively through use of daily e-diaries. It is notable also that the Hannawi study [Citation23] reported rates of fever of > 10% in the normal saline control groups, resulting in lower RR estimates for the active vaccine group. In another study, mRNA-1273 50ug was compared with saline in a transplant recipient population [Citation22], with significantly raised RR for fatigue (1.74 [95%CI 1.08–2.79]), pain at injection site (6.46 [95%CI 3.18–13.13]) and swelling (18.69 [95%CI 1.11–313.97]). Several other vaccines had a raised RR for pain compared to placebo: BNT162b2 30ug (8.29 [95%CI 6.58–10.45]), NVX-CoV2373 (7.36 [95%CI 3.51–15.41]), SCTV01C 20 µg (4.75 [95%CI 1.08–20.96]) and Spikogen (4.13 [95%CI 2.17–7.83]).
Studies comparing different COVID-19 vaccines (other than BNT162b2 30 µg), showed non-significant RRs for all adverse events. Studies comparing different doses of the same vaccine generally showed that half doses were associated with lower RRs than full doses. Nanthapisal et al. [Citation45], for example, compared 0.25 ml of ChAdOx-1 against a full dose of 0.5 ml and found significantly lower RR for systemic events: fever (0.27 [95%CI 0.16–0.47]), fatigue (0.85 [95%CI 0.74–0.97], headache (0.81 [95%CI 0.69–0.95]) but no differences in RRs for local events.
3.5. Sensitivity and publication bias analysis
Data from the two studies excluded from meta-analysis due to high RoB (CoronaVac compared with BNT162b2 30ug [Citation29], BNT162b2 15ug compared with 30ug [Citation30], were included in the sensitivity analysis forest plots. Trial heterogeneity did not change for either vaccine and there were no differences in reactogenicity RR for CoronaVac. For BNT162b2 15ug, RR for fever was 0.30 (95%CI 0.06–1.49) in the main analysis and 0.23 (95%CI 0.06–0.91) in the sensitivity analysis forest plots and no differences were observed for other symptoms (Supplement Forest Plot S1–6). Publication bias analysis results can be found in the supplement (Funnel Plots S1–6); there were imbalances within individual vaccine subgroups, which may be explained by a small number of papers within each subgroup.
3.6. Meta-regression analysis
Boosters with an mRNA vaccine were independently associated with increased risks for all six adverse events (RR for fever 7.44 [95%CI 3.06–18.12], fatigue 1.70 [95%CI 1.46–1.99], headache 1.69 [95%CI 1.39–2.06], injection site pain 2.01 [95%CI 1.77–2.27], redness 7.25 [95%CI 3.52–14.97] and swelling 1.64 [95%CI 1.37–1.97]; all p < 0.0001). Similarly, boosters with a viral vector vaccine were associated with an increased risk for all events except swelling (RR for fever 8.29 [95%CI 3.51–19.57], fatigue 1.65 [95%CI 1.39–1.96], headache 1.63 [95%CI 1.39–1.92], injection site pain 1.45 [95%CI 1.30–1.62]; all p < 0.0001; and redness 2.11 [95%CI 1.16–3.79]; p = 0.013).
Compared to a half-dose booster, a full dose booster was associated with increased risk for all events except swelling (RR for fever 2.40 [95%CI 1.33–4.35], p = 0.0004; fatigue 1.37 [95%CI 1.19–1.58], p < 0.0001; headache 1.28 [95%CI 1.19–1.58], p = 0.023; injection site pain 1.30 [95%CI 1.15–1.47], p < 0.0001; and redness 2.43 [95%CI 1.17–5.08], p = 0.017). Receipt of a viral vector vaccine or receipt of a mRNA vaccine as the primary schedule were both associated with reduced risks of local pain (RR 0.73 [95%CI 0.59–0.90], p = 0.0004 and 0.65 [95%CI 0.52–0.82], p < 0.0001) while primary vaccination with inactivated vaccines was associated with increased RR for fever only (2.12 [95%CI 1.01–4.49], p = 0.048).
