Abstract
Objective. Tissue factor (TF), the major activator of the extrinsic pathway of coagulation, is abundant in the placenta and decidua. The aim of this study was to determine the maternal plasma concentrations of TF and its primary inhibitor, tissue factor pathway inhibitor (TFPI), in women who delivered small for gestational age (SGA) neonates, and in pre-eclampsia.
Study design. A cross-sectional study included the following groups: 1) women with normal pregnancies (n = 86); 2) patients who delivered SGA neonates (n = 61) and 3) women with pre-eclampsia (n = 133). Maternal plasma concentrations of TF and TFPI were measured by a sensitive immunoassay. Non-parametric statistics were used for analysis.
Results. 1) Women with pre-eclampsia had a significantly higher median plasma concentration of TF than patients with a normal pregnancy (median: 1187 pg/mL; range: 69–11675 vs. median: 291.5 pg/mL; range: 6.3–2662.2; p < 0.0001, respectively); 2) Similarly, TFPI concentrations were higher in pre-eclampsia than in normal pregnancy (median: 87.5ng/mL; range 25.4–165.1 vs. median: 66.1 ng/mL; range: 14.3–86.5; p < 0.0001, respectively); 3) Surprisingly, mothers with SGA neonates had a lower median maternal plasma concentration of TF (median: 112.2 pg/mL; range: 25.6–1225.3) than women with a normal pregnancy (p < 0.0001).
Conclusion. 1) Maternal plasma concentrations of TF in patients with pre-eclampsia, but not in those who delivered an SGA neonate, were higher than in women with normal pregnancies; 2) Although the role of immunoreactive plasma TF in coagulation remains controversial, our observations suggest that changes are present in the context of complications of pregnancy.
Introduction
Pre-eclampsia and small for gestational age (SGA) are considered two of the ‘Great Obstetrical Syndromes’Citation[1] that complicate pregnancy, either as an isolated or a combined pathology. Moreover, the presence of fetal growth restriction in patients with pre-eclampsia is regarded as criteria for the severity of the disease Citation[2],Citation[3].
SGA and pre-eclampsia share similar underlying mechanisms of disease: 1) increased maternal leukocyte activation as a sign of systemic maternal inflammation has been reported in patients who developed pre-eclampsia Citation[4-20] and in women who delivered an SGA neonate Citation[21-25]; 2) an increased activation of the coagulation cascade, reflected by the higher maternal plasma concentrations of thrombin–antithrombin complexes Citation[10],Citation[26-30]; 3) abnormal placental implantation, manifested as a failure of transformation of the spiral arteries, shallow trophoblast invasion and spiral artery atherosis Citation[31-43]; 4) an antiangiogenic state Citation[44-70] characterized by elevated maternal plasma concentrations of soluble vascular endothelial growth factor receptor-1 Citation[71-78] and soluble endoglin Citation[44],Citation[79-81] that decrease the activity of vascular endothelial growth factor and reducing the angiogenic activity. However, there is also an approach suggesting that pre-eclampsia and SGA are different entities, and several mechanisms have been proposed to explain the differences between pre-eclampsia and SGA, including maternal infectious disease Citation[82-90], maternal obesity (which is associated with a higher degree of insulin resistance) Citation[91] and a different degree of systemic maternal inflammation Citation[25],Citation[91-93].
Tissue factor (TF), the major activator of the coagulation cascade, is involved also in the underlying mechanisms implicated in pre-eclampsia and SGA, such as systemic inflammation Citation[94-97], placental implantation Citation[98],Citation[99] and angiogenesis Citation[100-105]. During normal pregnancy, TF is abundant in the uterine decidua Citation[106],Citation[107], resulting in an efficient hemostatic mechanism that is activated both during implantation Citation[108] and after delivery Citation[109]. In addition to its tissue form, TF can be found in the maternal plasma as blood-born TF. The maternal plasma concentrations of TF during normal pregnancy are compatible with the non-pregnant state Citation[110],Citation[111] and increase during labour Citation[112].
Tissue factor pathway inhibitor (TFPI), the main physiological inhibitor of the TF pathway of coagulation, is a three Kunitz domain glycoprotein which inhibits thrombin generation through the inhibition of activated factor X and factor VIIa (FVIIa)/TF complex Citation[113],Citation[114]. The mean maternal plasma concentrations of total TFPI have been reported to increase during the first half of pregnancy until 20 weeks of gestation, subsequently staying relatively constant until term Citation[115], and to decrease during labour Citation[112].
There are two types of TFPI. TFPI-1 is found in the maternal circulation and fetal blood, platelets, endothelial cells and other organs Citation[116],Citation[117], while TFPI-2, the major form of TFPI in the placenta Citation[118-123], was first isolated as Placental Protein 5 (PP5) Citation[124],Citation[125]. During pregnancy, the maternal plasma TFPI-2 concentrations increase gradually, reach a plateau at 36 weeks of gestation, and subside after delivery Citation[124],Citation[126-130].
Maternal plasma concentrations of TF and free TFPI are higher in women with pre-eclampsia than in patients with a normal pregnancy Citation[131-133]. However, the differences in the maternal plasma concentrations of TF and TFPI between patients with pre-eclampsia and those who delivered an SGA neonate, as well as the differences between patients who delivered an SGA neonate and women with a normal pregnancy, have been poorly studied. Therefore, the aim of this study was to determine and compare the changes in the maternal plasma concentration of TF, TFPI and the TFPI/TF ratio in patients with pre-eclampsia, SGA neonate and women with normal pregnancies.
Methods
Study groups and inclusion criteria
A cross-sectional study was conducted and included patients in the following groups: 1) women with normal pregnancies (n = 86); 2) patients who delivered SGA neonates without pre-eclampsia (n = 61); and 3) patients with pre-eclampsia (n = 133). Women with normal pregnancies met the following criteria: 1) no medical, obstetrical or surgical complications at the time of the study; 2) gestational age ranging from 20 to 41 weeks; and 3) delivery of a term infant, appropriate for gestational age, without complications. Patients with multiple pregnancies or fetuses with congenital and/or chromosomal anomalies were excluded.
Samples and data were retrieved from our bank of biological samples and clinical databases. Many of these samples have previously been employed to study the biology of inflammation, hemostasis, angiogenesis regulation, and growth factor concentrations in non-pregnant women, normal pregnant women and those with pregnancy complications.
All women provided an informed consent prior to the collection of maternal blood. The Institutional Review Boards of both Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS) approved the collection and utilisation of samples for research purposes.
Clinical definitions
Pre-eclampsia was defined in the presence of hypertension (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg on at least two occasions, 4 h to 1 week apart) and proteinuria (≥300 mg in a 24 h urine collection or one dipstick measurement ≥2 + ) Citation[2]. A SGA neonate was defined as birthweight below the 10th percentile Citation[134]. Placental histologic findings were classified according to a diagnostic schema proposed by Redline et al. Citation[135].
Sample collection and human TF immunoassay
All blood samples were collected with a vacutainer into 0.109M trisodium citrate anticoagulant solution (BD; San Jose, CA, USA). The samples were centrifuged at 1300g for 10 min at 4°C and stored at −70°C until assay. Maternal plasma TF concentrations were determined by sensitive and specific immunoassays obtained from American Diagnostica (Greenwich, CT, USA), which recognises TF-apo, TF and TF-FVII complexes. The assays were conducted according to the manufacturer's recommendations. The calculated coefficient of variation (CV) in our laboratory was 5.3%, and the sensitivity is 10 pg/mL.
Human TFPI immunoassay
Concentrations of TFPI in maternal plasma were determined by sensitive and specific immunoassays obtained from American Diagnostica (Greenwich, CT, USA). The TFPI ELISA employs a murine anti-TFPI monoclonal as the capture antibody. This capturing antibody is directed against the Kunitz-1 domain of the TFPI molecule, therefore detecting both TFPI-1 and TFPI-2, and measuring the total TFPI plasma concentrations. The assay was conducted according to the manufacturer's recommendations. The calculated CV in our laboratory was 6.6%, and the sensitivity was approximately 10 ng/mL. The correlation between TFPI antigen concentrations and functional activity was approximately r2 = 0.785.
Statistical analysis
Plasma concentrations of TF and TFPI were not normally distributed. Thus, Kruskal–Wallis test with post-hoc analysis was used for comparisons of continuous variables. Comparison of proportions was performed by Chi-square and Fisher's exact tests. The Spearman's rho test was used to detect a correlation between the concentrations of TF, TFPI and TFPI/TF ratio to the gestational age at sample collection in women with a normal pregnancy. Multiple logistic regression analysis was performed to investigate the association between TF, TFPI and their ratio to pre-eclampsia. A p value < 0.05 was considered statistically significant. Analysis was performed with SPSS, version 12 (SPSS Inc., Chicago, IL, USA).
Results
displays the demographic and clinical characteristics of the study groups. Patients with pre-eclampsia had higher rates of primiparity and cesarean deliveries, as well as higher median gestational age at sample collection, lower median gestational age at delivery, and lower median birthweight, than women with normal pregnancies. Similarly, women who delivered an SGA neonate had a higher median gestational age at sample collection and a lower median gestational age at delivery and neonatal birthweight than women in the normal pregnancy group. Women with pre-eclampsia had a lower gestational age at delivery and a higher rate of cesarean section than women who delivered an SGA neonate. There was no correlation between maternal plasma TFPI/TF ratio to the gestational age at blood sample collection in patients with a normal pregnancy (r = 0.030, p = 0.79).
