ABSTRACT
Introduction
Transmissible vaccines offer a novel approach to suppressing viruses in wildlife populations, with possible applications against viruses that infect humans as zoonoses – Lassa, Ebola, rabies. To ensure safety, current designs propose a recombinant vector platform in which the vector is isolated from the target wildlife population. Because using an endemic vector creates the potential for preexisting immunity to block vaccine transmission, these designs focus on vector viruses capable of superinfection, spreading throughout the host population following vaccination of few individuals.
Areas covered
We present original theoretical arguments that, regardless of its R0 value, a recombinant vaccine using a superinfecting vector is not expected to expand its active infection coverage when released into a wildlife population that already carries the vector. However, if superinfection occurs at a high rate such that individuals are repeatedly infected throughout their lives, the immunity footprint in the population can be high despite a low incidence of active vaccine infections. Yet we provide reasons that the above expectation is optimistic.
Expert Opinion
High vaccine coverage will typically require repeated releases or release into a population lacking the vector, but careful attention to vector choice and vaccine engineering should also help improve transmissible vaccine utility.
1. Introduction
Vaccines have been immensely successful in suppressing infections transmitted between humans or infections transmitted between domestic animals. Vaccines are typically administered to the patient individually and have no lifespan beyond the patient. In principle, however, vaccines could transmit. Many vaccines (live attenuated virus vaccines in particular) are merely modified versions of the disease agents themselves [Citation1–3]. Since the disease agents transmit, transmission of the attenuated vaccine might also be possible. This is the case for the live attenuated Oral Polio Vaccine developed by Sabin, which was found to spread from vaccinated to naïve individuals [Citation4–6].
Genetic engineering and advances in virology have advanced the concept of a transmissible vaccine from a mere accident to a possibility that may be intentionally designed and potentially deployed. In the early 2000s, an Australian effort was initiated to engineer a sterilizing cytomegalovirus to transmit in mouse populations and thereby suppress reproduction on a large geographic scale [Citation7–10]. A vaccine was engineered but never released. At a similar time, an effort in Spain aimed to protect wild rabbits from two diseases by using a transmissible vaccine from an attenuated myxomavirus backbone engineered to carry a gene from rabbit hemorrhagic fever. This vaccine was released and shown to transmit on an island population [Citation11]. Recent efforts to develop an Ebola vaccine have used a platform that could provide transmission [Citation10,Citation12,Citation13].
Heightened public and scientific awareness of possible unintended consequences of genetic engineering has changed the political landscape for transmissible vaccine development [Citation14,Citation15]. Initially, the major issue was the technical feasibility of developing a vaccine that could both transmit and immunize. Now, however, a major concern is safety – prevention of vaccine evolution and uncontrollable escape that might harm rather than help. Thus, anticipated deployments of transmissible vaccines are limited to suppress infections in wildlife rather than in humans. In theory, wildlife vaccines could provide indirect protection to humans by reducing or eliminating zoonotic infections such as Lassa, Ebola, and rabies in the animal reservoir. Even here, safety warrants use of a vaccine virus that is avirulent in the wildlife population and unable to spread to humans and to non-target wildlife – thus excluding most applications of attenuated vaccines, for example.
One approach is to use a ‘transferable’ vaccine; in this design the vaccine infects only those who ingest it, and the method for vaccine spread is to apply it to wildlife individuals that will facilitate vaccine ingestion through their contacts (e.g [Citation16]). Our focus is instead on vaccines that can transmit – infect one individual which then transmits to and infects others, creating chains of transmission in the wildlife population [Citation10,Citation17–19]. While a transmissible vaccine raises more potential for unintended consequences than does a transferable vaccine, we will suggest that many proposals for transmissible vaccines have an intrinsic and previously unappreciated element of safety because, despite their transmission, they are unlikely to expand in the host population.
A transmissible vaccine design currently favored for its inherent safety is a recombinant vector platform consisting of two parts: a benign virus already endemic in the host population (the vector) and genetic material from the pathogen that elicits immunity (the antigenic ‘insert’) [Citation20]. Recombinant-vector vaccines using this design are proposed to be safe for several reasons. First, the vector is intentionally chosen as a nonpathogenic virus already established in the target population. Second, containment of the vaccine to its intended species can be ensured by selecting a vector already known to be confined to that species. Third, the insertion of a new gene encoding the vaccine antigen may invoke a fitness cost in the vector virus. (The vaccine antigen will need to be chosen to avoid increasing vaccine virulence or immune escape.) Consequently, the most likely path of any vaccine evolution is to eliminate expression of the insert, either through deletion or downregulation, converting the vaccine virus back to wild-type vector, which is already known to be benign and present in the host population.
But will recombinant vector vaccines work as hoped – transmit and immunize? Mathematical analyses have shown that an attenuated-virus vaccine released into a host population with the wild-type virus is expected to die out from competition with wild-type pathogen if the vaccine is in any way inferior [Citation17]. Similar considerations apply to some types of recombinant vector vaccines [Citation21]. However, vaccines using vectors that can superinfect present an interesting possibility: superinfection should enable the vaccine to transmit in the host population because of the vaccine’s ability to infect hosts that already harbor the wild-type vector [Citation7,Citation12,Citation13,Citation21]. This superinfection rationale – along with other desirable properties – has motivated designs using superinfecting vectors such as cytomegalovirus and other beta herpesviruses [Citation9,Citation10,Citation22–25].