Receipt of a homologous booster vaccine, compared to a heterologous BNT162b2 control, was associated with lower risk of fever (RR 0.42 [95%CI 0.20–0.88], p = 0.022); fatigue (RR 0.70 [95%CI 0.57–0.87], p = 0.0006); headache (RR 0.70 [95%CI 0.58–0.84] p < 0.0001); swelling (RR 0.51 [95%CI 0.31–0.82]). Of interest, studies in which there was a higher proportion of white compared with nonwhite participants reported an increased risk of injection site pain (RR 1.01 [95%CI 1.00–1.03] p = 0.024).
4. Discussion
This systematic review and meta-analysis focuses on the reactogenicity data reported from RCTs of COVID-19 vaccines when given as booster doses, compared with either placebo, control or other COVID-19 vaccines. Other published systematic reviews and meta-analyses have reported the efficacy and safety of COVID-19 boosters [Citation49–51] but not the detailed reactogenicity, nor an analysis by subgroups of vaccine combinations and doses. Our review adds a more granular analysis of the available reactogenicity data to the literature.
There are now fewer opportunities to assess COVID-19 vaccines in the context of a placebo-controlled trial and most comparisons are made against other approved COVID-19 vaccines. In fact, 12 of the 28 trials included in this review incorporated a BNT162b2 study arm and therefore this was selected as the ‘benchmark’ control for comparison, reflecting its predominance as a booster worldwide. This makes our results clinically relevant in many locations, although where BNT162b2 is not used as a booster, results may be less interpretable for providers. Nevertheless, a range of vaccine types were included in these comparisons, allowing useful conclusions to be drawn by individuals and populations faced with a choice of booster vaccines. As COVID-19 vaccine booster doses become routine, considerations around their effectiveness and safety will continue to be made; as COVID-19 becomes less frequent, it is likely that the acceptability of booster vaccines will be increasingly influenced by their safety and reactogenicity profiles.
We observed that mRNA vaccines are the most reactogenic boosters, followed by adenovirus vector vaccines, protein vaccines and inactivated vaccines. This is consistent with our previously published meta-analysis of primary COVID vaccine regimens [Citation5] and strongly suggests a ’class effect;’ a direct link between vaccine technology and reactogenicity profile. Inactivated vaccines were the least reactogenic across all adverse events included in the analysis. Adenovirus vector vaccines were associated with higher rates of fever, but lower rates of pain than the benchmark BNT162b2 30 ug vaccine, while protein sub-unit vaccines generally had lower rates of adverse events, especially of pain and swelling at the injection site. It is not clear which of the adverse events assessed are of most concern to vaccinees.
A dose-dependent effect on reactogenicity was seen for all vaccines where data for different doses were available (mRNA and ChAdOx-1 vaccines), with higher doses associated with more frequent reactions. This is consistent with immune responses, which are also generally proportional to the dose given. We consider the presence of this effect to add external validity to our study. It is notable that the half dose (50ug) of mRNA-1273 has reactogenicity which is comparable to the standard dose (30 ug) of BNT162b2, suggesting that reactogenicity has a common mechanism across mRNA vaccines.
The results from meta regression analysis were generally comparable to sub-group meta-analysis, where differences between vaccine type, individual vaccines and doses were apparent. Our meta-regression also demonstrated that receiving a booster dose of a homologous vaccine was associated with lower risks of solicited symptoms compared to receipt of a heterologous BNT162b2 booster. Our results add significantly more detail to the findings of a previous systematic review and meta-analysis of homologous compared to heterologous COVID-19 vaccine boosters in which fever, fatigue, myalgia, and ‘any systemic reaction’ were more frequently reported after a heterologous booster [Citation51]. Compared with this, our study has additional data sets which gives increased certainty regarding this association and allows us to add detail. Decision makers may find this information useful when recommending vaccine schedules. A consistent theme from surveys of those who are hesitant about receiving COVID-19 vaccines is wanting more information about side effects [Citation52]. Studies such as this one aggregate disparate trial data and give more specific and granular side-effect data which may aid academics and clinicians in addressing those concerns with the general public. It may also allow targeted recommendations to be made, for example, for groups with greater concerns about adverse events such as fever (e.g. pregnant women) or injection site pain (e.g. children), it may be possible to identify specific vaccines that minimize these unwanted effects.
