Introduction
The syndromic nature of spontaneous labour was a concept first proposed in 1994 Citation[1]. It has important implications for understanding the biology of preterm parturition, and also, setting realistic expectations about what programs for the prediction and prevention of preterm birth can and cannot do.
The traditional view of preterm parturition has been that it is fundamentally the same process as spontaneous labour at term, and that the crucial difference is the gestational age at which these processes occur Citation[2-4]. Another implicit assumption is that all causes of preterm labour are the same, because patients with preterm labour and intact membranes are treated with the combination of tocolytic agents and glucocorticoids worldwide. This article outlines the potential flaws in this concept, which we believe has halted progress in the prevention of preterm birth and in the treatment of preterm labour.
Preterm birth: definition, classification and burden of disease
A preterm birth is one that occurs between 24 weeks (fetal viability) and 37 weeks of gestation (menstrual age). The term “fetal viability” is intended to identify a fetus capable of extra-uterine life. When a conceptus is delivered before viability, this is considered a spontaneous abortion rather than a preterm birth. We have discussed the issues surrounding the definition of “viability” and the gold standards used for such a definition, and the interested reader is referred to such an article for further details Citation[5].
Approximately two-thirds of all preterm births occur after the spontaneous onset of labour. The remaining one-third is the result of “indicated preterm birth”. The term “indicated” connotes that labour and/or delivery has been the result of induction and/or abdominal delivery for maternal or fetal indications. The most common maternal indication is pre-eclampsia, and the fetal indication is a small-for-gestational-age fetus with evidence of compromise. Other causes of “indicated preterm birth” include congenital anomalies or maternal diseases that require delivery (e.g. acute fatty liver of pregnancy or other disorders, which would improve after delivery) Citation[6-10]. This distinction between spontaneous and indicated preterm birth is based upon the clinical circumstances which determine preterm delivery, namely, the presence of spontaneous labour. It is important to note that this classification does not take into account the cause of the “spontaneous” or “indicated preterm birth”, and indeed, there may be an overlap between the two Citation[9],Citation[11].
Preterm birth accounts for 5–12% of all births, and it is a leading cause of perinatal morbidity and mortality worldwide. The risk of death of a preterm neonate is 120 times greater than that of an infant born at term Citation[6],Citation[12]–Citation[16]. Moreover, survivors are at risk of short-term morbidity (respiratory distress syndrome, intraventricular haemorrhage, necrotizing enterocolitis, sepsis, retinopathy of prematurity) Citation[17-29], and long-term morbidity Citation[30,31] such as cerebral palsy Citation[32-36] learning disabilities Citation[23],Citation[37,38], blindness Citation[39,40] and crippling respiratory disease Citation[41-48]. Recent evidence suggests that preterm neonates may also be at risk for altered metabolic states in adult life Citation[49-52].
Preterm births have been classified according to the gestational age at which they occur, into very early (those with a gestational age at delivery of ≤28 weeks), early (≤32 weeks), and late preterm birth (33–36 weeks). The frequency of these three types is 0.82%, 2.2%, and 8.9%, respectively Citation[53]. Therefore, most preterm births occur late, yet serious morbidity and mortality affect disproportionately those born before the 28th week Citation[42],Citation[44,45],Citation[54-64]. Recent observations suggest that late preterm births are at a higher risk for health and developmental problems than infants born at term Citation[65-74]. Indeed, infant mortality, defined as “deaths at the age of one year” is significantly greater in late preterm birth than in term birth Citation[16],Citation[67,68]. Of interest is that this excess includes Sudden Infant Death Syndrome (SIDS) Citation[16], suggesting that a pathological process may operate in both inducing preterm birth and also increasing the susceptibility of infant mortality. The clinical implications of these observations is that late preterm birth should not be neglected as unimportant, or a short-term neonatal problem.
