?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background
The Japanese National Immunization Program currently includes the pediatric 13 valent pneumococcal conjugate vaccine (PCV13) to prevent pneumococcal infections. We aimed to evaluate the cost-effectiveness of 20-valent PCV (PCV20) as a pediatric vaccine versus PCV13.
Methods
A decision-analytic Markov model was used to estimate expected costs, quality-adjusted life-years (QALYs), and prevented cases and deaths caused by invasive pneumococcal disease, pneumonia, and acute otitis media over a ten-year time horizon from the societal and healthcare payer perspectives.
Results
PCV20 was dominant, i.e. less costly and more effective, over PCV13 (gained 294,599 QALYs and reduced Japanese yen [JPY] 352.6 billion [2.6 billion United States dollars, USD] from the societal perspective and JPY 178.9 billion [USD 1.4 billion] from the payer perspective). Sensitivity and scenario analyses validated the robustness of the base scenario results. When comparing PCV20 with PCV13, the threshold analysis revealed an incremental cost-effectiveness ratio that was within the threshold value (JPY 5 million/QALY) at a maximum acquisition cost of JPY 74,033 [USD 563] (societal perspective) and JPY 67,758 [USD 515] (payer perspective).
Conclusions
As a pediatric vaccine, PCV20 was dominant over PCV13 regardless of the study perspective.
1. Introduction
Pneumococcal disease (PD), caused by Streptococcus pneumoniae (S. pneumoniae), remains one of the most common vaccine-preventable diseases worldwide, causing both invasive pneumococcal disease (IPD) and noninvasive disease (e.g. acute otitis media [AOM]) [Citation1]. These infections are associated with high morbidity and mortality, specifically in infants with preexisting chronic conditions [Citation1]. In Japan, S. pneumoniae is the primary cause of bacterial meningitis in infants above the age of three months [Citation2,Citation3]. IPD conditions are recognized as category V infectious diseases and are the subject of all-case surveillance in Japan [Citation4].
The introduction of pneumococcal conjugate vaccines (PCVs) has been a major breakthrough in reducing the disease burden in Japan. Using PCVs is currently the most effective way to prevent pneumococcal infections. Currently, the pediatric 13-valent PCV (PCV13), which includes the purified capsular polysaccharide of 13 serotypes of S. pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9 V, 14, 18C, 19A, 19F, and 23F), is administered under the Japanese National Immunization Program (NIP) to prevent pneumococcal infections. According to a nationwide population-based surveillance of IPD in Japanese children, the percentage of IPD caused by PCV13-serotypes out of the total pediatric IPD cases was 53.2% in 2013 [Citation5]. Introduction of PCV13 into the NIP in 2013 resulted in lower cases of IPD caused by PCV13-serotypes, i.e. 8.2% in 2016 to 2.1% in 2019 and 2.3% in 2022 [Citation5], indicating the effectiveness of PCV13 against PCV13-serotypes. However, the non-PCV13-serotype had an increased composition ratio in pathogenic bacteria due to serotype replacement (the expansion of non-vaccine serotypes resulting from the removal of vaccine-type serotypes from the population). Therefore, new vaccines are expected to be developed that are active against current non-vaccine serotypes.
There are currently at least 100 identified pneumococcal serotypes [Citation6,Citation7], reflecting differences in the structure of the polysaccharide capsule [Citation8]; however, only a few result in the majority of the disease [Citation9,Citation10]. Higher-valent PCVs, 20-valent (PCV20) and 15-valent (PCV15), are expected to be introduced in Japan to expand serotype coverage and improve protection against PD. PCV20 includes PCV13 serotypes plus serotypes 8, 10A, 11A, 12F, 15B, 22F, and 33F [Citation11], and an application to approve its use in children has been submitted in Japan. In contrast, in 2023, PCV15 (including PCV13 serotypes plus 22F and 33F serotypes) was approved for children by the Ministry of Health, Labour and Welfare [Citation12]. Of note, 2022 surveillance data from 43 S. pneumoniae isolates collected from pediatric IPD cases in Japan showed that while only 2.3% and 9.3% were of PCV13 and PCV15 serotypes, respectively, a substantially higher number of serotypes were of PCV20 (30.2%) [Citation5]. Furthermore, the additional serotypes included in PCV20 are medically significant [Citation13–19]. To defend against these present non-vaccine serotypes, it would be advantageous to include higher-valent PCVs, particularly PCV20. This could potentially reduce the disease burden in this target population.
In Japan, in addition to the vaccine effectiveness and safety, cost-effectiveness is one of the key aspects of the inclusion of new vaccines in the NIP [Citation20]. In other countries, such as the United States (US) and Canada, economic evaluations have shown that PCV20 could be more cost-effective than PCV13 and PCV15 [Citation21,Citation22]. In Japan, a cost-effectiveness analysis of PCV15 compared with PCV13 was recently published [Citation23]. The study showed that PCV15 was dominant (less costly and more effective) over PCV13 as expected as the vaccine acquisition costs of PCV15 and PCV13 were parity although the serotype coverage of PCV15 was wider than PCV13. However, to the best of our knowledge, no cost-effectiveness analysis has been conducted to date for pediatric PCV20 in the Japanese population. Since the serotype distribution of different PCVs varies from country to country [Citation24], an evaluation of the health and economic impact of using PCV20 in Japan will inform the inclusion of PCV20 in the NIP, as well as price adjustments. Therefore, our study aimed to ascertain the cost-effectiveness of PCV20 versus the current routine vaccine, PCV13. In addition to this, we also evaluated the cost-effectiveness of PCV15 versus PCV13 and PCV20 versus PCV15.
2. Methods
2.1. Model description, settings, and outcomes
We adopted a previously published decision-analytic Markov (state-transition) model [Citation21] for Japanese settings to evaluate the potential health outcomes and economic impact of switching vaccination from PCV13 to PCV20 or PCV15 (). Additionally, we also compared PCV20 with PCV15. In this model, the three primary doses were administered at 2, 3, and 4 months postpartum, followed by a booster dose at 15 months (i.e. full 3 + 1 schedule), with all doses from the same vaccine type.
Figure 1. Model schematic.
