ABSTRACT
Background
Respiratory syncytial virus (RSV), a common respiratory pathogen, can lead to severe symptoms, especially in older adults (OA). A recently developed RSV prefusion F protein (RSVPreF3 OA) vaccine confers high protection against RSV lower respiratory tract disease (LRTD) over two full RSV seasons. The aim of this study was to assess the potential public health impact of RSVPreF3 OA vaccination in the Japanese OA population.
Research design and methods
A static Markov model was used to estimate the number of symptomatic RSV cases, hospitalizations and deaths in the Japanese population aged ≥ 60 years over a 3-year time horizon. Japan-specific RSV epidemiology and healthcare resource use parameters were used; vaccine efficacy was derived from a phase 3 randomized study (AReSVi-006, NCT04886596). Vaccination coverage was set to 50%.
Results
Without vaccination, >5 million RSV acute respiratory illness (ARI) would occur (2.5 million LRTD and 2.8 million upper respiratory tract infections) leading to ~ 3.5 million outpatient visits, >534,000 hospitalizations and ~ 25,500 RSV-related deaths over 3 years. Vaccination could prevent > 1 million RSV-ARI cases, 728,000 outpatient visits, 143,000 hospitalizations and 6,840 RSV-related deaths.
Conclusions
RSVPreF3 OA vaccination is projected to have a substantial public health impact by reducing RSV-related morbidity and mortality in the OA population.
Plain Language Summary
Respiratory syncytial virus (RSV) is one of the most frequent disease-causing agents that leads to common cold symptoms. In older adults, infection with RSV can result in severe complications including bronchitis/bronchiolitis, lung infection (pneumonia) and in rare cases death. Older people and people with chronic heart or lung disease are more likely to experience complications. We estimated that more than 5 million RSV cases occur in older adults (≥60 years) over a three-year period (1.8 million over one year). Many older adults (≥60 years) will see their treating physician because of an acute RSV infection or will be hospitalized.
Recently, a vaccine has been registered which protects older adults against RSV disease: the RSV prefusion F protein Older Adult (RSVPreF3 OA) vaccine. Vaccination with RSVPreF3 OA could prevent RSV infection in the older adult population and reduce the number of outpatient visits and hospitalizations; the impact is particularly high in Japan, where 35% of people are 60 years or older. We used a public health impact model to estimate how many RSV cases, hospitalizations and deaths could be prevented if 50% of people aged ≥ 60 years received the RSVPreF3 OA vaccine: We found that the vaccine could prevent about 1 million RSV infections, more than 728,000 outpatient visits, approximately 143,000 hospitalizations and 6,840 RSV-related deaths over a three-year period.
Adding RSVPreF3 OA vaccine to the national immunization program in Japan could protect older adults against RSV disease and reduce the burden on patients and the healthcare system.
1. Introduction
Respiratory syncytial virus (RSV) is one of the most frequent pathogens for acute respiratory illness (ARI) and a leading cause for lower respiratory tract disease (LRTD) in children aged <5 years [Citation1]. In immunocompetent adults, RSV infection typically causes mild symptoms such as rhinorrhea and cough. However, in older adults (≥60 years) and adults with preexisting conditions that put them at risk of severe infections, RSV can lead to severe LRTD, prolonged hospitalization, exacerbation of existing disease and even death [Citation2–5]. In Japan, RSV was shown to be a major cause for respiratory tract infection in older adults [Citation6]. In a retrospective Japanese medical claims analysis, median length of stay was 15 days for older adults aged >60 years hospitalized for RSV disease [Citation7]. In addition, cases of mass infection at elderly care facilities have been reported in Japan, underlining the need for protective measures in vulnerable populations [Citation8]. RSV exposure does not confer life-long immunity; therefore, individuals remain susceptible to repeated RSV infections throughout their entire life. Older adults have the highest RSV-related burden due to a higher risk of severe disease [Citation9].
In high-income countries, an estimated 5.2 million adults aged >60 years experienced RSV-related disease in 2019, leading to approximately 470,000 hospitalizations and 33,000 deaths [Citation3]. However, routine molecular testing for causative respiratory pathogens is usually not performed, challenging an accurate assessment of RSV-related disease burden in older adults. Due to the absence of routine testing and potential diagnostic insensitivity, the actual burden due to RSV might be significantly underestimated [Citation10,Citation11]. Recent observational studies and modeling analyses have contributed to a better understanding of RSV epidemiology and health impact in older adults. In a US observational cohort study over 12 seasons, 11% of outpatients aged ≥60 years presenting with respiratory disease tested positive for RSV [Citation12]. Among these patients, 19% experienced a serious outcome, defined as hospital admission, emergency department visit or pneumonia. People with preexisting conditions such as chronic obstructive pulmonary disease or congestive heart failure were at higher risk of serious outcomes. Several studies comparing outcomes in patients with RSV or influenza infection showed that RSV-related burden of disease equaled or exceeded that of influenza in older adults with chronic illnesses, such as chronic respiratory disease and congestive heart failure [Citation13–15]. Mortality rates of hospitalized patients were also comparable between RSV and influenza: Falsey et al. reported mortality rates of 8% and 7% in patients hospitalized for RSV and influenza, respectively [Citation13]. Similar results were reported by Lee et al. based on a prospective study enrolling hospitalized patients with RSV or influenza: unadjusted 30-day mortality rates were 9.1% in RSV patients compared with 8.0% in influenza patients [Citation15]. Finally, hospitalized RSV patients tended to be older compared with patients hospitalized for influenza [Citation14,Citation15]. Given the high morbidity and mortality of RSV-related disease, the development of RSV vaccines has been declared a priority by physicians and health organizations both at the national and global levels [Citation16,Citation17].