5. Limitations
All studies included in this review evaluated booster doses of the ‘original’ formulations of COVID-19 vaccines. More recently, bivalent vaccines targeting the Omicron variant have been developed [Citation53,Citation54]; no suitable randomized trials of these vaccines had been published at the time of our literature search. We attempted to gather missing reactogenicity data not reported in the included publications by contacting the corresponding authors, but we did not receive responses from all authors. Three papers were excluded from the main analysis due to high risk of bias; this approach may have led to selection bias. However, sensitivity analysis did not show any significant changes when these papers were added to the analysis.
We pooled reactogenicity information on aggregate data instead of patient-level data for meta-regression analyses. We acknowledge the statistically significant risk of reactogenicity and COVID-19 booster vaccines may not necessary reflect the true association in our findings. This is a common limitation for meta-regression in systematic review studies [Citation55]. To overcome this, we suggest that future research should use individual patient-level data for causality assessment for COVID-19 vaccines and reactogenicity. Publication bias analysis showed asymmetric funnel plots for redness and swelling, this may either due to small study number or data for these side effects were not available or not reported. In addition, several trials were sponsored by pharmaceutical companies; sponsored studies might be biased toward only publishing significant results via selected reporting [Citation56].
Finally, we selected several clinical variables for inclusion in our meta-regression model-based clinical relevance to COVID-19 disease epidemiology and vaccine safety as well as consistency of being reported by the manuscripts. There may be other confounding factors contributing to the heterogeneity that were not included in our model.
The majority of trials recruited adults over 18 years of age and therefore conclusions cannot be drawn about booster doses for children and adolescents. Similarly, there was limited data available for specific patient groups such as immunocompromised patients, while pregnant women were excluded from trial protocols. Although trials took place across all continents, resulting in a mix of ethnicities among participants, the ability to draw conclusions about whether ethnicity has an impact on reactogenicity was limited, due to over-representation of white Caucasian participants in the studies. Future trials should encourage diverse participant inclusion and expand the inclusion criteria for underrepresented patient groups. In addition, the safety and reactogenicity profile of newer bivalent COVID-19 boosters being employed as fourth or subsequent doses needs further characterization.
As with our previous review [Citation5], there was great variability in the number of solicited adverse events reported by the trials and so we focused on those symptoms most consistently reported. The lack of standardization of COVID-19 vaccine trials and the way data are reported make comparisons challenging. Greater standardization would facilitate more conclusive comparisons and should now be required of all studies. Finally, we have reported only reactogenicity, clearly decisions around choice of vaccines will also consider their effectiveness in preventing COVID-19.
6. Conclusions
Among COVID-19 vaccines currently available as booster doses, the four vaccine types (platforms) appear to have distinct reactogenicity profiles. mRNA vaccines are the most reactogenic, while the inactivated vaccines are the least reactogenic. Recommendations for COVID-19 vaccination need to balance multiple factors including clinical efficacy against circulating variants, reactogenicity, potential serious adverse events, availability and cost. Awareness of the reactogenicity profiles of different vaccine types may allow different vaccines to be recommended for specific clinical scenarios and, more generally, the availability of such detailed information can bolster public confidence in vaccination.
7. Expert opinion
The current COVID-19 vaccine trials have shifted their original focus on safety and efficacy of doses in unvaccinated participants to booster studies (third, fourth or subsequent doses) in those previously vaccinated. The adverse events reported from vaccine trials are still very heterogeneous, despite international efforts to unify the definitions such as through the Brighton collaboration [Citation57]. More effort should be made to standardize COVID-19 vaccine trial design: the choice of control, follow-up duration, definitions of adverse events and which to report, methods for collecting event data and choice of denominator for calculating adverse event rates.
Ultimately, the choice of COVID-19 vaccine will need to consider multiple factors, including reactogenicity, frequency of rare and serious adverse events, efficacy against circulating COVID-19 variant strains, availability of doses and costs.
It is likely that studies of the reactogenicity of COVID-19 vaccines as booster doses, as part of heterologous schedules and in other important groups such as children, immunocompromised populations and pregnant women will become more common over the next 5 years.