The common pathway of parturition
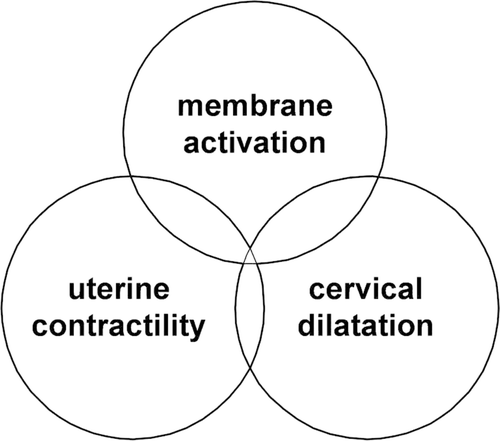
The common pathway of parturition has been defined as the anatomical, physiological, biochemical, endocrinological, immunological and clinical events that occur in the mother and/or fetus at the time of parturition regardless of whether this occurs at term or preterm. The most well-known components of the common pathway of parturition are the uterine components because they are clinically apparent to obstetricians and patients. Such components include: 1) increased myometrial contractility; 2) cervical ripening/dilatation and effacement; and 3) membrane/decidual activation Citation[1,2]. Each of these components is visibly and clinically activated in spontaneous labour at term, where uterine contractions become regular and painful, cervical dilatation and effacement easily detectable, and rupture of membranes is a well-recognised part of the parturitional process. Therefore, in most cases of spontaneous labour at term, there is synchronous activation of the common pathway.
We have proposed that the onset of spontaneous labour at term is the result of physiological signals that are set into motion when the mother cannot sustain fetal growth any longer. This is predicated on the basis that human gestation has an inherent limit related to the ability of the mother and/or placenta to sustain growth. When such a limit is reached, parturition is initiated. An unlimited duration of pregnancy would result in taxation of maternal resources, and the growth of a fetus that cannot pass through the birth canal. It is unclear if the duration of normal pregnancy is determined by the maternal and/or fetal genome. Clearly, there can be conflicting interests between these two genomes, and the time of the onset of labour most likely represents a balance between these conflicting interests. Whether this should be framed in terms of David Haig's Conflict Hypothesis, or alternatively, viewed as an issue of cooperation between the maternal and the fetal genome is an interesting biological question.
We view preterm parturition as the consequence of pathological signals that activate the common pathway of parturition. Moreover, the activation may be asynchronous. Therefore, some patients may present with preterm increased uterine contractility. Other patients may present with acute cervical insufficiency (a dilated cervix with membranes protruding through the cervix) and others may present with preterm premature rupture of membranes. It is also possible that preterm labour presents with synchronous activation of the common pathway. This represents the patient with increased uterine contractility, cervical dilatation, and membrane rupture. illustrates the components of the common pathway of parturition, and also the phenotypic expression of the preterm parturition syndromes (preterm uterine contractions, cervical insufficiency, preterm premature rupture of membranes).
Symptoms and signs of activation of the common pathway of parturition
The activation of the uterine components of the common pathway is manifested clinically with signs and symptoms. Mothers who have pain during uterine contractions, pelvic pressure in cases of cervical dilatation with prolapse of the membranes, or leakage of fluid have symptoms of the preterm parturition syndrome. The objective demonstration of these symptoms (uterine contractions demonstrated by palpation or a uterine strip, visual inspection with a speculum examination, or demonstration of fluid in the posterior fornix) would represent the signs. It is important to note that none of the signs and symptoms provide any information as to why preterm parturition has begun.
Subclinical evidence of activation of the common pathway
It is possible to detect activation of the different components of the pathway using sophisticated tools. For example, electromyography has been employed to detect changes in uterine electrical activity associated with labour Citation[75-78]. Cervical ultrasound can detect preterm effacement of the uterine cervix. The colloscope has been used to identify changes in collagen organization of the cervix underlining the process of cervical ripening. Finally, a positive fetal fibronectin or other markers represent evidence of degradation of the extracellular matrix. Such a process is important for membrane rupture, and therefore, it has been interpreted to be a biochemical marker of membrane/decidual activation. This conceptual framework allows a link between the clinical manifestations of the preterm labour syndrome, and the tools being developed to detect untimely activation of the different components of the common pathway of parturition.
