ABSTRACT
Introduction
Following the coronavirus disease pandemic, respiratory mucosal vaccines that elicit both mucosal and systemic immune responses have garnered increasing attention. However, human physiological characteristics pose significant challenges in the evaluation of mucosal immunity, which directly impedes the development and application of respiratory mucosal vaccines.
Areas Covered
This study summarizes the characteristics of immune responses in the respiratory mucosa and reviews the current status and challenges in evaluating immune response to respiratory mucosal vaccines.
Expert Opinion
Secretory Immunoglobulin A (S-IgA) is a major effector molecule at mucosal sites and a commonly used indicator for evaluating respiratory mucosal vaccines. However, the unique physiological structure of the respiratory tract pose significant challenges for the clinical collection and detection of S-IgA. Therefore, it is imperative to develop a sampling method with high collection efficiency and acceptance, a sensitive detection method, reference materials for mucosal antibodies, and to establish a threshold for S-IgA that correlates with clinical protection. Sample collection is even more challenging when evaluating mucosal cell immunity. Therefore, a mucosal cell sampling method with high operability and high tolerance should be established. Targets of the circulatory system capable of reflecting mucosal cellular immunity should also be explored.
1. Introduction
Influenza viruses, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and respiratory syncytial viruses (RSV) represent a category of respiratory viruses characterized by short incubation periods, rapid replication, and pathogenesis primarily in the respiratory mucosal tissues [Citation1]. They pose a challenge in inducing long-term protective immunity in humans, exhibit high reinfection rates, and significantly threaten global public health safety [Citation2–4]. Vaccination is one of the most effective and economical methods of controlling infectious diseases. Vaccines administered via intramuscular injection can effectively induce high levels of circulating antibodies, effector and memory B cells, and T cells, thereby reducing disease severity and mortality rates [Citation5–7]. However, they fail to induce mucosal immune responses; therefore, they do not effectively prevent infection [Citation8–11]. Respiratory mucosal vaccines not only trigger systemic immune responses, but also induce mucosal antibodies, tissue-resident memory lymphocytes, and trained immunity in the airways, forming a mucosal immune barrier against viral invasion and providing comprehensive protection to the body () [Citation12–14]. Additionally, respiratory mucosal vaccines are noninvasive and suitable for mass vaccination during infectious disease pandemics [Citation15,Citation16]. Therefore, they are considered as ideal vaccines for preventing influenza, SARS-CoV-2 and other respiratory pathogens. Although the development of respiratory mucosal vaccines has been ongoing for decades, only four types of influenza mucosal vaccines and five types of COVID-19 mucosal vaccines have been approved for mass distribution and emergency use () [Citation25]. Mucosal vaccine development has been hampered by several factors, including: 1) robust mucosal immunity hinders antigen delivery and immune response activation of mucosal vaccines, 2) the lack of standardized assays and sampling protocols implicates immunogenicity assessments, 3) concern over safety, and 4) lack of investment. Substantial research has been conducted on the antigen design, adjuvants and delivery systems to develop an effective mucosal vaccine [Citation26–29]. To assess the immunogenicity of mucosal vaccine, secretory immunoglobulin A (S-IgA), tissue residue memory lymphocytes, innate cells, circulating antibodies and cytotoxic T cells induced by mucosal vaccines are detected in clinical trials.
Figure 1. Immune characteristics of respiratory mucosal vaccines.
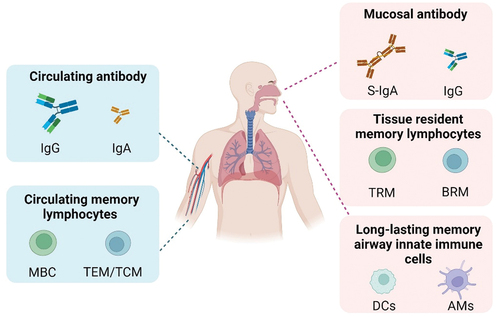
Table 1. Mucosal immunogenicity evaluation of approved respiratory mucosal vaccines.
This study reviews the characteristics of respiratory mucosal immune responses and summarizes the current status and challenges in the clinical assessment of immune responses to respiratory mucosal vaccines, thus providing a reference for the development of clinical evaluation methods for these vaccines.
2. Characteristics of respiratory mucosal immune responses
2.1. Mucosal antibodies
Antibodies are the main effector molecules at mucosal sites and originate from two primary sources: local S-IgA and circulatory system-infiltrating IgG. In the upper respiratory tract, S-IgA is the predominant immunoglobulin, exhibiting a level that is approximately three times higher level than that of IgG antibodies, and it plays a leading role in protection against upper respiratory tract infections [Citation30,Citation31]. In the lamina propria of mucosal sites, IgA produced by plasma cells can bind to the polymeric immunoglobulin receptor (pIgR) expressed on the basolateral side of the mucosal epithelial cells and is transported to the mucosal surface [Citation32]. Upon arrival at the mucosal surface, the extracellular portion of pIgR is cleaved and incorporated into the IgA structure as a secretory component (SC), referred to as S-IgA. Unlike IgA in serum, mucosal S-IgA primarily exists as a dimer [Citation33,Citation34]. Compared with monomeric IgA and IgG, S-IgA has a higher affinity and stronger neutralizing activity, effectively inhibiting pathogen adhesion, colonization, or invasion of mucosal surfaces and preserving the integrity of the mucosal barrier [Citation35–38]. This phenomenon may be related to the presence of multiple antigen-binding sites in S-IgA. Clinical studies on respiratory viruses like influenza and SARS-CoV-2 have shown that specific S-IgA levels rise rapidly between 7–15 days post-infection and then plateau, with S-IgA immune responses lasting for 3–9 months [Citation39–42]. There is evidence that antigen-specific S-IgA correlates with protection against infection by respiratory viruses [Citation41,Citation43–46]. Havervall et al. showed that participants with higher levels of SARS-CoV-2 spike-specific S-IgA had a significantly lower risk of subsequent Omicron breakthrough infections than those with lower levels. In a challenge study with Omicron BA.1.1 in mice, Wang et al. found that mice received nasal S-IgA from intranasal Ad5-S-Omicron vaccines had a lower viral load than mice that received nasal lavage fluids from a non-intranasal vaccine. Research by Sho et al. highlighted the importance of anti-spike S-IgA responses in individuals infected with SARS-CoV-2 to prevent viral shedding and SARS-CoV-2 transmission [Citation47]. Wagstaffe et al. Demonstrated the early IgA response strongly consistent with viral clearance [Citation48]. Therefore, the key to developing an effective mucosal vaccine is its ability to efficiently induce antigen-specific S-IgA.
