ABSTRACT
Background
A bivalent human papillomavirus vaccine (2vHPV) is currently used in the Netherlands; a nonavalent vaccine (9vHPV) is also licensed.
Research design and methods
We compared the public health and economic benefits of 2vHPV- and 9vHPV-based vaccination strategies in the Netherlands over 100 years using a validated deterministic dynamic transmission metapopulation model.
Results
Compared to 2vHPV, the 9vHPV strategy averted an additional 3,245 cases of and 825 deaths from 9vHPV-strain-attributable cancers, 4,247 cases of and 190 deaths from recurrent respiratory papillomatosis (RRP), and 1,009,637 cases of anogenital warts (AGWs), with an incremental cost-effectiveness ratio (ICER) of €4,975 per quality-adjusted life year (QALY) gained. The ICER increased in a scenario with increased HPV vaccination coverage rates and was relatively robust to one-way deterministic sensitivity analyses, with variation in the disease utility parameter having the most impact. When catch-up vaccination for individuals ≤26 years of age was added to the model, vaccinating with 9vHPV averted additional cancers and AGWs compared to 2vHPV vaccination.
Conclusion
Our analyses predict that transitioning from a 2vHPV- to a 9vHPV-based vaccination strategy would be cost-effective in the Netherlands.
1. Introduction
Vaccination against pathogenic strains of the human papillomavirus (HPV) provides a high degree of protection against acute infections and their long-term sequelae, which include cervical and various other cancers, anogenital warts (AGWs), and recurrent respiratory papillomatosis (RRP) [Citation1]. In the Netherlands, nationally funded HPV vaccination was introduced in 2010 for girls 12 years of age [Citation2,Citation3]. A catch-up program was also offered in 2009 to girls born between 1993 and 1996 [Citation3]. Since its inception, the Dutch HPV vaccination strategy has used a bivalent vaccine (2vHPV; Cervarix, GlaxoSmithKline Biologicals, S.A. [Citation4]) that protects against oncogenic viral strains HPV16 and 18, which together account for approximately 70% of cervical cancers, a majority of anal cancers, and smaller proportions of vaginal, vulval, and head and neck cancers [Citation3,Citation5–11]. The decision to incorporate 2vHPV into the Dutch National Immunisation Programme (NIP) was based in part on a 2007 study that used conservative health technology assessment tools and Markov models to estimate the cost-effectiveness of HPV vaccination in the Netherlands [Citation12]. The model predicted that vaccination of girls 12 years of age using 2vHPV would be cost-effective compared to no HPV vaccination [Citation12].
The ongoing analysis of long-term data on the benefits of HPV vaccination has provided evidence that the initial schedules implemented in many countries can be improved. For instance, a consensus is now emerging that HPV vaccination is more effective if it occurs earlier in life [Citation13–18]. Furthermore, while men and boys benefit indirectly from vaccination of girls (and vice versa) via reduced direct exposures to HPV (men who have sex with women) and the reduced overall population prevalence of HPV infections (all men), analyses conducted in the Netherlands and elsewhere have predicted that gender-neutral vaccination would generate additional direct public health and health economics benefits, including direct protection of men who exclusively have sex with men [Citation19–26]. Two recent modeling studies predicted that expanding the current Dutch 2vHPV-based vaccination strategy to include a male primary cohort or catch-up vaccination would prevent additional cases of HPV-associated cancer and have acceptable incremental cost-effectiveness ratios (ICERs) compared to the status quo [Citation25,Citation26].
In 2019, the Dutch Health Council reviewed the latest evidence relating to HPV vaccination and recommended several amendments to the original schedule, including expanding the primary vaccination cohort to include boys and offering catch-up vaccination to all individuals ≤26 years of age who had not already been vaccinated against HPV [Citation27]. They also recommended lowering the age of the primary vaccination cohort to as close to the 9th birthday as possible [Citation27]. These recommendations were implemented in 2022, and fully funded HPV vaccination is now available in the Netherlands to all individuals 9 years of age. A fully funded catch-up program for adults 18–26 years of age is also being offered through 2023 using the 2vHPV vaccine [Citation28].
Despite these efforts, there is still a substantial burden of HPV-associated disease in the Netherlands, including approximately 800 cases of and 200–250 deaths from cervical cancer, up to 2,720 cases of and 810 deaths from other HPV-associated cancers, and 45,600 cases of AGWs each year [Citation27,Citation29,Citation30]. This high burden of disease is partially attributable to the low HPV vaccination coverage rate, which as of 2019 was estimated to be 66% among girls and women, compared to the World Health Organization’s target of 90% [Citation29,Citation31–33]. However, pathogenic viral strains that are not covered by 2vHPV also contribute to the morbidity, mortality, and health care costs associated with diseases caused by HPV [Citation5–11,Citation34,Citation35].
Two vaccines that protect against additional strains of HPV compared to 2vHPV are licensed for use in the Netherlands, but have not yet been incorporated into the NIP [Citation3]. A quadrivalent vaccine (4vHPV; Gardasil, MSD, B.V. [Citation36]) provides protection against HPV16 and 18 as well as strains 6 and 11, which can cause AGWs. A nonavalent vaccine (9vHPV; Gardasil-9, MSD, B.V. [Citation37]) protects against the same four strains as 4vHPV as well as five additional oncogenic strains: HPV31, 33, 45, 52, and 58. The additional seven strains covered by 9vHPV compared to 2vHPV collectively account for around 20% of cervical cancers, substantial proportions of other HPV-associated cancers, and 85% of AGWs [Citation5–11,Citation34,Citation35,Citation37]. There is also evidence that 2vHPV and 4vHPV confer some degree of temporary protection against strains that are not included in the vaccine, notably genotypes 31, 33, and 45 [Citation38,Citation39]. All three of the approved vaccines have a proven safety record [Citation40].
The Dutch Health Council’s 2019 report and recommendations did not indicate a preference for vaccine type, stating that additional modeling would be required to make this decision [Citation27]. To date, however, very few studies have directly compared the public health and health economics benefits of HPV vaccination using 9vHPV versus 2vHPV. Modeling studies specific to the epidemiology and health care costs of HPV-associated diseases in Singapore and Germany have predicted that implementation of a 9vHPV-based vaccination strategy would prevent additional cases of HPV-associated cancers and AGWs and would be more cost-effective than use of the 2vHPV or 4vHPV vaccine in these countries [Citation41,Citation42]. In the Dutch context, a 2013 modeling study that compared the cost-effectiveness of 2vHPV- and 4vHPV-based vaccination strategies for a cohort of 100,000 women found that 2vHPV had a favorable ICER in terms of the prevention of cervical cancer, due to superior cross-protection against oncogenic strains other than HPV16 and 18; the 4vHPV-based strategy was slightly more favorable when both cervical cancer and AGW-related outcomes were considered [Citation43]. However, the model did not consider the costs associated with HPV-associated cancers at other sites, or among men [Citation43]. In addition, older cost-effectiveness models for HPV vaccination in the Netherlands need to be updated to incorporate a 2022 change in the free national cervical cancer screening program [Citation44]. The new program includes a unique conditional screening schedule whereby individuals with a negative HPV test are invited for their next test after 10 years rather than the usual 5 years, and has been shown to be cost-effective in an initial analysis [Citation44,Citation45].