In 2023, a AS01E-adjuvanted RSV prefusion F protein-based vaccine gained approval by several health authorities, including the Food and Drug Administration (FDA) in the US and the Ministry of Health, Labour and Welfare (MHLW) in Japan, for RSV-related disease prevention in adults aged ≥60 years (RSVPreF3 OA). Vaccination induces a robust humoral and cellular immune response to help protect older adults against RSV disease [Citation18]. An ongoing randomized, double-blind, phase 3 study of RSVPreF3 OA demonstrated vaccine efficacy during the first season of 82.6% against RSV-related LRTD, 94.1% against severe RSV-LRTD and 71.7% against RSV-ARI [Citation19,Citation20]. Follow-up data for two seasons showed that the protective effect was durable: after one dose of RSVPreF3 OA cumulative vaccine efficacy over two full seasons was 67.2% against RSV-LRTD and 78.8% against severe RSV-LRTD [Citation19].
The aim of this study was to determine the public health impact of introducing RSVPreF3 OA vaccination versus no vaccination in the Japanese adult population aged ≥60 years of age.
2. Methods
2.1. Model structure
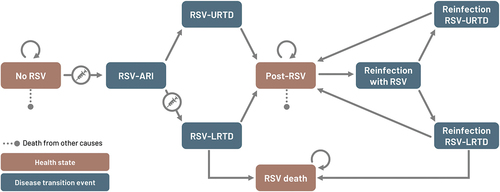
An existing, static, multi-cohort Markov model was adapted to the Japanese setting to estimate the number of RSV cases and RSV-related healthcare resource use in adults aged ≥60 years with and without RSVPreF3 OA immunization. No vaccination strategy was selected as the comparator since there is currently no RSV vaccine included in the Japan national immunization program (NIP). A cycle length of 1 month was used and considered appropriate to update health states since most RSV infections resolve within 1 month and the current model does not take into account long-term sequelae of RSV [Citation21]. A time horizon of 3 years was applied for the base-case analysis, however, results over 1 year were also reported without vaccination to estimate the annual burden of RSV in older adults. The three-year time horizon was selected because phase 3 efficacy results showed that 1 dose of RSVPreF3 OA provided durable protection for two full seasons and regression analysis based on phase 3 clinical data suggested that there was substantial residual vaccine efficacy throughout season 3. The model consists of three major health states, i.e. ‘No RSV,’ ‘Post-RSV’ and ‘RSV-death’ (). At the beginning, the entire population starts in the ‘No RSV’ health state. Thereafter, individuals can transition to other health states according to age-dependent transition probabilities. Different transition events account for the probability of having RSV-ARI, which develops either into RSV-upper respiratory tract disease (URTD) or RSV-LRTD. In addition, individuals could be re-infected with RSV since infection with RSV does not confer long-lasting immunity [Citation20]. All-cause mortality was integrated into the ‘No RSV’ and ‘Post-RSV’ health states, and RSV-related mortality occurs only in LRTD cases.
Figure 1. Schematic description of Markov model.
2.2. Model input parameters
2.2.1. Population
The size of the older adult population was obtained from 2022 Population Estimates published by Official Statistics in Japan (e-STAT) [Citation22]. The population was categorized into 7 age groups (60–64, 65–69, 70–74, 75–79, 80–84, 85–89 and ≥90 years) ().
Table 1. Epidemiologic and vaccine efficacy model input parameters.