Article highlights
The majority of booster vaccine trials compared COVID-19 vaccines against each other; placebo-controlled trials were rare.
Four main vaccine types were used as booster doses: mRNA vaccines were most reactogenic, followed by viral vector vaccines, protein sub-unit vaccines and inactivated vaccines.
Of the mRNA vaccines, 100ug (full dose) of mRNA-1273 was more reactogenic than 30ug of BNT162b2, but comparable when used at half-dose (50ug).
Compared to BNT162b2 (30ug), adenovirus-vector vaccines had an increased rate of fever but a lower rate of local adverse events while protein sub-unit vaccines had lower rates of pain and swelling at the injection site.
Inactivated vaccines had lower risks of local and systemic adverse events compared to BNT162b2.
Homologous boosters with the same vaccine received for primary immunization were less reactogenic than heterologous BNT162b2 boosters.
Reporting of solicited adverse events on vaccine trials is inconsistent, with differences in number and type of symptoms reported. This lack of standardization of reporting makes comparison of different vaccines challenging.
Vaccines with lower rates of reactogenicity may be preferable in specific settings and populations.
Declaration of interest
PT Heath coordinates research on behalf of St Georges, University of London, which is funded by vaccine manufacturers, including those that manufacture COVID-19 vaccines (Pfizer, AZ, Novavax, Moderna, Valneva, Janssen). He is also a member of the UK Joint Committee on Vaccination and Immunization (JCVI). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have received honoraria for their review work. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Author contributions
ASFR, PH, YH, DS, TBR and CLS conceptualized and designed the work. ASFR, TBR and CLS carried out the database search and identified eligible studies. ASFR, DS, TBR, TM, NT and CLS performed risk of bias assessments and data extraction. Tables and figures were created by ASFR. Data analysis and interpretation was performed by ASFR, guided by JH and PH. ASFR and PH drafted the manuscript. All authors contributed to, reviewed, and approved the final manuscript.
Supplemental Material
Download Zip (4.6 MB)Acknowledgments
We thank the library team at St George’s University of London for their input during the data search.
We would like to thank the core members of the COV-BOOST trial team (Saul Faust, Xinxue Liu, Victoria Cornelius, Leila Janani, Alasdair P. S. Munro) and the Oxford Vaccine Group team (Sue Ann Costa Clemens, Andrew J. Pollard) for facilitating access to adverse event data from their trials to be included in this review.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2024.2315089
Data availability statement
Study data is available via reasonable requests directed to the corresponding author.
Additional information
Funding
References
- World Health Organisation. WHO Coronavirus (COVID-19) Dashboard [Internet]. [cited 2023 Apr 26]. Available from: https://covid19.who.int/
- Watson OJ, Barnsley G, Toor J, et al. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22(9):1293–1302. doi: 10.1016/S1473-3099(22)00320-6
- World Health Organisation. COVID-19 vaccine tracker and landscape [internet]. [cited 2023 Apr 26]. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- UNICEF. COVID-19 Market Dashboard [Internet]. [cited 2023 Apr 26]. Available from: https://www.unicef.org/supply/covid-19-market-dashboard
- Sutton N, San Francisco Ramos A, Beales E, et al. Comparing reactogenicity of COVID-19 vaccines: a systematic review and meta-analysis. Expert Rev Vaccines. 2022;21(9):1301–1318. doi: 10.1080/14760584.2022.2098719
- Shaw RH, Stuart A, Greenland M, et al. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397(10289):2043–2046. doi: 10.1016/S0140-6736(21)01115-6