Table I. Demographic and clinical characteristics of the study population.
Changes in the median plasma concentrations of TF, TFPI and TFPI/TF ratio in the different study groups
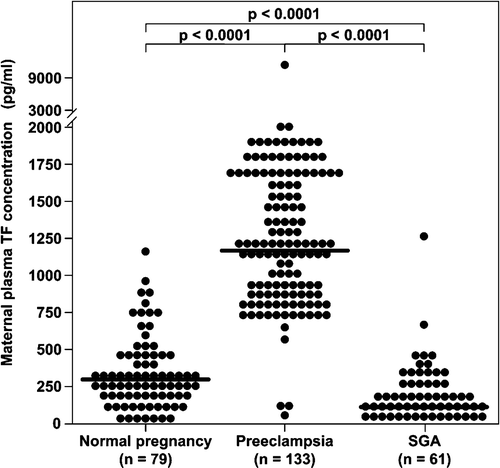
Of the 86 patients in the normal pregnancy group, 79 (91.9%) had detectable immunoreactive TF in the plasma. Maternal plasma TF concentrations were significantly higher in patients with pre-eclampsia than in women with a normal pregnancy (median: 1187 pg/mL; range: 69–11675 vs. median: 291.5 pg/mL; range: 6.3–2662.2; p < 0.0001, respectively), as well as from patients with an SGA neonate (median: 1187 pg/mL; range: 69–11675 vs. median: 112.2 pg/mL; range: 25.6–1225.3; p < 0.0001, respectively). In contrast, the median maternal plasma TF concentrations were significantly lower in women in the SGA group than in those in the normal pregnancy group (median: 112.2 pg/mL; range: 25.6–1225.3 vs. median: 291.5 pg/mL; range: 6.3–2662.2; p < 0.0001, respectively) ().
Figure 1. Comparison of median maternal plasma TF concentration between patients with normal pregnancy (n = 79), pre-eclampsia (n = 133), and women who delivered an SGA neonate (n = 61).
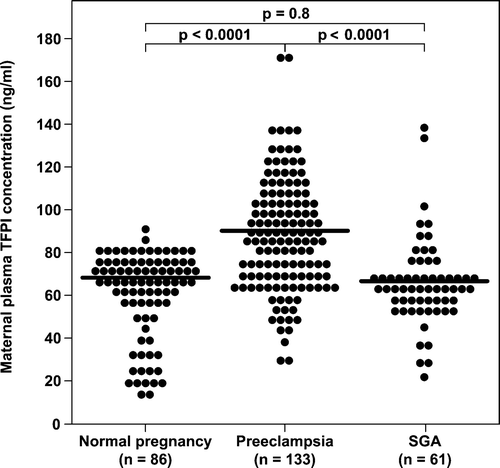
Maternal plasma TFPI concentrations were significantly higher in patients with pre-eclampsia than in women with a normal pregnancy (median: 87.5 ng/mL; range 25.4–165.1 vs. median: 66.1 ng/mL; range: 14.3–86.5; p < 0.0001, respectively). However, there were no significant differences in the median maternal TFPI concentrations between women in the SGA and normal pregnancy groups (median: 63.6 ng/mL; range 22.3–133.5 vs. median: 66.1 ng/mL; range: 14.3–86.5; respectively, p = 0.8) ().
Figure 2. Comparison of median maternal plasma TFPI concentration between patients with normal pregnancy (n = 86), pre-eclampsia (n = 133), and women who delivered an SGA neonate (n = 61).
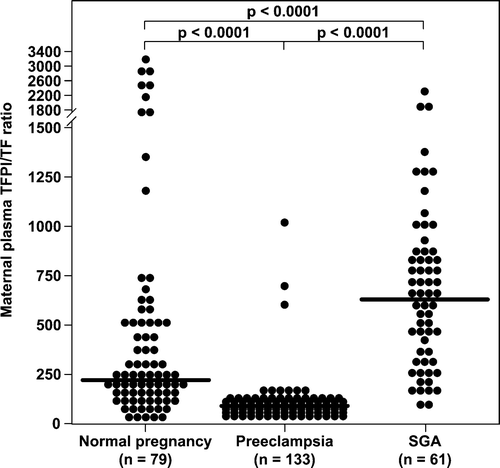
Patients with pre-eclampsia had a significantly lower median maternal plasma TFPI/TF ratio than both women with normal pregnancies (median: 68.9; range: 9.7–969.9 vs. median: 221.5; range: 25.4–3355.3; p < 0.0001, respectively) and women who delivered an SGA neonate (median: 68.9; range: 9.7–969.9 vs. median: 586.8; range: 53.7–2335.9; p < 0.0001, respectively). In contrast, women who delivered an SGA neonate had significantly higher median maternal plasma TFPI/TF ratio than women with a normal pregnancy (median: 586.8; range: 53.7–2335.9 vs. median: 221.5; range: 25.4–3355.3; p < 0.0001, respectively) ().
Figure 3. Comparison of maternal plasma TFPI/TF ratio between women with normal pregnancy (n = 79), pre-eclampsia (n = 133), and women who delivered an SGA neonate (n = 61).
We have constructed two multivariate logistic regression models to determine the association between maternal plasma TF and TFPI concentrations and pre-eclampsia. In the first model, maternal plasma concentrations of TF and TFPI, as well as the delivery of an SGA neonate, were all independently associated with the development of pre-eclampsia (). In the second model, the maternal plasma TFPI/TF ratio was introduced instead of the plasma concentrations of TF and TFPI (). The TFPI/TF ratio and the gestational age at delivery were negatively associated with the development of pre-eclampsia, while the gestational age at sample collection had a positive association with pre-eclampsia. The delivery of an SGA neonate was not associated with the development of pre-eclampsia in this model.
Table II. Multiple logistic regression analysis of the association of maternal plasma tissue factor pathway inhibitor and tissue factor concentrations and pre-eclampsia.
Table III. Multiple logistic regression analysis of the association of maternal plasma tissue factor pathway inhibitor/tissue factor ratio and pre-eclampsia.
Placental lesions in patients with pre-eclampsia and SGA and their association with the changes in TF, TFPI concentrations and their ratio (TFPI/TF)
Placental histology was available from 88% (117/133) of patients in the pre-eclampsia group and 80.3% (49/61) of patients from the SGA group. The specific histologic findings are presented in . Increased syncytial knots were more frequent in placentae of patients with pre-eclampsia than in those of patients in the SGA group [55.6% (65/117) vs. 32.7% (16/49), p = 0.01; respectively].
Table IV. A comparison of placental histologic lesions between patients with pre-eclampsia and patients who delivered an SGA neonate.
Changes in the median maternal plasma concentrations of TF, TFPI, and TFPI/TF ratio in patients with pre-eclampsia were associated with the following placental lesions: 1) Mural hypertrophy of decidual arteries (MHD) was associated with a higher median maternal plasma TF concentration [patients with MHD: median: 1678 pg/mL; range: 876–1876 pg/mL vs. patients without MHD: median: 1177 pg/mL; range: 69.8–11675 pg/mL, p = 0.042]; 2) Distal villous hypoplasia (DVH) was associated with a lower median maternal plasma TFPI concentration [patients with DVH: median: 70.4 ng/mL; range: 25.4–124.9 ng/mL vs. patients without DVH: median :88.7 ng/mL; range: 42.7–163.9 ng/mL, p = 0.011); 3) Remote villous infarcts were associated with a lower median maternal plasma TF concentration [patients with remote villous infarcts 845 pg/mL (102.5–1876 pg/mL) vs. patients without remote villous infarcts 1245 pg/mL (69.8–11675 pg/mL), p = 0.01] and a higher median TFPI/TF ratio [patients with remote villous infarcts 95.70 (31.3–661.5) vs. patients without remote villous infarcts 64.8 (9.7–969.9), p = 0.02]. In contrast, there was no association between the maternal plasma median concentrations of TF, TFPI and TFPI/TF ratio and specific placental lesions in the SGA group.
Discussion
Major findings of the study
1) Women with pre-eclampsia have a significantly higher median maternal plasma TF and TFPI concentrations than women with a normal pregnancy and women who delivered an SGA neonate. 2) The median maternal plasma TFPI/TF ratio was significantly lower in patients with pre-eclampsia than in patients with a normal pregnancy. 3) TF, TFPI and TFPI/TF ratio were independently associated with pre-eclampsia. 4) Among patients with pre-eclampsia, those who had MHD lesions had a higher median TF plasma concentration, and those with distal placental villous hypoplasia had a lower median maternal plasma TFPI concentration. 5) Women who delivered an SGA neonate had significantly lower maternal plasma TF concentrations than patients with a normal pregnancy.
Differences between pre-eclampsia and SGA and changes in maternal plasma TF concentrations
This study's observation that the median maternal plasma TF concentration of patients with pre-eclampsia are significantly higher than of those who delivered SGA neonates, and that the latter had a significantly lower median TF plasma concentrations than women with normal pregnancies are novel. Pre-eclampsia and SGA share many maternal and placental pathological features, and it was proposed that along with recurrent abortions, these obstetrical syndromes may be different phenotypes of the same underlying disease Citation[31],Citation[93]. However, it is not clear why some women will manifest the maternal phenotype of the disease (pre-eclampsia) with or without fetal involvement, while others will have only the fetal phenotype (growth restriction).