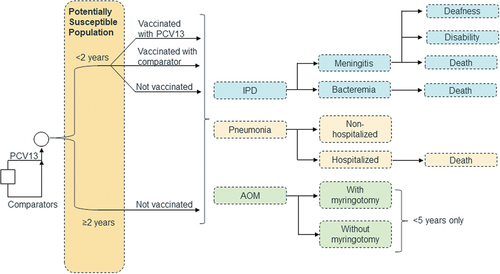
In each annual model cycle, individuals could develop multiple PDs, including IPD (meningitis and bacteremia), all-cause pneumonia (hospitalized and non-hospitalized), and AOM (with or without myringotomy), could not develp PD, or could die [Citation25]. The model considered direct vaccination effects (reduction in disease incidence caused by vaccine-type serotypes) and indirect effects (herd effects, i.e. protective effects observed in unvaccinated populations) to reflect post-vaccination transmission dynamics [Citation26]. A time horizon of ten years was adopted, as vaccine effects generally stabilize 5–9 years following PCV implementation [Citation27].
The model outcomes included: 1) health outcomes (number of cases by disease type and number of deaths); 2) economic outcomes (medical costs and productivity loss); and 3) cost-effectiveness outcomes (incremental costs, incremental quality-adjusted life-years [QALYs], and incremental cost-effectiveness ratios [ICERs] per QALY gained). Furthermore, the incremental net monetary benefit (INMB) was calculated as follows:
For Japan, a threshold of 5 million Japanese yen (JPY) (38,023.39 US dollars [USD]) (exchange rate: USD 1= JPY 131.498 [Citation28]) per QALY gained was used [Citation29,Citation30]. Health outcomes and costs were discounted at a rate of 2% per year [Citation31].
We did not model the unvaccinated population as a comparator because the pneumococcal vaccination uptake for children was nearly 100% in Japan, according to the most recent statistics in 2021 [Citation32].
2.2. Model inputs and data sources
The specific model parameters are presented in and further described in Supplemental –8. To the extent possible, relevant data from Japan were prioritized during the parameter search and used in the current analyses.
Table 1. Model input values for base scenario analysis.
2.2.1. Population
Population size stratified by age groups () and the size of the incoming birth cohort (Supplemental ) were obtained from the Japanese Statistics Bureau [Citation33] and the National Institute of Population and Social Security Research [Citation55], respectively.
2.2.2. Disease incidence and serotype distribution
Considering the impact of COVID-19 on PD, disease incidence rates before the COVID-19 pandemic were chosen for the base scenario analysis (). Incidence rates for IPD were calculated based on surveillance data from the National Institute of Infectious Diseases [Citation33,Citation37]. Incidence rates for noninvasive diseases were estimated from the claims database of JMDC Inc., which consists of claims data from 285 employer insurance associations, including the records of approximately 16 million people [Citation35]. The disease and procedure codes used for the analysis using the claims database are detailed in Supplemental Methods and Supplemental .
Table 2. Results of base scenario A.
The serotype distribution of IPD in children and elderly individuals was used in this analysis [Citation5,Citation56] (). In the absence of robust data on noninvasive diseases, the age-specific serotype distribution of IPD was used as a proxy.
2.2.3. Mortality and sequalae
Age-specific general mortality rates were taken from Japanese life tables [Citation57]. Published literature or publicly available data were used to estimate case fatality rates (CFRs) for meningitis [Citation2,Citation38], bacteremia [Citation53], and hospitalized pneumonia [Citation41], and the probability of developing sequalae (deafness and disability) from meningitis [Citation43,Citation53] (). CFRs related to non-hospitalized pneumonia or AOM and the probability of developing sequalae from other disease conditions were assumed to be zero based on prior evidence.
2.2.4. Vaccine uptake
Vaccine uptake for each vaccine under the full 3 + 1 schedule was assumed to be 97.9% for the primary doses and 97.1% for the booster dose, based on the current uptake of PCV13 for children <2 years of age in Japan [Citation32].
2.2.5. Vaccine effectiveness
Both direct and indirect effects were included for vaccine effectiveness (Supplemental Methods). The base scenario analysis included all diseases such as IPD, pneumonia and AOM. Based on previous estimates in Europe [Citation58,Citation59], each vaccine was assumed to have a full direct effect for five years after administration of the final dose, while the effects waned by 10% annually at Year 6 to 42% of the original vaccine effectiveness by Year 10 (i.e. maximum duration of protection). Indirect effects were applied as a reduction in disease incidence over time against serotypes unique to PCV20 and PCV15 (versus PCV13), as they provide maximum protection for the entire population. All disease states were assumed to have the same rate of accrual of indirect effects. Indirect effects against additionally covered serotypes were calculated using a reduction in disease incidence adjusted to the ratio of serotype distribution at the time of PCV13 introduction and the current serotype distribution. In addition, serotype 3 was excluded for estimating the indirect effects. Since this serotype is more relevant for adults aged ≥65 years [Citation60], the exclusion of serotype 3 was a conservative approach.
2.2.6. Utilities
Age- and sex-specific utilities for the general Japanese population [Citation61] and the proportion of females in the population [Citation33] are detailed in Supplemental Table S8. The model assumed a baseline utility of 1.0 for age groups <20 years due to a lack of data. Total utilities were calculated by considering QALY decrements for all disease states and a utility multiplier for lifetime events (disease sequalae) ().
2.2.7. Vaccination and medical costs
Vaccination costs consisted of vaccine acquisition costs and administration costs (). The vaccine acquisition cost for PCV15 was on par with that of PCV13 [Citation62,Citation63]. As of December 2023, PCV20 had not been approved in Japan. Therefore, for this study, we temporarily multiplied the cost of PCV13 by 1.13 to match the ratio of costs of pediatric PCV20 (USD 178.00 [JPY 23,407]) and PCV13 (USD 158.18 [JPY 20,800]) in the US [Citation64]. The vaccine administration cost was calculated based on a fee-for-service price list (2022) by the Ministry of Health, Labour and Welfare [Citation65] and included costs for initial consultation, infant addition, procedures, and biologics premiums.
Medical costs included the cost per episode of each disease condition and were estimated either from the JMDC database [Citation35] (for ages <65 years) or the MDV database [Citation52] (for ages ≥65 years) (). The MDV database contains claims data and discharge summaries from over 480 hospitals across Japan [Citation52]. Details on calculating costs are described in the supplemental material (Supplemental Methods). Management costs of long-term sequalae (deafness and disability) were estimated from previous literature [Citation53]. All vaccination and medical costs were adjusted to 2022 national tariffs [Citation65], wherever applicable.