- Atmar RL, Lyke KE, Deming ME, et al. Homologous and heterologous COVID-19 booster vaccinations. N Engl J Med. 2022;386(11):1046–1057. doi: 10.1056/NEJMoa2116414
- Nordström P, Ballin M, Nordström A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic covid-19 infection in Sweden: a nationwide cohort study. Lancet Reg Health Eur. 2021;11:100249. doi: 10.1016/j.lanepe.2021.100249
- Goncu Ayhan S, Oluklu D, Atalay A, et al. COVID‐19 vaccine acceptance in pregnant women. Int J Gynecol Obst. 2021;154(2):291–296. doi: 10.1002/ijgo.13713
- Patterson L, Berry E, Parsons C, et al. Using the COM-B framework to elucidate facilitators and barriers to COVID-19 vaccine uptake in pregnant women: a qualitative study. BMC Pregnancy Childbirth. 2023;23(1):640. doi: 10.1186/s12884-023-05958-y
- Tse A, Tseng HF, Greene SK, et al. Signal identification and evaluation for risk of febrile seizures in children following trivalent inactivated influenza vaccine in the vaccine safety datalink project, 2010–2011. Vaccine. 2012;30(11):2024–2031. doi: 10.1016/j.vaccine.2012.01.027
- Duffy J, Hambidge SJ, Jackson LA, et al. Febrile seizure risk after vaccination in children one to five months of age. Pediatr Neurol. 2017;76:72–78. doi: 10.1016/j.pediatrneurol.2017.08.005
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4
- Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019:l4898. doi: 10.1136/bmj.l4898
- Rohatgi A. WebPlotdigitizer [Internet]. [cited 2023 Apr 26]. Available from: https://automeris.io/WebPlotDigitizer
- Higgins JPT, Thomas J, Chandler J, (editors). Cochrane handbook for systematic reviews of interventions version 6.4 (updated August 2023). Cochrane; 2023.
- Mathieu E, Ritchie H, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19) [internet]. Our World In Data. 2020 [cited 2023 Apr 26]. Available from: https://ourworldindata.org/coronavirus
- Higgins JPT. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557
- Chowdhury MZI, Turin TC. Variable selection strategies and its importance in clinical prediction modelling. Fam Med Com Health. 2020;8(1):8. doi: 10.1136/fmch-2019-000262
- Ahi M, Hamidi Farahani R, Basiri P, et al. Comparison of the safety and immunogenicity of FAKHRAVAC and BBIBP-CorV vaccines when administrated as booster dose: a parallel two arms, randomized, double blind clinical trial. Vaccines (Basel) [Internet]. 2022;10(11):1800. doi: 10.3390/vaccines10111800
- Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med [Internet]. 2021;385(13):1244–1246. doi: 10.1056/NEJMc2111462
- Hannawi S, Saifeldin L, Abuquta A, et al. Safety and immunogenicity of a bivalent SARS-CoV-2 protein booster vaccine, SCTV01C, in adults previously vaccinated with mRNA vaccine: a randomized, double-blind, placebo-controlled phase 1/2 clinical trial. EBioMedicine [Internet]. 2023;87:104386. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2352396422005680
- Kumar D, Ferreira VH, Hall VG, et al. Neutralization of SARS-CoV-2 variants in transplant recipients after two and three doses of mRNA-1273 vaccine. Ann Intern Med [Internet]. 2022;175(2):226–233. doi: 10.7326/M21-3480
- Moreira ED, Kitchin N, Xu X, et al. Safety and efficacy of a third dose of BNT162b2 covid-19 vaccine. N Engl J Med [Internet]. 2022;386(20):1910–1921. doi: 10.1056/NEJMoa2200674
- Reindl-Schwaighofer R, Heinzel A, Mayrdorfer M, et al. Comparison of SARS-CoV-2 antibody response 4 weeks after homologous vs heterologous third vaccine dose in kidney transplant recipients. JAMA Intern Med [Internet]. 2022;182(2):165. doi: 10.1001/jamainternmed.2021.7372
- Tabarsi P, Anjidani N, Shahpari R, et al. Immunogenicity and safety of spikoGen®, an adjuvanted recombinant SARS‐CoV‐2 spike protein vaccine as a homologous and heterologous booster vaccination: a randomized placebo‐controlled trial. Immunology [Internet]. 2022;167(3):340–353. doi: 10.1111/imm.13540