Recent epidemiologic studies Citation[82],Citation[136] suggest that pre-eclampsia and SGA are distinct entities. The multinational epidemiologic study Citation[82] conducted by the World Health Organisation – included 39,615 pregnancies – compared the maternal risk factors and perinatal outcome of pregnancies complicated by pre-eclampsia, gestational hypertension, unexplained SGA neonates and a reference group of normal pregnancies Citation[82]. Maternal age above 40 years, pre-gestational maternal morbidity such as chronic hypertension, diabetes, renal and cardiac disease, as well as urinary tract infection during pregnancy were independent risk factors for pre-eclampsia, but not for SGA. In contrast, chronic respiratory disease was an independent risk factor only for the delivery of an SGA neonate. Pre-eclampsia was associated with increased risk for preterm delivery before 37 and 32 weeks of gestation. Unexplained SGA, however, had a protective effect against preterm delivery Citation[82]. The authors suggested that ‘pre-eclampsia and unexplained intrauterine growth restriction, often assumed to be related to placental insufficiency, seem to be independent biologic entities,’Citation[82] thus contradicting the notion that pre-eclampsia and SGA are a different spectrum of the same disease. This is in support of the current study; despite the similar placental histopathologic findings in the pre-eclampsia and SGA groups, a significant association between placental MHD arterioles and higher median maternal plasma TF concentration was observed only in the pre-eclampsia group.
Collectively, the evidence presented above suggests that pre-eclampsia is primarily a systemic maternal disease that in some cases is associated with fetal growth restriction, while SGA is primarily a fetal disease in which the systemic changes in the maternal compartment may not be as prominent as in pre-eclampsia. In fact, some of them are even in the opposite direction, as in the case of the maternal TF plasma concentrations reported herein.
Differences in the maternal systemic response between patients with pre-eclampsia and women who delivered SGA neonates
The maternal systemic inflammatory response of patients with pre-eclampsia includes changes in markers of endothelial cells Citation[137-140] and leukocyte activation Citation[4],Citation[25],Citation[93],Citation[141], complement split products, Citation[142] as well as thrombin generation that represent activation of the coagulation cascade Citation[10],Citation[26-29],Citation[143]. The following changes can also differentiate between patients with pre-eclampsia and those who delivered an SGA neonate:
Differences in the profile of maternal systemic leukocyte activation in patients with pre-eclampsia and those who delivered an SGA neonate
Maternal systemic leukocyte activation has been reported in women with a normal pregnancy, patients with pre-eclampsia Citation[4],Citation[25],Citation[93],Citation[141] and those with SGA neonates Citation[22],Citation[25],Citation[93]. Indeed, patients with pre-eclampsia had a significant delay in neutrophils apoptosis than patients who delivered SGA neonates, and those with normal pregnancies Citation[93]. However, maternal plasma concentrations of neutrophils activation markers, CD11b and CD62L, did not differ significantly between patients with pre-eclampsia and those who delivered SGA neonates Citation[25].
There is a substantial body of evidence showing the increased monocyte activation in pre-eclampsia in comparison to normal pregnancy Citation[4-6],Citation[144-151]. Peripheral blood monocytes from the uterine vein of patients with pre-eclampsia showed a higher degree of activation in comparison to those obtained from their cubital vein. The authors proposed that the passage through the placental bed activates the maternal monocyte in patients with pre-eclampsia Citation[144]. A recent study reported that pre-eclamptic patients have higher monocyte metabolic activity and oxidative burst than women who delivered an SGA neonate Citation[92]. This is in accord with our findings, since during systemic inflammation activated monocytes express TF on their membrane Citation[97],Citation[152-156] and shed micro-particles which contain TF into the plasma Citation[94],Citation[96],Citation[152],Citation[157-162]. Hence, the increased monocyte activation among patients with pre-eclampsia can be a possible source for the elevated maternal plasma TF concentrations in these individuals.
Differences in the profile of circulating endothelial cells adhesion molecules in patients with pre-eclampsia and those who delivered an SGA neonate
Circulating endothelial cell adhesion molecules were reported to be higher in the plasma of patients with pre-eclampsia Citation[137-140] and those who delivered an SGA neonate Citation[137],Citation[139] than in the case of women with normal pregnancies. However, patients with pre-eclampsia had a different expression pattern of endothelial cell adhesion molecules than women who delivered SGA neonates, and maternal plasma concentrations of intercellular cell adhesion molecule-1 were higher in pre-eclamptic patients than in patients who delivered an SGA neonate Citation[22] and women with a normal pregnancy Citation[137],Citation[139],Citation[140].
Differences in the maternal plasma complement split products profile in patients with pre-eclampsia and those who delivered an SGA neonate
Patients with pre-eclampsia had higher median maternal plasma concentrations of C5a than patients with SGA neonate and women with a normal pregnancy Citation[142]. Moreover, patients who delivered an SGA neonate had lower median maternal C4a plasma concentrations than women with a normal pregnancy Citation[142]. This correlates with the changes in maternal plasma TF concentrations observed in this study. The association between C5a and TF activation and expression has been previously reported Citation[163],Citation[164]: 1) C5a induces a 4.9-fold increase in TF activity and a 3.8-fold increase in TF mRNA expression by endothelial cells Citation[163]; 2) the administration of C5a to animals increases the procoagulant activity of alveolar macrophages by 5- to 6-fold through TF activation Citation[164]; and 3) serum from patients with anti-phospholipid syndrome induced the expression of TF by neutrophils of healthy individual and increased the extrinsic pathway procoagulant activity of these neutrophils, in a complement dependent manner through the C5a receptor Citation[165]. In addition, the C5a-induced TF expression by neutrophils contributes to the neutrophils oxidative burst, and was associated with antiphospholipid-related fetal injury in mice Citation[166]. Thus, the higher maternal plasma C5a concentrations reported in patients with pre-eclampsia when compared with those who deliver an SGA neonate may contribute to an increased TF expression and activation of neutrophils in these patients. Moreover, this association may serve as a possible explanation as to why the same placental lesions were associated with elevated median TF plasma concentration in patients with pre-eclampsia, but not in those who delivered an SGA neonate.
Placental microparticles and monocyte activation in patients with pre-eclampsia
It has been proposed that placental micro-particles may be the mediators of the increased maternal systemic inflammation observed during normal pregnancy, as well as the exaggerated systemic maternal inflammation reported in patients with pre-eclampsia Citation[167-169]. Microparticles are cellular particles of different sizes in the order of 100 nm that are shed into the plasma by platelets, leukocytes, granulocytes, erythrocytes, endothelial Citation[170] and trophoblast cells Citation[167],Citation[168],Citation[171]. Although present in the normal state, they are also associated with cellular activation, apoptosis, inflammation and coagulation Citation[172]. The smaller microparticles are called exosomes (30–100 nm) and are originated from intracellular multivesicular bodies that can be derived from dendritic cells and are part of their normal activity Citation[173]. A recent study reported that women with pre-eclampsia, particularly if the condition was developed before 34 weeks of gestation, had a significantly higher maternal plasma concentration of placental microparticles than women with a normal pregnancy who were matched for gestational age Citation[174]. In contrast, women in the fetal growth restriction group had a lower median plasma concentration of microparticles than women with a normal pregnancy, though this difference was not statistically significant Citation[174]. It has been proposed that apoptotic and necrotic placental debris may activate monocytes in normal pregnancy and that excessive placental debris may be associated with the systemic maternal inflammation observed in pre-eclampsia Citation[5],Citation[19]. Indeed, supernatants from endothelial cells co-cultured with syncytiotrophoblast microparticles activated monocytes in vitroCitation[93]. Thus, the differences in the concentrations of trophoblast microparticles in the maternal serum among patients with pre-eclampsia, SGA and women with a normal pregnancy may be related to the differences in maternal monocyte activation and in TF plasma concentrations observed in these patients.
Of note, VanWijkk et al. Citation[143] reported that the total number of microparticles presenting TF did not differ between patients with pre-eclampsia and those with a normal pregnancy. A post-hoc analysis of these results revealed that their study was under powered to detect a significant difference in the number of TF presenting microparticles Citation[143]. Moreover, the authors did not differentiate between the sources of the microparticles that were measured, which can influence the procoagulant activity of the microparticles Citation[143]. We therefore argue that a larger study is needed to determine whether patients with pre-eclampsia have a higher expression and secretion of TF expressing microparticles by activated monocytes than patients with SGA and those with a normal pregnancy.
What is the role of immunoreactive TF in the maternal plasma?
The procoagulant activity of immunoreactive TF in the maternal plasma (blood born TF) is a topic of debate Citation[97],Citation[152],Citation[175-178]. Blood born TF has very little or no procoagulant activity Citation[152], and only the administration of exogenous active TF generated a whole blood and plasma clot after the inhibition of the contact factor (factor XIIa) Citation[152]. On the other hand, it has been proposed that blood born TF does not initiate the coagulation cascade, but rather propagate clot formation by attaching to activated platelets and further enhancing the coagulation process Citation[175-178]. In addition, patients with pre-eclampsia, but not those with a normal pregnancy or non-pregnant women, had a significant reduction in their thrombin generation by microparticles after treatment with anti-FVII antibodies Citation[143]. The authors concluded that a higher proportion of thrombin generation is derived from the extrinsic pathway of coagulation in patients with pre-eclampsia Citation[143].