2.2. Tissue resident memory lymphocytes
Infections with respiratory pathogens or immunization with respiratory mucosal vaccines can induce tissue resident memory lymphocytes in the respiratory tract [Citation49–51]. These cells act as sentinels at the respiratory mucosal sites and promptly identify and proliferate upon secondary pathogen invasion.
Following viral infection or mucosal vaccine immunization, CD8+ tissue-resident memory (TRM) cells are characterized by the co-expression of CD69, CD103, and CXCR3, and the downregulation of CD62L, CCR7 and S1PR1, and are widespread throughout the respiratory tract and associated lymph nodes [Citation52–54]. Upon secondary exposure to pathogens, CD8+ TRM cells immediately recognize infected cells and antigenic epitopes presented by dendritic cells, which rapidly secrete effector molecules such as cytokines, perforin, and granzymes to kill infected cells [Citation55]. Studies have shown that respiratory CD8+ TRM plays an instrumental role in preventing respiratory viral infections. Based on a mouse model, Wu et al. emphasize the indispensable role of CD8+ TRM in facilitating cross-protective immunity against the influenza virus. Mice lacking influenza-specific CD8+ TRM in the lungs, even with abundant influenza-specific CD8+ TEM and TCM in the circulation, could not resist the attack of different influenza virus subtypes 6–7 months after the initial infection [Citation56].
CD4+ TRM cells located in the bronchus-associated lymphoid tissue and around the trachea play an important role in accelerating the clearance of respiratory pathogens [Citation57–59]. Shenoy et al. showed that CD4+ TRM cells secreting IL-17 promote the stable transcriptional expression of CXCL5 in lung epithelial cells, thereby recruiting neutrophils for the clearance of pneumococcus [Citation58]. Additionally, CD4+ TRM cells, through the secretion of IFN-γ, promote the differentiation of CD8+ TRM cells, speeding up the clearance of respiratory pathogens [Citation60].
In the late stages of immunization, B cell subsets in the germinal centers differentiate into tissue-resident memory B cells (BRM), which are rich in surface expressions of CXCR3, CCR6, and CD69, and settle in the bronchus-associated lymphoid tissue or the lung parenchyma near the alveoli [Citation61,Citation62]. Lung BRMs has a different expression profile compared with splenic memory B cells (MBC), characterized by increased expression of chemokine receptors; downregulation of lymphoid homing factors, G-protein-coupled receptors, and related regulatory proteins; and the absence of germinal center markers [Citation63]. Upon re-invasion by the same pathogen, BRMs rapidly differentiate into plasma cells and produce mucosal antibodies, thereby accelerating pathogen clearance. A study by Oh et al. highlighted the critical role of tissue-resident IgA-secreting B cells in the establishment of protective immunity in the lungs. Using a mouse model, they show that homologous and heterologous immunity against the influenza virus was maintained in mice previously immunized with intranasal influenza virus or recombinant influenza vaccine, even when circulating and tissue-resident T cells in the lungs were depleted [Citation64].
2.3. Trained immunity
During the induction of respiratory pathogens or mucosal vaccines, innate immune cells such as monocytes and macrophages undergo the epigenetic reprogramming of transcriptional pathways [Citation65]. When encountering the same or different antigens again, these cells can produce a more robust immune response, forming an innate immune memory, and exerting a stronger anti-infection protective effect [Citation65,Citation66]. Using murine models of acute respiratory viral infection, Yao et al. found that respiratory adenoviral infection elicited long-lived memory alveolar macrophages (AMs) with high MHC II expression, a defense-ready gene signature, increased glycolytic metabolism, and neutrophil chemokine production (MIP-2 and KC). Upon bacterial infection, memory AMs induce chemokines and neutrophilia to defend the infection within 48 h [Citation67]. The rapid response of innate memory cells at mucosal sites might be an ideal defense against respiratory viruses, which replicate locally and can be transmitted to others before encountering the full force of adaptive immune responses. Zhang et al. demonstrated that dNS1-receptor-binding domain (RBD) induced trained immunity that reprogrammed the chromatin accessibility of AMs, such as MHC II and several antiviral innate immune response genes, including Tlr3 and Tlr7. Trained immunity may provide a faster and stronger response to secondary infection [Citation68].
3. Current status and challenges in the clinical assessment of immune responses to respiratory mucosal vaccines
3.1. Current state and challenges in assessing mucosal antibody responses
S-IgA plays a critical role in mucosal immune responses [Citation69,Citation70]. It is an essential effector molecule in mucosal immunity and a common indicator for evaluating the immunogenicity of mucosal vaccines.
Mucosal vaccines, Ad5-nCoV-IH from CanSino Biologics (Tianjin, China) and BBV154 from Bharat Biotech (Hyderabad, India), based on Ad5 and Ad36 adenovirus vectors expressing the SARS-CoV-2 spike protein, have been approved for emergency use. In the phase IV clinical trial of Ad5-nCoV-IH and the phase III trial of BBV154, ELISA were used to measure SARS-CoV-2-specific IgA antibody titers in the saliva. The S-IgA seroconversion rates for Ad5-nCoV-IH at 14 and 28 days (39%, 42%) were significantly higher than those for the intramuscularly immunized Ad5-nCoV group (28%, 28%); the salivary IgA level at 42 days post-immunization with BBV154 was 12.3 EU/ml (95% CI: 8.4–13.5), slightly higher than the pre-immunization level of 10.7 EU/ml (95% CI: 8.7–17.4) [Citation20,Citation21]. dNS1-RBD, a nasal-spray vaccine based on a live-attenuated influenza virus vector expressing the SARS-CoV-2 RBD gene, has been approved for emergency use in China. ELISA results showed that the nasopharyngeal swab IgA antibody seroconversion rate for this vaccine was 13%, but its protective efficacy was 66.7% [Citation23,Citation71]. FluMist®, a seasonal influenza nasal spray vaccine produced by AstraZeneca (Cambridge, UK), was approved by the FDA in 2003 for use in individuals aged 2–49 years. In a human challenge study of 103 adults aged 18–45, ELISA was used to detect FluMist® and intramuscular influenza vaccine-induced nasal wash-specific IgA antibodies. Compared with the intramuscularly inactivated influenza vaccine, FluMist has a lower nasal wash-specific IgA antibody response rate. However, FluMist® showed a higher level of protection compared to the intramuscular inactivated vaccine [Citation18].