In response to this knowledge gap, the objective of the current study was to model and compare the cost-effectiveness of gender-neutral HPV vaccination strategies using 2vHPV or 9vHPV in the Netherlands among a primary cohort of individuals aged 9 years. To capture the impacts of the 2023 catch-up program, we also modeled scenarios that included catch-up vaccination of individuals ≤26 years of age with 2vHPV or 9vHPV.
2. Methods
2.1. Study design and model overview
This was a modeling study that used input data on population demographics, HPV vaccine efficacy (VE), vaccination coverage, health care costs, disease incidence and mortality rates, and utility decrements to assess the cost-effectiveness of four different HPV vaccination strategies in the Netherlands over the next 100 years. The primary intervention of interest was vaccination of individuals 9 years of age with 9vHPV, and the primary comparator was vaccination of individuals 9 years of age with 2vHPV (‘status quo’). For both vaccine types, we also assessed strategies that included catch-up vaccination of individuals ≤26 years of age over a 3-year period. Since this was a modeling study using deidentified published data as model inputs, no ethical approval or informed consent was required.
Public health and health economics outcomes were modeled, including cases of 9vHPV-strain-attributable diseases and resulting deaths averted, incremental costs, quality-adjusted life years (QALYs), and ICERs, i.e. vaccination program cost per QALY gained. The health economics analysis was from the payer perspective and did not account for societal costs such as days of work lost, reduced quality of life of caretakers, etc. As recommended by health economics modeling guidelines in the Netherlands, we assumed a discounting of 1.5% on effects and 4.0% on costs [Citation46].
2.2. Model structure
We used a deterministic dynamic transmission metapopulation model that captures the entire population of the Netherlands and accounts for its aging dynamics. The model has been previously published, validated, and used to assess the cost-effectiveness of different HPV vaccination strategies in other high-income countries [Citation47–52]. Within the model, the national population is divided into groups based on age, sex, and sexual activity level (i.e. average number of annual sexual partners); the latter variable was incorporated as in a previous adaptation of the same model [Citation47]. We amended the published version of the model to incorporate the January 2022 change to the Dutch cervical cancer screening program, which doubled the gap between tests to 10 years for individuals with a negative HPV test [Citation44].
2.2.1. HPV-associated cancers
The model’s flow diagram for HPV infections and HPV-associated cancers is shown in . All segments include all-cause death as an exit state, while the cancer segment also includes cancer-related death. New HPV infections are acquired by each group at a rate that is determined by their average sexual activity level and the prevalence of HPV among their likely sexual partners. A proportion of new infections are cleared by the immune system, which in some cases leads to seroconversion and partial protection against subsequent infections. New infections that are not cleared become persistent, and some persistent infections lead to HPV-associated pre-cancers.
Figure 1. Flow diagram of the model for cancers associated with human papillomavirus infection.
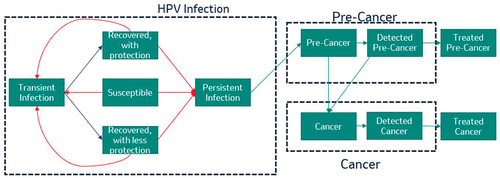
Cervical pre-cancers can be detected through screening programs, at which point treatment is attempted. Successfully treated cervical pre-cancers exit the model with no loss of quality of life. In the Netherlands, eligible individuals (cis women, transmen, and gender-diverse individuals with a cervix) 30–60 years of age were previously invited to participate in the national cervical cancer screening program every 5 years [Citation53]. Since January 2022, individuals 40–60 years of age with a negative previous HPV test result are invited every 10 years [Citation44]. In 2019, 56% of invitees participated in the program, and cervical pre-cancerous lesions were detected for 1.1% of participants [Citation53]. Testing was suspended between March and June 2020 due to the COVID-19 pandemic, but the resulting backlog was cleared by the end of 2021 [Citation44,Citation53]. Among the 55% of 2021 invitees who participated in screening, 43% opted for a test conducted at a doctor’s office and 12% for a newly available self-sampling kit [Citation44]. A high-risk strain of HPV was detected for 9.5% of all 2021 participants [Citation44]. The first HPV-vaccinated cohorts became eligible for cervical cancer screening in 2023; invitations for these cohorts include messaging that participation in screening is important even for vaccinated individuals [Citation44]. In the model, we included HPV DNA screening as currently implemented in the Netherlands. To exclude the impact of the COVID-19 pandemic, we used participation and referral rates from 2018, when 57.9% of eligible individuals participated in the screening program [Citation54].
The progression and regression parameters for pre-cancers varied by site and were based on previously calibrated values [Citation48]. Progression from pre-cancer to cancer was initially assumed to be undetected. Once a cancer is detected through screening programs (cervical cancer) or through the onset of symptoms (all cancers), treatment is attempted and loss of quality of life occurs. The cancer state is divided into three stages. No mortality or loss of quality of life is assumed for the first two stages of undetected cancer, but the third stage is associated with some risk of mortality. For detected cancers, the mortality rate increases and the quality of life decreases as the cancer becomes more severe.
2.2.2. Anogenital warts and recurrent respiratory papillomatosis
The model’s structure for AGWs and RRP is similar to that used for HPV-associated cancers. An exception is that the model for juvenile-onset RRP (JORRP) assumes that a proportion of the population is born infected with HPV6 or 11, with a risk of progressing to JORRP; the proportion of these individuals in each group is based on the prevalence of HPV infections among women of childbearing age during the group’s birth year(s). Acquired HPV infections can progress to adult-onset RRP (AORRP) or AGWs. A proportion of AGWs are assumed to remain untreated.