What are the plasma and placental changes in TFPI concentrations in normal and complicated pregnancies?
TFPI is the main inhibitor of TF, and the maternal plasma concentration of immunoreactive TFPI is 500–1000 times higher than that of TF Citation[179]. Our observation that total TFPI plasma concentrations are higher in patients with pre-eclampsia than in women with a normal pregnancy is in accord with previous reports Citation[131],Citation[133].
In contrast to the changes in the maternal plasma, a lower placental extract of total TFPI concentrations and TFPI mRNA expression was reported in pregnant women with vascular complications of pregnancy (pre-eclampsia, eclampsia, placental abruption, fetal growth restriction and fetal demise) in comparison to women with normal pregnancies Citation[180]. A different study also demonstrated a lower placental TFPI-2 immunoreactivity in patients with pre-eclampsia, though not in patients with fetal growth restriction Citation[181].
TFPI/TF ratio: an additional marker for coagulation activity?
The finding that the ratio of TFPI/TF in patients with pre-eclampsia is significantly lower than normal pregnancy and SGA is novel, as it suggests that the significant increase in TFPI plasma concentrations observed in pre-eclampsia may not be sufficient to compensate for the higher plasma TF concentration observed in these patients, and the overall balance is of a procoagulant state. Therefore, the TFPI/TF ratio may represent a good indicator for the severity of a procoagulant state in the context of pregnancy complications. The following evidence supporting this view was reported in patients with disseminated intravascular coagulation (DIC) and thrombotic thrombocytopenic purpura (TTP), which are both complicated by consumption coagulopathy resulting from a hypercoagulable state: 1) TFPI plasma concentrations are higher in patients with DIC than in healthy individuals Citation[178],Citation[179],Citation[182]; 2) patients with pre-DIC state have higher TF/TFPI ratio than patients with DIC, and patients with DIC who had a poor outcome also had a higher TF/TFPI ratio than those with a good outcome Citation[179] and 3) patients with TTP have lower TFPI concentrations than healthy controls Citation[178], as well as a significant increase in TFPI/TF ratio after treatment Citation[178]. The authors propose that this reflects an improvement in the hypercoagulable state associated with TTP Citation[178]. Therefore, the observation that an increase in TFPI plasma concentrations may be of benefit in reducing the activation of the coagulation cascade in the presence of a hypercoagulable state has relevant therapeutic implications.
A possible intervention that can change the TFPI/TF ratio and reduce its systemic effect is the administration of heparin/low molecular weight heparin (LMWH), which augments the secretion and production of TFPI by the endothelial cells Citation[183-188], leading to an increase in the plasma concentrations of TFPI-1 and TFPI-2 Citation[183-185],Citation[187-200]. Moreover, heparin binds factor Xa and TFPI-1 simultaneously, bringing them into proximity, which enhances factor Xa inhibition by TFPI-1 Citation[113],Citation[114],Citation[201],Citation[202]. Indeed, patients with recurrent abortions (of which 86.7% (26/30) had a thrombophilic mutation) that were treated with LMWH had a significantly higher total placental TFPI mRNA expression and protein concentrations than placentae of untreated patients with gestational vascular complications Citation[180]. Therefore, the TFPI/TF ratio may serve as a marker for an increased prothrombotic activity, and the administration of LMWH might be of benefit in the case of patients with low concentrations of TFPI or a low TFPI/TF ratio.
In summary, the marked increase of plasma TF concentrations observed in patients with pre-eclampsia may reflect the maternal systemic inflammatory response associated with pre-eclampsia. Moreover, although the median maternal plasma TFPI concentrations are higher in patients with pre-eclampsia than in women with a normal pregnancy, the ratio of TFPI/TF is significantly lower and can be considered as a marker for the presence of a hypercoagulable state in these patients.
Acknowledgement
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
References
- Romero R. The child is the father of the man. Prenat Neonat Med 1996; 1: 8–11
- ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol 2002; 99: 159–167
- F G Cunningham, Gant, N F, Leveno, K J, Gilstrap, L C, Hauth, J C, Wenstrom, K D. Williams obstetrics21st edn. McGraw-Hill, New York 2001; 567–618
- Gervasi M T, Chaiworapongsa T, Pacora P, Naccasha N, Yoon B H, Maymon E, Romero R. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol 2001; 185: 792–797
- Redman C W, Sacks G P, Sargent I L. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol 1999; 180: 499–506
- Redman C W, Sargent I L. The pathogenesis of pre-eclampsia. Gynecol Obstet Fertil 2001; 29: 518–522
- Gratacos E. Lipid-mediated endothelial dysfunction: a common factor to preeclampsia and chronic vascular disease. Eur J Obstet Gynecol Reprod Biol 2000; 92: 63–66
- Carr D B, McDonald G B, Brateng D, Desai M, Thach C T, Easterling T R. The relationship between hemodynamics and inflammatory activation in women at risk for preeclampsia. Obstet Gynecol 2001; 98: 1109–1116
- Wolf M, Kettyle E, Sandler L, Ecker J L, Roberts J, Thadhani R. Obesity and preeclampsia: the potential role of inflammation. Obstet Gynecol 2001; 98: 757–762
- Chaiworapongsa T, Yoshimatsu J, Espinoza J, Kim Y M, Berman S, Edwin S, Yoon B H, Romero R. Evidence of in vivo generation of thrombin in patients with small-for-gestational-age fetuses and pre-eclampsia. J Matern Fetal Neonatal Med 2002; 11: 362–367
- Chaiworapongsa T, Gervasi M T, Refuerzo J, Espinoza J, Yoshimatsu J, Berman S, Romero R. Maternal lymphocyte subpopulations (CD45RA+ and CD45RO+) in preeclampsia. Am J Obstet Gynecol 2002; 187: 889–893
- Saito S, Sakai M. Th1/Th2 balance in preeclampsia. J Reprod Immunol 2003; 59: 161–173
- Freeman D J, McManus F, Brown E A, Cherry L, Norrie J, Ramsay J E, Clark P, Walker I D, Sattar N, Greer I A. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension 2004; 44: 708–714
- Todros T, Bontempo S, Piccoli E, Ietta F, Romagnoli R, Biolcati M, Castellucci M, Paulesu L. Increased levels of macrophage migration inhibitory factor (MIF) in preeclampsia. Eur J Obstet Gynecol Reprod Biol 2005; 123: 162–166
- Redman C W, Sargent I L. Latest advances in understanding preeclampsia. Science 2005; 308: 1592–1594
- Luppi P, Deloia J A. Monocytes of preeclamptic women spontaneously synthesize pro-inflammatory cytokines. Clin Immunol 2006; 118: 268–275
- Borzychowski A M, Sargent I L, Redman C W. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med 2006; 11: 309–316
- Elovitz M A. Anti-inflammatory interventions in pregnancy: now and the future. Semin Fetal Neonatal Med 2006; 11: 327–332
- Sargent I L, Germain S J, Sacks G P, Kumar S, Redman C W. Trophoblast deportation and the maternal inflammatory response in pre-eclampsia. J Reprod Immunol 2003; 59: 153–160
- Redman C W, Sargent I L. Pre-eclampsia, the placenta and the maternal systemic inflammatory response – a review. Placenta 2003; 24(Suppl A)S21–S27
- Selvaggi L, Lucivero G, Iannone A, dell'Osso A, Loverro G, Antonaci S, Bonomo L, Bettocchi S. Analysis of mononuclear cell subsets in pregnancies with intrauterine growth retardation. Evidence of chronic B-lymphocyte activation. J Perinat Med 1983; 11: 213–217
- Johnston T A, Greer I A, Dawes J, Calder A A. Neutrophil activation in small for gestational age pregnancies. Br J Obstet Gynaecol 1991; 98: 105–106
- Bozzola M, Chirico G, Chiara A, Gasparoni A, Schimpff R M. Serum growth-promoting activity in human newborns. Relationship of thymidine activity with birth weight and the length of gestation. Acta Paediatr Scand 1985; 74: 534–538
- Girardi G, Yarilin D, Thurman J M, Holers V M, Salmon J E. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med 2006; 203: 2165–2175