2.2.8. Costs related to productivity losses
Productivity losses were considered in the analysis from the societal perspective. Productivity losses due to vaccination included half a day off by guardians for their children’s vaccination. Productivity losses due to the diseases were assumed to be incurred by guardians of patients aged <19 years, patients themselves (19–64 years), and caregivers (for patients aged ≥65 years). A human capital approach was adopted to estimate the costs associated with productivity losses based on work force participation, number of loss days, and average daily wages [Citation66]. Productivity losses were derived by multiplying the number of working days missed due to an episode of disease [Citation35,Citation53] and the wages lost due to this absence, as estimated by their average daily wages (JPY 18,809 [USD 143.04] in 2022) based on the basic survey on wage structure () [Citation54].
2.3. Analyses
The base scenario analyses compared PCV20 or PCV15 with PCV13 from the societal and healthcare payer perspectives. Additionally, we compared PCV20 versus PCV15. Two base scenarios were considered: A) the acquisition cost of PCV20 was JPY 8,102 and B) the acquisition cost of PCV20 was JPY 7,200. Furthermore, total 11 scenario analyses were conducted to assess uncertainty around specific model input parameters (Supplemental Table S9). A one-way sensitivity analysis (OWSA) was conducted for PCV20 versus PCV13 to identify drivers of model outcomes and examine key areas of uncertainty. The following variables were considered for the OWSA: direct and indirect vaccine effect, incidence of disease (including complication rates where relevant), CFRs, probability of sequalae, serotype coverage, PCV20 vaccine cost and other costs (except vaccine administration costs), utilities, and utility decrements. Upper and lower bounds were either based on the 95% confidence intervals derived from the data or calculated as ± 25% of the base scenario values where data were not available. The upper and lower bounds for the cost of the PCV20 vaccine were JPY 10,128 (JPY 8,102 × 1.25) and JPY 7,200 (parity to PCV13), respectively. A probabilistic sensitivity analysis (PSA) with 1000 iterations was performed to examine the impact of joint uncertainty on all input parameters. The distribution used for PSA are described in detail in Supplemental Table S10. Typical probability distributions were used in the analyses, following the guidance of Briggs et al. [Citation67]. Additionally, threshold analysis was conducted.
3. Results
3.1. Base scenario
In base scenario A, PCV20 was more effective in comparison with PCV13 for preventing pneumococcal infections, with a gain of 294,599 QALYs per population (0.036 QALYs per person) over a time horizon of ten years (). Additionally, PCV20 reduced JPY 352,637 million (USD 2,628 million) per population and JPY 44,506 (USD 338) per person in total costs from the societal perspective. Furthermore, PCV20 reduced JPY 178,901 million (USD 1,360 million) per population and JPY 22,579 (USD 172) per person from the payer perspective. These results suggested that PCV20 could be a dominant strategy over PCV13, regardless of the study perspective (). PCV20 prevented 9,298 cases of IPD (677 meningitis and 8,621 bacteremia); 1,508,487 cases of pneumonia (229,346 hospitalized and 1,279,141 non-hospitalized); 584,155 cases of AOM (25,839 with myringotomy and 558,316 without myringotomy); and 13,656 deaths compared with PCV13 ( and Supplemental Table S11). A cost-breakdown analysis showed that while PCV20 incurred an incremental vaccination cost of JPY 24,977 million (USD 190 million), the total treatment cost and productivity losses were reduced by JPY 203,878 million (USD 1,550 million) and JPY 173,737 million (USD 1,321 million), respectively. Similar results were obtained in base scenario B when the acquisition cost of PCV20 was set at par with those of PCV13 and PCV15 ().
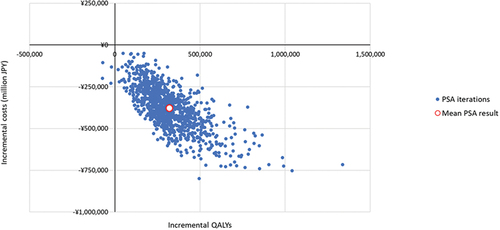
Figure 2. Cost-effectiveness plane for three vaccination programs (per population) in base scenario A.
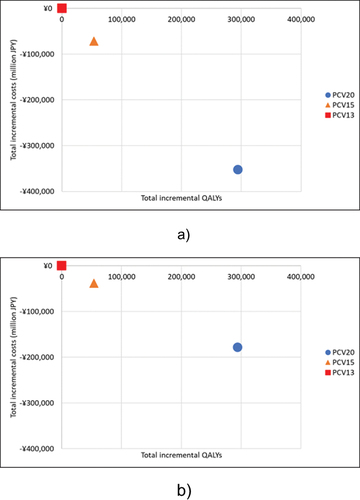
Table 3. Detailed incremental values in base scenarios and scenario 1 (indirect effects excluded) – societal perspective.
Table 4. Results of base scenario B and scenario 1.
Finally, when PCV20 was compared with PCV15, PCV20 was found to be more effective (gained 240,861 QALYs per population) and reduced costs by JPY 280,771 million (USD 2,135 million) and JPY 140,561 million (USD 1,069 million) per population from the societal and payer perspectives, respectively ().
3.2. Scenario analyses
show the results of scenario analyses, where nine input parameters were varied in 11 scenarios and their impact analyzed on the outcomes with PCV20 versus PCV13. In all scenario analyses expect scenario 1 from the payer perspective, PCV20 was dominant (i.e. less costly and more effective) regardless of the study perspective. In the scenario 1, the ICER for PCV20 compared to PCV13 was JPY 117,032/QALY which was below JPY 5 million/QALY (the threshold value in Japan [Citation29]) from the payer perspective. Thus, the results of the base scenarios were found to be robust.
Table 5. Results of scenario analyses for PCV20 versus PCV13.