Although various respiratory mucosal vaccines have established methods for detecting mucosal antibodies, the detection levels of specific mucosal antibodies are relatively low. This may be related to insufficient collection capacity of the sampling methods and the low sensitivity of detection methods.
Saliva, nasal wash and nasal lining fluid are the most used as mucosal sample from the upper respiratory tract. While saliva is easy to collect, its susceptibility to physiological rhythms and enzyme content affects its stability. Nasal washes can yield large sample volumes. However, the variability in the amount of specific mucosal IgA recovered from nasal washes is quite high. Because the same volume of saline is instilled into the nostril, and a variable amount of nasal wash is returned. To reduce the viability caused by nasal washing, researchers normalize the specific IgA content to the total IgA content. Although the nasal wash procedure is well tolerated, it is not suitable for children. In contrast, nasal lining fluid collected using flocked swabs and synthetic absorptive matrix (SAM) strips are more tolerate. In a comparative study of three nasal secretion collection methods (nasal wash, flocked swabs, and SAM), de Silva demonstrate that SAM strips had a stronger collection capacity, with total IgA and influenza-specific IgA titers being 4–6 times higher than those collected by flocked swabs and nasal washes [Citation72]. This result is consistence with that reported by Jochems. In addition, the total IgA content in samples collected from the left and right nostrils using SAM strips were similar. However, a clinical study by Bergin et al. showed that the titer of HIV antibodies obtained by flocked swabbing of nasal turbinate was higher than those collected by SAM absorption [Citation73]. The starkly different results of these two comparative studies may be related to the handling method, placement, duration of the matrix, and the site and frequency of swabbing.
Establishing robust and efficient sampling methods for mucosal antibody evaluation is challenging. To address this challenge, systematic comparative studies on nasal secretion collection methods that use Design of Experiment (DOE) tools are needed to deeply explore the impact of the operation details of a single method on antibody collection capacity. The factors that should be considered are: for nasal wash, factors such as the nasal wash device, buffer volume, number of rinses, and rinse intensity; for SAM strips, factors such as the pre-treatment method, placement, and duration of the strips; and for nasal swabs, factors such as swab material and size, scraping location, and frequency. After determining the optimal operations of each collection method, further comparisons of the collection capacity and robustness of the different nasal sampling methods can be conducted.
The titer of mucosal antibodies is low, approximately one two-hundredth to one five-hundredth that of serum antibodies [Citation74]. Therefore, ensuring high sensitivity of detection methods is also a challenge in mucosal antibody detection. As a commonly used antibody detection method, ELISA has the advantages of being accessible, easy to operate, and inexpensive. A phase III clinical trial of Ad5-nCoV-IH used a commercial SARS-CoV-2 RBD antibody ELISA kit to measure specific IgA titers in saliva with a linear range of 2–20 BAU/ml [Citation20]. However, whether this range meets the sensitivity requirements for clinical mucosal samples remain unknown. Meso Scale Discovery (Meso Scale Diagnostics LLC, Rockville, MD, U.S.A.) established a SARS-CoV-2 IgA antibody detection kit based on electrochemiluminescence technology, which can simultaneously detect specific IgA against 10 types of SARS-CoV-2 variants with high sensitivity and a linear detection range of 0.0109–1.56 BAU/ml for the original SARS-CoV-2 RBD [Citation75]. However, this kit is expensive, requires specific equipment, and lacks applicable data for mucosal antibodies. Currently, there is a lack of studies comparing ELISA and electrochemiluminescence technology for mucosal antibodies. Subsequent evaluations can be based on a certain number of negative and positive mucosal samples to assess the differences between the two methods in terms of positive detection rate and antibody detection consistency.
The use of standard materials is important to ensure accurate and comparable clinical evaluation of vaccines. The preparation of suitable standard materials is challenging. SARS-CoV-2 and pertussis mucosal IgA antibody detection methods based on electrochemiluminescence principles use serum antibodies as standard products [Citation76]. Serum IgA is monomeric, while mucosal S-IgA is polymeric, and their binding or neutralization abilities with antigens differ. Additionally, serum matrix components differ significantly from mucosal components. Therefore, it is necessary to explore the impact of serum antibodies on the accurate quantification of mucosal S-IgA antibodies.
Setting standards for evaluating mucosal antibodies is also challenging. Different respiratory mucosal vaccines have set varying standards for mucosal antibody evaluation (). In the phase IV clinical trial of the Ad5-nCoV-IH vaccine, researchers defined SARS-CoV-2-specific S-IgA of 5 RU/ml or higher as positive response [Citation20]; dNS1-RBD and FluMist® clinical studies both defined a change greater than 2 in SARS-CoV-2-specific S-IgA antibodies before and after immunization as a significant response [Citation18,Citation23]; AstraZeneca defined a fold change in anti-S IgA normalized for total IgA of more than 3 as a significant antibody response of AZD1222 [Citation77]. Establishment of protection-related S-IgA thresholds still have long way to go.