The first main point of our paper is to argue that a superinfecting vector does not endow the vaccine with any special potential for expansion in a population where the wild-type vector is already established – on average, vaccine presence (in terms of active infections) will at best remain at its introduced level because of competition with the wildtype vector. The second main point is that vector (and thus vaccine) superinfection can offer an advantage in enabling a large footprint of latent infections and thus of immunity, but only if the rate of reinfection is high relative to host lifespan. There are thus two properties that contribute to the coverage achieved by a superinfecting vaccine: active vaccine level in the host population and repeated infection of the same host. When released into the very population from which the vector was isolated, there is a strong a priori expectation that any vectored vaccine will be self-contained and not expand – neutral at best, but typically disappearing without repeated or ongoing vaccine releases. Such containment may limit vaccine efficacy, but it also promotes safety – the vaccine should not escape. Instead, to ensure a large footprint of immunity, vector choice should focus on the rate of reinfection, not superinfection per se. We will emphasize, however, that the expectation of containment is theoretical and applies strictly to a vaccine released into the population already containing the vector strain used in the vaccine.
This paper focuses on the ecological and population framework underlying recombinant vector vaccine spread in a population. Although vaccine spread will depend on many details specific to the vaccine, there are basic population principles that transcend all details and put limits on vaccine spread. Most of our arguments are presented verbally, but they have mathematical bases, often provided in supplements. For convenience, we use (wild-type) ‘vector’ to denote the virus isolated from the host population that is used as the vaccine’s genetic backbone and use ‘vaccine’ to denote the engineered vector carrying the antigen-gene. We will refer to the ‘pathogen’ as the agent against which the vaccine is designed, regardless of whether the pathogen actually causes harm in the wildlife reservoir.
1.1. Caveats and qualifiers
This paper should not be construed to advocate for or against transmissible vaccines. They are controversial because of safety concerns: they combine genetic engineering with self-replication and transmission. They thus have a greater-than-average potential for unintended consequences and for evolution to change the dynamics unpredictably. Transmissible vaccines also raise ethical concerns, especially so when the vaccine may infect humans. Arguments on different sides of the controversy are sometimes presented here for context, but the focus of this paper is specifically to challenge the supposition that, using current design proposals, transmissible vaccines will have the intended consequences of spreading throughout the host population. Our arguments thus identify a reason to question their utility, not their safety or ethics, albeit there are safety implications of a vaccine with limited utility. Indeed, the decision to release a transmissible vaccine will be based on the balance of positives and negatives. Vaccine spread in the host population is key to both.
We offer what may be thought of as a kind of null model for transmissible vaccine behavior – idealized in many ways, but a starting point to inspire further investigations. Thus, the models here neglect evolution and recombination. We accept that transmissible vaccines may evolve and that their evolution may be difficult to predict until we have more experience. Likewise, release of a vaccine into the population endemic for the vector virus will often lead to recombination between the vaccine and vector, leading to unintended changes in the vaccine. But our purpose is to consider whether a transmissible vaccine taken at face value, thus endowed with the hoped-for transmission and immunization properties, will have the expected outcome of immunizing a population. Evaluating this null model should be an essential step in predicting the consequences of a vaccine release. Vaccine evolution and recombination is not being relied upon to attain that goal. Only when we understand vaccine behavior in the absence of evolution and recombination is there motivation to consider deviations from ideal behavior.
2. Population neutrality is the baseline expectation for a recombinant vaccine – in a population with the vector
This section establishes one main point of the paper: under current plans, competition of a vaccine with its wild-type vector will be the principal factor that limits its expansion in the host population. This expectation is based on first principles and applies when introducing a vectored vaccine into the population from which the vector was isolated. The vaccine will transmit to other hosts in the population, but any single introduction of vaccine will not result in a tendency for the vaccine to increase or decrease its level of active infections beyond that at which it was introduced. The vaccine will be intrinsically contained through competition with the wild-type vector, just as the vector contains itself at steady state – the infection will spread to some individuals and be lost from or suppressed in others with no net growth of active infections. Later sections present factors that can modify this expectation up or down.
2.1. Vaccine equivalence means that the vaccine can do no better than the wild-type vector
Two concepts are essential to understanding the arguments here, and they are easily confounded. One concept is based on a relative comparison of the growth properties of vector and vaccine viruses: for many arguments and models, we will assume equivalence of vector and vaccine growth properties. That means the vaccine transmits as if it is an unaltered wild-type virus. This is a best case for a vaccine, as the engineering is likely to at least slightly impair its growth, and host immunity against the antigenic insert may also slow vaccine transmission. But the best case is easiest for developing understanding. The second concept addresses whether the transmissible vaccine declines, expands, or maintains a constant population abundance. We will refer to vaccine population neutrality to indicate the case where the vaccine maintains a constant proportion of active infections in the population (on average). Lipsitch et al. have considered the relationships between viral infection properties and neutrality in several different contexts; their application and definitions differ somewhat from those here [Citation26].
In epidemiology, the reproductive number R is a measure that describes the growth of individuals, which in our case is the spread of the virus in a population [Citation27]. R is the average number of secondary infections arising from one infected individual. When a typical virus is first introduced into a naïve population, its growth is denoted by the special term R0, known as the basic reproductive number [Citation28,Citation29]. R0 applies specifically to the hypothetical case of a single infected individual in a naïve population: the number of secondary infections it creates. As the infection spreads, the number of susceptible hosts declines, and viral spread slows from the ensuing shortage of suitable hosts. Ultimately, viral expansion stops when an infected host is only able to create one new infection, R = 1. This limit on viral expansion may have many causes: host immunity, altered host behavior affecting transmission, saturation of virus-compatible sites within the host, or elevated host death (which we assume is absent in this case). The value of R0 does not change during the epidemic because it is defined for a fully naïve population, but R does change. R = 1 does not mean that the vaccine stops transmitting, only that the number of active infections are no longer increasing. The identity of who is infected continues to change as some hosts clear the infection (or go latent), others gain infection; host births and deaths also contribute to changes in infection identities.