Considering only direct effects for all diseases such as IPD, pneumonia and AOM (i.e. exclusion of indirect effects) (Scenario 1), considering direct effects for all disease and indirect effects only for IPD (Scenario 2), and utilizing the incidence and serotype distribution from the COVID-19 era (Scenario 3) had the highest impact on INMB. In the base case, INMB was calculated as JPY 1,826 billion (USD 14 billion) [incremental QALYs (294,599 QALYs) × threshold (JPY 5 million/QALY) – incremental costs (JPY −352.6 billion)] from the societal perspective. INMB was JPY 64 billion (USD 487 million) in Scenario 1, JPY 212 billion (USD 2 billion) in Scenario 2 and JPY 1,164 billion (USD 9 billion) in Scenario 3, respectively. The results of Scenario 11, where incidence and cost data of AOM were used from Yamanaka et al. [Citation69], prevented a greater number of cases of AOM at a higher cost benefit compared with the base scenario results (Supplemental Table S12).
3.3. Sensitivity analyses
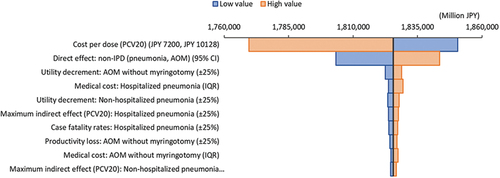
The INMB values in OWSA for PCV20 versus PCV13 from the societal perspective are presented as a tornado diagram (). The factors that had the highest impact on the INMB for PCV20 versus PCV13 were PCV20 vaccine cost, followed by the vaccine’s direct effects on pneumonia and AOM. Positive INMB values in all cases confirmed the robustness of our base scenario results, indicating that PCV20 is a dominant strategy over PCV13.
Figure 3. Tornado plot for PCV20 vs. PCV13 (INMB).
PSA results showed that when PCV20 was compared with PCV13, most simulations were in the southeast quadrant (99.6%), indicating higher QALYs at lower costs (). The PSA results were complemented with a cost-effectiveness acceptability curve that showed the probability of PCV20 being accepted was 98.5% under a threshold of JPY 5 million/QALY when comparing the three vaccines (Supplemental Figure S2).
3.4. Threshold analysis
Threshold analysis of the acquisition cost of PCV20 showed that the ICER values for PCV20 versus PCV13 continued to be dominant at a maximum cost of JPY 20,837 (USD 158) from the societal perspective and JPY 14,562 (USD 111) from the payer perspective. Moreover, the cost remained below the threshold value for Japan (JPY 5 million/QALY) at a maximum acquisition cost for PCV20 of JPY 74,033 (USD 563) from the societal perspective and JPY 67,758 (USD 515) from the payer perspective.
4. Discussion
Our base scenario results suggested that vaccination with PCV20 prevented pneumococcal infections and deaths while incurring lower costs in comparison with PCV13 and PCV15. Therefore, PCV20, at the assumed acquisition cost of JPY 8,102, can be considered a dominant strategy over PCV13 and PCV15 from both the societal and payer perspectives, as it provides wider serotype coverage than lower-valent vaccines. The robustness of our base scenario results was further validated by scenario and sensitivity analyses.
The scenario that had the greatest impact on health and cost outcomes was the exclusion of indirect effects. The estimation of the indirect effects of vaccines may not always be easy to capture [Citation70]. Moreover, due to a lack of published data in Japan, global data were used to estimate these effects in the base scenario analysis. Therefore, we evaluated an alternate scenario where indirect effects were not included as a conservative approach. Athough this scenario had the greatest impact on ICER, PCV20 was still dominant over PCV13 from a societal perspective and resulted in an ICER less than the threshold of JPY 5 million per QALY gained from a payer perspective. However, it is evident from several reports that PCVs exert an indirect effect (herd immunity) [Citation26,Citation71], and therefore this scenario can be considered an extreme example.
During the COVID-19 pandemic (2020–2022), the incidence rate of community-acquired pneumonia, including pneumococcal pneumonia, was markedly reduced due to several safety measures that were applied globally to curb the spread of coronavirus infection [Citation72,Citation73]. However, as COVID-19 converges gradually, pneumococcal infection rates are anticipated to increase [Citation74]. Therefore, this study employed the incidence and serotype distribution data before the COVID-19 period (2017–2019) for the base scenario analysis. In contrast, after experiencing COVID-19, residents’ awareness of preventing infectious diseases may have been altered, and the incidence of pneumococcal infections may continue to be low [Citation72]. We assessed the impact of these changes in disease incidence on our base scenario results by performing a scenario analysis that used the lower PD incidence during the COVID-19 era (2020–2022). The results of this scenario showed that the base scenario cost-effectiveness outcomes remained unchanged as a dominant strategy.
For the base scenario analysis in this study, AOM was defined as either with or without myringotomy, which was different than the categorization used in a previous study in Japan [Citation69] (complex AOM and simple AOM). Therefore, we conducted a scenario analysis using incidence and cost data for complex and simple AOM from Yamanaka et al. [Citation69]. This analysis showed that PCV20 was a dominant strategy over PCV13. However, since Yamanaka et al. used incidence data from a previous study conducted in the US during 2000–2004 [Citation75], caution should be exercised when applying this result to Japanese settings.
As of December 2023, the application for approval to use of PCV20 as a pediatric vaccine has been submitted in Japan, and the acquisition cost for PCV20 was still unknown. In this study, we temporarily set it as JPY 8,102 for base scenario A and JPY 7,200 (parity to PCV13) for base scenario B. In both base scenarios, PCV20 was found to be dominant over PCV13. Furthermore, threshold analysis revealed that the ICER was still under JPY 5 million/QALY in comparison with PCV13 when the acquisition cost of PCV20 was set at JPY 74,033 from the societal perspective and JPY 67,758 from the payer perspective. These results will be informative for future discussions about the PCV20 price and its inclusion in the Japanese NIP.
There are several ways to value lost productivity. The human capital approach was used in this study because the approach is commonly used and recommended in many pharmacoeconomic guidelines including Japan [Citation31,Citation76]. One of the other interesting approaches is to estimate the society’s loss because of less productivity caused by the absence of adults or of parents caring for sick children and losing days from work. However, our study result indicated that PCV20 was dominant, being associated with less cost and greater effectiveness, in both societal and payer perspectives (i.e. with and without productivity loss). Therefore, scenario analysis with another approach for evaluation of productivity losses was not conducted in this study.