- Sabatier F, Bretelle F, D'Ercole C, Boubli L, Sampol J, gnat-George F. Neutrophil activation in preeclampsia and isolated intrauterine growth restriction. Am J Obstet Gynecol 2000; 183: 1558–1563
- Cadroy Y, Grandjean H, Pichon J, Desprats R, Berrebi A, Fournie A, Boneu B. Evaluation of six markers of haemostatic system in normal pregnancy and pregnancy complicated by hypertension or pre-eclampsia. Br J Obstet Gynaecol 1993; 100: 416–420
- de Boer K, ten Cate J W, Sturk A, Borm J J, Treffers P E. Enhanced thrombin generation in normal and hypertensive pregnancy. Am J Obstet Gynecol 1989; 160: 95–100
- Tanjung M T, Siddik H D, Hariman H, Koh S C. Coagulation and fibrinolysis in preeclampsia and neonates. Clin Appl Thromb Hemost 2005; 11: 467–473
- Bonnar J, Redman C W, Denson K W. The role of coagulation and fibrinolysis in preeclampsia. Perspect Nephrol Hypertens 1976; 5: 85–93
- Schjetlein R, Haugen G, Wisloff F. Markers of intravascular coagulation and fibrinolysis in preeclampsia: association with intrauterine growth retardation. Acta Obstet Gynecol Scand 1997; 76: 541–546
- Burton G J, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig 2004; 11: 342–352
- De Wolf F, Carreras L O, Moerman P, Vermylen J, Van A A, Renaer M. Decidual vasculopathy and extensive placental infarction in a patient with repeated thromboembolic accidents, recurrent fetal loss, and a lupus anticoagulant. Am J Obstet Gynecol 1982; 142: 829–834
- Brosens I, Renaer M. On the pathogenesis of placental infarcts in pre-eclampsia. J Obstet Gynaecol Br Commonw 1972; 79: 794–799
- Rogers B B, Bloom S L, Leveno K J. Atherosis revisited: current concepts on the pathophysiology of implantation site disorders. Obstet Gynecol Surv 1999; 54: 189–195
- Lala P K, Chakraborty C. Factors regulating trophoblast migration and invasiveness: possible derangements contributing to pre-eclampsia and fetal injury. Placenta 2003; 24: 575–587
- Brosens I, Dixon H G, Robertson W B. Fetal growth retardation and the arteries of the placental bed. Br J Obstet Gynaecol 1977; 84: 656–663
- Sheppard B L, Bonnar J. An ultrastructural study of utero-placental spiral arteries in hypertensive and normotensive pregnancy and fetal growth retardation. Br J Obstet Gynaecol 1981; 88: 695–705
- Gerretsen G, Huisjes H J, Elema J D. Morphological changes of the spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation. Br J Obstet Gynaecol 1981; 88: 876–881
- Hustin J, Foidart J M, Lambotte R. Maternal vascular lesions in pre-eclampsia and intrauterine growth retardation: light microscopy and immunofluorescence. Placenta 1983; 4: 489–498
- Woods D L. Relative placental size in growth-retarded infants. Am J Obstet Gynecol 1986; 154: 1170
- Lyall F, Simpson H, Bulmer J N, Barber A, Robson S C. Transforming growth factor-beta expression in human placenta and placental bed in third trimester normal pregnancy, preeclampsia, and fetal growth restriction. Am J Pathol 2001; 159: 1827–1838
- Egbor M, Ansari T, Morris N, Green C J, Sibbons P D. Pre-eclampsia and fetal growth restriction: how morphometrically different is the placenta. Placenta 2006; 27: 727–734
- Egbor M, Ansari T, Morris N, Green C J, Sibbons P D. Morphometric placental villous and vascular abnormalities in early- and late-onset pre-eclampsia with and without fetal growth restriction. BJOG 2006; 113: 580–589
- Levine R J, Lam C, Qian C, Yu K F, Maynard S E, Sachs B P, Sibai B M, Epstein F H, Romero R, Thadhani R, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 2006; 355: 992–1005
- Malamitsi-Puchner A, Boutsikou T, Economou E, Makrakis E, Iliodromiti Z, Kouskouni E, Hassiakos D. The role of the anti-angiogenic factor endostatin in intrauterine growth restriction. J Soc Gynecol Investig 2005; 12: 195–197
- Ahmed A, Perkins J. Angiogenesis and intrauterine growth restriction. Baillieres Best Pract Res Clin Obstet Gynaecol 2000; 14: 981–998
- Boutsikou T, Malamitsi-Puchner A, Economou E, Boutsikou M, Puchner K P, Hassiakos D. Soluble vascular endothelial growth factor receptor-1 in intrauterine growth restricted fetuses and neonates. Early Hum Dev 2006; 82: 235–239
- Cetin I, Foidart J M, Miozzo M, Raun T, Jansson T, Tsatsaris V, Reik W, Cross J, Hauguel-de-Mouzon S, Illsley N, et al. Fetal growth restriction: a workshop report. Placenta 2004; 25: 753–757
- Maynard S E, Min J Y, Merchan J, Lim K H, Li J, Mondal S, Libermann T A, Morgan J P, Sellke F W, Stillman I E, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003; 111: 649–658
- Wolf M, Hubel C A, Lam C, Sampson M, Ecker J L, Ness R B, Rajakumar A, Daftary A, Shakir A S, Seely E W, et al. Preeclampsia and future cardiovascular disease: potential role of altered angiogenesis and insulin resistance. J Clin Endocrinol Metab 2004; 89: 6239–6243
- Bdolah Y, Sukhatme V P, Karumanchi S A. Angiogenic imbalance in the pathophysiology of preeclampsia: newer insights. Semin Nephrol 2004; 24: 548–556
- Karumanchi S A, Bdolah Y. Hypoxia and sFlt-1 in preeclampsia: the “chicken-and-egg” question. Endocrinology 2004; 145: 4835–4837
- Davison J M, Homuth V, Jeyabalan A, Conrad K P, Karumanchi S A, Quaggin S, Dechend R, Luft F C. New aspects in the pathophysiology of preeclampsia. J Am Soc Nephrol 2004; 15: 2440–2448
- Thadhani R, Ecker J L, Mutter W P, Wolf M, Smirnakis K V, Sukhatme V P, Levine R J, Karumanchi S A. Insulin resistance and alterations in angiogenesis: additive insults that may lead to preeclampsia. Hypertension 2004; 43: 988–992
- Levine R J, Maynard S E, Qian C, Lim K H, England L J, Yu K F, Schisterman E F, Thadhani R, Sachs B P, Epstein F H, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004; 350: 672–683
- Thadhani R, Mutter W P, Wolf M, Levine R J, Taylor R N, Sukhatme V P, Ecker J, Karumanchi S A. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab 2004; 89: 770–775
- Bdolah Y, Karumanchi S A, Sachs B P. Recent advances in understanding of preeclampsia. Croat Med J 2005; 46: 728–736
- Lam C, Lim K H, Karumanchi S A. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension 2005; 46: 1077–1085
- Nadar S K, Karalis I, Al Y E, Blann A D, Lip G Y. Plasma markers of angiogenesis in pregnancy induced hypertension. Thromb Haemost 2005; 94: 1071–1076
- Maynard S E, Venkatesha S, Thadhani R, Karumanchi S A. Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr Res 2005; 57: 1R–7R
- Rajakumar A, Michael H M, Rajakumar P A, Shibata E, Hubel C A, Karumanchi S A, Thadhani R, Wolf M, Harger G, Markovic N. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta 2005; 26: 563–573
- Levine R J, Thadhani R, Qian C, Lam C, Lim K H, Yu K F, Blink A L, Sachs B P, Epstein F H, Sibai B M, et al. Urinary placental growth factor and risk of preeclampsia. JAMA 2005; 293: 77–85
- Levine R J, Karumanchi S A. Circulating angiogenic factors in preeclampsia. Clin Obstet Gynecol 2005; 48: 372–386
- Aggarwal P K, Jain V, Sakhuja V, Karumanchi S A, Jha V. Low urinary placental growth factor is a marker of pre-eclampsia. Kidney Int 2006; 69: 621–624
- Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim Y M, Bdolah Y, Lim K H, Yuan H T, Libermann T A, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 2006; 12: 642–649
- Levine R J, Qian C, Maynard S E, Yu K F, Epstein F H, Karumanchi S A. Serum sFlt1 concentration during preeclampsia and mid trimester blood pressure in healthy nulliparous women. Am J Obstet Gynecol 2006; 194: 1034–1041
- Tjoa M L, Levine R J, Karumanchi S A. Angiogenic factors and preeclampsia. Front Biosci 2007; 12: 2395–2402
- Mayhew T M, Wijesekara J, Baker P N, Ong S S. Morphometric evidence that villous development and fetoplacental angiogenesis are compromised by intrauterine growth restriction but not by pre-eclampsia. Placenta 2004; 25: 829–833
- Rutland C S, Mukhopadhyay M, Underwood S, Clyde N, Mayhew T M, Mitchell C A. Induction of intrauterine growth restriction by reducing placental vascular growth with the angioinhibin TNP-470. Biol Reprod 2005; 73: 1164–1173
- Maulik D, Frances E J, Ragolia L. Fetal growth restriction: pathogenic mechanisms. Clin Obstet Gynecol 2006; 49: 219–227
- Wathen K A, Tuutti E, Stenman U H, Alfthan H, Halmesmaki E, Finne P, Ylikorkala O, Vuorela P. Maternal serum-soluble vascular endothelial growth factor receptor-1 in early pregnancy ending in preeclampsia or intrauterine growth retardation. J Clin Endocrinol Metab 2006; 91: 180–184