- Wang XY, Mahmood SF, Jin F, et al. Efficacy of heterologous boosting against SARS-CoV-2 using a recombinant interferon-armed fusion protein vaccine (V-01): a randomized, double-blind and placebo-controlled phase III trial. Emerg Microbes Infect. 2022;11(1):1910–1919. doi: 10.1080/22221751.2022.2088406
- Mok CKP, Chen C, Yiu K, et al. A randomized clinical trial using CoronaVac or BNT162b2 vaccine as a third dose in adults vaccinated with two doses of CoronaVac. Am J Respir Crit Care Med [Internet]. 2022;205(7):844–847. doi: 10.1164/rccm.202111-2655LE
- Intapiboon P, Seepathomnarong P, Ongarj J, et al. Immunogenicity and safety of an intradermal BNT162b2 mRNA vaccine booster after two doses of inactivated SARS-CoV-2 vaccine in healthy population. Vaccines (Basel) [Internet]. 2021;9(12):1375. doi: 10.3390/vaccines9121375
- Shinkai M, Sonoyama T, Kamitani A, et al. Immunogenicity and safety of booster dose of S-268019-b or BNT162b2 in Japanese participants: an interim report of phase 2/3, randomized, observer-blinded, noninferiority study. Vaccine [Internet]. 2022;40(32):4328–4333. doi: 10.1016/j.vaccine.2022.06.032
- Nantanee R, Jantarabenjakul W, Jaru-Ampornpan P, et al. A randomized clinical trial of a fractional low dose of BNT162b2 booster in adults following AZD1222. Vaccines (Basel) [Internet]. 2022;10(6):914. doi: 10.3390/vaccines10060914
- Launay O, Cachanado M, Luong Nguyen LB, et al. Immunogenicity and safety of beta-adjuvanted recombinant booster vaccine. N Engl J Med [Internet]. 2022;387(4):374–376. doi: 10.1056/NEJMc2206711
- Munro APS, Feng S, Janani L, et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): a multicentre, blinded, phase 2, randomised trial. Lancet Infect Dis [Internet]. 2022;22(8):1131–1141. doi: 10.1016/S1473-3099(22)00271-7
- Poh XY, Tan CW, Lee IR, et al. Antibody response of heterologous vs homologous messenger RNA vaccine boosters against the severe acute respiratory syndrome coronavirus 2 omicron variant: interim results from the PRIBIVAC study, a randomized clinical trial. Clinl Infect Dis [Internet]. 2022;75(12):2088–2096. doi: 10.1093/cid/ciac345
- Bonelli M, Mrak D, Tobudic S, et al. Additional heterologous versus homologous booster vaccination in immunosuppressed patients without SARS-CoV-2 antibody seroconversion after primary mRNA vaccination: a randomised controlled trial. Ann Rheum Dis [Internet]. 2022;81(5):687–694. doi: 10.1136/annrheumdis-2021-221558
- Munro APS, Janani L, Cornelius V, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet [Internet]. 2021;398(10318):2258–2276. doi: 10.1016/S0140-6736(21)02717-3
- Costa Clemens SA, Weckx L, Clemens R, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet [Internet]. 2022;399(10324):521–529. doi: 10.1016/S0140-6736(22)00094-0
- Chuang C-H, Huang C-G, Huang C-T, et al. Titers and breadth of neutralizing antibodies against SARS-CoV-2 variants after heterologous booster vaccination in health care workers primed with two doses of ChAdOx1 nCov-19: a single-blinded, randomized clinical trial. J Clin Virol [Internet]. 2022;157:105328. doi: 10.1016/j.jcv.2022.105328
- Mallory RM, Formica N, Pfeiffer S, et al. Safety and immunogenicity following a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373): a secondary analysis of a randomised, placebo-controlled, phase 2 trial. Lancet Infect Dis [Internet]. 2022;22(11):1565–1576. doi: 10.1016/S1473-3099(22)00420-0
- Zhang Y, Ma X, Yan G, et al. Immunogenicity, durability, and safety of an mRNA and three platform-based COVID-19 vaccines as a third dose following two doses of CoronaVac in China: a randomised, double-blinded, placebo-controlled, phase 2 trial. EClinicalMedicine [Internet]. 2022;54:101680. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2589537022004102