To the best of our knowledge, this is the first analysis evaluating the cost-effectiveness of the pediatric PCV20 vaccination strategy using epidemiologic data, including serotype distribution, in Japan. Our study strengthens previous evidence from other countries on the impact of vaccinating with PCV20 despite using a relatively different population setting, epidemiological information, and vaccine prices [Citation21,Citation22,Citation77–80]. For example, in the US, vaccination with PCV20 was shown to result in an ICER ranging from cost-saving to USD 125,000/QALY compared with PCV13 in children aged <2 years, and therefore, the Pneumococcal Vaccines Work Group recommended the routine use of PCV20 in this population in June 2023 [Citation77]. Similar to PCV20, our study results are also in alignment with the recently published cost-effectiveness analysis of PCV15 compared with PCV13 in Japan, which reported PCV15 to be a dominant strategy over PCV13 for pediatric vaccination from both the societal and payer perspectives [Citation23].
The results of this study must be interpreted in the context of a few limitations related to the study design, particularly around the availability of inputs used to populate the model. First, the decision-analytic model is a simplified representation of disease transmission and outcomes in PD and may not represent the complete heterogeneity of the health care delivery system. We mitigated this by using age stratification and by including the indirect effects of the vaccines. Second, in line with other published studies, multiple sources were used to derive the direct and indirect effects of vaccines for each health state, thereby introducing some bias regarding interpretation and data synthesis. As the direct effectiveness data of PCV15 and PCV20 was limited, it was assumed that the direct effectiveness of PCV13, PCV15, and PCV20 would be same against the serotypes included in the vaccines, i.e. serotype-specific effectiveness was not considered in the model due to the lack of robust data. It is therefore essential to review the vaccine effectiveness as more evidence on PCV15 and PCV20 accumulates. Third, we assumed the serotype distribution for each vaccine to be the same for IPD and noninvasive diseases; however, there could be unaccounted differences. Sensitivity analyses confirmed that the outcomes remained unchanged after using alternative parameters. Fourth, our study did not consider the serotype replacement, which is the reduction in cases of PD caused by vaccine-type serotypes of PCV15 and PCV20 after their introduction. The outcomes could have been overestimated as we used the same values for the serotype distribution and incidence over the ten years of analysis. In the future, it will be crucial to closely monitor the distribution of emerging serotypes after the introduction of the PCV15 and PCV20 vaccines. Last, the probability distributions around a few parameters in PSA might be subjective because they were based on extrapolation and secondary data synthesis. Nonetheless, the combination of scenario and sensitivity analyses provided evidence that the conclusions reported in this study were robust.
5. Conclusions
From the societal and payer perspectives, PCV20 was estimated to be a dominant vaccination strategy over PCV13 for protecting children against pneumococcal infections in Japan. In the future, PCV20 is expected to replace PCV13 in the routine vaccination program in Japan, where there are fiscal challenges to curb rapidly increasing medical costs.
Declaration of interest
M Shinjoh has no relevant affiliations or financial involvement with any other organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. However, outside this study, M Shinjoh has received speaking honoraria from Meiji Seika Pharma Co., Ltd., Shionogi & Co., Ltd., MSD K.K., FUJIREBIO Inc., Mitsubishi Tanabe Pharma Corporation, Taisho Pharmaceutical Co., Ltd., FUJIFILM Toyama Chemical Co., Ltd., The Mainichi Newspapers Co., Ltd., Maruho Co., Ltd., Astellas Pharma Inc., Sumitomo Pharma Co., Ltd., Daiichi Sankyo Co., Ltd., Takeda Pharmaceutical Co., Ltd., Japan Vaccine, a consultation fee from Pfizer Japan Inc., and grant support from JSPS KAKENHI, Japan (Grant Number JP20K10546, 2020–2023), none of which was in connection with the work presented here. K Togo, N Yonemoto, T Hayamizu, and K Kamei are employees of Pfizer Japan Inc. J Morii is an employee of IQVIA Solutions G.K., and J Perdrizet is an employee of Pfizer Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All the authors (M Shinjoh, K Togo, T Hayamizu, N Yonemoto, J Morii, J Perdrizet, K Kamei) (1) made substantial contributions to the study concept or the data analysis or interpretation, (2) drafted the manuscript or revised it critically for important intellectual content, (3) approved the final version of the manuscript to be published, and (4) agreed to be accountable for all aspects of the work.
Ethical approval
This study used publicly available or anonymized data; therefore, ethical approval was not required.
Supplemental Material
Download MS Word (281.8 KB)Acknowledgments
The authors thank Dr. Ataru Igarashi of Yokohama City University School of Medicine and The University of Tokyo for providing valuable scientific advice. Medical writing support was provided by Anshika Singhal and Saurabh Trikha of IQVIA, India, and was funded by Pfizer Japan Inc. The analysis was supported by Des Dillon-Murphy and Ruth Chapman of Evidera. A claims database analysis was supported by Masashi Mikami of Pfizer Japan Inc. Yawen Dai of IQVIA Solutions G.K. assisted with data collection and the literature review.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article or its supplemental materials.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2024.2345670
Additional information
Funding
References
- Centers for Disease Control and Prevention, Hall E, Wodi AP, Hamborsky J, et al., editors. Epidemiology and prevention of vaccine-preventable diseases. 14th ed. Washington, D.C: Public Health Foundation; 2021.
- Shinjoh M, Yamaguchi Y, Furuichi M, et al. Recent trends in pediatric bacterial meningitis in Japan, 2016-2018 - S. agalactiae has been the most common pathogen. J Infect Chemother. 2020 Oct;26:(10):1033–1041.