- Bujold E, Romero R, Chaiworapongsa T, Kim Y M, Kim G J, Kim M R, Espinoza J, Goncalves L F, Edwin S, Mazor M. Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. J Matern Fetal Neonatal Med 2005; 18: 9–16
- Chaiworapongsa T, Romero R, Kim Y M, Kim G J, Kim M R, Espinoza J, Bujold E, Goncalves L, Gomez R, Edwin S, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med 2005; 17: 3–18
- Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res 2004; 95: 884–891
- Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee K Y, Goncalves L F, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young investigator award. Am J Obstet Gynecol 2004; 190: 1541–1547
- Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab 2003; 88: 2348–2351
- Padavala S, Pope N, Baker P, Crocker I. An imbalance between vascular endothelial growth factor and its soluble receptor in placental villous explants of intrauterine growth-restricted pregnancies. J Soc Gynecol Investig 2006; 13: 40–47
- Regnault T R, Orbus R J, de V B, Davidsen M L, Galan H L, Wilkening R B, Anthony R V. Placental expression of VEGF, PlGF and their receptors in a model of placental insufficiency-intrauterine growth restriction (PI-IUGR). Placenta 2002; 23: 132–144
- Stepan H, Kramer T, Faber R. Maternal plasma concentrations of soluble endoglin in pregnancies with intrauterine growth restriction. J Clin Endocrinol Metab 2007; 92: 2831–2834
- Lopez-Novoa J M. Soluble endoglin is an accurate predictor and a pathogenic molecule in pre-eclampsia. Nephrol Dial Transplant 2007; 22: 712–714
- Luft F C. Soluble endoglin (sEng) joins the soluble fms-like tyrosine kinase (sFlt) receptor as a pre-eclampsia molecule. Nephrol Dial Transplant 2006; 21: 3052–3054
- Villar J, Carroli G, Wojdyla D, Abalos E, Giordano D, Ba'aqeel H, Farnot U, Bergsjo P, Bakketeig L, Lumbiganon P, et al. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions. Am J Obstet Gynecol 2006; 194: 921–931
- von Dadelszen P, Magee L A. Could an infectious trigger explain the differential maternal response to the shared placental pathology of preeclampsia and normotensive intrauterine growth restriction. Acta Obstet Gynecol Scand 2002; 81: 642–648
- Mittendorf R, Lain K Y, Williams M A, Walker C K. Preeclampsia. A nested, case-control study of risk factors and their interactions. J Reprod Med 1996; 41: 491–496
- Banhidy F, Acs N, Puho E H, Czeizel A E. Pregnancy complications and birth outcomes of pregnant women with urinary tract infections and related drug treatments. Scand J Infect Dis 2007; 39: 390–397
- Lee C J, Hsieh T T, Chiu T H, Chen K C, Lo L M, Hung T H. Risk factors for pre-eclampsia in an Asian population. Int J Gynaecol Obstet 2000; 70: 327–333
- Schieve L A, Handler A, Hershow R, Persky V, Davis F. Urinary tract infection during pregnancy: its association with maternal morbidity and perinatal outcome. Am J Public Health 1994; 84: 405–410
- Hill J A, Devoe L D, Bryans C I, Jr. Frequency of asymptomatic bacteriuria in preeclampsia. Obstet Gynecol 1986; 67: 529–532
- Savige J A, Gilbert G L, Fairley K F, McDowall D R. Bacteriuria due to Ureaplasma urealyticum and Gardnerella vaginalis in women with preeclampsia. J Infect Dis 1983; 148: 605
- Gilbert G L, Garland S M, Fairley K F, McDowall D M. Bacteriuria due to ureaplasmas and other fastidious organisms during pregnancy: prevalence and significance. Pediatr Infect Dis 1986; 5: S239–S243
- Ness R B, Sibai B M. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol 2006; 195: 40–49
- Chaiworapongsa T, Gervasi M T, Espinoza J, Bujold E, Kim Y M, Blackwell S, Romero R. Preeclampsia and SGA differ by the extent of monocyte, but not neutrophil, metabolic activity and oxidative burst. Am J Obstet Gynecol 2003; 187: S220
- von Dadelszen P, Watson R W, Noorwali F, Marshall J C, Parodo J, Farine D, Lye S J, Ritchie J W, Rotstein O D. Maternal neutrophil apoptosis in normal pregnancy, preeclampsia, and normotensive intrauterine growth restriction. Am J Obstet Gynecol 1999; 181: 408–414
- Osterud B. The role of platelets in decrypting monocyte tissue factor. Semin Hematol 2001; 38: 2–5
- Osterud B. Tissue factor expression by monocytes: regulation and pathophysiological roles. Blood Coagul Fibrinolysis 1998; 9(Suppl 1)S9–S14
- Osterud B, Bjorklid E. Sources of tissue factor. Semin Thromb Hemost 2006; 32: 11–23
- Osterud B. Cellular interactions in tissue factor expression by blood monocytes. Blood Coagul Fibrinolysis 1995; 6(Suppl 1)S20–S25
- Lockwood C J, Krikun G, Rahman M, Caze R, Buchwalder L, Schatz F. The role of decidualization in regulating endometrial hemostasis during the menstrual cycle, gestation, and in pathological states. Semin Thromb Hemost 2007; 33: 111–117
- Dusse L M, Carvalho M G, Cooper A J, Lwaleed B A. Tissue factor and tissue factor pathway inhibitor: a potential role in pregnancy and obstetric vascular complications. Clin Chim Acta 2006; 372: 43–46
- Ruf W, Mueller B M. Tissue factor in cancer angiogenesis and metastasis. Curr Opin Hematol 1996; 3: 379–384
- Wojtukiewicz M Z, Sierko E, Klement P, Rak J. The hemostatic system and angiogenesis in malignancy. Neoplasia 2001; 3: 371–384
- Nash G F, Walsh D C, Kakkar A K. The role of the coagulation system in tumour angiogenesis. Lancet Oncol 2001; 2: 608–613
- Brodsky S V. Coagulation, fibrinolysis and angiogenesis: new insights from knockout mice. Exp Nephrol 2002; 10: 299–306
- Schatz F, Krikun G, Caze R, Rahman M, Lockwood C J. Progestin-regulated expression of tissue factor in decidual cells: implications in endometrial hemostasis, menstruation and angiogenesis. Steroids 2003; 68: 849–860
- Belting M, Ahamed J, Ruf W. Signaling of the tissue factor coagulation pathway in angiogenesis and cancer. Arterioscler Thromb Vasc Biol 2005; 25: 1545–1550
- Lockwood C J, Krikun G, Schatz F. The decidua regulates hemostasis in human endometrium. Semin Reprod Endocrinol 1999; 17: 45–51
- Lockwood C J, Krikun G, Schatz F. Decidual cell-expressed tissue factor maintains hemostasis in human endometrium. Ann N Y Acad Sci 2001; 943: 77–88
- Lockwood C J, Schatz F. A biological model for the regulation of peri-implantational hemostasis and menstruation. J Soc Gynecol Investig 1996; 3: 159–165
- Hahn L. On fibrinolysis and coagulation during parturition and menstruation. Acta Obstet Gynecol Scand Suppl 1974; 28: 7–40
- Holmes V A, Wallace J M. Haemostasis in normal pregnancy: a balancing act. Biochem Soc Trans 2005; 33: 428–432
- Bellart J, Gilabert R, Miralles R M, Monasterio J, Cabero L. Endothelial cell markers and fibrinopeptide A to D-dimer ratio as a measure of coagulation and fibrinolysis balance in normal pregnancy. Gynecol Obstet Invest 1998; 46: 17–21
- Uszynski M, Zekanowska E, Uszynski W, Kuczynski J. Tissue factor (TF) and tissue factor pathway inhibitor (TFPI) in amniotic fluid and blood plasma: implications for the mechanism of amniotic fluid embolism. Eur J Obstet Gynecol Reprod Biol 2001; 95: 163–166
- Broze G J, Jr, Girard T J, Novotny W F. Regulation of coagulation by a multivalent Kunitz-type inhibitor. Biochemistry 1990; 29: 7539–7546
- Broze G J, Jr, Warren L A, Novotny W F, Higuchi D A, Girard J J, Miletich J P. The lipoprotein-associated coagulation inhibitor that inhibits the factor VII-tissue factor complex also inhibits factor Xa: insight into its possible mechanism of action. Blood 1988; 71: 335–343
- Sarig G, Blumenfeld Z, Leiba R, Lanir N, Brenner B. Modulation of systemic hemostatic parameters by enoxaparin during gestation in women with thrombophilia and pregnancy loss. Thromb Haemost 2005; 94: 980–985
- Tay S P, Cheong S K, Boo N Y. Circulating tissue factor, tissue factor pathway inhibitor and D-dimer in umbilical cord blood of normal term neonates and adult plasma. Blood Coagul Fibrinolysis 2003; 14: 125–129
- Edstrom C S, Calhoun D A, Christensen R D. Expression of tissue factor pathway inhibitor in human fetal and placental tissues. Early Hum Dev 2000; 59: 77–84