2.2.2. Epidemiology
RSV incidence in the Japanese older population was derived from a prospective, multicenter, observational cohort study in Japan, which evaluated the occurrence of RSV infection in older adult participants, aged ≥65 years during one season [Citation6]. RSV-ARI was detected in 2.4% of participants. This value was adjusted using an under-ascertainment multiplier of 1.75 (range: 1.5 to 2.2) to account for missed cases due to using a specific sampling methodology [Citation10,Citation11]. Systematic literature reviews showed that reported RSV detection rates, which were based on conventional methods that used viral culture and antigen testing of nasopharyngeal or nasal swabs, are biased toward lower values because positive cases could be missed when relying on one diagnostic method only. Adding either paired serology or sputum/saliva polymerase chain reaction (PCR) to conventional nasal swab PCR, led to an increase of RSV detection rate of 50% each [Citation10,Citation11]. We assumed that there was an overlap of 50% among samples that tested positive with either paired serology and nasal swab PCR or paired sputum/saliva and nasal swab PCR; hence, the reported RSV incidence was adjusted by a factor of 1.75, which is within the range of reported under-ascertainment factors (range: 1.5 to 2.2) [Citation10,Citation11]. The proportion of RSV-ARI patients, developing RSV-LRTD (47.60%) was derived from the phase 3 AReSVi-006 study; the remainder of RSV-ARI were assumed to have RSV-URTD () [Citation23]. The proportion of medically attended RSV (51.8%), including outpatient and inpatient visits, was estimated based on the healthcare-seeking behavior of patients with influenza-like illness (ILI) symptoms in Japan (75.5%) which was adjusted using the ratio of medically-attended ARIs vs ILI in Hong Kong (0.687) [Citation24,Citation25]. The overall medically attended RSV cases were distributed among RSV-URTD and RSV-LRTD, based on the ratio of medically attended LRTD and URTD proportions in the AReSVi-006 study (medically attended RSV-LRTD proportion: 63/139 [45.3%]; medically attended RSV-URTD proportion: 31/153 [20.3%] and ratio of medically attended RSV-LRTD vs RSV-URTD: 2.24) [Citation26].
Seasonality of RSV incidence in older adults was assumed to be similar to that reported for pediatric patients but with a two-month shift, such that the RSV peak occurs two months later, i.e., in November () [Citation27,Citation28]. Seasonal shifts of RSV infections in the older adult population vs infant and pediatric populations have been reported previously [Citation28]. Since the natural RSV epidemiology was perturbed during the coronavirus disease 2019 (COVID-19) pandemic, surveillance data between 2016 and 2019 were used to obtain the typical seasonal pattern [Citation29].
RSV-LRTD mortality rates were estimated using multiple data sources: inpatient mortality rates reported for prospectively enrolled patients (in- and outpatients) with RSV-related pneumonia in Japan (3.37%); the proportion of patients with medically attended RSV, who developed pneumonia (27.44%); the proportion of patients with RSV-LRTD (47.60%); and the proportion of medically attended RSV (51.8%) () [Citation23–25,Citation30,Citation31]. The resulting RSV-LRTD mortality rate (1.01%) was converted into an age-dependent mortality distribution such as to match that reported in a US burden-of-illness model [Citation32]. All-cause mortality was sourced from 2021 Japanese Abridged Life Tables contained in official Japanese statistics [Citation33].
2.2.3. Vaccine efficacy
Peak vaccine efficacy against RSV-ARI (74.17%) and RSV-LRTD (88.02%) and the monthly waning of efficacy were modeled using linear regression to fit data from a phase 3 clinical study with RSVPreF3 OA (AReSVi-006) including data from season 1 and season 2 [Citation34]. During the first month after vaccination, vaccine efficacy was assumed to be 50% of peak vaccine efficacy. From the start of month 2, a monthly waning rate was applied to vaccine efficacy. Estimated monthly waning rates were 2.26% for vaccine efficacy against RSV-ARI and 2.10% for vaccine efficacy against RSV-LRTD (). Vaccination coverage was set to 50% in line with influenza vaccine coverage observed in older adults in Japan prior to the COVID-19 pandemic, which varied between 47.9% and 55.9% from 2005 to 2019 [Citation35]. In the base case analysis, people were vaccinated in September, two months prior to the assumed seasonal RSV peak, and no re-vaccination took place during the following three years.
2.2.4. Healthcare resource use (HCRU)
The proportion of medically attended RSV episodes was estimated at 51.8% (72.9% for LRTD and 32.6% for URTD as described above) [Citation24–26]. Number of outpatient visits (e.g. visits to healthcare centers, physicians, or ambulatory care) and hospitalizations for RSV-URTD and RSV-LRTD were estimated from non-RSV specific Statistics of 2021 Medical Activities in Public Health Insurance contained in official Japanese statistics [Citation36]. Health insurance data included the number of claims, total medical fee points, and total medical treatment days. Data were divided into outpatient/inpatient, <75 years/≥75 years and by disease. Diseases described as pneumonia and acute bronchitis/acute bronchiolitis were categorized as LRTDs, while acute nasopharyngitis, acute pharyngitis/acute tonsillitis and other acute upper respiratory tract infections were categorized as URTDs. The number of claims was assumed to be the number of patients. The number of patients aged 60–74 years was adjusted by the estimated number of patients by age reported in Patient Survey 2020 contained in official Japanese statistics [Citation37]. The frequency of outpatient visits and hospitalizations related to RSV-URTD and RSV-LRTD was weighted by the number of patients for each disease and multiplied by the medically attended rate for RSV-related URTD and LRTD ().