- Furuichi M, Yaginuma M, Shinjoh M, et al. Extended-spectrum β-lactamase-producing Escherichia coli in neonates and listeria monocytogenes in young children with bacterial meningitis in Japan. J Pediatric Infect Dis Soc. 2023 Apr 18;12(3):165–168. doi: 10.1093/jpids/piac135
- Yanagihara K, Kosai K, Mikamo H, et al. Serotype distribution and antimicrobial susceptibility of streptococcus pneumoniae associated with invasive pneumococcal disease among adults in Japan. Int J Infect Dis. 2021 Jan;102:260–268. doi: 10.1016/j.ijid.2020.10.017
- Epidemiological information of invasive pneumococcal infection in children and adults. [overview of FY2013~FY2022] 2022. Available from: https://ipd-information.com/?page_id=46
- Pimenta F, Moiane B, Gertz RE Jr., et al. New pneumococcal serotype 15D. J Clin Microbiol. 2021 Apr 20;59(5):e00329–21. doi: 10.1128/JCM.00329-21
- Ganaie F, Saad JS, McGee L, et al. A new pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral streptococcus. MBio. 2020 May 19;11(3):e00937–20. doi: 10.1128/mBio.00937-20
- Hausdorff WP, Hanage WP. Interim results of an ecological experiment - conjugate vaccination against the pneumococcus and serotype replacement. Hum Vaccin Immunother. 2016;12(2):358–374. doi: 10.1080/21645515.2015.1118593
- Yahiaoui RY, Bootsma HJ, den Heijer CD, et al. Distribution of serotypes and patterns of antimicrobial resistance among commensal streptococcus pneumoniae in nine European countries. BMC Infect Dis. 2018 Aug 29;18(1):440. doi: 10.1186/s12879-018-3341-0
- Kay EJ, Yates LE, Terra VS, et al. Recombinant expression of streptococcus pneumoniae capsular polysaccharides in Escherichia coli. Open Biol. 2016 Apr;6:(4):150243.
- Pfizer. Full prescribing information-PREVNAR 20® 2023. 2023 Apr. Available from: https://labeling.pfizer.com/ShowLabeling.aspx?id=15428
- MSD Corporation. 15-valent pneumococcal conjugate vaccine “vacnyvance ® aqueous suspension syringe” obtained additional approval for pediatric indication 2023. 2023 Aug 31. Available from: https://www.msd.co.jp/news/product-news-20230626-2/
- Levy C, Varon E, Ouldali N, et al. Bacterial causes of otitis media with spontaneous perforation of the tympanic membrane in the era of 13 valent pneumococcal conjugate vaccine. PLOS ONE. 2019;14(2):e0211712. doi: 10.1371/journal.pone.0211712
- Garcia Quesada M, Yang Y, Bennett JC, et al. Serotype distribution of remaining pneumococcal meningitis in the mature PCV10/13 period: findings from the PSERENADE project. Microorganisms. 2021 Apr 1;9(4):738. doi: 10.3390/microorganisms9040738
- Ekinci E, Desmet S, Van Heirstraeten L, et al. Streptococcus pneumoniae serotypes carried by young children and their association with acute otitis media during the period 2016-2019. Front Pediatr. 2021;9:664083. doi: 10.3389/fped.2021.664083
- De Miguel S, Latasa P, Yuste J, et al. Age-dependent serotype-associated case-fatality rate in invasive pneumococcal disease in the autonomous community of Madrid between 2007 and 2020. Microorganisms. 2021 Nov 3;9(11):2286. doi: 10.3390/microorganisms9112286
- Cho EY, Choi EH, Kang JH, et al. Early changes in the serotype distribution of invasive pneumococcal isolates from children after the introduction of extended-valent pneumococcal conjugate vaccines in Korea, 2011-2013. J Korean Med Sci. 2016 Jul;31:(7):1082–1088.
- Balsells E, Guillot L, Nair H, et al. Serotype distribution of streptococcus pneumoniae causing invasive disease in children in the post-PCV era: a systematic review and meta-analysis. PLOS ONE. 2017;12(5):e0177113. doi: 10.1371/journal.pone.0177113
- Amin-Chowdhury Z, Collins S, Sheppard C, et al. Characteristics of invasive pneumococcal disease caused by emerging serotypes after the introduction of the 13-valent pneumococcal conjugate vaccine in England: a prospective observational cohort study, 2014-2018. Clin Infect Dis. 2020 Nov 5;71(8):e235–e243. doi: 10.1093/cid/ciaa043
- Ministry of Health Labour and Welfare (MHLW). [Basic plan for immunization] 2014. 21 Nov 2014 [2023 Sep 7]. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/kekkaku-kansenshou/kihonteki_keikaku/index.html
- Lytle D, Grajales Beltrán AG, Perdrizet J, et al. Cost-effectiveness analysis of PCV20 to prevent pneumococcal disease in the Canadian pediatric population. Hum Vaccin Immunother. 2023 Aug;19:(2):2257426.
- Warren S, Barmpouni M, Kossyvaki V, et al. Estimating the clinical and economic impact of switching from the 13-valent pneumococcal conjugate vaccine (PCV13) to higher-valent options in Greek infants. Vaccines (Basel). 2023 Aug 15;11(8):1369. doi: 10.3390/vaccines11081369
- Tajima A, Abe M, Weaver J, et al. Cost-effectiveness analysis of pediatric immunization program with 15-valent pneumococcal conjugate vaccine in Japan. J Med Econ. 2023 Jan;26:(1):1034–1046.
- Grabenstein JD, Weber DJ. Pneumococcal serotype diversity among adults in various countries, influenced by pediatric pneumococcal vaccination uptake. Clin Infect Dis. 2014 Mar;58(6):854–864. doi: 10.1093/cid/cit800
- Ministry of Health Labour and Welfare (MHLW). Pneumococcal vaccine working team. Pneumococcal conjugate vaccine for children working team report. 2018 [2023 Sep 4]. Available from: https://www.mhlw.go.jp/file/05-Shingikai-10601000-Daijinkanboukouseikagakuka-Kouseikagakuka/0000207088_1.pdf
- Løchen A, Anderson RM. Dynamic transmission models and economic evaluations of pneumococcal conjugate vaccines: a quality appraisal and limitations. Clin Microbiol Infect. 2020 Jan;26(1):60–70. doi: 10.1016/j.cmi.2019.04.026
- Shiri T, Datta S, Madan J, et al. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: a systematic review and meta-analysis. Lancet Glob Health. 2017 Jan;5:(1):e51–e59.
- OECD. Exchange rates 2022. Available from: https://data.oecd.org/conversion/exchange-rates.htm
- Fukuda T, Shiroiwa T. Cost effectiveness evaluation of health care technologies in Japan: new HTA system and the role of C2H. J Natl Inst Public Health. 2021;70(1):22–27.
- Hasegawa M, Komoto S, Shiroiwa T, et al. Formal implementation of cost-effectiveness evaluations in Japan: a unique health technology assessment system. Value Health. 2020 Jan;23:(1):43–51.