3.2. Current state and challenges in assessing mucosal cellular immune responses
T cells in mucosal sites play crucial roles in clearing virus-infected cells and regulating B cell functions [Citation55,Citation78]. Innate immune cells in the airways also play a significant role in the defense against respiratory pathogens. Therefore, mucosal cellular immunity is an important indicator for respiratory mucosal vaccines. Currently, only a few clinical studies of respiratory mucosal vaccines have evaluated mucosal cellular immunity. In clinical studies targeting the aerosolized inhalation of a tuberculosis adenovirus vector vaccine, Jeyanathan et al. collected bronchoalveolar lavage fluid for mucosal cellular immunity evaluation, in addition to PBMCs for assessing systemic cellular immune response. They found that aerosolized inhalation not only induced circulatory cellular immunity but also induced cellular responses and trained immunity at mucosal sites. This was evidenced by a significant increase in the proportion of specific CD4 cells with homing markers α4/β1 integrins in PBMCs and tissue-resident T cells expressing α4/β1 integrins at mucosal sites. Alveolar macrophages in the lavage fluid significantly upregulated genes in antigen-stimulated inflammatory responses, STAT protein tyrosine phosphorylation, IL-10 synthesis regulation, and other signaling pathways when stimulated by Mycobacterium tuberculosis lysates [Citation51].
The challenge in evaluating mucosal cellular immunity lies in the collection of bronchoalveolar samples. This method is difficult to perform, has relatively low acceptance, and is challenging to use in large-scale clinical studies. Currently, researchers are attempting to use curettes or flock swabs to collect mucosal cell samples from the nasal turbinate to assess mucosal cellular immune responses induced by respiratory viruses [Citation79–81]. The novel noninvasive nasal sampling method established by Jochems et al. used curettage to collect nasal cells from the inferior turbinate. This novel method can generate a median of 4367 immune cells (interquartile range: 1511–10348), demonstrating excellent tolerability and reproducibility [Citation80]. Importantly, this approach does not induce any microlesions in the nasal mucosa, which could potentially result in blood contamination of the specimens. Compared with the nasal wash, the most commonly used method for collecting nasopharyngeal cells, the curette isolates a larger fraction of T cells and epithelial cells. Based on the research by Jochems et al., Lim et al. established a simple localized sampling method to obtain nasal sample using flocked swabs [Citation82]. Thus, more immune cells were obtained. Up to 200,000 lymphocytes were isolated from each donor. Further experiments, such as an IFN-γ ELISpot assay and activation-induced markers assay, which require at least 15,000 lymphocytes, could be performed. This method may suitable for assessing the efficacy of respiratory mucosal vaccines via nasal immunization. However, additional clinical research are required to evaluate their robustness and suitability.
3.3. Current state and challenges in assessing circulating antibody and cellular immune responses
Serum antibody titer is a commonly used indicator for evaluating vaccine immunogenicity [Citation64]. In a phase 4 randomized trial of Ad5-nCoV-IH, the RBD-specific IgG and neutralizing antibodies were measured to assess immunogenicity. Compared to homologous boosting with an intramuscularly inactivated vaccine, aerosolized Ad5-CoV elicited higher levels of RBD-specific IgG and neutralizing antibodies.
Seroconversion of specific serum antibodies is one of the indicators to evaluate the immunogenicity of a live attenuated influenza vaccine (LAIV) and live-attenuated influenza. For the Russian-backbone live-attenuated influenza vaccine with an updated pandemic H1NA strain, the seroconversion rate was 38% on day 28 after vaccination [Citation83]. In the human challenge test, the FluMist immunization group exhibited a lower HI antibody response rate against three different types of influenza viruses, A/H1N1, A/H3N2 and B/Harbin, on day 28 (23%, 33%, 3%, respectively) than that of the intramuscular inactivated vaccine (91%, 76%, 76%, respectively). However, the estimated protective efficacies of FluMist® (80%, 78%, 100%, respectively) were higher than those of the intramuscularly inactivated vaccine (60%, 67%, 100%, respectively). dNS1-RBD and FluMist demonstrated the same pattern. They had low specific serum seroconversion rates and exhibited high protection rates. A similar scenario was observed in a clinical trial on dNS1-RBD. In the dNS1-RBD group, the seroconversion rate was 22%; however the protective efficacy was only 66.7%. Therefore, the use of serum antibodies to evaluate mucosal vaccine immunogenicity requires further investigation.
Given that respiratory mucosal vaccines can induce the production of circulating TEM and TCM, researchers have evaluated mucosal vaccine-induced cellular immune responses by collecting and testing specific cellular immune responses in the participants’ PBMCs. After quantifying T cell responses in the circulation, Lindsey found that the live attenuated influenza vaccine predominantly induced CD4+ responses [Citation83]. Furthermore, on day 21, there was a significant induction of pH1 hemagglutinin specific CD4+ T cells. In comparing the immune responses induced by oral and intramuscular BCG vaccination, Hoft et al. found that the proportion of CD8 + T cells with α4/β1 integrins in circulation also significantly increased in the oral BCG group [Citation84]. Additionally, α4/β1 integrins have been shown to play an important role in the transport of CD4+ Th1 and CD8+ T cells to the lungs in multiple models [Citation78,Citation85]. This suggests that detecting changes in the proportion of T cells with α4/β1 integrin markers in the circulatory system may indicate the mucosal immune response induced by mucosal vaccines. However, there is a risk that circulating cellular immune evaluation may not accurately reflect mucosal cellular immunity. In a human challenge study with RSV, Jozwik et al. found that the alleviation of lower respiratory tract symptoms and reduction in viral load were related to virus-specific CD8+ T cells in the trachea, not to specific T cells in the circulatory system [Citation86]. It is recommended to assess the feasibility of using circulating cellular immunity as a surrogate for mucosal cellular immune evaluation through extensive clinical studies and identify targets that reflect mucosal cellular immunity.
3.3.1. Expert opinion
Respiratory mucosal vaccines can induce antigen specific S-IgA, CD8+ effector T cells, and tissue-resident memory T/B cells at mucosal sites, thereby forming a powerful barrier against viral invasion. The World Health Organization (WHO) has proposed the development of COVID-19 vaccines that can elicit mucosal immunity [Citation87]. In 2023, the US government listed the development of a promising mucosal vaccine as a key project for the next generation of COVID-19 vaccines [Citation88]. The key challenges in developing mucosal vaccines against respiratory viruses have been described by Morens et al. [Citation1]. The limitation of mucosal immune response evaluations in mucosal vaccines development remain a concern. However, S-IgA, tissue-resident memory cells, innate cells, circulating antibody and T cells induced by mucosal vaccines can serve as evaluation effectors.