- Iino M, Foster D C, Kisiel W. Quantification and characterization of human endothelial cell-derived tissue factor pathway inhibitor-2. Arterioscler Thromb Vasc Biol 1998; 18: 40–46
- Sprecher C A, Kisiel W, Mathewes S, Foster D C. Molecular cloning, expression, and partial characterization of a second human tissue-factor-pathway inhibitor. Proc Natl Acad Sci USA 1994; 91: 3353–3357
- Udagawa K, Miyagi Y, Hirahara F, Miyagi E, Nagashima Y, Minaguchi H, Misugi K, Yasumitsu H, Miyazaki K. Specific expression of PP5/TFPI2 mRNA by syncytiotrophoblasts in human placenta as revealed by in situ hybridization. Placenta 1998; 19: 217–223
- Udagawa K, Yasumitsu H, Esaki M, Sawada H, Nagashima Y, Aoki I, Jin M, Miyagi E, Nakazawa T, Hirahara F, et al. Subcellular localization of PP5/TFPI-2 in human placenta: a possible role of PP5/TFPI-2 as an anti-coagulant on the surface of syncytiotrophoblasts. Placenta 2002; 23: 145–153
- Kamei S, Kazama Y, Kuijper J L, Foster D C, Kisiel W. Genomic structure and promoter activity of the human tissue factor pathway inhibitor-2 gene. Biochim Biophys Acta 2001; 1517: 430–435
- Hube F, Reverdiau P, Iochmann S, Trassard S, Thibault G, Gruel Y. Demonstration of a tissue factor pathway inhibitor 2 messenger RNA synthesis by pure villous cytotrophoblast cells isolated from term human placentas. Biol Reprod 2003; 68: 1888–1894
- Butzow R, Virtanen I, Seppala M, Narvanen O, Stenman U H, Ristimaki A, Bohn H. Monoclonal antibodies reacting with placental protein 5: use in radioimmunoassay, Western blot analysis, and immunohistochemistry. J Lab Clin Med 1988; 111: 249–256
- Kisiel W, Sprecher C A, Foster D C. Evidence that a second human tissue factor pathway inhibitor (TFPI-2) and human placental protein 5 are equivalent. Blood 1994; 84: 4384–4385
- Chand H S, Foster D C, Kisiel W. Structure, function and biology of tissue factor pathway inhibitor-2. Thromb Haemost 2005; 94: 1122–1130
- Seppala M, Wahlstrom T, Bohn H. Circulating levels and tissue localization of placental protein five (PP5) in pregnancy and trophoblastic disease: absence of PP5 expression in the malignant trophoblast. Int J Cancer 1979; 24: 6–10
- Than G N, Bohn H, Szabo D G. Advances in pregnancy- related protein research. 1993; 1–333
- Obiekwe B C, Chard T. Placental protein 5: circulating levels in twin pregnancy and some observations on the analysis of biochemical data from multiple pregnancy. Eur J Obstet Gynecol Reprod Biol 1981; 12: 135–141
- Obiekwe B C, Sooby J, Salem H T, Chard T. Placental protein 5: disappearance from the circulation after delivery. Eur J Obstet Gynecol Reprod Biol 1982; 13: 1–5
- Abdel Gader A M, Al-Mishari A A, Awadalla S A, Buyuomi N M, Khashoggi T, Al-Hakeem M. Total and free tissue factor pathway inhibitor in pregnancy hypertension. Int J Gynaecol Obstet 2006; 95: 248–253
- Bellart J, Gilabert R, Angles A, Piera V, Miralles R M, Monasterio J, Cabero L. Tissue factor levels and high ratio of fibrinopeptide A:D-dimer as a measure of endothelial procoagulant disorder in pre-eclampsia. Br J Obstet Gynaecol 1999; 106: 594–597
- Schjetlein R, Abdelnoor M, Haugen G, Husby H, Sandset P M, Wisloff F. Hemostatic variables as independent predictors for fetal growth retardation in preeclampsia. Acta Obstet Gynecol Scand 1999; 78: 191–197
- Alexander G R, Himes J H, Kaufman R B, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol 1996; 87: 163–168
- Redline R W, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation–a workshop report. Placenta 2005; 2(Suppl A)S114–S117
- Grisaru-Granovsky S, Halevy T, Eidelman A, Elstein D, Samueloff A. Hypertensive disorders of pregnancy and the small for gestational age neonate: not a simple relationship. Am J Obstet Gynecol 2007; 196: 335
- Bretelle F, Sabatier F, Blann A, D'Ercole C, Boutiere B, Mutin M, Boubli L, Sampol J, gnat-George F. Maternal endothelial soluble cell adhesion molecules with isolated small for gestational age fetuses: comparison with pre-eclampsia. BJOG 2001; 108: 1277–1282
- Chaiworapongsa T, Romero R, Yoshimatsu J, Espinoza J, Kim Y M, Park K, Kalache K, Edwin S, Bujold E, Gomez R. Soluble adhesion molecule profile in normal pregnancy and pre-eclampsia. J Matern Fetal Neonatal Med 2002; 12: 19–27
- Coata G, Pennacchi L, Bini V, Liotta L, Di Renzo G C. Soluble adhesion molecules: marker of pre-eclampsia and intrauterine growth restriction. J Matern Fetal Neonatal Med 2002; 12: 28–34
- Krauss T, Kuhn W, Lakoma C, Augustin H G. Circulating endothelial cell adhesion molecules as diagnostic markers for the early identification of pregnant women at risk for development of preeclampsia. Am J Obstet Gynecol 1997; 177: 443–449
- Sacks G P, Studena K, Sargent K, Redman C W. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol 1998; 179: 80–86
- Richani K, Soto E, Romero R, Espinoza J, Chaiworapongsa T, Nien J K, Edwin S, Kim Y M, Hong J S, Goncalves L, et al. Preeclampsia and SGA differ in the maternal plasma complement split products profile. J Soc Gynecol Investig 2005; 12: 148A
- VanWijk M J, Boer K, Berckmans R J, Meijers J C, van der Post J A, Sturk A, Vanbavel E, Nieuwland R. Enhanced coagulation activation in preeclampsia: the role of APC resistance, microparticles and other plasma constituents. Thromb Haemost 2002; 88: 415–420
- Mellembakken J R, Aukrust P, Olafsen M K, Ueland T, Hestdal K, Videm V. Activation of leukocytes during the uteroplacental passage in preeclampsia. Hypertension 2002; 39: 155–160
- Schiessl B. Inflammatory response in preeclampsia. Mol Aspects Med 2007; 28: 210–219
- Saito S, Shiozaki A, Nakashima A, Sakai M, Sasaki Y. The role of the immune system in preeclampsia. Mol Aspects Med 2007; 28: 192–209
- Holthe M R, Lyberg T, Staff A C, Berge L N. Leukocyte-platelet interaction in pregnancies complicated with preeclampsia. Platelets 2005; 16: 91–97
- Sakai M, Tsuda H, Tanebe K, Sasaki Y, Saito S. Interleukin-12 secretion by peripheral blood mononuclear cells is decreased in normal pregnant subjects and increased in preeclamptic patients. Am J Reprod Immunol 2002; 47: 91–97
- Daniel Y, Kupferminc M J, Baram A, Jaffa A J, Fait G, Wolman I, Lessing J B. Plasma interleukin-12 is elevated in patients with preeclampsia. Am J Reprod Immunol 1998; 39: 376–380
- Haeger M, Unander M, Norder-Hansson B, Tylman M, Bengtsson A. Complement, neutrophil, and macrophage activation in women with severe preeclampsia and the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol 1992; 79: 19–26
- Bailey K, Herrod H G, Younger R, Shaver D. Functional aspects of T-lymphocyte subsets in pregnancy. Obstet Gynecol 1985; 66: 211–215
- Butenas S, Bouchard B A, Brummel-Ziedins K E, Parhami-Seren B, Mann K G. Tissue factor activity in whole blood. Blood 2005; 105: 2764–2770
- Butenas S, Mann K G. Blood coagulation. Biochemistry (Mosc.) 2002; 67: 3–12
- Rivers R P, Hathaway W E, Weston W L. The endotoxin-induced coagulant activity of human monocytes. Br J Haematol 1975; 30: 311–316
- Bach R R, Moldow C F. Mechanism of tissue factor activation on HL-60 cells. Blood 1997; 89: 3270–3276
- Egorina E M, Sovershaev M A, Bjorkoy G, Gruber F X, Olsen J O, Parhami-Seren B, Mann K G, Osterud B. Intracellular and surface distribution of monocyte tissue factor: application to intersubject variability. Arterioscler Thromb Vasc Biol 2005; 25: 1493–1498
- Satta N, Toti F, Feugeas O, Bohbot A, chary-Prigent J, Eschwege V, Hedman H, Freyssinet J M. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J Immunol 1994; 153: 3245–3255
- Sabatier F, Roux V, Anfosso F, Camoin L, Sampol J, gnat-George F. Interaction of endothelial microparticles with monocytic cells in vitro induces tissue factor-dependent procoagulant activity. Blood 2002; 99: 3962–3970
- Shet A S, Aras O, Gupta K, Hass M J, Rausch D J, Saba N, Koopmeiners L, Key N S, Hebbel R P. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood 2003; 102: 2678–2683
- Eilertsen K E, Osterud B. The role of blood cells and their microparticles in blood coagulation. Biochem Soc Trans 2005; 33: 418–422
- Baroni M, Pizzirani C, Pinotti M, Ferrari D, Adinolfi E, Calzavarini S, Caruso P, Bernardi F, Di V F. Stimulation of P2 (P2X7) receptors in human dendritic cells induces the release of tissue factor-bearing microparticles. FASEB J 2007; 21: 1926–1933
