Abstract
Objective. The fetus is thought to play a central role in the onset of labor. Pulmonary surfactant protein (SP)-A, secreted by the maturing fetal lung, has been implicated in the mechanisms initiating parturition in mice. The present study was conducted to determine whether amniotic fluid concentrations of SP-A and SP-B change during human parturition.
Study design. Amniotic fluid SP-A and SP-B concentrations were measured with a sensitive and specific ELISA in the following groups of pregnant women: (1) mid-trimester of pregnancy, between 15 and 18 weeks of gestation (n = 29), (2) term pregnancy not in labor (n = 28), and (3) term pregnancy in spontaneous labor (n = 26). Non-parametric statistics were used for analysis.
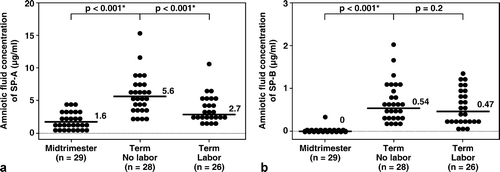
Results. SP-A was detected in all amniotic fluid samples. SP-B was detected in 24.1% (7/29) of mid-trimester samples and in all samples at term. The median amniotic fluid concentrations of SP-A and SP-B were significantly higher in women at term than in women in the mid-trimester (SP-A term no labor: median 5.6 μg/mL, range 2.2–15.2 μg/mL vs. mid-trimester: median 1.64 μg/mL, range 0.1–4.7 μg/mL, and SP-B term no labor: median 0.54 μg/mL, range 0.17–1.99 μg/mL vs. mid-trimester: median 0 μg/mL, range 0–0.35 μg/mL; both p < 0.001). The median amniotic fluid SP-A concentration in women at term in labor was significantly lower than that in women at term not in labor (term in labor: median 2.7 μg/mL, range 1.2–10.1 μg/mL vs. term no labor: median 5.6 μg/mL, range 2.2–15.2 μg/mL; p < 0.001). There was no significant difference in the median amniotic fluid SP-B concentrations between women in labor and those not in labor (term in labor: median 0.47 μg/mL, range 0.04–1.32 μg/mL vs. term no labor: median 0.54 μg/mL, range 0.17–1.99 μg/mL; p = 0.2).
Conclusion. The amniotic fluid concentration of SP-A decreases in spontaneous human parturition at term.
Introduction
Pulmonary surfactant, a complex molecule matrix containing lipids (90%) and specific proteins (10%), reduces surface tension at the air–liquid interface of the lung Citation[1-3]. Lipids in surfactant are critical for pulmonary function, and attention has shifted to the role of the surfactant proteins (SPs). Currently, four types of SP have been characterized: SP-A, SP-B, SP-C, and SP-D. SP-A and SP-D are relatively hydrophilic, C-type collagenous lectins, involved in host defense and the regulation of surfactant structure or homeostasis Citation[4-9]. In contrast, SP-B and SP-C are relatively hydrophobic and play important roles in surfactant homeostasis and function after birth Citation[10-12]. SP-A is the most abundant of the SPs in human lung.
Recently, SP-A was implicated in a mechanism initiating parturition in mice Citation[13]. The experimental basis for this is that: (1) the amniotic fluid concentration of SP-A increased with advancing gestational age in mice; (2) the administration of SP-A into the amniotic cavity on E15 of gestation caused preterm delivery; (3) intra-amniotic injection of a neutralizing antibody to SP-A was associated with prolonged gestation Citation[13]. The proposed hypothesis is that SP-A produced by the maturing fetal lung is secreted into the amniotic cavity where it induces local macrophage activation and infiltration of these cells into the uterine wall, leading to a pro-inflammatory response, suspension of progesterone action, and parturition Citation[13].
It is unclear whether SP-A plays a role in the initiation of parturition in humans. The present study was conducted to determine whether human spontaneous parturition at term is associated with changes in amniotic fluid concentrations of SP-A and SP-B.