At this steady state of R = 1, any vector or equivalent vaccine released into the population will also be subject to R = 1. It will transmit but not tend to increase its abundance – it will exhibit population neutrality on average (it is to be expected that random, temporal and spatial variation will affect the specifics somewhat). In technical terms, the ‘force of infection’ will remain the same. Thus, a transmissible vaccine released into a population where the wild-type vector is already established will also not expand its presence (measured by active infections that contribute to the virus transmission). On average, it will create new infections at the same rate that hosts recover from or clear currently active infections. This point is self-evident for viruses that do not superinfect, but we will next argue that it must also apply with superinfection.
This argument is robust – insensitive to countless details that would be included in a specific model. It would, however, be violated by evolution of the vaccine to become a ‘new’ virus that escapes the limits faced by wild-type vectors endemic in the population. However, that evolution would also already be occurring in the vector population, unless the engineering endowed the vaccine with a unique ability to evolve escape. If such vector and vaccine evolution was occurring, the viral population would be a heterogeneous mix of different types. Section [Citation4] addresses many types of heterogeneity.
2.2. Superinfection does not allow a vaccine to escape ecological constraints on expansion
Although the preceding point about neutrality is well established for many kinds of viruses, it is less obvious for superinfecting viruses such as cytomegalovirus (CMV [Citation30–32]. Hosts infected with CMV are often observed to be infected with multiple strains of CMV, as if a first infection does not elicit immunity that blocks subsequent infections. The ability to superinfect might seem to endow a virus with a capacity for unlimited expansion and thus an escape from neutrality. Indeed, we can prove that allowing unlimited and unconstrained superinfection by a virus results in indefinite constant exponential growth of the virus (Supplement S1). Such unconstrained growth is not sustainable in any biological system, however, as viral numbers would then quickly exceed even the number of molecules in the host population – much less the number of hosts. Consequently, there must be a limit on superinfection such that the number of infections per host stops increasing.
This limit could be biologically imposed in different ways while still allowing high levels of superinfection. As two examples, immunity may progressively block new infections as the host acquires more infections, or the total transmission rate of a multiply-infected host may be the same as for a singly infected host (i.e. if two strains infect a host, their combined rate of transmission is equal to that observed when infecting a host alone, either equally split, or dominated by one strain or the other). Regardless of mechanism, a limit is assured, and once the vector has reached this limit in the host population, any ‘equivalent’ vaccine using this vector will be unable to expand its active infections beyond those released. Supplement S2 presents one model of superinfection in which transmission is dominated by the most recent infection; a steady state ensues in which the vaccine experiences population neutrality determined by the level at which it was introduced.
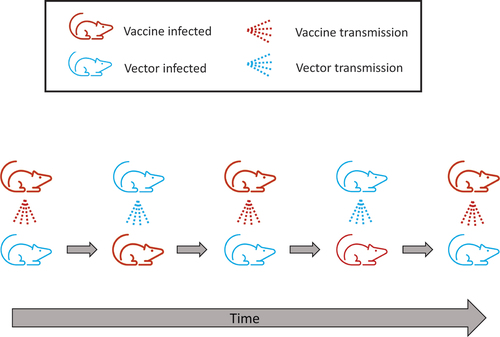
One subtle factor that works to maintain vaccine population neutrality despite superinfection is that, for every new host the vaccine infects, a host with the vaccine is being superinfected upon by the wild-type vector (a simple example is offered in ). This gain-balanced-by-loss of infections is a consequence of the vector having achieved endemicity in the population to which the vaccine is introduced (along with vaccine equivalence to the vector).
Figure 1. Cartoon illustration of a superinfecting vaccine being introduced into the same population from which the vector came. In actuality, the vaccine is superinfected upon as often as it transmits, but the figure only follows a ‘recipient’ host being repeatedly superinfected upon through time. Colors distinguish vector-infected from vaccine-infected hosts. The bottom row follows one host; the top row depicts its contacts that transmit to it and change its infection state. If, for example, the population is initially 50% wild-type and 50% vaccine, half of encounters are each type, and the host spends 50% of its time in each state. This is an extreme case of superinfection dominating prior infections, but it is especially suited to illustrating how population neutrality applies despite the superinfection.
This constraint on vaccine expansion can be overcome in various ways. One is to introduce the vaccine into a vector-naïve population (or perhaps a population with a different strain of the vector), whence the vaccine R0 dictates spread [Citation17]. The other is to supplement – continue releasing the vaccine. The first approach has the potential of vaccine escape with unintended consequences, the latter far less so.