- Center for Outcomes Research and Economic Evaluation for Health- National Institute of Public Health (C2H Japan). Guideline for preparing cost-effectiveness evaluation to the Central Social Insurance Medical Council. Version 3 2022. 2022 Jan 19 [cited 2022 Apr 12]. Available from: https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf
- Ministry of Health Labour and Welfare (MHLW). [Number of people who received routine immunizations] 2021. 2023 Aug 29. Available from: https://www.mhlw.go.jp/topics/bcg/other/5.html
- Ministry of Internal Affairs and Communications. Population estimates (as of October 1st, 2021): Statistics Bureau; 2022. 2023 Jun 7. Available from: https://www.stat.go.jp/data/jinsui/2021np/index.html
- National Institute of Infectious Diseases. [Annual report of the National Epidemiological Surveillance of infectious diseases (NESID): 2014–2021] 2022. 2022 Feb 3 [2023 Jul 6]. Available from: https://www.niid.go.jp/niid/en/component/search/?searchword=NESID%E5%B9%B4%E5%A0%B1%E9%9B%86%E8%A8%88%E8%A1%A8&ordering=newest&searchphrase=all&limit=50
- JMDC. JMDC Real World Tokyo: JMDC; 2022 [2023 Jun 7]. Available from: https://www.eng.phm-jmdc.com/
- Morimoto K, Suzuki M, Ishifuji T, et al. The burden and etiology of community-onset pneumonia in the aging Japanese population: a multicenter prospective study. PLOS ONE. 2015 Mar 30;10(3):e0122247. doi: 10.1371/journal.pone.0122247
- National Institute of Infectious Diseases and Health Service Bureau. [Infectious agents surveillance report (IASR) Vol.44 No.1 2023 January]. 2023 Jun 7. Available from: https://www.niid.go.jp/niid/images/idsc/iasr/44/515.pdf
- Chang B, Tamura K, Fujikura H, et al. Pneumococcal meningitis in adults in 2014-2018 after introduction of pediatric 13-valent pneumococcal conjugate vaccine in Japan. Sci Rep. 2022 Feb 23;12(1):3066. doi: 10.1038/s41598-022-06950-w
- Shinjoh M, Yamaguchi Y, Iwata S. Pediatric bacterial meningitis in Japan, 2013-2015 - 3-5 years after the wide use of Haemophilus influenzae type b and Streptococcus pneumoniae conjugated vaccines. J Infect Chemother. 2017 Jul;23(7):427–438. doi: 10.1016/j.jiac.2017.02.014
- Nakano S, Fujisawa T, Ito Y, et al. Nationwide surveillance of paediatric invasive and non-invasive pneumococcal disease in Japan after the introduction of the 13-valent conjugated vaccine, 2015-2017. Vaccine. 2020 Feb 11;38(7):1818–1824. doi: 10.1016/j.vaccine.2019.12.022
- Ministry of Health Labour and Welfare (MHLW). [Overview of patient survey 2020] 2022. 2023 Jun 7. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/kanja/20/index.html
- Ministry of Health Labour and Welfare (MHLW). [Vital statistics 2014–2020] 2022. 2023 May 26 [2023 Jun 7]. Available from: https://www.mhlw.go.jp/toukei/list/81-1.html
- Iwata S, Takata M, Morozumi M, et al. Drastic reduction in pneumococcal meningitis in children owing to the introduction of pneumococcal conjugate vaccines: longitudinal analysis from 2002 to 2016 in Japan. J Infect Chemother. 2021 Apr;27:(4):604–612.
- Iwata S, Hanada S, Takata M, et al. Risk factors and pathogen characteristics associated with unfavorable outcomes among adults with pneumococcal meningitis in Japan, 2006 to 2016. J Infect Chemother. 2023 Jul;29:(7):637–645.
- Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine. 2004 Oct 22;22(31–32):4203–4214.
- Rozenbaum MH, van Hoek AJ, Fleming D, et al. Vaccination of risk groups in England using the 13 valent pneumococcal conjugate vaccine: economic analysis. BMJ. 2012 Oct 26;345(oct26 1):e6879. doi: 10.1136/bmj.e6879
- Stoecker C, Kim L, Gierke R, et al. Incremental cost-effectiveness of 13-valent pneumococcal conjugate vaccine for adults age 50 years and older in the United States. J Gen Intern Med. 2016 Aug;31:(8):901–908.
- Oostenbrink R, AM HA, Essink-Bot ML. The EQ-5D and the health utilities index for permanent sequelae after meningitis: a head-to-head comparison. J Clin Epidemiol. 2002 Aug;55(8):791–799. doi: 10.1016/S0895-4356(02)00448-1
- Ikeda T, Yanagawa S, Murakami K, et al. Cost-utility analysis of a disability prevention program for community-dwelling older adults with mild disability in the urban area of Tokyo, Japan. Japanese J Human Sci Health-Soc Serv. 2017 Mar 21;23(2):3–12.
- Ministry of Health Labour and Welfare (MHLW). [Guidelines for evaluating the cost-effectiveness of vaccination]. Available from: https://www.mhlw.go.jp/stf/shingi/2r98520000014ryv-att/2r98520000014sdi.pdf
- Ministry of Health Labour and Welfare (MHLW). Pneumococcal 13-valent conjugate vaccine [diphtheria CRM197 protein] suspension for intramuscular injection. (in Japanese). Available from: https://www.mhlw.go.jp/stf/shingi/2r985200000371fc-att/2r985200000371td.pdf
- MDV. MDV Database Overview Tokyo: MDV; 2023 Jun 7. Available from: https://en.mdv.co.jp/about-mdv-database/mdv-database-overview/
- Suaya JA, Ohno T, Hilton B, et al. Cost-effectiveness analysis of 13-valent versus 10-valent pneumococcal conjugate vaccines as part of routine infant pneumococcal vaccination program in Japan. Jpn J Pediatr. 2015 06;68(6):1197–1217.