Materials and methods
Study design
A cross-sectional study was conducted to measure SP-A, SP-B, and total protein concentration in amniotic fluid samples obtained from women during the second trimester (15–18 weeks) of pregnancy (n = 29), at term (≥37 weeks of gestation) not in labor (n = 28), and at term gestation in spontaneous labor (n = 26). Amniotic fluid was obtained by trans-abdominal amniocentesis. The fluid was centrifuged and the supernatant stored. Patients with meconium-stained amniotic fluid samples were excluded. Second trimester amniotic fluid samples were obtained from patients who had consented to have an amniocentesis for prenatal diagnosis of genetic disorders. All patients from this group delivered a normal neonate at term. Women at term, but not in labor, underwent amniocentesis for the assessment of fetal lung maturity prior to cesarean section, whereas those in labor underwent amniocentesis because of either uncertain gestational age or for the diagnosis of intra-amniotic infection. All women at term had no evidence of medical or obstetrical complications, had negative amniotic fluid cultures for microorganisms, and delivered a normal infant with neonatal birth weight of more than 2500 g. Amniotic fluid not required for clinical assessment was centrifuged for 10 min at 4°C and the supernatant was aliquoted and stored at −70°C until analysis.
All women provided informed consent prior to the collection of amniotic fluid. The collection and utilization of the samples was approved by the Human Investigation Committee of the Sótero del Río Hospital, Santiago, Chile (a major affiliate of the Catholic University of Santiago), the Pennsylvania Hospital, and the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Many of these samples were used in previous studies of cytokines, matrix metalloproteinases and chemokines in human parturition Citation[14-17].
ELISA for determining the SP-A and SP-B concentrations in amniotic fluid
SP-A and SP-B concentrations in amniotic fluid were determined by a sandwich ELISA system, which has been validated for amniotic fluid as previously described in detail Citation[18,], Citation[19]. The inter-assay coefficient of variation (CV) for SP-A was 33.5% and for SP-B was 34.2%, and the intra-assay CV was 7.7% for SP-A and 18.7% for SP-B. The sensitivity for SP-A was 10 ng/mL and for SP-B was 2 ng/mL.
Statistical analysis
The Mann–Whitney U-test was utilized to determine differences in the median between groups. Contingency tables and Chi-square tests were employed for comparisons of proportions. The statistical package used was SPSS 12.0 (SPSS Inc., Chicago, IL, USA).
Results
SP-A was detected in all amniotic fluid samples. SP-B was detected in 24.1% (7/29) of mid-trimester samples (as early as 15 weeks of gestation) and in all samples at term. summarizes demographic and clinical characteristics of the three study groups. As expected, the median maternal age of women in the mid-trimester group was significantly higher than women at term not in labor. Among women at term, there was no significant difference in median maternal age, gestational age at amniocentesis, and neonatal birth weight.
Table I. Demographic and clinical characteristics.
The median amniotic fluid concentrations of SP-A and of SP-B were significantly higher in women at term not in labor than those in the mid-trimester (SP-A term no labor: median 5.6 μg/mL, range 2.2–15.2 μg/mL vs. mid-trimester: median 1.64 μg/mL, range 0.1–4.7 μg/mL; p < 0.001 (see ), and SP-B term no labor: median 0.54 μg/mL, range 0.17–1.99 μg/mL vs. mid-trimester: median 0 μg/mL, range 0–0.35 μg/mL; p < 0.001 (see )).
Figure 1. The median concentrations of SP-A and of SP-B in amniotic fluid were significantly higher at term than in the mid-trimester of pregnancy (SP-A term no labor: median 5.6 μg/mL, range 2.2–15.2 μg/mL vs. mid-trimester: median 1.64 μg/mL, range 0.1–4.7 μg/mL; p < 0.001, and SP-B term no labor: median 0.54 μg/mL, range 0.17–1.99 μg/mL vs. mid-trimester: median, 0 μg/mL, range 0–0.35 μg/mL; p < 0.001). The median amniotic fluid concentration of SP-A in women at term in spontaneous labor was significantly lower than in those not in labor (term in labor: median 2.7 μg/mL, range 1.2–10.1 μg/mL vs. term no labor: median 5.6 μg/mL, range 2.2–15.2 μg/mL; p < 0.001). There was no significant difference in the median amniotic fluid concentration of SP-B between women with and without labor (term in labor: median 0.47 μg/mL, range 0.04–1.32 μg/mL vs. term no labor: median 0.54 μg/mL, range 0.17–1.99 μg/mL; p = 0.2). *p < 0.05.