3. The footprint of vaccine latency – and thus immunity – may be much larger than active infections
The two concepts introduced above, vaccine equivalence and population neutrality, are generic to essentially all populations, whether viral or not, with or without superinfection. We now introduce a third concept that is specific to infections: the difference between active and latent or suppressed infections. Viruses capable of superinfection can exist in states of active infection, but many can also switch from active to latent infections – a dormant state in which the viral genome is present in a host but not actively transmitting. Importantly, a host with a latent vaccine infection may have become immune to the pathogen by virtue of the prior active vaccine infection, with that immunity persisting long after the vaccine transmission from that host ceased. The key point here is that population neutrality ensures that the vaccine will not expand its active infections beyond the level introduced – its force of infection will remain the same, whether large or small. But while moving through the population at this constant level of active infections, the superinfecting vaccine leaves behind hosts whose infections have cleared or gone latent. Depending on rates of infection and conversion to latency, this legacy or ‘footprint’ of latent infections can be substantially larger than that of active infections. If hosts with latent vaccine infections are immune to the pathogen, then the fraction of the population infected will underrepresent the fraction of the population immune. This latency footprint may provide a substantial benefit of using a superinfecting vector for a transmissible vaccine. We elaborate.
Superinfection can attain a large immunity footprint despite a small active vaccine presence if hosts are repeatedly superinfected over their lifetimes. If a host is infected k times during its lifetime and the frequency of vaccine infections is f, then to first order, the probability that an individual will get infected at some point during their lifespan is given by 1-(1-f)^k. To take an extreme, suppose that the average host experienced 100 infections over its lifespan, each infection remaining active long enough to elicit immunity against the pathogen antigen. Then even if only 1% of the host population was actively infected by vaccine at any one time – the constraint of population neutrality – most hosts (~63%) would be exposed to the vaccine at some point their lifetime and become immune. In essence, vaccine latency allows the vaccine to bootstrap immunizations potentially well above the tyranny of a low active vaccine presence. Superinfection allows the repeated infections on which this benefit rests, but the benefit depends on rates.
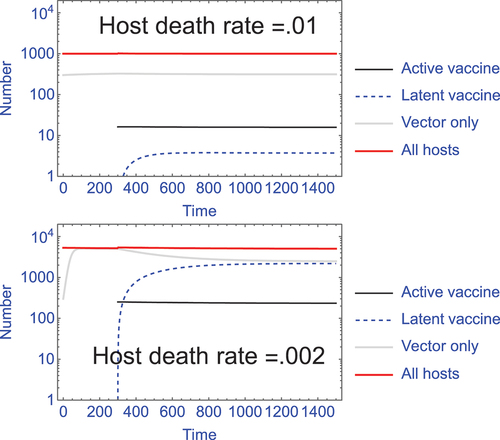
Superinfection alone does not ensure a large footprint of immunity. Using our model (S2.3) of unlimited superinfection in Supplement S2, we find that host longevity relative to the frequency of superinfections appears to be important (). We also note that superinfection is not the only possible driver of this benefit; any vector that can repeatedly infect the same host should have the same effect. Thus, viruses that experience waning immunity – such as respiratory viruses – should work similarly to superinfecting viruses as vectors for transmissible vaccines [Citation33]. We conjecture that vaccine design will benefit by using vectors with rapid turnover of active infection in their hosts, but this problem needs a formal and comprehensive theoretical analysis before any firm conclusions can be drawn. Furthermore, extensive background work may be necessary to measure the super- and reinfection rates that would foretell vaccine footprint.
Figure 2. The footprint of vaccine latency, hence immunity, can vary dramatically with changes in host longevity. With superinfection, hosts with latent vaccine contribute to population immunity but are not part of the active vaccine infections and thus can far exceed the population neutrality limit on active infections. This latency ‘footprint’ can be a major benefit to use of a superinfecting vector for a transmissible vaccine. However, superinfection alone does not ensure a large latency footprint – the magnitude depends on infection and host parameters. Two trials of Supplement S2 model (S2.3) are shown, differing in background host death rate (δ); in this model, ‘latent’ infections are considered to be those in which a vaccine infection has been superceded by infection with the wild-type vector. In both trials, vaccine infections at time 300 are introduced at 1/20 the level of active vector infections, and they remain at this level throughout – this is the population neutrality effect whereby the vaccine cannot expand. In contrast, the latency footprint is not determined by just the active vaccine infections but depends – profoundly – on host longevity (the inverse of host death rate). In the top panel, the latent infections (dashed blue) comprise only 0.4% of the total population (measured at time 2000), whereas in the lower panel with reduced host death, latent infections comprise 43% of the total population. Other parameters in the trials are b = 10, β = 1.5 x 10−5. The death rate parameter (δ) also affects the overall level of infections, so the numbers of vaccinated individuals introduced differs between the two trials to achieve 1/20 the level of active vector infections.
4. Accommodating vector strain variation
Population neutrality applies to a vaccine released in a population with the same ‘strain’ of vector as used in the vaccine. Virus populations often exist as a collection of multiple strains (e.g [Citation7,Citation22,Citation23,Citation26,Citation34,Citation35]. How is strain variation in the vector population likely to affect vaccine performance?
One challenge in confronting this question is in knowing what constitutes a different strain. Two vector isolates that differ in genome sequence need not behave as different strains with regard to vaccine performance. From the perspective here, vaccine performance defines strains regardless of genomic sequence. Even more problematic is that strains may not have discrete boundaries with respect to vaccine behavior. Thus, even members of what are considered the same vector strain may vary in the cross immunity they elicit and differ in the degree to which they suppress each other when in the same host. The arguments above as regards population neutrality were thus presented in the very specific context of a vaccine released into the same population that was the source of the vector used in the vaccine, since in that case it is assured that the vaccine has the same vector strain as exists in the host population. But we can make no claim about the vaccine released into a geographically distinct host population carrying a slightly different vector; this is a topic ripe for formal analysis.