Preterm parturition as a syndrome with multiple aetiologies
We have proposed that preterm parturition should not be considered a monolithic entity, but rather as a syndrome. The term “syndrome” is defined in the Oxford Medical Dictionary as “a combination of symptoms and signs that form a distinct clinical picture indicative of a particular disorder”. Therefore, these symptoms and signs tend to occur together. The crucial point is that the signs and symptoms that define the preterm parturition syndrome are caused by multiple aetiologies or pathological processes.
Many have referred to preterm parturition as a multifactorial condition. This term is used to indicate that there may be more than one factor responsible for the disorder. However, we find this characterization incomplete, because there are no unifactorial diseases in medicine. For example, sickle cell disease, which is caused by a single base pair mutation in the nucleotides encoding for haemoglobin, may be expressed with a wide range of phenotypes which depend to a large extent on environmental factors.
We have argued that obstetrical disorders are really syndromes, and refer to them as “The Great Obstetrical Syndromes”Citation[79]. The features of “The Great Obstetrical Syndromes” are the following: 1) multiple aetiology; 2) long preclinical stage; 3) frequent fetal involvement; 4) clinical manifestations which are often adaptive in nature; and 5) predisposition to a particular syndrome is influenced by gene–environment interaction and/or complex gene–gene interactions involving maternal and/or fetal genotypes Citation[80-108].
Preterm parturition meets all the criteria for the syndrome, and we refer the reader to a previous article for the examination of the arguments employed in support of this thesis Citation[109].
Aetiologies of the preterm parturition syndrome
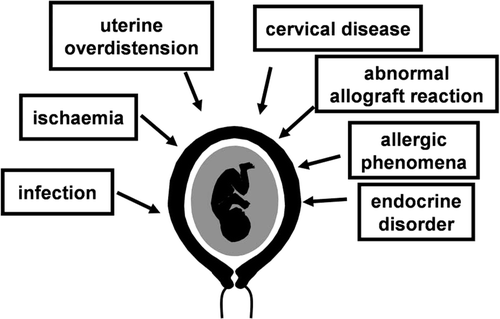
The following mechanisms of disease have been implicated: 1) intrauterine infection/inflammation Citation[110-292]; 2) uterine ischaemia Citation[293-307]; 3) uterine overdistension Citation[308-327]; 4) abnormal allogenic recognition Citation[328-335]; 5) allergic-like reaction Citation[336-340]; 6) cervical disease [341,342]; and 7) endocrine disorders Citation[343-359]. Most of these mechanisms of disease operate in non-pregnant women. For example, ischaemia is a major pathophysiologic process in cardiovascular disease. Fluid overload may be responsible for acute congestive heart failure. Rejection is the main problem in transplantation medicine. Allergy and endocrine disorders are well-established mechanisms of disease in childhood and adult life. It is also possible that preterm parturition may be caused by mechanisms of disease that are unique to the maternal–fetal relationship, because of its unique anatomy, physiology, immunology and metabolic demands. Consequently, we remain open to the discovery of undescribed mechanisms of disease during pregnancy.
illustrates the causes of the preterm labour syndrome. Thus far, infection is the only mechanism of disease for which a causal link has been established. However, there is substantial evidence in support of the others, but causality still needs to be proven. A full discussion of the scientific evidence which supports a role for each mechanism of disease in the preterm parturition syndrome is available in recent publications by our group Citation[109].
What are the consequences of the concept that preterm parturition is a syndrome?
The implicit consequence that preterm parturition is a syndrome with multiple aetiologies is that there would not be a single treatment, diagnostic method or preventive strategy that will work for all preterm births. For example, tocolysis has been tested in women with preterm labour and intact membranes, and has been shown to prolong pregnancy for 48 hours to 7 days Citation[360-364]. This prolongation of pregnancy has not been associated with a change in perinatal morbidity and mortality Citation[360-364]. It is now clear that tocolytic agents do not work in this setting of subclinical intra-amniotic infection, and indeed, that treatment with beta-adrenergic agents may increase the risk of pulmonary oedema in such patients Citation[365]. It is possible that some of the judgements made about tocolytic effectiveness could be the result of administering these drugs to patients who may not benefit (patients with subclinical infection). This does not mean that there may not be a subgroup that would benefit from efforts to arrest uterine contractions and prolong pregnancy.