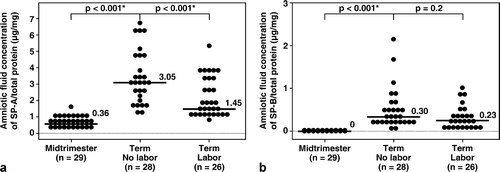
The median amniotic fluid concentration of SP-A in women at term in spontaneous labor was approximately 50% of that found in women at term not in labor (term in labor: median 2.7 μg/mL, range 1.2–10.1 μg/mL vs. term no labor: median 5.6 μg/mL, range 2.2–15.2 μg/mL; p < 0.001; see ). There was no significant difference in the median amniotic fluid SP-B concentration between the two groups of women at term (term in labor: median 0.47 μg/mL, range 0.04–1.32 μg/mL vs. term no labor: median 0.54 μg/mL, range 0.17–1.99 μg/mL; p = 0.2; see ). The results remained similar after adjusting for total amniotic fluid protein content (p = 0.001 and p = 0.2, respectively; see ).
Figure 2. After adjusting for total protein concentration, the median amniotic fluid concentrations of SP-A and of SP-B were significantly higher at term than during the mid-trimester of pregnancy (SP-A/total protein, term no labor: median 3.05 μg/mg, range 1.15–6.87 μg/mg vs. mid-trimester: median 0.36 μg/mg, range 0.09–1.59 μg/mg; p < 0.001, and SP-B/total protein, term no labor: median 0.30 μg/mg, range 0.03–2.11 μg/mg vs. mid-trimester: median 0 μg/mg, range 0–0.08 μg/mg; p < 0.001). Amniotic fluid SP-A concentration after adjusting for total protein concentration in women at term in labor was lower than that for those not in labor (term labor: median 1.45 μg/mg, range 0.8–25.18 μg/mg vs. term not in labor: median 3.05 μg/mg, range 1.15–6.87 μg/mg; p < 0.001). There was no significant difference in the median amniotic fluid concentration of SP-B/total protein in women with and without labor (term labor: median 0.23 μg/mg, range 0.04–5.58 μg/mg vs. term not in labor: median 0.30 μg/mg, range 0.03–2.11 μg/mg; p = 0.2). One data point in the term labor group was an outlier (SP-B/total protein: 5.58 μg/mg) and is not shown in the figure. *p < 0.05.
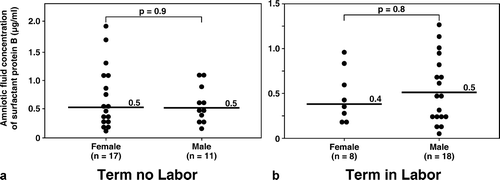
Although more male fetuses were included in the group of women at term in labor than in those not in labor (in labor: 69.2% (18/26) vs. not in labor: 39.3% (11/28); p = 0.03) (), there was no significant difference in the median amniotic fluid concentration of SP-A and SP-B stratified by fetal gender in both the term in labor and term not in labor groups (all p > 0.05; see and ).
Figure 3. Amniotic fluid concentrations of SP-A in women at term with and without labor stratified by gender of the fetus. There was no significant difference in the median amniotic fluid concentration of SP-A stratified by fetal gender in both the term in labor and term not in labor groups (term no labor SP-A, female: median 5.7 μg/mL, range 2.2–15.2 μg/mL vs. male: median 5.3 μg/mL, range 2.6–11.7 μg/mL; p = 0.8, and term in labor SP-A, female: median 2.4 μg/mL, range 1.7–4.1 μg/mL vs. male: median 3.0 μg/mL, range 1.2–10.1 μg/mL; p = 0.2).
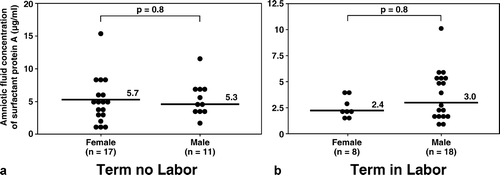
Figure 4. Amniotic fluid concentrations of SP-B in women at term with and without labor stratified by gender of the fetus. There was no significant difference in the median amniotic fluid concentration of SP-B stratified by fetal gender in both the term in labor and term not in labor groups (term no labor SP-B, female: median 0.5 μg/mL, range 0.2–1.9 μg/mL vs. male: median 0.5 μg/mL, range 0.2–1.1 μg/mL; p = 0.9, and term in labor SP-B, female: median 0.4 μg/mL, range 0.2–0.9 μg/mL vs. male: median 0.5 μg/mL, range 0.04–1.3 μg/mL; p = 0.8).