With these caveats, we can offer tentative theoretical arguments of the impact of vector genetic variation on vaccine performance. All of these arguments address vector variation within a single host population. For the most part, these arguments suggest that vector variation within a host population either changes little or works against the vaccine. There are four cases to consider.
Case 1: genetic variation in the vector population is neutral, merely maintained by genetic drift. Under this case, nothing changes in the arguments above.
Case 2: the different viral strains are maintained as a balanced polymorphism, whereby strains 1, 2, … are being maintained at possibly different abundances. This case works somewhat against transmissible vaccine goals because it reduces the population size to which population neutrality applies: a vaccine that uses vector strain 1 can at best achieve equivalence only to vector strain 1 and will be limited to the fraction of the population occupied by strain 1. Thus, if strain 1 comprises 10% of the vector population, a vaccine using strain 1 as its genetic backbone can at best expect to displace only 10% of the vector population.
Case 3: strains undergo evolutionary turnover because of selection. Thus, strain 1 is gradually displacing strains 2, 3, … , which themselves are legacies of past strain superiorities. In this case, vector choice matters greatly, because choice of any strain other than 1 dooms the vaccine. Predicting strain turnover has yet to be mastered for any virus (Citation36–38). However, if the turnover is slow, vector choice will matter little in the short term.
Case 4: strains are independent, experiencing no interference or cross immunity. In this case, either strain can be used as a vector. Provided that the vaccine is released into a population harboring the vector of the same strain used in the vaccine, the vaccine will be subject to the rules above.
5. Empirical measures that foretell vaccine fate prior to a release
Despite the preceding logic that recombinant vectored vaccines are unlikely to spread widely from single introductions (in populations endemic for the vector), there are enough unknowns in the details to warrant a few key empirical measures of vaccine properties prior to a release. We suggest the following questions need to be evaluated. Answers to these questions should be experimentally tractable in a laboratory or other closed-environment setting and will provide evidence on whether to expect vaccine neutrality, expansion or decline.
What restrictions operate on vector superinfection between two infected individuals? Can one vector genotype superinfect a host already carrying that genotype, or is there a benefit to a novel virus strain? How does this change over time as immunity wanes?
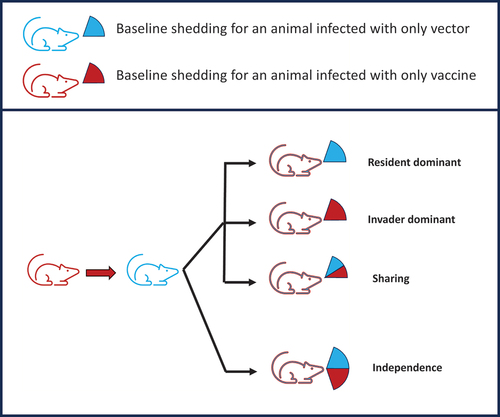
How much virus of each genotype is transmitted from superinfected individuals? In particular, is there independence among different superinfections or a limit on total transmission from one host (e.g. )? Does superinfection with a new virus block recrudescence of earlier viruses? Models that lead to population neutrality require a limit on the total transmission per host regardless of the number of superinfections.
Figure 3. Different possible transmission consequences for a superinfecting transmissible vaccine. The top part of the figure is a key, the lower part depicts a host that started with a vaccine infection (red state) and then became superinfected with wild-type vector (state changed to blue). Qualitatively, there are four cases of possible outcomes from this host regarding the rate it transmits vaccine and vector to new hosts. In the top three cases, the total transmission rate is the same as if it had been infected only once, but the cases differ in whether the transmission is shared between the vaccine and vector or whether the most recent infection dominates the transmission. Those three cases obey population neutrality. At the bottom, the case of independence violates population neutrality because each new superinfection transmits the same regardless of the number of prior infections. These possibilities can be measured experimentally to determine whether a superinfecting transmissible vaccine may escape the expectation of population neutrality.
How does the magnitude and duration of immunity to the pathogen depend on the previous infection history of the individual with the pathogen as well as the circulating wild-type and vaccine viruses?
This is a minimal list but one that is also potentially manageable. Even preliminary answers to these questions will be fundamental to predicting transmissible vaccine success prior to a release. Parallel questions can be asked for vaccines using other types of multiply-infecting viruses as vector (e.g. respiratory viruses).
6. Limitations of a null model
This paper has imposed a breathtakingly simple model onto a highly complex process of vrial transmission and superinfection resulting in conclusions of broad generality about transmissible vaccine success. The biological complexity of viral dynamics may seem to render our approach highly suspect, if not hopeless. However, the model simplicity rests on an inherent physical reality that has been evident at least since Malthus: populations cannot grow indefinitely. When they stop growing, reproduction and survival are reduced to merely maintain the numbers. Whatever messy details underlie the actual viral dynamics, viruses that invade a host population will reach a limit to their numbers, and that limit is the basis of our proposed generality.
At the same time, we acknowledge that the dynamics and evolution of superinfecting viruses such as CMV remain almost completely obscure. Superinfection has been inferred by isolating multiple viral strains from a single host, not from measuring rates of viral turnover in hosts. Recombination among strains within a host may be rampant, resulting in deletions that create subtypes and potentially lead to transmission of different viral genotypes than those initiating the infection. Empirical studies of CMV within-host dynamics (or of other superinfecting viruses) would go far in framing expectations for a transmissible vaccine using the virus as vector. Even attempts to merely model such complexities in the absence of empirical work would help shed light on whether our null model is valid. Indeed, if any model is found to contradict our null model, such a result could lead to a search for appropriate viruses that may improve transmissible vaccine success.