2.3. Reported outputs
The model estimated the number of the following RSV-related events: ARI, URTD, and LRTD. The number of outpatient visits, hospitalizations and LRTD-related deaths was estimated, and the number needed to vaccinate (NNV) to prevent one event was calculated. All outcomes were reported over a three-year time horizon.
2.4. Sensitivity and scenario analyses
Deterministic sensitivity analyses (DSA) were carried out by varying epidemiology, vaccine efficacy, and HCRU input parameters along pre-specified confidence intervals. These intervals were derived from published literature; if data regarding variance and uncertainty were not available, the parameters (number of hospitalizations and vaccination coverage) were varied by ≥ 50% ().
Scenario analyses were carried out by varying the age at vaccination (i.e. ≥65 years, ≥70 years, ≥ 75 years), coverage (i.e. 25%, 75% and 100%) and vaccination timing (i.e. 6 or 3 months before the base case and 3 months after the base case).
3. Results
The total population consisted of 43,681,000 adults aged ≥60 years. Without vaccination, the model projects that over the next three years, approximately 5.3 million RSV-ARI cases (1.8 million in the first year) occur. Among these symptomatic RSV-ARI cases, approximately 2.8 million can be attributed to RSV-URTD (951,000 in the first year) and 2.5 million to RSV-LRTD (864,000 in the first year). RSV-URTD and RSV-LRTD would lead to approximately 1.3 and 2.1 million outpatient visits, respectively (454,000 and 726,000 in the first year), while an estimated 534,000 RSV-related hospitalizations would occur (184,000 in the first year) ().
Table 2. PHI of RSVPreF3 OA vaccine over 3 years (base case).
In the base-case scenario assuming a vaccination coverage of 50%, approximately 22 million older adults aged ≥60 years would receive RSVPreF3 OA vaccine. With RSVPreF3 OA vaccination, >1,008,000 RSV-ARI cases could be prevented over a time period of 3 years. As a consequence, healthcare resource use would be reduced: approximately 728,000 RSV-related outpatient visits and 143,000 RSV-related hospitalizations would be avoided over a three-year time period. In addition, RSV deaths could be reduced by 6,840 cases (). The estimated NNV to avoid one RSV infection was 22, and NNVs to prevent one hospitalization and one death are 153 and 3,194, respectively ().
The results of the DSA are presented as tornado diagram in . DSA results were most sensitive to variations in RSV incidence. In all one-way sensitivity analyses considered, the number of cases avoided by vaccination varied between 504,250 and 1,845,616 for RSV-ARI 71,594 and 261,180 for hospitalizations, and 3,420 and 12,475 for RSV-deaths.
Figure 2. Tornado diagram – DSA results.
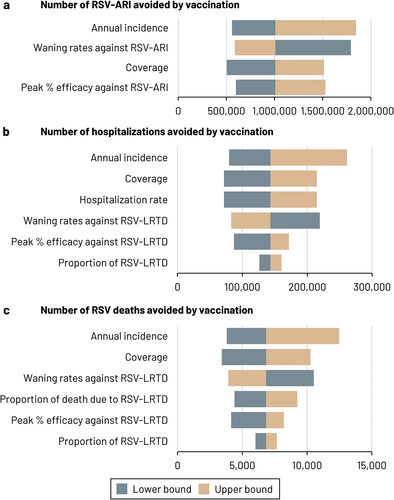
Scenario analyses show that vaccinating people at older age (e.g. ≥75 years) would limit the potential positive public health impact of the RSV vaccine, since fewer people would be protected against RSV infection, thereby decreasing the numbers of RSV hospitalizations and deaths avoided by 29 and 37% points, respectively (). Increasing coverage has a beneficial effect on the numbers of cases avoided and 100% coverage could prevent approximately 2 million RSV-ARI over a three-year time horizon. Based on model results, the most favorable vaccination timing would be 2 months prior to peak month (seasonal peak is expected in November based on pre-pandemic data). Vaccinating people later (1 month after seasonal peak) or earlier (5 or 8 months prior to seasonal peak) would diminish the number of RSV cases, hospitalizations and deaths avoided by approximately 20%.
Table 3. Scenario analyses.
4. Discussion
Development of an RSV vaccine has been a long-standing public health priority due to the high disease burden imposed by RSV on patients and the healthcare sector. The potential impact of circulating RSV on the healthcare system became particularly evident during the post-COVID-19 era. After protective health measures imposed during the pandemic were gradually lifted, a surge in RSV and other respiratory infections was observed, favored by increased testing and awareness for respiratory infections, leading to increased affluence to hospitals [Citation38]. In Japan and elsewhere, out-of-season resurgence of RSV infections was observed [Citation39,Citation40]. As there are no specific treatments available against RSV, vaccination against RSV could not only reduce the disease burden on patients but may effectively reduce the pressure on hospitals during seasonal RSV peaks.