2.3. Model inputs, parameters, calculations, and calibration
2.3.1. Demographics and sexual behavior
The model assumes a steady-state age distribution with constant population size. Since health economics outcomes are computed on a per-capita basis, only the public health outcomes would be affected by any changes in population size. All-cause mortality probabilities for each age group were taken from the United Nations Demographics Yearbook for 2019 [Citation55]. The size of each demographic group was then inferred from these rates using published methods [Citation56].
The sizes of the sexual activity level groups were based on a 2017 survey of sexual health in the Netherlands in which 42.0% of men and 46.0% of women reported low sexual activity (0–2 partners per year), 24.0% of men and 25.0% of women reported medium sexual activity (3–5 partners per year), and the remainder of each group reported high sexual activity (≥6 partners per year) [Citation57]. Sexual behavior data were computed using a standard approach that uses partnership data and assumptions about sexual mixing structure to estimate the number of partners between various age, sex, and sexual activity level groups [Citation51,Citation58]. Dutch sexual partnership data were not available, and so proxy data from the United Kingdom (UK) were used as input [Citation59]. Similarly, the parameters used to model sexual mixing between different demographic groups were based on proxy data from the United States (US) [Citation52]. The mixing parameter for age was set at 0.6 between debut and cessation and at 0.1 after cessation, while the extent of mixing between different sexual activity groups was set at 0.7 [Citation52].
Individuals who have undergone a hysterectomy were assumed to still be able to acquire and transmit HPV infections, but were excluded from the model’s calculations relating to cervical cancer screening and progression to cervical disease. The rate of hysterectomies for each age group was based on published Dutch data from 1995 to 1998 [Citation60].
2.3.2. Clinical parameters
While rates of symptom recognition are almost certainly affected by cancer site, age, and geographic, demographic, and socioeconomic factors, a lack of suitable data compelled us to assume a uniform rate of symptom recognition across all cancers and groups, but varying by disease severity. These values were estimated from previous calibrations of the model and assumed an annual probability of symptom recognition of 4% for local cancers, 18% for regional cancers, and 95% for distant cancers [Citation48]. Other model parameters relating to the percentage of cervical, vaginal, and vulvar pre-cancers and carcinomas in situ (CIS) that were treated were previously estimated in a US study through model calibration [Citation48]. It was assumed that 50% of Stage 1 cervical intraepithelial neoplasias (CINs) were treated, as were 100% of Stage 2 and 3 CINs and cervical CIS. We assumed that no other pre-cancers were treated. For AGWs and RRP, the likelihood of progression to disease (by age) and health care-seeking behavior (by sex) were taken from the same US modeling study mentioned above, as were the mortality rates for RRP [Citation48]. Treatment success and mortality rates for detected cancers varied with cancer stage, as previously modeled using an independent competing risk framework [Citation48]. Cancer mortality rates are presented in Supplementary Table S1 [Citation48].
For several clinical parameters, we calibrated the model by adjusting parameters to optimize an objective function that compared model output with real-world data from a series of global meta-analyses and other relevant studies [Citation5,Citation7–11,Citation34,Citation47]. Calibrated HPV transmission, seroconversion, extent of protection conferred by seroconversion, and infection persistence parameters are provided in Supplementary Table S2 for oncogenic viral strains 16, 18, 31, 33, 45, 52, and 58 (by infection site) and in Supplementary Table S3 for AGW-associated strains 6 and 11. The calibrated attribution of relevant diseases to each strain of HPV is provided in Supplementary Table S4.
2.3.3. Vaccine parameters
The degree of 9vHPV-mediated protection against diseases caused by each viral strain was based on clinical trial data (Supplementary Table S5, Supplementary Table S6) [Citation61–66]. The VE of the comparator bivalent vaccine against strains 16 and 18 was assumed to be the same as for 9vHPV. In the base case, all vaccine-mediated protection was assumed to be lifelong. Historical vaccination coverage rates from 2011 to 2018 ranged from 45.5% in 2018 to 61.0% in 2015–2016; based on these data, assumed coverage rates of 70% (female) and 50% (male) were selected for the base-case analysis (see below) [Citation67].
2.3.4. Health economics parameters
The model used the most recent Dutch list prices per dose for 2vHPV (€118.11) and 9vHPV (€139.95) [Citation68]. The administration cost per dose was assumed to be identical for both vaccines and was therefore set to €0 in the model. Baseline utility decrement values for each age group ranged from 0.95 for individuals 0–24 years of age to 0.83 for those ≥75 years of age [Citation69]. The health care costs and utility decrement values associated with treating each episode of HPV-associated disease are provided in Supplementary Table S7, organized by disease site and stage [Citation43,Citation48,Citation70,Citation71].
2.3.5. Model outputs
The public health outputs of the model comprised the number of cases of and deaths from all diseases (pre-cancers/cancers at each site, AGWs, RRP) associated with strains of HPV covered by 9vHPV. The model’s economic outputs incorporated the total costs to the payer of detecting and treating/managing each case of each of the above diseases, as well as the costs of vaccine administration, cytology screening, etc., under each vaccination strategy. Survival outcomes were estimated as both crude and quality-adjusted life years; the latter values were obtained by weighting survival times by the relevant health utility decrements. The ICER of each strategy compared to the status quo of 2vHPV with no catch-up was calculated as the strategy’s incremental cost divided by the incremental gain in QALYs.
2.4. Analyses
2.4.1. Base case
The base-case analysis assessed four gender-neutral vaccination strategies, all with an assumed vaccination coverage of 70% (female)/50% (male):
2vHPV (status quo): continuation of 2vHPV vaccination with an immediate transition to vaccination of individuals 9 years of age (change from 12 years of age), and a year of catch-up vaccination for individuals 11 years of age in the year of transition.
2vHPV plus catch-up: as for Strategy 1, but with the addition of vaccination of individuals ≤26 years of age for 3 years, such that vaccination coverage among this group increases to 70% (female)/50% (male) by the end of the 3-year catch-up period. These vaccination rates were based on the results of a survey conducted by the Dutch National Institute for Public Health and the Environment in preparation for the 2023 catch-up vaccination campaign, which estimated a participation rate of 60% for boys and men and 50% for unvaccinated girls and women [Citation72].
9vHPV: as for Strategy 1, but immediately replacing 2vHPV with 9vHPV.
9vHPV plus catch-up: as for Strategy 2, but immediately replacing 2vHPV with 9vHPV.
2.4.2. Additional analyses
We conducted deterministic sensitivity analyses to explore the impact of varying several uncertainty parameters on the ICERs of strategies 2–4 (Supplementary Table S8). A premium 9vHPV vaccine price threshold analysis was also run for each strategy.