- Poitevin S, Cochery-Nouvellon E, Dupont A, Nguyen P. Monocyte IL-10 produced in response to lipopolysaccharide modulates thrombin generation by inhibiting tissue factor expression and release of active tissue factor-bound microparticles. Thromb Haemost 2007; 97: 598–607
- Ikeda K, Nagasawa K, Horiuchi T, Tsuru T, Nishizaka H, Niho Y. C5a induces tissue factor activity on endothelial cells. Thromb Haemost 1997; 77: 394–398
- Sitrin R G, Kaltreider H B, Ansfield M J, Webster R O. Procoagulant activity of rabbit alveolar macrophages. Am Rev Respir Dis 1983; 128: 282–287
- Ritis K, Doumas M, Mastellos D, Micheli A, Giaglis S, Magotti P, Rafail S, Kartalis G, Sideras P, Lambris J D. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol 2006; 177: 4794–4802
- Redecha P, Tilley R, Tencati M, Salmon J E, Kirchhofer D, Mackman N, Girardi G. Tissue factor: a link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood 2007; 110: 2423–2431
- Germain S J, Sacks G P, Sooranna S R, Sargent I L, Redman C W. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol 2007; 178: 5949–5956
- Redman C W, Sargent I L. Microparticles and immunomodulation in pregnancy and pre-eclampsia. J Reprod Immunol 2007; 76: 61–67
- Rusterholz C, Holzgreve W, Hahn S. Oxidative stress alters the integrity of cell-free mRNA fragments associated with placenta-derived syncytiotrophoblast microparticles. Fetal Diagn Ther 2007; 22: 313–317
- Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak M Z. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 2006; 20: 1487–1495
- Gupta A K, Holzgreve W, Huppertz B, Malek A, Schneider H, Hahn S. Detection of fetal DNA and RNA in placenta-derived syncytiotrophoblast microparticles generated in vitro. Clin Chem 2004; 50: 2187–2190
- Diamant M, Tushuizen M E, Sturk A, Nieuwland R. Cellular microparticles: new players in the field of vascular disease. Eur J Clin Invest 2004; 34: 392–401
- Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002; 2: 569–579
- Goswami D, Tannetta D S, Magee L A, Fuchisawa A, Redman C W, Sargent I L, von D P. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta 2006; 27: 56–61
- Balasubramanian V, Grabowski E, Bini A, Nemerson Y. Platelets, circulating tissue factor, and fibrin colocalize in ex vivo thrombi: real-time fluorescence images of thrombus formation and propagation under defined flow conditions. Blood 2002; 100: 2787–2792
- Balasubramanian V, Vele O, Nemerson Y. Local shear conditions and platelet aggregates regulate the incorporation and activity of circulating tissue factor in ex-vivo thrombi. Thromb Haemost 2002; 88: 822–826
- Chou J, Mackman N, Merrill-Skoloff G, Pedersen B, Furie B C, Furie B. Hematopoietic cell-derived microparticle tissue factor contributes to fibrin formation during thrombus propagation. Blood 2004; 104: 3190–3197
- Kobayashi M, Wada H, Wakita Y, Shimura M, Nakase T, Hiyoyama K, Nagaya S, Minami N, Nakano T, Shiku H. Decreased plasma tissue factor pathway inhibitor levels in patients with thrombotic thrombocytopenic purpura. Thromb Haemost 1995; 73: 10–14
- Shimura M, Wada H, Wakita Y, Nakase T, Hiyoyama K, Nagaya S, Mori Y, Shiku H. Plasma tissue factor and tissue factor pathway inhibitor levels in patients with disseminated intravascular coagulation. Am J Hematol 1997; 55: 169–174
- Aharon A, Lanir N, Drugan A, Brenner B. Placental TFPI is decreased in gestational vascular complications and can be restored by maternal enoxaparin treatment. J Thromb Haemost 2005; 3: 2355–2357
- Ogawa M, Yanoma S, Nagashima Y, Okamoto N, Ishikawa H, Haruki A, Miyagi E, Takahashi T, Hirahara F, Miyagi Y. Paradoxical discrepancy between the serum level and the placental intensity of PP5/TFPI-2 in preeclampsia and/or intrauterine growth restriction: possible interaction and correlation with glypican-3 hold the key. Placenta 2007; 28: 224–232
- Shimura M, Wada H, Wakita Y, Nakase T, Hiyoyama K, Nagaya S, Mori Y, Shiku H. Plasma tissue factor and tissue factor pathway inhibitor levels in patients with disseminated intravascular coagulation. Am J Hematol 1996; 52: 165–170
- Jones G R, Davey M W, Sinosich M, Grudzinskas J G. Specific interaction between placental protein 5 and heparin. Clin Chim Acta 1981; 110: 65–70
- Li Y, Rodriquez M, Spencer F A, Becker R C. Comparative effects of unfractionated heparin and low molecular weight heparin on vascular endothelial cell tissue factor pathway inhibitor release: a model for assessing intrinsic thromboresistance. J Thromb Thrombolysis 2002; 14: 123–129
- Menabawey M, Silman R, Rice A, Chard T. Dramatic increase of placental protein 5 levels following injection of small doses of heparin. Br J Obstet Gynaecol 1985; 92: 207–210
- Hansen J B, Svensson B, Olsen R, Ezban M, Osterud B, Paulssen R H. Heparin induces synthesis and secretion of tissue factor pathway inhibitor from endothelial cells in vitro. Thromb Haemost 2000; 83: 937–943
- Meisser A, Bischof P, Bohn H. Placental protein 5 (PP5) inhibits thrombin-induced coagulation of fibrinogen. Arch Gynecol 1985; 236: 197–201
- Nisbet A D, Brenner R D, Horne C H, Bohn H. Placental protein 5 (PP5) in pregnancy and malignant disease: the influence of heparin binding. Clin Chim Acta 1982; 119: 21–29
- Jeske W, Fareed J. Pharmacodynamic considerations in the selection of dosage of tinzaparin for various indications: experimental studies in primates. Semin Thromb Hemost 2004; 30(Suppl 1)41–47
- Kemme M J, Burggraaf J, Schoemaker R C, Kluft C, Cohen A F. Quantification of heparin-induced TFPI release: a maximum release at low heparin dose. Br J Clin Pharmacol 2002; 54: 627–634
- Kaiser B, Hoppensteadt D A, Fareed J. Tissue factor pathway inhibitor: an update of potential implications in the treatment of cardiovascular disorders. Expert Opin Investig Drugs 2001; 10: 1925–1935
- Sandset P M, Bendz B, Hansen J B. Physiological function of tissue factor pathway inhibitor and interaction with heparins. Haemostasis 2000; 30(Suppl 2)48–56
- Kaiser B, Glusa E, Hoppensteadt D A, Breddin H K, Amiral J, Fareed J. A supersulfated low-molecular-weight heparin (IK-SSH) increases plasma levels of free and total tissue factor pathway inhibitor after intravenous and subcutaneous administration in humans. Blood Coagul Fibrinolysis 1998; 9: 517–523
- Hansen J B, Sandset P M, Huseby K R, Huseby N E, Bendz B, Ostergaard P, Nordoy A. Differential effect of unfractionated heparin and low molecular weight heparin on intravascular tissue factor pathway inhibitor: evidence for a difference in antithrombotic action. Br J Haematol 1998; 101: 638–646
- Jeske W, Hoppensteadt D, Klauser R, Kammereit A, Eckenberger P, Haas S, Wyld P, Fareed J. Effect of repeated Aprosulate and Enoxaparin administration on tissue factor pathway inhibitor antigen levels. Blood Coagul Fibrinolysis 1995; 6: 119–124
- Jesty J, Wun T C, Lorenz A. Kinetics of the inhibition of factor Xa and the tissue factor-factor VIIa complex by the tissue factor pathway inhibitor in the presence and absence of heparin. Biochemistry 1994; 33: 12686–12694
- Huang Z F, Wun T C, Broze G J, Jr. Kinetics of factor Xa inhibition by tissue factor pathway inhibitor. J Biol Chem 1993; 268: 26950–26955
- Wun T C. Lipoprotein-associated coagulation inhibitor (LACI) is a cofactor for heparin: synergistic anticoagulant action between LACI and sulfated polysaccharides. Blood 1992; 79: 430–438
- Lindahl A K, Abildgaard U, Larsen M L, Aamodt L M, Nordfang O, Beck T C. Extrinsic pathway inhibitor (EPI) and the post-heparin anticoagulant effect in tissue thromboplastin induced coagulation. Thromb Res Suppl 1991; 14: 39–48
- Ostergaard P, Nordfang O, Petersen L C, Valentin S, Kristensen H. Is tissue factor pathway inhibitor involved in the antithrombotic effect of heparins? Biochemical considerations. Haemostasis 1993; 23(Suppl 1)107–111
- Wesselschmidt R, Likert K, Huang Z, MacPhail L, Broze G, Jr. Structural requirements for tissue factor pathway inhibitor interactions with factor Xa and heparin. Blood Coagul Fibrinolysis 1993; 4: 661–669
- Broze G J, Jr. Tissue factor pathway inhibitor and the revised theory of coagulation. Annu Rev Med 1995; 46: 103–112