In this study, assuming a coverage of 50% based on influenza vaccine uptake in Japan, RSVPreF3 OA vaccine was estimated to reduce RSV-hospitalizations and deaths by 27% in the older adult population (≥60 years) over a time period of 3 years. The number of RSV-ARI, hospitalizations and deaths were most sensitive to the annual RSV incidence. Estimates of RSV incidence published in the literature were in general lower compared with the RSV incidence used in this study (4.2%), since previous studies did not consider the potential for under-detection of RSV cases [Citation3]. In our model, recent insight from systematic literature reviews was used to adjust reported RSV incidence rate for under-ascertainment, i.e. missed cases due to specific testing or sampling method used [Citation10,Citation11,Citation41]. The number of medically attended RSV cases and hospitalizations might be higher in Japan compared with other regions of the world, due to universal health insurance in Japan which promotes accessibility to healthcare services [Citation42]. Adjusting RSV incidence and healthcare resource use by considering RSV under-ascertainment, may better reflect the potential public health impact of RSVPreF3 OA vaccine. In a recent model for predicting trends of excess mortality in the overall population in Japan, it was estimated that RSV was associated with approximately 34,000 deaths annually, exceeding the 8,760 RSV-related deaths in older adults aged ≥60 years estimated in this study [Citation43]. Although direct comparison between these results is difficult due to differences in methodology and population, results emphasize the high mortality burden imposed by RSV.
There are only few studies evaluating the impact of RSV vaccination on disease burden and therefore, comparison to similar studies is limited. In the US context, using the same static Markov model, vaccination with RSVPreF3 OA in older adults aged ≥60 years resulted in a 53% reduction of RSV-LRTD cases and a 55.6% reduction of RSV-related deaths over a 1-year time horizon [Citation44]. The higher benefit observed in the US study may stem from higher coverage rates (52.4% in the 60–64 years age group; 73.9% in the ≥65 years age group) and higher vaccine efficacy during season 1 compared with cumulative vaccine efficacy over several seasons. In a scenario analysis with coverage set to 75%, RSVPreF3 OA immunization would lead to a 40% reduction of RSV hospitalizations and RSV-related deaths in the Japanese setting. Thus, a high coverage is essential to maximize public health impact. Inclusion of RSV vaccines in the Japanese NIP is likely to increase uptake. Indeed, a survey in older adults in Japan showed that eligibility for subsidy for pneumococcal vaccination under the routine NIP was highly correlated with vaccine uptake [Citation45]. In addition, immunization of the older adult population is of particular importance in Japan, which has one of the highest proportions of older adults worldwide. Another factor to consider by policy makers is the duration of vaccine efficacy. Vaccine efficacy was assessed over two seasons during the AReSVi-006 phase 3 clinical study, and regression analysis using these data suggested that protection against RSV disease extended into season 3 [Citation19]. Thus, the public health impact was estimated over 3 years (without re-vaccination) in the base-case analysis.
In scenario analyses, the age at vaccination was varied from ≥60 years in the base-case analysis, to ≥ 65, ≥70 and ≥75 years. Raising the age at vaccination leads to a reduction in overall case avoidance and diminishes the potential positive public health impact. This result is highly dependent on age-specific input parameters and further studies are needed to determine age-specific incidence of RSV-related infections, hospitalizations and deaths.
There are several limitations to this study. First, an indirect protection was not included. Vaccination with RSVPreF3 OA may indirectly protect unvaccinated individuals in the case that enough people in their entourage receive the vaccine and disease transmission is interrupted. Incorporation of herd effect would require the use of a dynamic rather than a static model and is expected to lead to higher estimates of public health impact. Second, there is uncertainty regarding the incidence of symptomatic and/or medically attended RSV-ARI overall and across age groups in Japan. Reasons for this uncertainty are several-fold: routine testing for RSV infection is not performed as part of daily practice in Japan, given that available treatment options are for symptom relief and not RSV-specific [Citation4]. Consequently, symptomatic RSV incidence in Japan was derived from a single observational study over one season only. Medically attended RSV cases were estimated based on the reported RSV incidence and published healthcare seeking behavior of Japanese and Chinese patients with ILI and ARI. Conventional RSV testing may be affected by significant false negative rates. An under-ascertainment factor of 1.75 was applied to reported incidence data, to account for missed cases; this assumption is supported by results from two systematic reviews that indicated that the true RSV incidence might be up to 2.2-fold higher compared with reported RSV incidence [Citation10,Citation11]. Furthermore, an accurate description of the seasonal pattern of RSV incidence in older adults is missing. In this study, the seasonal pattern was based on that observed in pediatric patients before the COVID-19 pandemic as RSV incidence was unstable during the pandemic. Surveillance data post-pandemic suggests that the seasonal RSV pattern still differs from that prior to the pandemic [Citation39]. Further studies or routine surveillance, using sensitive detection methods, would be needed to obtain accurate estimates of annual RSV incidence rates and seasonal variations in the post-pandemic era for the older adult population in Japan. There remains uncertainty regarding the actual RSV-related HCRU in Japan. Claims data used in this study are typically not RSV-specific but records only contain information regarding URTD or LRTD, regardless of causative pathogens. Additional studies are needed to accurately describe RSV-specific epidemiology and HRCU in older adults in Japan.