We also assessed the public health and health economics impacts of two additional scenarios: increased vaccination coverage, and temporary partial cross-protection from 2vHPV against HPV31, 33, and 45 (Supplementary Table S8, Supplementary Table S9) [Citation38]. For the cross-protection scenario, time-dependent clinical trial data were used to fit a simple vaccine trial model with two parameters (efficacy against persistent infection and duration of protection), using Markov chain Monte Carlo methods [Citation73,Citation74]. The level of protection against transient infections with these strains was conservatively assumed to be the same as that provided by 9vHPV.
3. Results
3.1. Model fit
To assess the calibrated parameters used in the model, we compared modeled and actual data on the incidence in each age group of AGWs and of anal, cervical, head and neck, penile, vaginal, and vulvar cancers, as well as deaths from cervical cancer, that are attributable to the HPV strains covered by 9vHPV (Supplementary Figure S1, Supplementary Figure S2).
3.2. Base-case analyses
3.2.1. Public health outcomes
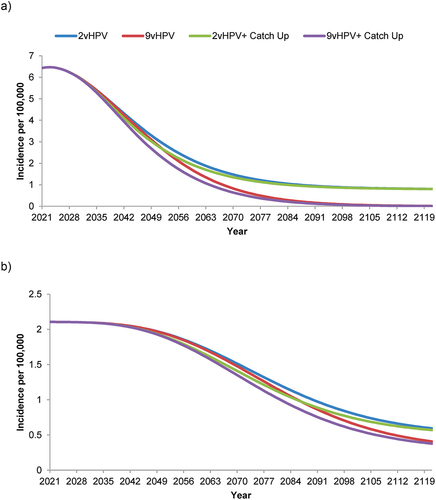
All four strategies were predicted to decrease the incidence of 9vHPV-strain-attributable cervical cancer over time from the 2021 level of 6.46/100,000 people, but the incidence decreased more rapidly and to a greater extent with the 9vHPV and especially the 9vHPV plus catch-up strategies compared to the 2vHPV and 2vHPV plus catch-up strategies (). The incidence of 9vHPV-strain-attributable cervical cancer was projected to reach <0.1/100,000 by 2121 with both 9vHPV-based strategies, and plateaued at ~0.8/100,000 by 2121 with both 2vHPV-based strategies. Similar overall trends were observed for all other 9vHPV-strain-attributable cancers combined, although the estimated incidence had not yet plateaued with any strategy by the end of the 100-year time horizon (). The projected incidence of these cancers fell from 2.11/100,000 in 2021 to 0.59/100,000 (2vHPV), 0.56/100,000 (2vHPV plus catch-up), 0.41/100,000 (9vHPV), or 0.38/100,000 (9vHPV plus catch-up) by 2121.
Figure 2. Forecasted incidence of nonavalent human papillomavirus vaccine-strain-attributable (a) cervical cancer and (b) all other anogenital plus head & neck cancers in the Netherlands with strategies using a bivalent or nonavalent human papillomavirus vaccine for individuals ≥9 years of age before sexual debut, with or without catch-up vaccination programs for individuals ≤26 years of age.
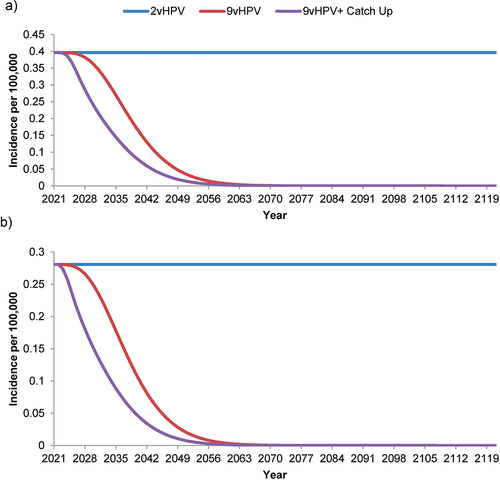
For both the 9vHPV and the 9vHPV plus catch-up strategies, the projected incidence of 9vHPV-strain-attributable AGWs (), JORRP (), and AORRP () decreased more rapidly than that of 9vHPV-strain-attributable cancers. The incidence of AGWs was estimated to reach ~0.1/100,000 by 2066 with a catch-up program, compared to 2071 without. Similarly, incorporation of a catch-up program into the 9vHPV strategy was estimated to accelerate the effective elimination (i.e. reduction to ~0.1/100,000) of JORRP by 6 years (by 2053 compared to 2059 with no catch-up program) and of AORRP by 5 years (by 2050 compared to 2055).
Figure 3. Forecasted incidence of nonavalent human papillomavirus vaccine-strain-attributable anogenital warts in the Netherlands with strategies using a bivalent or nonavalent human papillomavirus vaccine for individuals ≥9 years of age before sexual debut, with or without catch-up vaccination programs for individuals ≤26 years of age.
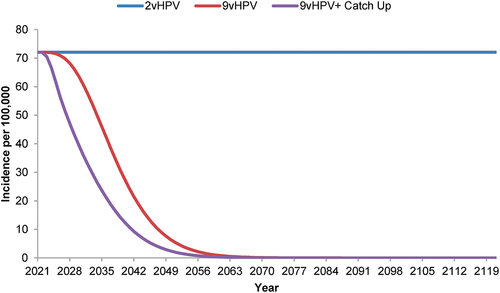
Figure 4. Forecasted incidence of nonavalent human papillomavirus vaccine-strain-attributable (a) juvenile-onset and (b) adult-onset recurrent respiratory papillomatosis in the Netherlands with strategies using a bivalent or nonavalent human papillomavirus vaccine for individuals ≥9 years of age before sexual debut, with or without catch-up vaccination programs for individuals.
Compared to the 2vHPV status quo, the 9vHPV strategy was projected to avert an additional 1,009,637 cases of 9vHPV-strain-attributable AGWs in the Dutch population over the 100-year modeled period, 3,245 cases of HPV-associated cancer, 4,247 cases of RRP, and 1,015 deaths (825 from cancer and 190 from RRP; ). The majority (81.3%) of the 790 averted female cancer-related deaths were from cervical cancer, while 27/36 (77.1%) averted male cancer-related deaths were from penile cancer. When a catch-up program for individuals ≥26 years of age was added to both strategies, the 9vHPV plus catch-up strategy averted 1,083,060 additional cases of 9vHPV-strain-attributable AGWs, 3,661 of cancer, and 4,558 of RRP as well as 1,131 deaths (926 from cancer and 205 from RRP), compared to 2vHPV plus catch-up.