Discussion
Principal findings of this study
(1) The median amniotic fluid SP-A concentration, but not SP-B, is lower in women with spontaneous labor at term than in women not in labor. (2) SP-A and SP-B amniotic fluid concentrations are higher in term gestation than in the mid-trimester of pregnancy.
Physiology of SP-A and SP-B
SP-A is a large, oligomeric protein from the collectin family of C-type lectins, encoded by two genes (SP-A1 and SP-A2) located on chromosome 10. SP-A plays important roles in host defense against infection and is required for the formation of tubular myelin, the major form of alveolar surfactant. SP-A binds bacteria, viruses, allergens, and apoptotic cells and functions as an opsonin, enhancing uptake by alveolar macrophages, monocytes, neutrophils, and dendritic cells Citation[4,], Citation[7,], Citation[11,], Citation[12]. SP-A may also induce or inhibit the production of cytokines and inflammatory mediators (e.g., tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β), reactive oxygen species, and nitric oxide metabolites depending on the types of pathogens or stimuli, activation state of cells, and cellular receptors. The CD91–calreticulin complex, signal-inhibitory regulatory protein-α (SIRP-alpha), or Toll-like receptors (TLRs) 2 and 4 have been proposed as mediators of cellular responses to SP-A Citation[7,], Citation[12,], Citation[20]. In the context of the host response of the lung, the immunomodulatory properties of SP-A might benefit the host Citation[7,], Citation[12]. For example, in the resting state, clearance of apoptotic cells or pathogens without invoking a robust inflammatory response may serve as a protective role in the lung. However, in the context of infection, the recruitment of immune cells or inflammatory mediators might be warranted Citation[7,], Citation[12]. While SP-A-deficient mice are susceptible to infection and lack tubular myelin, surfactant function does not significantly alter by the lack of SP-A Citation[21,], Citation[22]. SP-A has been detected in amniotic fluid and maternal and umbilical cord blood Citation[23,], Citation[24].
SP-B is a hydrophobic protein encoded by a single gene located on chromosome 2 Citation[25]. SP-B plays a critical role in surfactant homeostasis by promoting adsorption of lipid surfactant into the expanding surface film and maintaining its stability during respiratory cycles to lower the surface tension within the alveolar spaces Citation[1,], Citation[26]. Infants with inherited deficiency of SP-B develop severe respiratory distress at birth Citation[27]. SP-B has been detected in amniotic fluid and in adult serum Citation[28].
Alveolar type II cells synthesize all four surfactant proteins and surfactant lipids. Surfactant proteins are also synthesized by airway cells, including Clara cells and submucosal cells. SP-A, but not SP-B, has been localized to non-pulmonary sites Citation[12] including brain, trachea, testis, salivary glands, lacrimal glands, heart, prostate, kidney, pancreas, vagina Citation[29], and human chorio-amniotic membranes Citation[30], supporting the role of SP-A in host defense rather than reducing surface tension.
SP-A and SP-B in mid-trimester amniotic fluid
The finding that amniotic fluid concentrations of SP-A and SP-B in women at term are higher than in the mid-trimester of pregnancy is consistent with that of previous studies Citation[18],Citation[30-33]. Pryhuber and colleagues Citation[18] reported that amniotic fluid concentrations of SP-A and SP-B, and the lecithin–sphingomyelin (L/S) ratio increased with advancing gestational age. The mean amniotic fluid concentration of SP-A gradually increased after 19–23 weeks of gestation, followed by a quadratic increase between 30–40 weeks of gestation. In the current study, SP-A was detectable in the amniotic fluid at 15–18 weeks of gestation. SP-A expression has been detected by in situ hybridization in the human fetal respiratory tract by 16 weeks Citation[18,], Citation[34]. At that time SP-A protein and mRNA expression were observed only in the tracheal epithelium. However, expression by bronchiolar cells and pre-type II lining terminal airways were not observed until 19–20 weeks of gestation Citation[18,], Citation[34]. It is possible that amniotic fluid SP-A in the early mid-trimester derives from an extrapulmonary origin, most likely chorioamniotic membranes. The presence of SP-A in the amniotic cavity at 17 weeks in a patient with fetal tracheal atresia (without tracheo-esophageal fistula) supports this view Citation[35].