The same concept applies to methods for risk assessment or preventive strategies. A positive fetal fibronectin test identified patients at risk for preterm delivery Citation[366-372]. Similarly, a short sonographic cervical length is a risk factor for early preterm delivery Citation[373-381]. Moreover, there is evidence that patients with a short cervix tend to have a positive fetal fibronectin test in vaginal fluid, and such patients are more likely to deliver preterm Citation[369],Citation[382]–Citation[385]. We interpret this evidence as suggesting that the insult responsible for preterm birth may act in an asynchronous fashion, and affect one component of the pathway of parturition more than the others. Similarly, such insult may be of a temporary nature, and may be resolved without resorting to preterm parturition. For example, patients with a positive fibronectin often have a second test which is negative Citation[386]. Although the first test may be interpreted as a false positive, an alternative explanation is that an insult to the utero-placental unit resulted in activation of matrix degradation detectable by an increased concentration of fibronectin in vaginal fluid. However, if such insult is self-limited, this would allow homeostasis to handle the insult and return the membrane-decidual state to its baseline. On the other hand, persistence of the stimulus and further insult may lead not only to persisting activation of one pathway, but recruitment of the others. This would explain why some patients begin with a positive fibronectin, develop a short cervix, and eventually have increased uterine contractility. The more components activated, the more likely it is that the process will evolve from a reversible to an irreversible state of preterm labour.
The methods of prevention may also have a different effectiveness, depending upon the mechanism of disease. For example, a cervical cerclage may be effective in the subpopulation with cervical disease, which is amenable to early diagnosis (e.g. a sonographic short cervix and a prior history of preterm birth, or a patient with active shortening of the cervix in the current pregnancy) Citation[387,388]. However, this intervention may be totally ineffective in patients who have had a previous preterm delivery unrelated to cervical disease. Indeed, a randomized clinical trial of cervical cerclage in women with a cervical length of ≤15 mm demonstrated that this operation is not effective in reducing preterm birth in nulliparous women with an isolated short cervix Citation[389]. If the primary mechanism of disease is infection/inflammation, these patients may benefit from antimicrobial/anti-inflammatory treatment. However, if infection/inflammation is not the mechanism of disease, there is little hope that antimicrobial treatment would prevent preterm birth. This is probably at the heart of why antimicrobial treatment to prevent preterm birth has largely failed. Patients with Group B streptococcus (GBS) or Ureaplasma urealyticumCitation[390] are not at risk for preterm delivery, and therefore, treatment of colonized women has not reduced the rate of preterm birth. The same applies to patients with asymptomatic Trichomonas vaginalisCitation[391]. The odds ratio for preterm delivery is only 1.4, which means that most women with asymptomatic Trichomonas vaginalis will deliver at term, and therefore, cannot benefit from antibiotic administration. Bacterial vaginosis also represents a complex story in which the problems include the adequacy of the diagnosis and the host response Citation[255],Citation[392]–Citation[395]. The interested reader is referred to a previous article in which we have explored the question in depth Citation[109].
We predict that a successful strategy to prevent preterm birth must be based on an understanding of the mechanisms of disease for a particular patient, the development of a method for early detection of this specific pathologic process, as well as treatment targeted to this process. In other words, diagnosis, treatment and prevention aimed at the specific cause of the syndrome rather than to the manifestations of the syndrome.
We do not exclude that there may be primary disorders of the terminal components of the pathway. For example, cervical insufficiency may result from subclinical intrauterine infection, but may also be a primary disease of the cervix. The latter can be identified with cervical sonography and treated with a cervical cerclage, which may prevent preterm delivery even though it does not cure the underlying mechanism of disease responsible for the primary disorder. Additionally, we believe that a primary myometrium disorder may be operative in some patients. Professor Stephen Lye has described that the myometrium undergoes an organized and sequential change of phenotype as it progresses from early pregnancy to labour Citation[317,318],Citation[320],Citation[327], Citation[396-400]. Acceleration of such a timetable may be a cause for preterm delivery. We envision that if a similar program exists in humans, pharmacologic interventions may be used to treat and prevent preterm delivery. For example, tocolysis may be used acutely to achieve uterine rest, while progesterone could be employed to reverse the untimely progression away from a contractile phenotype of the myometrium and towards a quiescent one.