Table 1. Nonavalent human papillomavirus vaccine-strain-attributable disease cases and deaths in the Netherlands over 100 years with use of 9vHPV versus 2vHPV, with and without catch-up vaccination.
3.2.2. Health economics outcomes
Compared to the status quo, the 9vHPV strategy had an ICER of €4,975/QALY and the 9vHPV plus catch-up strategy had an ICER of €40,331/QALY (). The Netherlands generally uses cost-effectiveness thresholds of €20,000, €50,000, and €80,000/QALY depending on the severity of illness [Citation75]; other cost-effectiveness analyses have compared the cost per QALY to the gross domestic product (GDP) of the Netherlands, which in 2021 was ~€49,000 per capita [Citation76]. The 2vHPV + catch-up strategy was dominated by the 9vHPV strategy.
Table 2. Cost-effectiveness outcomes of four base-case human papillomavirus vaccination strategies in the Netherlands over 100 years.
3.3. Deterministic sensitivity analyses
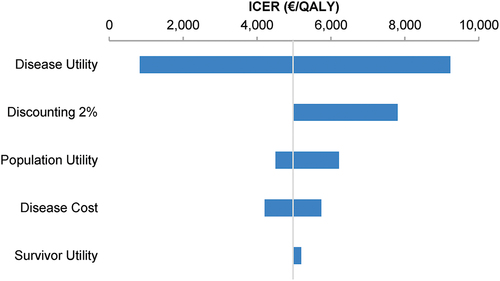
In the 9vHPV versus 2vHPV analysis, the parameter with the greatest impact on the ICER of the 9vHPV strategy was disease utility; varying this parameter resulted in a range of €829–9,233/QALY, compared to €4,975/QALY in the base-case analysis (). The survivor utility parameter had the least impact on the ICER. The premium 9vHPV vaccine price threshold analysis revealed that compared to 2vHPV, 9vHPV was a cost-saving strategy when the premium was <€9.50 (Supplementary Figure S3). At a premium of <€59.12, the ICER of the 9vHPV strategy remained under €20,000/QALY.
Figure 5. Deterministic sensitivity analysis of the incremental cost-effectiveness ratios of 9vHPV versus 2vHPV human papillomavirus vaccination strategies for the Netherlands over 100 years.
3.4. Scenario analyses
In the scenario of increased vaccination coverage rates (90% among females and 70% among males), the 9vHPV strategy had an ICER of €7,067/QALY compared to the status quo, and the 9vHPV plus catch-up strategy had an ICER of €47,837 (). In the scenario in which 2vHPV conferred partial cross-protection against HPV31, 33, and 45, the 9vHPV strategy had an ICER of €4,991/QALY compared to the status quo, while the 9vHPV plus catch-up strategy had an ICER of €40,331/QALY.
Table 3. Cost-effectiveness outcomes of four human papillomavirus vaccination strategies in the Netherlands over 100 years in scenarios of increased vaccination coverage or cross-protection against additional viral strains.
4. Discussion
In this modeling study, we compared the impact of four different gender-neutral vaccination strategies on the incidence, mortality, and health care costs associated with diseases attributable to strains of HPV that are covered by 9vHPV. Both 9vHPV-based strategies were predicted to avert additional cases of and deaths from HPV-associated diseases over 100 years, compared to the respective 2vHPV strategy; there was a greater difference in all public health outcomes between the 9vHPV and 2vHPV-based strategies when catch-up vaccination was included in the model. In addition, catch-up vaccination using 9vHPV was predicted to avert additional cases of AGWs and to accelerate the effective elimination of AGWs and RRP by 5–6 years, compared to 9vHPV vaccination without catch-up.
In the primary cost-effectiveness analysis, the ICER of the 9vHPV strategy versus 2vHPV was low, i.e. ~10% of the 2021 per-capita GDP of the Netherlands. Our model thus predicted that vaccination using 9vHPV would be cost-effective over 100 years in the Netherlands compared to continued use of 2vHPV in the primary cohort. Notably, the strategy that incorporated catch-up vaccination using 2vHPV (as implemented in 2023 in the Netherlands) was dominated by the strategy in which 2vHPV was replaced by 9vHPV in the primary cohort with no catch-up program. However, the maximum public health benefits were achieved when the 9vHPV primary cohort strategy was augmented with a catch-up program that also used the 9vHPV vaccine, although this strategy exceeded the lowest of the three cost-effectiveness thresholds used in the Netherlands. In the case that catch-up HPV vaccination were to become a permanent feature of the Dutch NIP, which would then become the base case, the incremental value of the 9vHPV vaccine would still be substantial: the ICER of 9vHPV + catch-up versus 2vHPV + catch-up was €5,665/QALY, demonstrating that the bulk of the value realized by the 9vHPV vaccine is retained in the case of a permanent catch-up program.
The estimated Dutch ICERs compared favorably to the ICER of a gender-neutral 9vHPV vaccination strategy versus the status quo of 4vHPV vaccination of girls in Germany, which was predicted to be €22,987/QALY [Citation41]. A similar study in Singapore estimated that 9vHPV vaccination of girls would have an ICER of SGD929/QALY (~€642 at the 31 March 2023 exchange rate) compared with 2vHPV vaccination of girls; however, only cervical cancer and AGW-related outcomes were included in the model [Citation42].
The introduction of the 9vHPV vaccine was predicted to decrease the incidence of cervical cancer to a greater extent than that of other HPV-associated cancers, due to the larger proportion of cancers of the cervix that are attributable to non-2vHPV strains HPV31, 33, 45, 52, and 58 [Citation3,Citation5–11]. The 9vHPV strategy was also predicted to cause a particularly large and rapid decrease in the incidence of AGWs and RRP, which are largely caused by non-2vHPV strains HPV6 and 11 [Citation34,Citation35]. Further, the benefits of incorporating catch-up vaccination into the 9vHPV strategy were more pronounced for AGWs and RRP than for HPV-associated cancers.
In the current study, the ICER of 9vHPV versus 2vHPV was most sensitive to the disease utility parameter. In previous studies conducted in Germany and Singapore, similar models were most sensitive to the duration of vaccine-mediated protection; this parameter was not included in the current sensitivity analyses as there is now strong evidence for prolonged protection [Citation41,Citation42].
In a scenario of higher HPV vaccination coverage rates than have been achieved to date in the Netherlands, the ICERs of the 9vHPV-based strategies were predicted to increase due to the reduction in marginal value at higher coverage rates. If efforts to increase the Dutch HPV vaccination coverage rate are successful, further analyses may therefore be needed to ensure the chosen strategy remains cost-effective. In contrast, incorporating cross-protection against non-vaccine strains into the model had little impact on the cost-effectiveness analyses. This was likely due to our assumption that cross-protection was not lifelong and to the 4% discounting of costs, which meant that the savings in vaccination costs with the 2vHPV strategy did not greatly reduce the incremental value of the 9vHPV strategy.