Specific S-IgA is commonly used as an effector for clinical immunogenicity evaluation in respiratory mucosal vaccines. However, the uneven distribution and low levels of mucosal antibodies pose significant sampling and detection challenges. Nasal secretions are commonly used; however, collection methods differ among studies, and systematic comparative studies are lacking. Therefore, DOE should be used to design combinations for each sampling step, and the optimal combinations of these methods should be clarified through clinical trials. Systematic comparative studies should follow. The relevance and usefulness of salivary samples to respiratory immunization can be explored through clinical trials. In terms of detection, existing ELISA methods may not meet the high sensitivity requirements for mucosal antibodies. Therefore, enzyme-labeled antibodies should be screened and standardized S-IgA antibody detection methods established based on the analytical quality by design concept. Although highly sensitive electrochemiluminescence kits may be more beneficial for specific S-IgA detection and evaluation, these kits lack applicability for mucosal clinical samples. Systematic validation using negative and positive mucosal samples is required to clarify the detection sensitivity. Mucosal S-IgA is the ideal standard for mucosal evaluation. High-titer mucosal standard materials can be prepared by collecting large numbers of nasal washes and concentrating them. Recombinant-like antibodies can be used to establish S-IgA standards, however, their interchangeability must be proven through collaborative calibration. Regarding evaluation indicators of specific S-IgA antibody responses, analysis of the impact of sample collection bias, the health status of subjects during sampling, and other factors on sample quality and test results should be considered, and then indicators conducive to the accurate analysis of specific S-IgA should be selected. Regarding the evaluation standards of specific S-IgA antibody responses, mucosal samples from individuals with no history of corresponding vaccine administration or virus infection should be used to determine the baseline level of antibodies and thresholds for specific S-IgA related to protection.
Respiratory mucosal cellular immune responses play an important role in clearing virus-infected cells infected by viruses. Although assessing mucosal cellular immunity by collecting bronchoalveolar lavage fluid is feasible, this sampling method is demanding, low tolerant, not suitable for large-scale clinical studies. The reliability of nasal curettage and flocked swab as methods for studying the nasal cell immune response during infection has been demonstrated. However, the applicability of this noninvasive sampling method in vaccine clinical evaluation requires further evaluation.
Neutralizing antibodies, polyfunctional T cells and cytotoxic T cells may contribute to protection against respiratory viruses. Further research on the correlation between these components and respiratory virus prevention is required.
Article highlights
Respiratory mucosal vaccines, which can elicit both mucosal and systemic immune responses, have garnered increasing attention.
Currently, four influenza mucosal vaccines have been approved for marketing and five COVID-19 mucosal vaccines have been approved for emergency use. Clinical studies have confirmed the efficacy of respiratory mucosal vaccines.
The complexity of mucosal immunity and uniqueness of its physiological structure impedes the evaluation of mucosal immune responses, thus limiting the development and application of mucosal vaccines.
The unique physiological structure and low levels of antibodies in the respiratory tract pose challenges for the detection of S-IgA, a crucial effector molecule in the mucosa. To address this, standard sampling methods, sensitive detection method, and reference materials should be established, along with an investigation of the clinical protection threshold associated with S-IgA.
Obtaining clinical samples for mucosal cellular immunity evaluation poses significant challenges. A highly efficient and acceptable method for sample collection should be established
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Xuanxuan Zhang, Jialu Zhang and Si Chen conceived and drafted the manuscript; Qian He, Yu Bai, Jianyang Liu, Zhongfang Wang, Zhenglun Liang provided valuable discussion; Ling Chen, Qunying Mao and Miao Xu revised the manuscript. All authors have read and approved the article.
Additional information
Funding
References
- Morens DM, Taubenberger JK, Fauci AS. Rethinking next-generation vaccines for coronaviruses, influenzaviruses, and other respiratory viruses. Cell Host Microbe. 2023;31(1):146–157. doi:10.1016/j.chom.2022.11.016
- Timeline: WHO’s COVID-19 response; WHO; 2024 Jan 24 [cited 2024 Jan 24]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline.
- Sullivan SJ, Jacobson RM, Dowdle WR, et al. 2009 H1N1 influenza. Mayo Clin Proc. 2010;85(1):64–76. doi:10.4065/mcp.2009.0588
- Confirmed death cases of COVID-19; WHO; 2024 Jan 24 [cited 2024 Jan 24]. Available from: https://covid19.who.int/.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577
- Halperin SA, Ye L, MacKinnon-Cameron D, et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet. 2022;399(10321):237–248. doi: 10.1016/S0140-6736(21)02753-7
- Tarke A, Coelho CH, Zhang Z, et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022;185(5):847–859.e11. doi: 10.1016/j.cell.2022.01.015
- Bleier BS, Ramanathan M Jr., Lane AP. COVID-19 vaccines may not prevent nasal SARS-CoV-2 infection and asymptomatic transmission. Otolaryngol Head Neck Surg. 2021;164(2):305–307. doi:10.1177/0194599820982633
- Sano K, Bhavsar D, Singh G, et al. SARS-CoV-2 vaccination induces mucosal antibody responses in previously infected individuals. Nat Commun. 2022;13(1):5135. doi: 10.1038/s41467-022-32389-8
- Tang J, Zeng C, Cox TM, et al. Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination. Sci Immunol. 2022;7(76):eadd4853. doi: 10.1126/sciimmunol.add4853
- Nickel O, Rockstroh A, Wolf J, et al. Evaluation of the systemic and mucosal immune response induced by COVID-19 and the BNT162b2 mRNA vaccine for SARS-CoV-2. PloS One. 2022;17(10):e0263861. doi: 10.1371/journal.pone.0263861