7. Conclusion
Transmissible vaccines of wildlife populations are being entertained to suppress zoonoses [Citation10]. In such applications, the a priori safest design is considered to be use of a recombinant, vectored vaccine in which the vector is isolated from the wildlife population, engineered to carry an insert from the pathogen, and released back into the wildlife population. While it has been appreciated that interference from the wild-type vector is likely to suppress vaccine spread, use of a vector capable of superinfection has been supposed to overcome this constraint. We find evidence both for and against an advantage of superinfection.
Various theoretical lines of argument suggest that essentially any recombinant-vectored transmissible vaccine will not expand its numbers if released into the population from which the vector was isolated. This conclusion is independent of the vaccine R0, a value which accrues to its spread in a naïve population. This point has been clear for platforms such as attenuated transmissible vaccines [Citation17]. Here we have specifically made the same case for vectors capable of superinfection. The key to this result is that even a superinfecting vector will have come to a constant level in its host population, and whatever now limits the wild-type vector will also limit the vaccine when introduced into the same population.
These considerations imply that transmissible vaccines should be self-contained in any population to which they are likely to be released. From a safety perspective, vaccine escape should not be a problem if the vaccine does not spread – mitigating concerns about a release. But a vaccine with limited coverage yields less impact – reducing the very justification for a release. To have their desired effect of widely immunizing the host population, such vaccines will need to be repeatedly introduced to offset their intrinsic containment. The extent of such releases will depend on details of the specific system. Release into vector- and pathogen-naïve populations will not face any of these constraints on spread, but containment then becomes a concern. A priori, host populations with vector strains differing genetically from that used in the vaccine could go either way – behave as if they are vector-naïve or vector-neutral with respect to vaccine dynamics.
8. Expert opinion
We emphasize that our arguments can be justified only in the narrow context of releasing the transmissible vaccine into the same population from which the vector was obtained. This is the specific context which is most likely to apply to any actual release, as it ensures the greatest safety against unintended consequences from any kind of vaccine-vector differences. But without knowing how vector strains from different host populations interact, we offer no predictions for vaccine performance in populations with vector strains not used in the vaccine.
The biological details of any implementation may often lead to deviations from a ‘neutral’ expectation. One complication with a possibly a large effect that was not covered above is vaccine interference from host immunity to the pathogen [Citation21]. The pathogen is the agent that the vaccine is intended to suppress. If host immunity to the pathogen blocks vaccine infection or transmission, the transmissible vaccine is expected to decline in a population that already has both vector and pathogen present (Supplement S3). Such interference might be measured experimentally in advance of any release. A second possible deviation from neutrality comes from the engineering used to insert foreign genetic material into the vector. The naïve expectation is that any such engineering will be detrimental to the vector. However, such costs to engineering are not always observed [Citation39]. In contrast to these two effects, vaccine abundance may get a boost if the vaccine protects the host against a lethal pathogen, but to benefit, the vaccine must transmit from the host for long periods. Last, we have ignored vaccine recombination and evolution. The limited work with engineered viruses suggests that the most likely evolutionary outcome is loss or down-regulation of the insert [Citation39]. But assurances beyond ‘the most likely’ may be warranted for a transmissible engineered virus.
These considerations suggest that vector choice and vaccine design will have a profound effect on vaccine efficacy. Resting on prior work as well as that here, we can now offer three features of transmissible vaccine design that will maximize vaccine persistence and immunity coverage in the population: (A) vaccine equivalence to the vector in transmission properties, (B) repeated infection of the same hosts by the vector and thus by vaccine, and (C) immunity to the pathogen minimally interfering with vaccine infection and transmission. In principle, vaccine engineering can play crucial roles in (A) and (C); property (B) is likely determined by properties intrinsic to the vector. Of course, if the vaccine is introduced into a population lacking the vector strain from which the vaccine was constructed and the pathogen, it may expand according to its R0, and most of the factors identified in this paper may not apply.
The arguments here rest on assumptions about vaccine and vector behavior under superinfection. The key properties of these assumptions are testable with simple experiments such that the potential for vaccine spread and expansion can be known at least approximately prior to any wild release. Furthermore, the models here are gross simplifications of any actual system. Their purpose has been to demonstrate properties that should apply widely and affect vaccine success. Even so, we note that the models omit details that are likely to be important in any implementation. For example, an important omission from our models is vaccine interference when superinfecting a host previously vaccinated, and such interference will impede vaccine spread even beyond the factors identified above. Our models have also assumed constant host population sizes and that viral abundances attain steady states. Many wild host populations and viral abundances exhibit periodicity. We do not expect that such fluctuations will affect the qualitative behaviors described here, but a formal analysis of vaccine dynamics under fluctuations would be welcome. More broadly, we appreciate that any actual transmissible vaccine will face its own set of constraints; the factors identified here may well be necessary considerations, but they are not sufficient considerations for any comprehensive effort in developing a transmissible vaccine.
Article highlights
Transmissible vaccines for wildlife could block zoonoses with minimal investment.
Proposed vaccine designs use a recombinant platform with a vector that can superinfect to overcome host immunity and an antigenic insert against the target pathogen. Safety dictates that the vaccine be released into the host population from which the vector was obtained.