- Ministry of Health Labour and Welfare (MHLW). [Basic survey on wage structure in 2022] (in Japanese). 2022. Available from: https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00450091&tstat=000001011429&cycle=0&tclass1=000001202310&tclass2=000001202312&tclass3=000001202328&tclass4val=0
- National Institute of population and social security research. [population projections for Japan (2023 revision): median birth (median death)]: National Institute of population and social security Research; 2023. 2023 Jun 7. Available from: https://www.ipss.go.jp/pp-zenkoku/j/zenkoku2023/db_zenkoku2023/db_r5_suikeikekka_1.html
- Epidemiological information of invasive pneumococcal infection in children and adults. Epidemiological status in FY2022. 2022. Available from: https://ipd-information.com/?page_id=55
- Ministry of Health Labour and Welfare (MHLW). [Overview of the simplified life tables in 2021] 2021. 2023 Jun 7. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/life/life21/index.html
- Savulescu C, Krizova P, Valentiner-Branth P, et al. Effectiveness of 10 and 13-valent pneumococcal conjugate vaccines against invasive pneumococcal disease in European children: SpIDnet observational multicentre study. Vaccine. 2022 Jun 23;40(29):3963–3974. doi: 10.1016/j.vaccine.2022.05.011
- Patterson S, Webber C, Patton M, et al. A post hoc assessment of duration of protection in CAPiTA (community acquired pneumonia immunization trial in adults). Trials Vaccinol. 2016 Mar 7;5:92–96.
- Perdrizet J, Horn EK, Hayford K, et al. Historical population-level impact of infant 13-valent pneumococcal conjugate vaccine (PCV13) national immunization programs on invasive pneumococcal disease in Australia, Canada, England and Wales, Israel, and the United States. Infect Dis Ther. 2023 May;12:(5):1351–1364.
- Shiroiwa T, Noto S, Fukuda T. Japanese population norms of EQ-5D-5L and health utilities index mark 3: disutility catalog by disease and symptom in community settings. Value Health. 2021 Aug;24(8):1193–1202. doi: 10.1016/j.jval.2021.03.010
- Ministry of Health Labour and Welfare (MHLW). [Precipitated 13-valent pneumococcal conjugate vaccine (non-toxic mutant diphtheria toxin conjugate) Prevenar 13Ⓡ Aqueous Suspension Injection] 2013. 2023 Sep 2. Available from: https://www.mhlw.go.jp/stf/shingi/2r985200000371fc.att/2r985200000371td.pdf
- Ministry of Health Labour and Welfare (MHLW). [Pneumococcal vaccine for children] 2023. 2023 Sep 2. Available from: https://www.mhlw.go.jp/content/10900000/001139474.pdf
- Centers for Disease Control and Prevention. Vaccines for children program (VFC). CDC vaccine price list. 2023. Available from: https://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html
- Ministry of Health Labour and Welfare (MHLW). [Medical service fee information] 2023. 2023 Jun 7. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/iryouhoken/newpage_21053.html
- Igarashi A, Hirose E, Kobayashi Y, et al. Cost-effectiveness analysis for PCV13 in adults 60 years and over with underlying medical conditions which put them at an elevated risk of pneumococcal disease in Japan. Expert Rev Vaccines. 2021 Sep;20:(9):1153–1165.
- Briggs A, Schulpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Ministry of Health Labour and Welfare (MHLW). Health labour sciences research grant. [description of invasive pneumococcus and Haemophilus influenzae infections in the infectious disease trend survey and comparison with the urushibara group in children]. 2015. Available from: https://mhlw-grants.niph.go.jp/system/files/2014/143121/201420044A/201420044A0001.pdf
- Yamanaka N, Hotomi M, Sugita R. Disease-burden of acute otitis media on children and cost-effectiveness of pneumococcal conjugate vaccine in Japan. J Pediat Infect Dis Immuno. 2009;21:37–48.
- Goldblatt D. The indirect effect of pneumococcal conjugate vaccine. Lancet Glob Health. 2017 Jan;5(1):e6–e7. doi: 10.1016/S2214-109X(16)30338-2
- Nymark LS, Sharma T, Miller A, et al. Inclusion of the value of herd immunity in economic evaluations of vaccines. A systematic review of methods used. Vaccine. 2017 Dec 14;35(49 Pt B):6828–6841. doi: 10.1016/j.vaccine.2017.10.024
- Yamamoto T, Komiya K, Fujita N, et al. COVID-19 pandemic and the incidence of community-acquired pneumonia in elderly people. Respir Investig. 2020 Nov;58:(6):435–436.
- Yan Y, Tomooka K, Naito T, et al. Decreased number of inpatients with community-acquired pneumonia during the COVID-19 pandemic: a large multicenter study in Japan. J Infect Chemother. 2022 May;28:(5):709–713.
- Kitano T, Aoki H. The incremental burden of invasive pneumococcal disease associated with a decline in childhood vaccination using a dynamic transmission model in Japan: a secondary impact of COVID-19. Comput Biol Med. 2021 Jun;133:104429. doi: 10.1016/j.compbiomed.2021.104429
- Ray GT, Whitney CG, Fireman BH, et al. Cost-effectiveness of pneumococcal conjugate vaccine: evidence from the first 5 years of use in the United States incorporating herd effects. Pediatr Infect Dis J. 2006 Jun;25:(6):494–501.
- Jiang S, Wang Y, Si L, et al. Incorporating productivity loss in health economic evaluations: a review of guidelines and practices worldwide for research agenda in China. BMJ Glob Health. 2022 Aug;7:(8):e009777.
- Kobayashi M Centers for Disease Control and Prevention, national center for immunization & respiratory diseases. Evidence to recommendations framework and policy options: use of 20-valent pneumococcal conjugate vaccine in U.S. Children. 2023. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/04-Pneumococcal-Kobayashi-508.pdf
- Huang L, McDade CL, Perdrizet JE, et al. Cost-effectiveness analysis of the South African infant national immunization program for the prevention of pneumococcal disease. Infect Dis Ther. 2023 Mar;12:(3):933–950.
- Wilson M, McDade C, Beby-Heijtel AT, et al. Assessing public health impact of four pediatric pneumococcal conjugate vaccination strategies in the Netherlands. Infect Dis Ther. 2023 Jul;12:(7):1809–1821.
- Wilson M, Lucas A, Mendes D, et al. Estimating the cost-effectiveness of switching to higher-valency pediatric pneumococcal conjugate vaccines in the United Kingdom. Vaccines (Basel). 2023 Jun 28;11(7):1168. doi: 10.3390/vaccines11071168