The observation that amniotic fluid SP-B was detectable in 24% of the mid-trimester samples and as early as 15 weeks of gestation was unexpected. However, all of the samples except one had low concentrations of SP-B. Contrary to the present findings, SP-B has been previously reported to be first detectable in amniotic fluid at 31 weeks of gestation Citation[18]. SP-B mRNA and pro-protein have been detected in lung tissue as early as 13–15 weeks of gestation Citation[36-38]. The mature SP-B protein has been detected after 19 weeks Citation[37,], Citation[38] and not consistently detectable until the 24th week when the epithelial cell lining begins to differentiate into type II cells containing lamellar bodies.
Amniotic fluid concentrations of SP-A and SP-B in women at term with and without labor
Animal studies suggest that SP-A is incorporated into myelinic and vesicular forms of extracellular surfactant Citation[39] and that labor can stimulate the release of surfactant into the pulmonary airways Citation[40,], Citation[41]. In humans, the concentration of SP-A in umbilical cord blood is higher in newborns delivered after spontaneous labor than in those delivered by elective cesarean section Citation[24]. Cho et al. Citation[24] suggested that this could be due to an increased transfer of pulmonary SP-A to the fetal circulation during labor. Our finding that the median amniotic fluid SP-A concentration was lower in women at term in labor than in those not in labor was unexpected and not consistent with the view that SP-A secreted by the lung may play a role in the onset of parturition. Condon et al. Citation[13,], Citation[42] proposed that SP-A could initiate parturition by directly stimulating amniotic fluid fetal macrophages to migrate into the uterine wall, where they would produce pro-inflammatory cytokines and activate nuclear factor kappa B leading to suspension of the progesterone action and uterine quiescence. However, there is no information available about the amniotic fluid concentrations of SP-A in mice in labor compared to those not in labor. A recent study in placental bed biopsy and postpartum hysterectomy specimens from pregnant patients with male fetuses reported that macrophages in human myometrium after labor are all maternal origin Citation[43]. This study suggested that the trafficking of fetal macrophages in labor seems to be different between humans and mice. Although in vitro experiments provided evidence that surfactant lipids and SP-A could stimulate prostaglandin production in human amnion Citation[44,], Citation[45], there was no significant difference in SP-A mRNA expression in the chorioamniotic membranes of women at term not in labor and those in labor Citation[35]. Finally, the observation that mice with a genetic deletion Citation[46] or an over-expression Citation[47] of the SP-A gene reproduce normally contradicts the role of SP-A as an essential factor of labor.
The current study does not exclude a role for SP-A in the mechanism of parturition. Human labor is associated with an increased production of prostaglandins and pro-inflammatory cytokines by intrauterine tissue, amniotic fluid, and invading macrophages Citation[48-51]. The decreased amniotic fluid concentration of SP-A may be due to the uptake by macrophages and other cells including amnion. However, there is no experimental evidence at this point that this is the case. Alternatively, SP-A could potentially suppress an inflammatory response in intrauterine tissues during normal gestation Citation[7]. A decrease in amniotic fluid SP-A could be associated with an acute intrauterine inflammatory response and parturition Citation[7]. A recent study indicated that human fetal membranes from women in spontaneous labor, but without histologic chorioamnionitis, had a gene expression pattern consistent with inflammation Citation[52].
Another possible explanation for the observed lower amniotic fluid concentration of SP-A in women at term in labor than in those without labor is that SP-A may be incorporated into tubular myelin Citation[39] in preparation for neonatal exposure to the noxious elements present in room air after birth. Incorporation of SP-A into the complex surfactant molecule matrix present in the lung leads to a decrease in amniotic fluid SP-A concentration. In an animal experiment, a time-dependent movement of exogenous SP-A from extracellular components into pneumocyte type II cells was observed after intra-tracheal instillation of SP-A Citation[39] suggesting that alveolar clearance and recycling of SP-A into type II cells or tubular myelin could occur in humans. Finally, since there is an increased concentration of neutrophil elastase in the amniotic fluid of women at term in labor Citation[53], and the incubation of bronchoalveolar lavage fluid from patients with cystic fibrosis (that contained high concentration of protease) with SP-A caused a time-dependent degradation of SP-A Citation[54], it is also possible that the decreased concentrations of SP-A in the amniotic fluid are due to the action of proteases during term labor Citation[53,], Citation[54].