We show that vaccines using these designs are, under the most ideal conditions for vaccine spread, expected to maintain a constant level of active infections in the host population.
A vaccine is expected to decline if it cannot infect or transmit from hosts immune to the pathogen or if the antigenic insert interferes with vaccine transmission.
Even with a low active vaccine presence, a vaccine can create a large immunity footprint in the population if it repeatedly superinfects hosts; superinfection rates are unknown, so it is not clear if a large immunity footprint is attainable.
On balance, transmissible vaccine success will require careful choice of vector and vaccine engineering. Ongoing vaccine releases are likely to be needed to maintain vaccine presence.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download Zip (779.8 KB)Data availability statement
All data in this study are numbers generated by iteration of equations given in the supplements and displayed in the figures. Initial conditions for these numerical trials are given in the figures. As such, the data are fully reproducible from the information provided.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2024.2320845
Additional information
Funding
References
- Fenner F, Cairns J. Variation in virulence in relation to adaptaton to new hosts. In: Burnet F Stanley W, editors The viruses. Animal viruses. Vol. 3. NY: Academic Press; 1959. p. 225–249.
- Hanley KA. The double-edged sword: how evolution can make or break a live-attenuated virus vaccine. Evolution. 2011;4(4):635–643. Cited: in: PMID: 22468165. doi: 10.1007/s12052-011-0365-y
- Lauring AS, Jones JO, Andino R. Rationalizing the development of live attenuated virus vaccines. Nat Biotechnol. 2010;28(6):573–579. Cited: in: PMID: 20531338. doi: 10.1038/nbt.1635
- Sabin AB. Paralytic poliomyelitis: old dogmas and new perspectives. Rev Infect Dis. 1981;3:543–564. Cited: in: PMID: 6269169. doi: 10.1093/clinids/3.3.543
- Sabin AB. Oral poliovirus vaccine: history of its development and use and current challenge to eliminate poliomyelitis from the world. J Infect Dis. 1985;151(3):420–436. Cited: in: PMID: 2982959. doi: 10.1093/infdis/151.3.420
- Nathanson N, Kew OM. From emergence to eradication: the epidemiology of poliomyelitis deconstructed. Am J Epidemiol. 2010;172:1213–1229. Cited: in: PMID: 20978089. doi: 10.1093/aje/kwq320
- Farroway LN, Gorman S, Lawson MA, et al. Transmission of two Australian strains of murine cytomegalovirus (MCMV) in enclosure populations of house mice (mus domesticus). Epidemiol Infect. 2005;133(4):701–710. Cited: in: PMID: 16050517. doi: 10.1017/S0950268805003717
- Hardy CM, Hinds LA, Kerr PJ, et al. Biological control of vertebrate pests using virally vectored immunocontraception. J Reprod Immunol. 2006;71(2):102–111. doi: 10.1016/j.jri.2006.04.006
- Hardy CM. Current status of virally vectored immunocontraception for biological control of mice. Soc Reprod Fertil Suppl. 2007;63:495–506. Cited: in: PMID: 17566294.
- Murphy AA, Redwood AJ, Jarvis MA. Self-disseminating vaccines for emerging infectious diseases. Expert Rev Vaccines. 2016;15(1):31–39. Cited: in: PMID: 26524478 doi: 10.1586/14760584.2016.1106942
- Torres JM, Sánchez C, Ramírez MA, et al. First field trial of a transmissible recombinant vaccine against myxomatosis and rabbit hemorrhagic disease. Vaccine. 2001;19(31):4536–4543. Cited: in: PMID: 11483281. doi: 10.1016/s0264-410x(01)00184-0
- Tsuda Y, Caposio P, Parkins CJ, et al. A replicating cytomegalovirus-based vaccine encoding a single Ebola virus nucleoprotein CTL epitope confers protection against Ebola virus. PloS Negl Trop Dis. 2011;5(8):e1275. Cited: in: PMID: 21858240. doi: 10.1371/journal.pntd.0001275
- Tsuda Y, Parkins CJ, Caposio P, et al. A cytomegalovirus-based vaccine provides long-lasting protection against lethal Ebola virus challenge after a single dose. Vaccine. 2015;33:2261–2266. Cited: in: PMID: 25820063.doi: 10.1016/j.vaccine.2015.03.029
- Sandbrink JB, Watson MC, Hebbeler AM, et al. Safety and security concerns regarding transmissible vaccines. Nat Ecol Evol. 2021;5(4):405–406. Cited: in: PMID: 33542476. doi: 10.1038/s41559-021-01394-3
- Lentzos F, Rybicki EP, Engelhard M, et al. Eroding norms over release of self-spreading viruses. Science. 2022;375(6576):31–33. Cited: in: PMID: 34990258. doi: 10.1126/science.abj5593
- Bakker KM, Rocke TE, Osorio JE, et al. Fluorescent biomarkers demonstrate prospects for spreadable vaccines to control disease transmission in wild bats. Nat Ecol Evol. 2019;3(12):1697–1704. Cited: in: PMID: 31740844. doi: 10.1038/s41559-019-1032-x