- Ascough S, Vlachantoni I, Kalyan M, et al. Local and systemic immunity against respiratory syncytial virus induced by a novel intranasal vaccine. A randomized, double-blind, placebo-controlled clinical trial. Am J Respir Crit Care Med. 2019;200(4):481–492. doi: 10.1164/rccm.201810-1921OC
- Eiden J, Fierro C, Schwartz H, et al. Intranasal M2SR (M2-deficient single replication) H3N2 influenza vaccine provides enhanced mucosal and serum antibodies in adults. J Infect Dis. 2022;227(1):103–112. doi: 10.1093/infdis/jiac433
- Pilapitiya D, Wheatley AK, Tan HX. Mucosal vaccines for SARS-CoV-2: triumph of hope over experience. EBioMedicine. 2023;92:104585. doi:10.1016/j.ebiom.2023.104585
- Nakahashi-Ouchida R, Fujihashi K, Kurashima Y, et al. Nasal vaccines: solutions for respiratory infectious diseases. Trends Mol Med. 2023;29(2):124–140. doi:10.1016/j.molmed.2022.10.009
- Chavda VP, Vora LK, Pandya AK, et al. Intranasal vaccines for SARS-CoV-2: from challenges to potential in COVID-19 management. Drug Discov Today. 2021;26(11):2619–2636. doi:10.1016/j.drudis.2021.07.021
- Rudenko L, van den Bosch H, Kiseleva I, et al. Live attenuated pandemic influenza vaccine: clinical studies on A/17/California/2009/38 (H1N1) and licensing of the Russian-developed technology to WHO for pandemic influenza preparedness in developing countries. Vaccine. 2011;29(Suppl 1):A40–44. doi: 10.1016/j.vaccine.2011.04.122
- Treanor JJ, Kotloff K, Betts RF, et al. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza a (H1N1), a (H3N2), and B viruses. Vaccine. 1999;18(9–10):899–906. doi: 10.1016/S0264-410X(99)00334-5
- Rudenko L, Yeolekar L, Kiseleva I, et al. Development and approval of live attenuated influenza vaccines based on Russian master donor viruses: process challenges and success stories. Vaccine. 2016;34(45):5436–5441. doi:10.1016/j.vaccine.2016.08.018
- Tang R, Zheng H, Wang BS, et al. Safety and immunogenicity of aerosolised Ad5-nCov, intramuscular Ad5-nCov, or inactivated COVID-19 vaccine CoronaVac given as the second booster following three doses of CoronaVac: a multicentre, open-label, phase 4, randomised trial. Lancet Respir Med. 2023;11(7):613–623. doi: 10.1016/S2213-2600(23)00049-8
- Singh C, Verma S, Reddy P, et al. Phase III Pivotal comparative clinical trial of intranasal (iNCOVACC) and intramuscular COVID-19 vaccine (Covaxin((R))). NPJ Vaccin. 2023;8(1):125. doi: 10.1038/s41541-023-00717-8
- Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8
- Zhu F, Zhuang C, Chu K, et al. Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Respir Med. 2022;10(8):749–760. doi: 10.1016/S2213-2600(22)00131-X
- Banihashemi SR, Es-Haghi A, Fallah Mehrabadi MH, et al. Safety and efficacy of combined intramuscular/intranasal RAZI-COV PARS vaccine candidate against SARS-CoV-2: a preclinical study in several animal models. Front Immunol. 2022;13:836745. doi: 10.3389/fimmu.2022.836745
- Xu H, Cai L, Hufnagel S, et al. Intranasal vaccine: factors to consider in research and development. Int J Pharm. 2021;609:121180. doi:10.1016/j.ijpharm.2021.121180
- Zhao B, Yang J, He B, et al. A safe and effective mucosal RSV vaccine in mice consisting of RSV phosphoprotein and flagellin variant. Cell Rep. 2021;36(3):109401. doi: 10.1016/j.celrep.2021.109401
- Yang J, Liu MQ, Liu L, et al. A triple-RBD-based mucosal vaccine provides broad protection against SARS-CoV-2 variants of concern. Cell Mol Immunol. 2022;19(11):1279–1289. doi: 10.1038/s41423-022-00929-3
- Ye T, Jiao Z, Li X, et al. Inhaled SARS-CoV-2 vaccine for single-dose dry powder aerosol immunization. Nature. 2023;624(7992):630–638. doi: 10.1038/s41586-023-06809-8
- Lei H, Alu A, Yang J, et al. Cationic crosslinked carbon dots-adjuvanted intranasal vaccine induces protective immunity against omicron-included SARS-CoV-2 variants. Nat Commun. 2023;14(1):2678. doi: 10.1038/s41467-023-38066-8
- Twigg HL 3rd. Humoral immune defense (antibodies): recent advances. Proc Am Thorac Soc. 2005;2(5):417–421. doi:10.1513/pats.200508-089JS
- Kirkeby L, Rasmussen TT, Reinholdt J, et al. Immunoglobulins in nasal secretions of healthy humans: structural integrity of secretory immunoglobulin A1 (IgA1) and occurrence of neutralizing antibodies to IgA1 proteases of nasal bacteria. Clin Diagn Lab Immunol. 2000;7(1):31–39. doi:10.1128/CDLI.7.1.31-39.2000
- Rojas R, Apodaca G. Immunoglobulin transport across polarized epithelial cells. Nat Rev Mol Cell Biol. 2002;3(12):944–955. doi:10.1038/nrm972
- Woof JM, Russell MW. Structure and function relationships in IgA. Mucosal Immunol. 2011;4(6):590–597. doi:10.1038/mi.2011.39
- Lindh E. Increased risistance of immunoglobulin A dimers to proteolytic degradation after binding of secretory component. J Immunol. 1975;114(1 Pt 2):284–286. doi:10.4049/jimmunol.114.1_Part_2.284
- Suzuki T, Kawaguchi A, Ainai A, et al. Relationship of the quaternary structure of human secretory IgA to neutralization of influenza virus. Proc Natl Acad Sci U S A. 2015;112(25):7809–7814. doi: 10.1073/pnas.1503885112
- Marcotte H, Cao Y, Zuo F, et al. Conversion of monoclonal IgG to dimeric and secretory IgA restores neutralizing ability and prevents infection of omicron lineages. Proc Natl Acad Sci U S A. 2024;121(3):e2315354120. doi: 10.1073/pnas.2315354120
- Renegar KB, Small PA Jr., Boykins LG, et al. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173(3):1978–1986. doi:10.4049/jimmunol.173.3.1978
- Renegar KB, Jackson GD, Mestecky J. In vitro comparison of the biologic activities of monoclonal monomeric IgA, polymeric IgA, and secretory IgA. J Immunol. 1998;160(3):1219–1223. doi:10.4049/jimmunol.160.3.1219
- Brokstad KA, Cox RJ, Eriksson JC, et al. High prevalence of influenza specific antibody secreting cells in nasal mucosa. Scand J Immunol. 2001;54(1–2):243–247. doi:10.1046/j.1365-3083.2001.00947.x
- Wright PF, Murphy BR, Kervina M, et al. Secretory immunological response after intranasal inactivated influenza A virus vaccinations: evidence for immunoglobulin A memory. Infect Immun. 1983;40(3):1092–1095. doi:10.1128/iai.40.3.1092-1095.1983
- Marking U, Bladh O, Havervall S, et al. 7-month duration of SARS-CoV-2 mucosal immunoglobulin-A responses and protection. Lancet Infect Dis. 2023;23(2):150–152. doi: 10.1016/S1473-3099(22)00834-9
- Liew F, Talwar S, Cross A, et al. SARS-CoV-2-specific nasal IgA wanes 9 months after hospitalisation with COVID-19 and is not induced by subsequent vaccination. EBioMedicine. 2023;87:104402. doi: 10.1016/j.ebiom.2022.104402
- Zuo F, Marcotte H, Hammarstrom L, et al. Mucosal IgA against SARS-CoV-2 omicron infection. N Engl J Med. 2022;387(21):e55.