Strengths and limitations of the study
This is the first study examining the question whether the concentrations of SP-A and SP-B in amniotic fluid change in human parturition. Since this is a retrospective cross-sectional study, the chronological events or causal relationships would be difficult to specify. Moreover, the ELISA assay system validated for amniotic fluid Citation[18,], Citation[19] had slightly high inter- and intra-assay coefficients of variation. So far, there has been no commercially available assay for surfactant proteins (except SP-D).
Conclusion
In summary, our study demonstrates that the amniotic fluid concentrations of SP-A, but not of SP-B, are lower in normal pregnancies at term in spontaneous labor than in those not in labor.
Acknowledgement
This research was supported in part by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Whitsett J A, Weaver T E. Hydrophobic surfactant proteins in lung function and disease. N Engl J Med 2002; 347: 2141–2148
- Weaver T E, Whitsett J A. Function and regulation of expression of pulmonary surfactant-associated proteins. Biochem J 1991; 273(Pt 2)249–264
- Jobe A H, Ikegami M. Surfactant and acute lung injury. Proc Assoc Am Physicians 1998; 110: 489–495
- McCormack F X, Whitsett J A. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J Clin Invest 2002; 109: 707–712
- Cockshutt A M, Weitz J, Possmayer F. Pulmonary surfactant-associated protein A enhances the surface activity of lipid extract surfactant and reverses inhibition by blood proteins in vitro. Biochemistry 1990; 29: 8424–8429
- Dobbs L G, Wright J R, Hawgood S, Gonzalez R, Venstrom K, Nellenbogen J. Pulmonary surfactant and its components inhibit secretion of phosphatidylcholine from cultured rat alveolar type II cells. Proc Natl Acad Sci U S A 1987; 84: 1010–1014
- Kishore U, Greenhough T J, Waters P, Shrive A K, Ghai R, Kamran M F, Bernal AL, Reid KB, Madan T, Chakraborty T. Surfactant proteins SP-A and SP-D: Structure, function and receptors. Mol Immunol 2006; 43: 1293–1315
- Rice W R, Ross G F, Singleton F M, Dingle S, Whitsett J A. Surfactant-associated protein inhibits phospholipid secretion from type II cells. J Appl Physiol 1987; 63: 692–698
- Suzuki Y, Fujita Y, Kogishi K. Reconstitution of tubular myelin from synthetic lipids and proteins associated with pig pulmonary surfactant. Am Rev Respir Dis 1989; 140: 75–81
- Whitsett J A, Nogee L M, Weaver T E, Horowitz A D. Human surfactant protein B: Structure, function, regulation, and genetic disease. Physiol Rev 1995; 75: 749–757
- Whitsett J A. Surfactant proteins in innate host defense of the lung. Biol Neonate 2005; 88: 175–180
- Wright J R. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 2005; 5: 58–68
- Condon J C, Jeyasuria P, Faust J M, Mendelson C R. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci U S A 2004; 101: 4978–4983
- Romero R, Gomez R, Galasso M, Munoz H, Acosta L, Yoon B H, Svinarich D, Cotton DB. Macrophage inflammatory protein-1 alpha in term and preterm parturition: Effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol 1994; 32: 108–113
- Espinoza J, Chaiworapongsa T, Romero R, Gomez R, Kim J C, Yoshimatsu J, Edwin S, Rathnasabapathy C, Yoon B H. Evidence of participation of soluble CD14 in the host response to microbial invasion of the amniotic cavity and intra-amniotic inflammation in term and preterm gestations. J Matern Fetal Neonatal Med 2002; 12: 304–312
- Park K H, Chaiworapongsa T, Kim Y M, Espinoza J, Yoshimatsu J, Edwin S, Gomez R, Yoon B H, Romero R. Matrix metalloproteinase 3 in parturition, premature rupture of the membranes, and microbial invasion of the amniotic cavity. J Perinat Med 2003; 31: 12–22