- Nuismer SL, Althouse BM, May R, et al. Eradicating infectious disease using weakly transmissible vaccines. Proc Biol Sci. 2016;283:20161903. Cited: in: PMID: 27798311. 1841. doi: 10.1098/rspb.2016.1903
- Nuismer SL, May R, Basinski A, et al. Controlling epidemics with transmissible vaccines. PLoS One. 2018;13(5):e0196978. Cited: in: PMID: 29746504. doi: 10.1371/journal.pone.0196978
- Nuismer SL, Bull JJ. Self-disseminating vaccines to suppress zoonoses. Nat Ecol Evol. 2020;4(9):1168–1173. Cited: in: PMID: 32719452. doi: 10.1038/s41559-020-1254-y
- Streicker DG, Bull JJ, Nuismer SL. Self-spreading vaccines: base policy on evidence. Science. 2022;375(6587):1362–1363. Cited: in: PMID: 35324312. doi: 10.1126/science.abo0238
- Basinski AJ, Varrelman TJ, Smithson MW, et al. Evaluating the promise of recombinant transmissible vaccines. Vaccine. 2018;36(5):675–682. Cited: in: PMID: 29279283. doi: 10.1016/j.vaccine.2017.12.037
- Nikolovski S, Lloyd ML, Harvey N, et al. Overcoming innate host resistance to vaccination: employing a genetically distinct strain of murine cytomegalovirus avoids vector-mediated resistance to virally vectored immunocontraception. Vaccine. 2009;27(38):5226–5232. Cited: in: PMID: 19591797. doi: 10.1016/j.vaccine.2009.06.064
- Griffiths ME, Broos A, Bergner LM, et al. Longitudinal deep sequencing informs vector selection and future deployment strategies for transmissible vaccines. PLoS Biol. 2022;20(4):e3001580. Cited: in: PMID: 35439242. doi: 10.1371/journal.pbio.3001580
- Varrelman TJ, Remien CH, Basinski AJ, et al. Quantifying the effectiveness of betaherpesvirus-vectored transmissible vaccines. Proc Natl Acad Sci U S A. 2022;119:e2108610119. Cited: in: PMID: 35046024. doi: 10.1073/pnas.2108610119
- Griffiths ME, Meza DK, Haydon DT, et al. Inferring the disruption of rabies circulation in vampire bat populations using a betaherpesvirus-vectored transmissible vaccine. Proc Natl Acad Sci U S A. 2023;120:e2216667120. Cited: in: PMID: 36877838. doi: 10.1073/pnas.2216667120
- Lipsitch M, Colijn C, Cohen T, et al. No coexistence for free: Neutral null models for multistrain pathogens. Epidemics. 2009;1(1):2–13. Cited: in: PMID: 21352747. doi: 10.1016/j.epidem.2008.07.001
- Adam D. A guide to R — the pandemic’s misunderstood metric. Nature. 2020;583(7816):346–348. doi: 10.1038/d41586-020-02009-w
- Anderson RM, May RM. Infectious diseases of humans: dynamics and control. Oxford: Oxford University Press. 1992.
- Keeling MJ, Rohani P. Modeling infectious diseases in humans and animals. Princeton (NJ): Princeton University Press; 2008.
- Quinn M, Erkes DA, Snyder CM. Cytomegalovirus and immunotherapy: opportunistic pathogen, novel target for cancer and a promising vaccine vector. Immunotherapy. 2016;8(2):211–221. Cited: in: PMID: 26786895. doi: 10.2217/imt.15.110
- Gorman S, Lloyd ML, Smith LM, et al. Prior infection with murine cytomegalovirus (MCMV) limits the immunocontraceptive effects of an MCMV vector expressing the mouse zona-pellucida-3 protein. Vaccine. 2008;26(31):3860–3869. Cited: in: PMID: 18573574. doi: 10.1016/j.vaccine.2008.05.020
- Méndez AC, Rodríguez-Rojas C, Del Val M. Vaccine vectors: the bright side of cytomegalovirus. Med Microbiol Immunol. 2019;208(3–4):349–363. Cited: in: PMID: 30900089. doi: 10.1007/s00430-019-00597-7
- Yewdell JW.Individuals cannot rely on COVID-19 herd immunity: durable immunity to viral disease is limited to viruses with obligate viremic spread. PLoS Pathog. 2021;17(4):e1009509. Cited: in: PMID: 33901246. doi: 10.1371/journal.ppat.1009509
- Kucharski AJ, Andreasen V, Gog JR. Capturing the dynamics of pathogens with many strains. J Math Biol. 2016;72(1–2):1–24. Cited: in: PMID: 25800537. doi: 10.1007/s00285-015-0873-4
- LaTourrette K, Garcia-Ruiz H. Determinants of virus variation, evolution, and Host adaptation. Pathogens. 2022;11(9):1039. Cited: in: PMID: 36145471. doi: 10.3390/pathogens11091039
- Bush RM, Bender CA, Subbarao K, et al. Predicting the evolution of human influenza a. Science. 1999;286(5446):1921–1925. Cited: in: PMID: 10583948 doi: 10.1126/science.286.5446.1921
- Neher RA, Russell CA, Shraiman BI. Predicting evolution from the shape of genealogical trees. Elife. 2014;3:e03568. Cited: in: PMID: 25385532. doi: 10.7554/eLife.03568
- Barrat-Charlaix P, Huddleston J, Bedford T, et al. Limited predictability of amino acid substitutions in seasonal influenza viruses. Mol Biol Evol. 2021;38(7):2767–2777. Cited: in: PMID: 33749787. doi: 10.1093/molbev/msab065
- Willemsen A, Zwart MP. On the stability of sequences inserted into viral genomes. Virus evol. 2019;5(2): vez045. Cited: in: PMID: 31741748. doi: 10.1093/ve/vez045