5. Conclusion
Inclusion of RSVPreF3 OA vaccination in the Japan NIP is predicted to have a beneficial public health impact, helping protect the older adult population (≥60 years) against RSV-related respiratory disease and alleviating the burden to the healthcare system.
Abbreviations
| ARI | = | acute respiratory infection |
| DSA | = | deterministic sensitivity analysis |
| FDA | = | Food and Drug Administration |
| HCRU | = | healthcare resource use |
| LRTD | = | lower respiratory tract disease |
| NIP | = | national immunization program |
| NNV | = | number needed to vaccinate |
| RSV | = | respiratory syncytial virus |
| RSVPreF3 OA | = | RSV prefusion F protein-based vaccine for older adults |
| URTD | = | upper respiratory tract disease |
Declaration of interest
A Mizukami, F Verelst, D Molnar and YF Ho are employed by GSK. F Verelst, D Molnar and Y Ho also hold shares in GSK. V Preckler was employed by and held shares in GSK during the study conduct. T Matsuki was employed and held shares by GSK during the study conduct and is currently employed by MSD. D Kurai received consulting fees from GSK in the context of this work. D Kurai also received consulting fees from GSK, Janssen Pharmaceutical K.K., Daiichi Sankyo Company, Limited and Asahi Kasei Pharma Corporation outside of this work and honoraria from GSK, Shionogi & Co., Ltd., Gilead Sciences, Janssen Pharmaceutical K.K., Taisho Pharmaceutical Co., Ltd., Kaken Pharmaceutical CO., LTD., AstraZeneca K.K., Kyorin Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd. and Alfresa Corporation outside of this work. D Kurai’s institution also received donation from Asahi Kasei Pharma Corporation, Maruishi Pharmaceutical. Co., Ltd., Shionogi & Co., Ltd. and Kyorin Pharmaceutical Co., Ltd. outside of this work and research funding from Daiichi Sankyo Company, Limited outside of this work. A Igarashi received payment from GSK for the conduct of this work and payments from Takeda Pharmaceuticals, Pfizer, Moderna and MSD, outside of this work. These authors declare no other financial and non-financial relationships and activities and no conflict of interest.
Reviewer disclosures
Peer reviewers on this manuscript have received honoraria for their review work. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Author contributions
All authors participated in the design or implementation or analysis, and interpretation of the study, and the development of this manuscript. All authors had full access to the results and gave final approval before submission.
Trademark
AS01 is a trademark owned by or licensed to GSK.
Previous presentations
JSVAC | 20–21 October 2023 |Shizuoka, Japan
Geolocation information
Japan.
Acknowledgments
The authors would like to thank Shoko Akiyama who provided support for the study. The authors would also like to thank Business & Decision Life Sciences Medical Communication Service Center for editorial assistance and manuscript coordination, on behalf of GSK. Katrin Spiegel provided medical writing support.
Data availability statement
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to https://www.gsk-studyregister.com/en. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an enquiry via the website.
Additional information
Funding
References
- Lambert L, Sagfors AM, Openshaw PJ, et al. Immunity to RSV in early-life. Front Immunol. 2014;5:466. doi: 10.3389/fimmu.2014.00466
- Shi T, Denouel A, Tietjen AK, et al. Global disease burden estimates of respiratory syncytial virus–associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis. 2020;222(Supplement_7):S577–S583. doi: 10.1093/infdis/jiz059
- Savic M, Penders Y, Shi T, et al. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: a systematic literature review and meta-analysis. Influenza Other Respir Viruses. 2023 Jan;17(1):e13031.
- Nam HH, Ison MG. Respiratory syncytial virus infection in adults. BMJ. 2019;366(I5021):l5021. doi: 10.1136/bmj.l5021
- Villanueva DH, Arcega V, Rao M. Review of respiratory syncytial virus infection among older adults and transplant recipients. Ther Adv Infect Dis. 2022 Jan;9:20499361221091413. doi: 10.1177/20499361221091413
- Kurai D, Natori M, Yamada M, et al. Occurrence and disease burden of respiratory syncytial virus and other respiratory pathogens in adults aged ≥65 years in community: a prospective cohort study in Japan. Influenza Resp Viruses. 2022 Mar;16(2):298–307. doi: 10.1111/irv.12928
- Igarashi A, Togo K, Kobayashi Y, et al. Inpatient and outpatient costs associated with respiratory syncytial virus in Japanese infants and older adults. Future Virol. 2023;18(10):643–657. doi: 10.2217/fvl-2023-0069
- National Institute of Infectious Diseases (NIID). A case of RSV-B outbreak in a nursing home during the new coronavirus epidemic. IASR. 2022;43(4):87–88.