- Sheikh-Mohamed S, Isho B, Chao GYC, et al. Systemic and mucosal IgA responses are variably induced in response to SARS-CoV-2 mRNA vaccination and are associated with protection against subsequent infection. Mucosal Immunol. 2022;15(5):799–808. doi: 10.1038/s41385-022-00511-0
- Bagga B, Harrison L, Roddam P, et al. Unrecognized prolonged viral replication in the pathogenesis of human RSV infection. J Clin Virol. 2018;106:1–6. doi:10.1016/j.jcv.2018.06.014
- Habibi MS, Jozwik A, Makris S, et al. Impaired antibody-mediated protection and defective IgA B-cell memory in experimental infection of adults with respiratory syncytial virus. Am J Respir Crit Care Med. 2015;191(9):1040–1049. doi: 10.1164/rccm.201412-2256OC
- Miyamoto S, Nishiyama T, Ueno A, et al. Infectious virus shedding duration reflects secretory IgA antibody response latency after SARS-CoV-2 infection. Proc Natl Acad Sci U S A. 2023;120(52):e2314808120. doi: 10.1073/pnas.2314808120
- Wagstaffe HR, Thwaites RS, Reynaldi A, et al. Mucosal and systemic immune correlates of viral control after SARS-CoV-2 infection challenge in seronegative adults. Sci Immunol. 2024;9(92):eadj9285. doi: 10.1126/sciimmunol.adj9285
- Poon MML, Rybkina K, Kato Y, et al. SARS-CoV-2 infection generates tissue-localized immunological memory in humans. Sci Immunol. 2021;6(65):eabl9105. doi: 10.1126/sciimmunol.abl9105
- Grau-Exposito J, Sanchez-Gaona N, Massana N, et al. Peripheral and lung resident memory T cell responses against SARS-CoV-2. Nat Commun. 2021;12(1):3010. doi: 10.1038/s41467-021-23333-3
- Jeyanathan M, Fritz DK, Afkhami S, et al. Aerosol delivery, but not intramuscular injection, of adenovirus-vectored tuberculosis vaccine induces respiratory-mucosal immunity in humans. JCI Insight. 2022;7(3). doi: 10.1172/jci.insight.155655
- Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol. 2016;16(2):79–89. doi:10.1038/nri.2015.3
- Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41(6):886–897. doi:10.1016/j.immuni.2014.12.007
- Heeg M, Goldrath AW. Insights into phenotypic and functional CD8(+) T(RM) heterogeneity. Immunol Rev. 2023;316(1):8–22. doi:10.1111/imr.13218
- Schenkel JM, Fraser KA, Beura LK, et al. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346(6205):98–101. doi:10.1126/science.1254536
- Wu T, Hu Y, Lee YT, et al. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukocyte Biol. 2014;95(2):215–224. doi: 10.1189/jlb.0313180
- Zhao J, Zhao J, Mangalam AK, et al. Airway memory CD4(+) T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity. 2016;44(6):1379–1391. doi: 10.1016/j.immuni.2016.05.006
- Shenoy AT, Wasserman GA, Arafa EI, et al. Lung CD4(+) resident memory T cells remodel epithelial responses to accelerate neutrophil recruitment during pneumonia. Mucosal Immunol. 2020;13(2):334–343. doi: 10.1038/s41385-019-0229-2
- Teijaro JR, Turner D, Pham Q, et al. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187(11):5510–5514. doi:10.4049/jimmunol.1102243
- Son YM, Cheon IS, Wu Y, et al. Tissue-resident CD4(+) T helper cells assist the development of protective respiratory B and CD8(+) T cell memory responses. Sci Immunol. 2021;6(55). doi: 10.1126/sciimmunol.abb6852
- Allie SR, Bradley JE, Mudunuru U, et al. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat Immunol. 2019;20(1):97–108. doi: 10.1038/s41590-018-0260-6
- Barker KA, Etesami NS, Shenoy AT, et al. Lung-resident memory B cells protect against bacterial pneumonia. J Clin Invest. 2021;131(11). doi: 10.1172/JCI141810
- Tan HX, Juno JA, Esterbauer R, et al. Lung-resident memory B cells established after pulmonary influenza infection display distinct transcriptional and phenotypic profiles. Sci Immunol. 2022;7(67):eabf5314. doi: 10.1126/sciimmunol.abf5314
- Oh JE, Song E, Moriyama M, et al. Intranasal priming induces local lung-resident B cell populations that secrete protective mucosal antiviral IgA. Sci Immunol. 2021;6(66):eabj5129. doi: 10.1126/sciimmunol.abj5129
- Netea MG, Dominguez-Andres J, Barreiro LB, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20(6):375–388. doi: 10.1038/s41577-020-0285-6
- Netea MG, Joosten LA, Latz E, et al. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098. doi: 10.1126/science.aaf1098
- Yao Y, Jeyanathan M, Haddadi S, et al. Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell. 2018;175(6):1634–1650 e1617. doi: 10.1016/j.cell.2018.09.042
- Zhang L, Jiang Y, He J, et al. Intranasal influenza-vectored COVID-19 vaccine restrains the SARS-CoV-2 inflammatory response in hamsters. Nat Commun. 2023;14(1):4117. doi: 10.1038/s41467-023-39560-9