As with any modeling study, our analyses are subject to some known limitations. For instance, the unavailability of suitable real-world data for some of the model’s parameters necessitated some assumptions. First, we assumed that sexual behavior, cervical cancer screening rates, vaccine dosing regimens and formulations, and vaccination coverage rates would not change during the study’s 100-year time horizon. As demonstrated by the vaccination coverage scenario analysis, changes over time to these parameters may affect the model’s predictions. In addition, data were not available on inter-group differences in several model parameters. Our assumption of equal cancer symptom recognition/disease treatment rates and clinical outcomes among different demographic groups was likely inaccurate, as was our assumption that HPV transmission rate data for heterosexual partnerships also applied to other partnership types. Finally, we were unable to identify suitable data on the proportion of pre-cancers (including unsuccessfully treated cervical pre-cancers) that progress to cancer, and so assumed that the progression rate was 100%.
For certain other parameters, proxy data from other countries were used (e.g. UK data for sexual partnerships and US data for sexual mixing between age groups, pre-cancer treatment rates, and treatment success rates); the use of Netherlands-specific data would likely result in more accurate estimates of the relative costs and benefits of each vaccination strategy. Further, some of the Netherlands-specific data incorporated into the model may be out of date, such as the hysterectomy rate data for each age group, which were from 1995 to 1998. Changes to the hysterectomy rate since 1998 could influence the cervical cancer-related outcomes of the model. Finally, the societal costs of HPV-associated diseases were not considered in the cost-effectiveness analysis due to a lack of suitable data. The payer-only perspective of the analysis results in a conservative estimate of vaccine cost-effectiveness, but it is in line with previous Dutch cost-effectiveness studies [Citation12,Citation25,Citation26,Citation43].
5. Conclusions
Our analyses predict that replacing 2vHPV with 9vHPV in the Dutch NIP would prevent additional cases of and deaths from HPV-associated diseases and would be highly cost-effective in the primary cohort, with or without a permanently established catch-up vaccination program. Additionally, a catch-up vaccination strategy would avert additional cases of cancer and AGWs and accelerate the elimination of AGWs and RRP compared to 9vHPV vaccination without catch-up.
Declaration of interests
C Palmer, W Wang, and K Saxena are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, U.S.A. and own stock in Merck & Co., Inc., Rahway, NJ, U.S.A. C Dolk is an employee of MSD, the Netherlands and owns stock in Merck & Co., Inc., Rahway, NJ, U.S.A. U Sabale is an employee of MSD, Lithuania and owns stock in Merck & Co., Inc., Rahway, NJ, U.S.A. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they were an investigator on the Phase II and III studies for the bivalent vaccine (2HPV). Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Ethics statement
Since this was a modeling study using deidentified published data as model inputs, no ethical approval or informed consent was required.
Author contributions
Conception and design: CP, CD, US, WW, and KS. Analysis and interpretation of the data: CP, CD, US, WW, and KS. Drafting of the paper or revising it critically for intellectual content: CP, CD, US, WW, and KS. All authors made a substantial contribution to the study and were involved in drafting, writing, and/or revising the article. All authors approved and agree to be accountable for the final version of the article approved for publication.
Supplemental Material
Download (199 KB)Acknowledgments
A version of this study was previously presented as a conference poster: Dolk C, Sabale U, Palmer C. The public health impact and cost-effectiveness of gender neutral 9vHPV vaccination in the Netherlands. EUROGIN International Multidisciplinary HPV Congress 2022, Düsseldorf, Germany. Abstract #3493. The authors thank Cath Ennis, PhD, in collaboration with ScribCo for medical writing assistance.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2024.2322543.
Additional information
Funding
References
- Illah O, Olaitan A. Updates on HPV Vaccination. Diagnostics (Basel). 2023;13(2):243. doi: 10.3390/diagnostics13020243
- Health council of the Netherlands. Vaccination against cervical cancer 2008. Available from: https://www.gezondheidsraad.nl/documenten/adviezen/2008/04/01/vaccinatie-tegen-baarmoederhalskanker
- Schurink T, de Melker H. HPV vaccination: background information for the Dutch Health Council. Rijksinstituut voor Volksgezondheid en Milieu. 2017. https://www.rivm.nl/bibliotheek/rapporten/2017-0020.pdf
- European Medicines Agency. Cervarix. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/cervarix
- de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11). doi: 10.1016/S1470-2045(10)70230-8
- Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–527. doi: 10.1056/NEJMoa021641
- Alemany L, Cubilla A, Halec G, et al. Role of human papillomavirus in penile carcinomas worldwide. Eur Urol. 2016;69(5):953–961. doi: 10.1016/j.eururo.2015.12.007
- Alemany L, Saunier M, Alvarado-Cabrero I, et al. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer. 2015;136(1):98–107. doi: 10.1002/ijc.28963
- Alemany L, Saunier M, Tinoco L, et al. Large contribution of human papillomavirus in vaginal neoplastic lesions: a worldwide study in 597 samples. Eur J Cancer. 2014;50(16):2846–2854. doi: 10.1016/j.ejca.2014.07.018
- Castellsague X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108:6. doi: 10.1093/jnci/djv403
- de Sanjose S, Alemany L, Ordi J, et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer. 2013;49(16):3450–3461. doi: 10.1016/j.ejca.2013.06.033
- Boot HJ, Wallenburg I, de Melker HE, et al. Assessing the introduction of universal human papillomavirus vaccination for preadolescent girls in the Netherlands. Vaccine. 2007;25(33):6245–6256. doi: 10.1016/j.vaccine.2007.05.061
- Drolet M, Benard E, Perez N, et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. The Lancet. 2019;394(10197):497–509. doi: 10.1016/S0140-6736(19)30298-3
- Falcaro M, Castanon A, Ndlela B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet. 2021;398(10316):2084–2092. doi: 10.1016/S0140-6736(21)02178-4
- Garland SM, Kjaer SK, Munoz N, et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin Infect Dis. 2016;63(4):519–527. doi: 10.1093/cid/ciw354
- Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:22. doi: 10.1001/jama.2016.17615
- Palmer T, Wallace L, Pollock KG, et al. Prevalence of cervical disease at age 20 after immunisation with bivalent HPV vaccine at age 12-13 in Scotland: retrospective population study. BMJ. 2019;36510. doi: 10.1136/bmj.l1161