- Stephens LM, Varga SM. Considerations for a respiratory syncytial virus vaccine targeting an elderly population. Vaccines (Basel). 2021 Jun 9;9(6):624. doi: 10.3390/vaccines9060624
- Li Y, Kulkarni D, Begier E, et al. Adjusting for case under-ascertainment in estimating RSV hospitalisation burden of older adults in high-income countries: a systematic review and modelling study. Infect Dis Ther. 2023 Apr;12(4):1137–1149. doi: 10.1007/s40121-023-00792-3
- McLaughlin JM, Khan F, Begier E, et al. Rates of medically attended RSV among US adults: a systematic review and meta-analysis. Open Forum Infect Dis. 2022 Jul;9(7):ofac300.
- Belongia EA, King JP, Kieke BA, et al. Clinical features, severity, and incidence of RSV illness during 12 consecutive seasons in a community cohort of adults >/=60 years old. Open Forum Infect Dis. 2018 Dec;5(12):ofy316.
- Falsey AR, Hennessey PA, Formica MA, et al. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–1759. doi: 10.1056/NEJMoa043951
- Ackerson B, Tseng HF, Sy LS, et al. Severe morbidity and mortality associated with respiratory syncytial virus versus influenza infection in hospitalized older adults. Clin Infect Dis. 2019 Jul 2;69(2):197–203. doi: 10.1093/cid/ciy991
- Lee N, Lui GC, Wong KT, et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infect Dis. 2013 Oct;57(8):1069–77.
- Anderson LJ, Dormitzer PR, Nokes DJ, et al. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine. 2013 Apr 18;31(Suppl 2):B209–B215. doi: 10.1016/j.vaccine.2012.11.106
- World Health Organization. WHO preferred product characteristics for respiratory syncytial virus (RSV) vaccines. Organization WH, editor. Geneva: Department of Immunization, Vaccines and Biologicals; 2017.
- Leroux-Roels I, Davis MG, Steenackers K, et al. Safety and immunogenicity of a respiratory syncytial virus prefusion F (RSVPreF3) Candidate vaccine in older adults: phase 1/2 randomized clinical trial. J Infect Dis. 2023 Mar 28;227(6):761–772. doi: 10.1093/infdis/jiac327
- GSK. GSK shares positive data for Arexvy, its respiratory syncytial virus (RSV) older adult vaccine, indicating protection over two RSV seasons. 2023 [cited 2023 Jun]. Available from: https://www.gsk.com/en-gb/media/press-releases/gsk-shares-positive-data-for-arexvy-its-respiratory-syncytial-virus-older-adult-vaccine-indicating-protection-over-two-rsv-seasons/
- Papi A, Ison MG, Langley JM, et al. Respiratory syncytial virus prefusion F protein vaccine in older adults. N Engl J Med. 2023;388(7):595–608. doi: 10.1056/NEJMoa2209604
- Colosia A, Costello J, McQuarrie K, et al. Systematic literature review of the signs and symptoms of respiratory syncytial virus. Influenza Other Respir Viruses. 2023 Feb;17(2):e13100.
- Portal site of Official Statistics of Japan (e-STAT). Estimation of population 2022. [cited 2023 Jun]. Available from: https://www.e-stat.go.jp/stat-search/files?page=1&toukei=00200524&tstat=000000090001
- Ison MG, Papi A, Langley JM, et al. editors. Efficacy of one dose of the respiratory syncytial virus (RSV) prefusion F protein vaccine (RSVPreF3 OA) in adults IDWeeK. Boston MA (US): Oxford University Press; 2023.
- Tsuzuki S, Yoshihara K. The characteristics of influenza-like illness management in Japan. BMC Public Health. 2020 Apr 28;20(1):568. doi: 10.1186/s12889-020-08603-x
- Zhang Q, Feng S, Wong IOL, et al. A population-based study on healthcare-seeking behaviour of persons with symptoms of respiratory and gastrointestinal-related infections in Hong Kong. BMC Public Health. 2020 Mar 27;20(1):402. doi: 10.1186/s12889-020-08555-2
- GSK Data on File. 2023N539973_00.
- National Institute of Infectious Diseases. Annual report (2020): sentinel-reporting diseases (monthly) - number of cases and number of cases per sentinel by year, sex, and month. 2020 [cited 2023 Jun]. Available from: https://www.niid.go.jp/niid/images/idwr/ydata/2020/Syuukei/Syu_22_3.xlsx
- Takahashi H, Sato Y, Yajima T, editors. #193 diagnostic status and clinical picture of adult RSV infection cases after the COVID-19 pandemic. The 71st Japanese Association for Infectious Diseases East Japan meeting/The 69th Japanese Society of Chemotherapy East Japan branch meeting 2022; Sapporo.