- Jin P, Guo X, Chen W, et al. Safety and immunogenicity of heterologous boost immunization with an adenovirus type-5-vectored and protein-subunit-based COVID-19 vaccine (convidecia/ZF2001): a randomized, observer-blinded, placebo-controlled trial. Beeson JG, editor. PLoS Med [Internet]. 2022;19(5):e1003953. doi: https://doi.org/10.1371/journal.pmed.1003953
- Al Kaabi N, Yang YK, Du LF, et al. Safety and immunogenicity of a hybrid-type vaccine booster in BBIBP-CorV recipients in a randomized phase 2 trial. Nat Commun [Internet]. 2022;13(1):3654. doi: 10.1038/s41467-022-31379-0
- Li J, Hou L, Guo X, et al. Heterologous AD5-nCOV plus CoronaVac versus homologous CoronaVac vaccination: a randomized phase 4 trial. Nat Med [Internet]. 2022;28(2):401–409. doi: 10.1038/s41591-021-01677-z
- Nanthapisal S, Puthanakit T, Jaru-Ampornpan P, et al. A randomized clinical trial of a booster dose with low versus standard dose of AZD1222 in adult after 2 doses of inactivated vaccines. Vaccine [Internet]. 2022;40(18):2551–2560. doi: 10.1016/j.vaccine.2022.03.036
- Omma A, Batirel A, Aydin M, et al. Safety and immunogenicity of inactive vaccines as booster doses for COVID-19 in Türkiye: a randomized trial. Hum Vaccin Immunother [Internet]. 2022;18(6). doi: 10.1080/21645515.2022.2122503
- Sablerolles RSG, Rietdijk WJR, Goorhuis A, et al. Immunogenicity and reactogenicity of vaccine boosters after Ad26.COV2.S Priming. N Engl J Med [Internet]. 2022;386(10):951–963. doi: 10.1056/NEJMoa2116747
- Zhang Z, He Q, Zhao W, et al. A heterologous V-01 or variant-matched bivalent V-01D-351 booster following primary series of inactivated vaccine enhances the neutralizing capacity against SARS-CoV-2 delta and omicron strains. J Clin Med. 2022;11(14):11. doi: 10.3390/jcm11144164
- Graña C, Ghosn L, Evrenoglou T, et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst Rev. John Wiley and Sons Ltd; 2022.
- Petrelli F, Luciani A, Borgonovo K, et al. Third dose of SARS-CoV-2 vaccine: a systematic review of 30 published studies. J Med Virol. 2022;94(6):2837–2844. doi: 10.1002/jmv.27644
- Deng J, Ma Y, Liu Q, et al. Comparison of the effectiveness and safety of heterologous booster doses with homologous booster doses for SARS-CoV-2 vaccines: a systematic review and meta-analysis. Int J Environ Res Public Health MDPI. 2022;19(17):10752. doi: 10.3390/ijerph191710752
- Office for National Statistics (ONS). COVID-19 vaccine refusal, UK: February to March 2021. [Internet] 2021 [cited 2023 Dec 18]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandwellbeing/bulletins/covid19vaccinerefusaluk/februarytomarch2021#main-themes-for-concern-about-or-unwillingness-to-receive-a-covid-19-vaccine
- Mayr FB, Talisa VB, Shaikh O, et al. Effectiveness of homologous or heterologous COVID-19 boosters in veterans. N Engl J Med. 2022;386(14):1375–1377. doi: 10.1056/NEJMc2200415
- Chalkias S, Harper C, Vrbicky K, et al. A bivalent omicron-containing booster vaccine against COVID-19. N Engl J Med. 2022;387(14):1279–1291. doi: 10.1056/NEJMoa2208343
- Thompson SG, Higgins JPT. How should meta‐regression analyses be undertaken and interpreted? Stat Med. 2002;21(11):1559–1573. doi: 10.1002/sim.1187
- Melander H, Ahlqvist-Rastad J, Meijer G, et al. Evidence b(i)ased medicine–selective reporting from studies sponsored by pharmaceutical industry: review of studies in new drug applications. BMJ [Internet]. 2003;326(7400):1171–1173. Available from: https://www.bmj.com/lookup/doi/10.1136/bmj.326.7400.1171
- Bonhoeffer J, Kohl K, Chen R, et al. The brighton collaboration: addressing the need for standardized case definitions of adverse events following immunization (AEFI). Vaccine. 2002;21(3–4):298–302. doi: 10.1016/S0264-410X(02)00449-8