- Esplin M S, Romero R, Chaiworapongsa T, Kim Y M, Edwin S, Gomez R, Gonzalez R, Adashi E Y. Amniotic fluid levels of immunoreactive monocyte chemotactic protein-1 increase during term parturition. J Matern Fetal Neonatal Med 2003; 14: 51–56
- Pryhuber G S, Hull W M, Fink I, McMahan M J, Whitsett J A. Ontogeny of surfactant proteins A and B in human amniotic fluid as indices of fetal lung maturity. Pediatr Res 1991; 30: 597–605
- McMahan M J, Mimouni F, Miodovnik M, Hull W M, Whitsett J A. Surfactant associated protein (SAP-35) in amniotic fluid from diabetic and nondiabetic pregnancies. Obstet Gynecol 1987; 70: 94–98
- Guillot L, Balloy V, McCormack F X, Golenbock D T, Chignard M, Si-Tahar M. Cutting edge: The immunostimulatory activity of the lung surfactant protein-A involves Toll-like receptor 4. J Immunol 2002; 168: 5989–5992
- LeVine A M, Kurak K E, Wright J R, Watford W T, Bruno M D, Ross G F, Whitsett JA, Korfhagen TR. Surfactant protein-A binds group B Streptococcus enhancing phagocytosis and clearance from lungs of surfactant protein-A-deficient mice. Am J Respir Cell Mol Biol 1999; 20: 279–286
- LeVine A M, Bruno M D, Huelsman K M, Ross G F, Whitsett J A, Korfhagen T R. Surfactant protein A-deficient mice are susceptible to group B streptococcal infection. J Immunol 1997; 158: 4336–4340
- Doyle I R, Hermans C, Bernard A, Nicholas T E, Bersten A D. Clearance of Clara cell secretory protein 16 (CC16) and surfactant proteins A and B from blood in acute respiratory failure. Am J Respir Crit Care Med 1998; 158: 1528–1535
- Cho K, Matsuda T, Okajima S, Matsumoto Y, Sagawa T, Fujimoto S, Kobayashi K. Factors influencing pulmonary surfactant protein A levels in cord blood, maternal blood and amniotic fluid. Biol Neonate 1999; 75: 104–110
- Nogee L M. Genetics of the hydrophobic surfactant proteins. Biochim Biophys Acta 1998; 1408: 323–333
- Hawgood S. Surfactant protein B: Structure and function. Biol Neonate 2004; 85: 285–289
- Nogee L M. Alterations in SP-B and SP-C expression in neonatal lung disease. Annu Rev Physiol 2004; 66: 601–623
- Hermans C, Dong P, Robin M, Jadoul M, Bernard A, Bersten A D, Doyle IR. Determinants of serum levels of surfactant proteins A and B and Clara cell protein CC16. Biomarkers 2003; 8: 461–471
- MacNeill C, Umstead T M, Phelps D S, Lin Z, Floros J, Shearer D A, Weisz J. Surfactant protein A, an innate immune factor, is expressed in the vaginal mucosa and is present in vaginal lavage fluid. Immunology 2004; 111: 91–99
- Miyamura K, Malhotra R, Hoppe H J, Reid K B, Phizackerley P J, Macpherson P, Lopez B A. Surfactant proteins A (SP-A) and D (SP-D): Levels in human amniotic fluid and localization in the fetal membranes. Biochim Biophys Acta 1994; 1210: 303–307
- King R J, Ruch J, Gikas E G, Platzker A C, Creasy R K. Appearance of apoproteins of pulmonary surfactant in human amniotic fluid. J Appl Physiol 1975; 39: 735–741
- Shelley S A, Balis J U, Paciga J E, Knuppel R A, Ruffolo E H, Bouis P J, Jr. Surfactant ‘apoproteins’ in human amniotic fluid: An enzyme-linked immunosorbent assay for the prenatal assessment of lung maturity. Am J Obstet Gynecol 1982; 144: 224–228
- Dilger I, Schwedler G, Dudenhausen J W. Determination of the pulmonary surfactant-associated protein SP-B in amniotic fluid with a competition ELISA. Gynecol Obstet Invest 1994; 38: 24–27
- Khoor A, Gray M E, Hull W M, Whitsett J A, Stahlman M T. Developmental expression of SP-A and SP-A mRNA in the proximal and distal respiratory epithelium in the human fetus and newborn. J Histochem Cytochem 1993; 41: 1311–1319
- Han Y M, Romero R, Kim Y M, Kim J S, Richani K, Friel L A, Kusanovic JP, Jeanty C, Vitale S, Nien JK, et al. Surfactant protein-A mRNA expression by human fetal membranes is increased in histological chorioamnionitis but not in spontaneous labour at term. J Pathol 2007; 211: 489–496