- Miyama T, Iritani N, Nishio T, et al. Seasonal shift in epidemics of respiratory syncytial virus infection in Japan. Epidemiol Infect. 2021 Feb 11;149:e55. doi: 10.1017/S0950268821000340
- Katsurada N, Suzuki M, Aoshima M, et al. The impact of virus infections on pneumonia mortality is complex in adults: a prospective multicentre observational study. BMC Infect Dis. 2017 Dec 6;17(1):755. doi: 10.1186/s12879-017-2858-y
- Nguyen-Van-Tam JS, O’Leary M, Martin ET, et al. Burden of respiratory syncytial virus infection in older and high-risk adults: a systematic review and meta-analysis of the evidence from developed countries. Eur Respir Rev. 2022 Dec 31;31(166):220105. doi: 10.1183/16000617.0105-2022
- Molnar D, La E, Verelst F, editors. 33 - modeling the clinical and economic burden of respiratory syncytial virus (RSV) among adults ≥60 years of age in the United States. A Global Conference on Novel RSV Preventative and Therapeutic Interventions - RSVVW’23 7th ReSViNET Conference; 2023; Lisbon, Portugal: ReSViNET - Respiratory Syncytial Virus Foundation.
- Portal site of Official Statistics of Japan (e-STAT). Abridged life tables 2021 2021 [cited 2023 Apr]. Available from: https://www.e-stat.go.jp/stat-search/files?page=1&query=%E7%B0%A1%E6%98%93%E7%94%9F%E5%91%BD%E8%A1%A8&layout=dataset
- Ortega Sanchez I Advisory Committee on Immunization Practices (ACIP) held on June 21, 2023. 2023 [cited 2023 Jun]. Available from: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/05-RSV-Adults-Ortega-Sanchez-508.pdf
- Ministry of Health Labour and Welfare (MHLW). The number (%) of those who got vaccinated in the national immunization program [cited 2023 Apr]. Available from: https://www.mhlw.go.jp/topics/bcg/other/5.html
- Portal site of official statistics of Japan (e-STAT). Statistics Of Medical Activities In Public Health Insurance 2021. 2021 [cited 2023 Apr]. Available from: https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00450048&tstat=000001029602&cycle=7&tclass1=000001166295&tclass2=000001166326&tclass3=000001166327&tclass4val=0
- Portal site of official statistics of Japan (e-STAT). Patient Survey 2020. 2020 [cited 2023 Apr]. Available from: https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00450022&tstat=000001031167&cycle=7&tclass1=000001166809&tclass2=000001166811&tclass3=000001166812&tclass4=000001166813&cycle_facet=tclass1%3Acycle&tclass5val=0&metadata=1&data=1
- European Centre for Disease Prevention and Control (ECDC). Intensified circulation of respiratory syncitial virus (RSV) and associated hospital burden in the EU/EEA Stockholm 2022. [cited 2023 Jun]. Available from: https://www.ecdc.europa.eu/en/publications-data/intensified-circulation-respiratory-syncytial-virus-rsv-and-associated-hospital
- National Institute of Infectious Diseases (NIID). Infectious disease - surveillance. 2023 [cited 2023 Jul]. Available from: https://www.niid.go.jp/niid/en/iasr-e.html
- Liu P, Xu M, Lu L, et al. The changing pattern of common respiratory and enteric viruses among outpatient children in Shanghai, China: two years of the COVID-19 pandemic. J Med Virol. 2022 Oct;94(10):4696–4703.
- Onwuchekwa C, Moreo LM, Menon S, et al. Underascertainment of respiratory syncytial virus infection in adults due to diagnostic testing limitations: a systematic literature review and meta-analysis. J Infect Dis. 2023;228(2):173–184. doi: 10.1093/infdis/jiad012
- Interac. Japan’s Healthcare System vs The US | Statistics & Cost Comparison. 2023. Available from: https://interacnetwork.com/japans-healthcare-system-vs-the-us-statistics-cost-comparison/#:~:text=Comparative%20statistics,healthy%20lifestyles%2C%20and%20preventing%20disease
- Mizumoto K, Chowell G, Simonsen L, et al. The burden of influenza and respiratory syncytial viruses in Japan, 2006–2014: a regionand age-specific excess mortality study. Inter J Infect Dis. 2019;79:1–150. doi: 10.1016/j.ijid.2018.11.241
- Molnar D, La E, Verelst F, et al. editors. Assessing the public health impact of the adjuvanted respiratory syncytial virus prefusion F protein vaccine among older adults in the United States (US). Boston (US): ISPO RRTI(h)(s); 2023.
- Shono A, Hoshi SL, Kondo M. The impact on vaccination coverage following introduction of a routine pneumococcal vaccination programme for the elderly in Japan. Vaccine. 2018 Sep 18;36(39):5886–5890. doi: 10.1016/j.vaccine.2018.08.023