- Petaja T, Pedersen C, Poder A, et al. Long-term persistence of systemic and mucosal immune response to HPV-16/18 AS04-adjuvanted vaccine in preteen/adolescent girls and young women. Int J Cancer. 2011;129(9):2147–2157. doi: 10.1002/ijc.25887
- Bogaards JA, Wallinga J, Brakenhoff RH, et al. Direct benefit of vaccinating boys along with girls against oncogenic human papillomavirus: bayesian evidence synthesis. BMJ. 2015;350(may12 7):h2016–h2016. doi: 10.1136/bmj.h2016
- Suijkerbuijk AW, Donken R, Lugner AK, et al. The whole story: a systematic review of economic evaluations of HPV vaccination including non-cervical HPV-associated diseases. Expert Rev Vaccines. 2017;16(4):361–375. doi: 10.1080/14760584.2017.1256778
- Stanley M. HPV vaccination in boys and men. Hum Vaccin Immunother. 2014;10(7):2109–2111. doi: 10.4161/hv.29137
- Lehtinen M, Apter D. Gender-neutrality, herd effect and resilient immune response for sustainable impact of HPV vaccination. Curr Opin Obstet Gynecol. 2015;27(5):326–332. doi: 10.1097/GCO.0000000000000208
- Morais E, El Mouaddin N, Schuurman S, et al. Landscape assessment for gender neutral human papillomavirus vaccination recommendations including head and neck cancer burden data. Vaccine. 2021;39(39):5461–5473. doi: 10.1016/j.vaccine.2021.08.043
- Spinu AD, Anghel RF, Marcu DR, et al. HPV vaccine for men: where to? (review). Exp Ther Med. 2021;22:5. doi: 10.3892/etm.2021.10701
- Simons JJM, Vida N, Westra TA, et al. Cost-effectiveness analysis of a gender-neutral human papillomavirus vaccination program in the Netherlands. Vaccine. 2020;38(30):4687–4694. doi: 10.1016/j.vaccine.2020.05.031
- Simons JJM, Westra TA, Postma MJ. Cost-effectiveness of a male catch-up human papillomavirus vaccination program in the Netherlands. Prev Med Rep. 2022;28:2810. doi: 10.1016/j.pmedr.2022.101872
- Health Council of the Netherlands. Vaccination against HPV. Available from: https://www.healthcouncil.nl/documents/advisory-reports/2019/06/19/vaccination-against-hpv
- National Institute for Public Health and the Environment. More than two million invitations to get vaccinated against HPV in 2023 2023. Available from: https://www.rivm.nl/en/news/more-than-two-million-invitations-to-get-vaccinated-against-hpv-in-2023
- Bruni L, Albero G, Serrano B, et al. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in Netherlands. Summary Report. 2023 Mar 10 [cited 2024 Feb 27]. Available from: https://hpvcentre.net/statistics/reports/NLD.pdf
- Staritsky LE, van Aar F, Visser M, et al. Sexually transmitted infections in the Netherlands in 2019. the Netherlands: National Institute for Public Health and the Environment; 2020 [cited 2024 Feb 27]. Available from:https://www.rivm.nl/bibliotheek/rapporten/2020-0052.pdf
- Bruni L, Saura-Lazaro A, Montoliu A, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med. 2021;144:14410. doi: 10.16/j.ypmed.2020.106399
- World Health Organization. Accelerating the elimination of cervical cancer as a global public health problem. World Health Organization. 2019 [cited 2024 Feb 27]. Available from:https://apps.who.int/gb/ebwha/pdf_files/EB146/B146_9-en.pdf
- van Lier EA, Oomen PJ, Giesbers H, et al. Vaccinatiegraad en jaarverslag: Rijksvaccinatieprogramma Nederland 2021. Nederland: Rijksinstituut voor Volksgezondheid en Milieu; 2022 [cited 2024 Feb 27]. Available from: https://www.rivm.nl/bibliotheek/rapporten/2022-0017.pdf
- Aubin F, Pretet JL, Jacquard AC, et al. Human papillomavirus genotype distribution in external acuminata condylomata: a large French national study (EDiTH IV). Clin Infect Dis. 2008;47(5):610–615. doi: 10.1086/590560
- Garland SM, Steben M, Sings HL, et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199(6):805–814. doi: 10.1086/597071
- European Medicines Agency. Gardasil. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/gardasil
- European Medicines Agency. Gardasil 9. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/gardasil-9
- Brown DR, Joura EA, Yen GP, et al. Systematic literature review of cross-protective effect of HPV vaccines based on data from randomized clinical trials and real-world evidence. Vaccine. 2021;39(16):2224–2236. doi: 10.1016/j.vaccine.2020.11.076
- Wheeler CM, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16–26 years. J Infect Dis. 2009;199(7):936–944. doi: 10.1086/597309
- Phillips A, Patel C, Pillsbury A, et al. Safety of human papillomavirus vaccines: an updated review. Drug Saf. 2018;41(4):329–346. doi: 10.1007/s40264-017-0625-z
- Largeron N, Petry KU, Jacob J, et al. An estimate of the public health impact and cost-effectiveness of universal vaccination with a 9-valent HPV vaccine in Germany. Expert Rev Pharmacoecon Outcomes Res. 2017;17(1):85–98. doi: 10.1080/14737167.2016.1208087
- Tay SK, Hsu TY, Pavelyev A, et al. Clinical and economic impact of school-based nonavalent human papillomavirus vaccine on women in Singapore: a transmission dynamic mathematical model analysis. BJOG. 2018;125(4):478–486. doi: 10.1111/1471-0528.15106
- Westra TA, Stirbu-Wagner I, Dorsman S, et al. Inclusion of the benefits of enhanced cross-protection against cervical cancer and prevention of genital warts in the cost-effectiveness analysis of human papillomavirus vaccination in the Netherlands. BMC Infect Dis. 2013;13(1):1–11. doi: 10.1186/1471-2334-13-75
- National Institute for Public Health and the Environment. Newsletter: Dutch population screening for cervical cancer 2022. 2022 [cited 2024 Feb 27]. Available from: https://www.rivm.nl/sites/default/files/2023-01/Newsletter%20Dutch%20population%20screening%20for%20cervical%20cancer%202022%20DEF2.pdf
- Jansen E, Naber SK, Aitken CA, et al. Cost-effectiveness of HPV-based cervical screening based on first year results in the Netherlands: a modelling study. BJOG. 2021;128(3):573–582. doi: 10.1111/1471-0528.16400
- Hakkaart-van Roijen L, van der Linden N, Bouwmans C, et al. Richtlijn voor het uitvoeren van economische evaluaties in de gezondheidszorg. Kostenhandleiding: Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg Zorginstituut Nederland. 2015 [cited 2024 Feb 27]. Available from: https://www.zorginstituutnederland.nl/binaries/zinl/documenten/publicatie/2016/02/29/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-de-gezondheidszorg/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-de-gezondheidszorg.pdf
- Cody P, Tobe K, Abe M, et al. Public health impact and cost effectiveness of routine and catch-up vaccination of girls and women with a nine-valent HPV vaccine in Japan: a model-based study. BMC Infect Dis. 2021;21(1):1–13. doi: 10.1186/s12879–020–05632–0
- Daniels V, Prabhu VS, Palmer C, et al. Public health impact and cost-effectiveness of catch-up 9-valent HPV vaccination of individuals through age 45 years in the United States. Hum Vaccin Immunother. 2021;17(7):1943–1951. doi: 10.1080/21645515.2020.1852870
- Dasbach EJ, Insinga RP, Elbasha EH. The epidemiological and economic impact of a quadrivalent human papillomavirus vaccine (6/11/16/18) in the UK. BJOG. 2008;115(8). doi: 10.1111/j.1471-0528.2008.01743.x
- Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28(42):6858–6867.
- Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis. 2007;13(11):28–41.
- Elbasha EH, Dasbach EJ, Insinga RP. A multi-type HPV transmission model. Bull Math Biol. 2008;70(8). doi: 10.1007/s11538-008-9338-x
- National Institute for Public Health and the Environment. Cervical cancer population screening in the Netherlands: factsheet 2021. 2021 [cited 2024 Feb 27]. Available from: https://www.rivm.nl/sites/default/files/2022-03/013673%20Factsheet%20Cervical%20Cancer%20Screening.pdf
- Rijksinstituut voor Volksgezondheid en Milieu. monitor bevolkingsonderzoek baarmoederhalskanker 2018. 2019. Available from: https://www.rivm.nl/sites/default/files/2019-12/Monitor%20BMHK%202018.pdf
- United Nations Statistics Division. Demographic Yearbook – 2019. 2019. Available from: https://unstats.un.org/unsd/demographic-social/products/dyb/dyb_2019/
- Hethcote HW. An age-structured model for pertussis transmission. Math Biosci. 1997;145(2). doi: 10.1016/s0025-5564(97)00014-x
- de Graaf H, Wijsen C. Sexuele Gezondheid in Nederland 2017. 2017 [cited2024 Feb 27]. Available from: https://rutgers.nl/wp-content/uploads/2021/03/Seksuele-Gezondheid-in-Nederland-2017.pdf
- Garnett GP, Anderson RM. Factors controlling the spread of HIV in heterosexual communities in developing countries: patterns of mixing between different age and sexual activity classes. Philos Trans R Soc Lond B Biol Sci. 1993;342(1300). doi: 10.1098/rstb.1993.0143
- Mercer CH, Tanton C, Prah P, et al. Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the national surveys of sexual attitudes and lifestyles (natsal). Lancet. 2013;382(9907). doi: 10.1016/S0140-6736(13)62035-8
- Hanstede MM, Burger MJ, Timmermans A, et al. Regional and temporal variation in hysterectomy rates and surgical routes for benign diseases in the Netherlands. Acta Obstet Gynecol Scand. 2012;91(2):220–225. doi: 10.1111/j.1600-0412.2011.01309.x
- Ault KA, IISG F. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. The Lancet. 2007;369(9576):1861–1868. doi: 10.1016/S0140-6736(07)60852-6
- Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–1943. doi: 10.1056/NEJMoa061760
- Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364(5):401–411. doi: 10.1056/NEJMoa0909537
- Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–723. doi: 10.1056/NEJMoa1405044
- Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369(9574). doi: 10.1016/S0140-6736(07)60777-6
- Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365(17):1576–1585. doi: 10.1056/NEJMoa1010971
- Rijksinstituut voor Volksgezondheid en Milieu. Vaccinatiegraad en jaarverslag Rijksvaccinatieprogramma Nederland 2018 2019. Available from: https://www.rivm.nl/publicaties/vaccinatiegraad-en-jaarverslag-rijksvaccinatieprogramma-nederland-2018
- Medicijnkosten.nl. GARDASIL 9 INJSUSP WWSP 0,5ML. Available from: https://www.medicijnkosten.nl/medicijn?artikel=GARDASIL+9+INJSUSP+WWSP+0%2C5ML&id=fc25241a7318c7a927bd91e42edf846f
- Szende A, Janssen B, Cabases J, Editors. Self-reported population health: an international perspective based on EQ-5D [Internet]. Dordrecht (NL): Springer; 2014. PMID: 29787044.
- Luttjeboer J, Westra TA, Wilschut JC, et al. Cost–effectiveness of the prophylactic HPV vaccine: an application to the Netherlands taking non-cervical cancers and cross-protection into account. Vaccine. 2013;31(37):3922–3927. doi: 10.1016/j.vaccine.2013.06.044
- Qendri V, Bogaards JA, Berkhof J. Health and economic impact of a tender-based, sex-neutral human papillomavirus 16/18 vaccination program in the Netherlands. J Infect Dis. 2017;216(2):210–219. doi: 10.1093/infdis/jix272
- Ministry of Finance. 3.1 Article 1 public health 2022. Available from: https://www.rijksfinancien.nl/memorie-van-toelichting/2022/1SUPP/XVI/onderdeel/1276156
- Apter D, Wheeler CM, Paavonen J, et al. Efficacy of human papillomavirus 16 and 18 (HPV-16/18) AS04-adjuvanted vaccine against cervical infection and precancer in young women: final event-driven analysis of the randomized, double-blind PATRICIA trial. Clin Vaccine Immunol. 2015;22(4):361–373. doi: 10.1128/CVI.00591-14
- Gamerman D, Lopes HF. Markov Chain Monte Carlo: stochastic simulation for bayesian inference. Second ed. Chapman & Hall; 2006.
- Zwaap J, Knies S, van der Meijden C, et al. Kosteneffectiviteit in de praktijk Zorginstituut Nederland. 2015 [cited 2024 Feb 27]. Available from: https://www.zorginstituutnederland.nl/publicaties/rapport/2015/06/26/kosteneffectiviteit-in-de-praktijk
- Statista. Gross domestic product (GDP) per capita in the Netherlands from 1960 to 2021 2022. Available from: https://www.statista.com/statistics/530398/netherlands-gdp-per-capita/