- Liley H G, White R T, Warr R G, Benson B J, Hawgood S, Ballard P L. Regulation of messenger RNAs for the hydrophobic surfactant proteins in human lung. J Clin Invest 1989; 83: 1191–1197
- Pryhuber G S. Regulation and function of pulmonary surfactant protein B. Mol Genet Metab 1998; 64: 217–228
- Stahlman M T, Gray M E, Whitsett J A. The ontogeny and distribution of surfactant protein B in human fetuses and newborns. J Histochem Cytochem 1992; 40: 1471–1480
- Savov J, Wright J R, Young S L. Incorporation of biotinylated SP-A into rat lung surfactant layer, type II cells, and Clara cells. Am J Physiol Lung Cell Mol Physiol 2000; 279: L118–126
- Rooney S A, Gobran L I, Wai-Lee T S. Stimulation of surfactant production by oxytocin-induced labor in the rabbit. J Clin Invest 1977; 60: 754–759
- Marino P A, Rooney S A. The effect of labor on surfactant secretion in newborn rabbit lung slices. Biochim Biophys Acta 1981; 664: 389–396
- Mendelson C R, Condon J C. New insights into the molecular endocrinology of parturition. J Steroid Biochem Mol Biol 2005; 93: 113–119
- Kim C J, Kim J S, Kim Y M, Cushenberry E, Richani K, Espinoza J, Romero R. Fetal macrophages are not present in the myometrium of women with labor at term. Am J Obstet Gynecol 2006; 195: 829–833
- Lopez B A, Newman G E, Phizackerley P J, Turnbull A C. Surfactant stimulates prostaglandin E production in human amnion. Br J Obstet Gynaecol 1988; 95: 1013–1017
- Sun K, Brockman D, Campos B, Pitzer B, Myatt L. Induction of surfactant protein A expression by cortisol facilitates prostaglandin synthesis in human chorionic trophoblasts. J Clin Endocrinol Metab 2006; 91: 4988–4994
- Korfhagen T R, Bruno M D, Ross G F, Huelsman K M, Ikegami M, Jobe A H, Wert SE, Stripp BR, Morris RE, Glasser SW, et al. Altered surfactant function and structure in SP-A gene targeted mice. Proc Natl Acad Sci U S A 1996; 93: 9594–9959
- Elhalwagi B M, Zhang M, Ikegami M, Iwamoto H S, Morris R E, Miller M L, Dienger K, McCormack FX. Normal surfactant pool sizes and inhibition-resistant surfactant from mice that overexpress surfactant protein A. Am J Respir Cell Mol Biol 1999; 21: 380–387
- Mitchell M D, Chang M C, Chaiworapongsa T, Lan H Y, Helliwell R J, Romero R, Sato T A. Identification of 9alpha,11beta-prostaglandin F2 in human amniotic fluid and characterization of its production by human gestational tissues. J Clin Endocrinol Metab 2005; 90: 4244–4248
- Romero R, Munoz H, Gomez R, Parra M, Polanco M, Valverde V, Hasbun J, Garrido J, Ghezzi F, Mazor M, et al. Increase in prostaglandin bioavailability precedes the onset of human parturition. Prostaglandins Leukot Essent Fatty Acids 1996; 54: 187–191
- Keski-Nisula L T, Aalto M L, Kirkinen P P, Kosma V M, Heinonen S T. Myometrial inflammation in human delivery and its association with labor and infection. Am J Clin Pathol 2003; 120: 217–224
- Keelan J A, Marvin K W, Sato T A, Coleman M, McCowan L M, Mitchell M D. Cytokine abundance in placental tissues: Evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J Obstet Gynecol 1999; 181: 1530–1536
- Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim Y M, Mazor M, Romero R. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol 2006; 195: 394.e1–24
- Helmig B R, Romero R, Espinoza J, Chaiworapongsa T, Bujold E, Gomez R, Ohlsson K, Uldbjerg N. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med 2002; 12: 237–246
- Rubio F, Cooley J, Accurso F J, Remold-O′Donnell E. Linkage of neutrophil serine proteases and decreased surfactant protein-A (SP-A) levels in inflammatory lung disease. Thorax 2004; 59: 318–323