- Strugnell RA, Wijburg OL. The role of secretory antibodies in infection immunity. Nat Rev Microbiol. 2010;8(9):656–667. doi:10.1038/nrmicro2384
- Corthésy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front Immunol. 2013;4:185. doi:10.3389/fimmu.2013.00185
- Zhu F, Huang S, Liu X, et al. Safety and efficacy of the intranasal spray SARS-CoV-2 vaccine dNS1-RBD: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2023;11(12):1075–1088. doi: 10.1016/S2213-2600(23)00349-1
- de Silva TI, Gould V, Mohammed NI, et al. Comparison of mucosal lining fluid sampling methods and influenza-specific IgA detection assays for use in human studies of influenza immunity. J Immunol Methods. 2017;449:1–6. doi: 10.1016/j.jim.2017.06.008
- Bergin PJ, Langat R, Omosa-Manyonyi G, et al. Assessment of anti-HIV-1 antibodies in oral and nasal compartments of volunteers from 3 different populations. J Acquir Immune Defic Syndr. 2016;73(2):130–137. doi: 10.1097/QAI.0000000000001094
- DeFrancesco L. COVID-19 antibodies on trial. Nat Biotechnol. 2020;38(11):1242–1252. doi:10.1038/s41587-020-0732-8
- DISCOVERY MS. V-PLEX COVID-19 serology assays insert. Rockville (MD): Mesoscale;2023.
- Keech C, Miller VE, Rizzardi B, et al. Immunogenicity and safety of BPZE1, an intranasal live attenuated pertussis vaccine, versus tetanus-diphtheria-acellular pertussis vaccine: a randomised, double-blind, phase 2b trial. Lancet. 2023;401(10379):843–855. doi: 10.1016/S0140-6736(22)02644-7
- Madhavan M, Ritchie AJ, Aboagye J, et al. Tolerability and immunogenicity of an intranasally-administered adenovirus-vectored COVID-19 vaccine: an open-label partially-randomised ascending dose phase I trial. EBioMedicine. 2022;85:104298. doi: 10.1016/j.ebiom.2022.104298
- Feng CG, Britton WJ. CD4+ and CD8+ T cells mediate adoptive immunity to aerosol infection of mycobacterium bovis bacillus Calmette-Guérin. J Infect Dis. 2000;181(5):1846–1849. doi:10.1086/315466
- Lim JME, Tan AT, Le Bert N, et al. SARS-CoV-2 breakthrough infection in vaccinees induces virus-specific nasal-resident CD8+ and CD4+ T cells of broad specificity. J Exp Med. 2022;219(10). doi: 10.1084/jem.20220780
- Jochems SP, Piddock K, Rylance J, et al. Novel analysis of immune cells from nasal microbiopsy demonstrates reliable, reproducible data for immune populations, and superior cytokine detection compared to nasal wash. PloS One. 2017;12(1):e0169805. doi: 10.1371/journal.pone.0169805
- Roukens AHE, Pothast CR, Konig M, et al. Prolonged activation of nasal immune cell populations and development of tissue-resident SARS-CoV-2-specific CD8(+) T cell responses following COVID-19. Nat Immunol. 2022;23(1):23–32. doi: 10.1038/s41590-021-01095-w
- Lim JME, Tan AT, Bertoletti A. Protocol to detect antigen-specific nasal-resident T cells in humans. STAR Protoc. 2023;4(1):101995. doi:10.1016/j.xpro.2022.101995
- Lindsey BB, Jagne YJ, Armitage EP, et al. Effect of a Russian-backbone live-attenuated influenza vaccine with an updated pandemic H1N1 strain on shedding and immunogenicity among children in the Gambia: an open-label, observational, phase 4 study. Lancet Respir Med. 2019;7(8):665–676. doi: 10.1016/S2213-2600(19)30086-4
- Hoft DF, Xia M, Zhang GL, et al. PO and ID BCG vaccination in humans induce distinct mucosal and systemic immune responses and CD4(+) T cell transcriptomal molecular signatures. Mucosal Immunol. 2018;11(2):486–495. doi: 10.1038/mi.2017.67
- Walrath JR, Silver RF. The α4β1 integrin in localization of Mycobacterium tuberculosis-specific T helper type 1 cells to the human lung. Am J Respir Cell Mol Biol. 2011;45(1):24–30. doi:10.1165/rcmb.2010-0241OC
- Jozwik A, Habibi MS, Paras A, et al. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat Commun. 2015;6(1):10224. doi: 10.1038/ncomms10224
- Interim statement on COVID-19 vaccines in the context of the circulation of the omicron SARS-CoV-2 variant from the WHO Technical Advisory Group on COVID-19 vaccine composition (TAG-CO-VAC). WHO; 2022 Mar 8 [cited 2024 Jan 4]. Available from: https://www.who.int/news/item/08-03-2022-interim-statement-on-covid-19-vaccines-in-the-context-of-the-circulation-of-the-omicron-sars-cov-2-variant-from-the-who-technical-advisory-group-on-covid-19-vaccine-composition-(tag-co-vac)-08-march-2022
- Fact sheet: HHS details $5 billion ‘project NextGen’ initiative to stay ahead of COVID-19; HHS Press Office; 2023 May 11 [cited 2024 Jan 4]. Available from: https://www.hhs.gov/about/news/2023/05/11/fact-sheet-hhs-details-5-billion-project-nextgen-initiative-stay-ahead-covid.html