Inflammation as a mechanism for term and preterm parturition
Liggins Citation[401] was the first to liken cervical ripening to an inflammatory response. Since then, accumulating evidence supports the idea that inflammation can be detected in the cervix, myometrium, chorioamniotic membranes and amniotic cavity of women in labour Citation[402-409].
Spontaneous labour at term is associated with the infiltration of inflammatory cells in these tissues and increased production of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α and IL-8) Citation[202],Citation[410] and chemokines (GROα, granulocyte colony stimulating factor [GCSF], GMCSM, neutrophil attractant/activating peptide –1/IL-8, etc) Citation[410]. Recently, using a genome-wide screen, we have been able to demonstrate that genes involved in the control of inflammation are upregulated in the chorioamniotic membranes after labour at term, even in the absence of histological chorioamnionitis Citation[403,404]. Of interest is that an inflammatory signature was not observed in the maternal circulation of patients with spontaneous labour at term Citation[403,404]. This suggests that an inflammatory process is localized to the membranes. These findings may reflect the fact that spontaneous parturition is associated with an increased pro-inflammatory cytokine and chemokine response. We have made similar observations in the myometrium (Romero, unpublished observations), and this is consistent with those made by Professor Roger Smith from Newcastle, Australia Citation[402], as well as Professor Murray Mitchell from Auckland, New Zealand (in the membranes) Citation[410-414]. Thus, the emerging picture is that inflammation is important in term spontaneous labour.
In addition, pathologic inflammation, which is that elicited by the presence of microbial products, microorganisms and other mechanisms of tissue injury, has been implicated in preterm parturition. Thus, the evidence is now compelling that inflammation in the uterine components of the common pathway is present in term and preterm labour, although the severity of the inflammatory process is far greater in the context of subclinical intrauterine infection in preterm labour Citation[406,407].
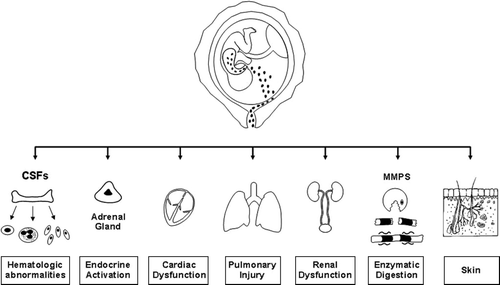
Microbial invasion of the amniotic cavity and intrauterine inflammation have been associated with microbial invasion of the human fetus Citation[415] and the development of the Fetal Inflammatory Response Syndrome (FIRS) Citation[249],Citation[407],Citation[416-418]. This condition has been operationally defined as an elevation in fetal plasma IL-6 concentration, and is a risk factor for spontaneous preterm labour, neonatal morbidity and mortality, as well as multisystemic involvement (cardiac dysfunction Citation[419,420], adrenal stress response Citation[421], neurologic injury Citation[422-438], as well as lung disease Citation[128],Citation[439-445]) (). The relevance of this is that the identification of the patient with intra-amniotic inflammation has become a priority to select a patient in whom tocolysis would be futile, and who may benefit from antimicrobial and anti-inflammatory agents. Of interest is that the lower the gestational age at presentation, the higher the frequency of intra-amniotic infection and inflammation Citation[110],Citation[446]. This has implications for the management of patients with preterm labour, because we have demonstrated that treatment with a tocolytic agent was less effective at gestational ages of <28 weeks of gestation, when infection is more common Citation[360,361]. A full discussion of the role of infection and inflammation in preterm labour and fetal injury is available in recent reviews by our group Citation[407],Citation[418],Citation[447].
Acknowledgement
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), NIH, DHHS.
References
- Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer D M. The preterm labor syndrome. Ann N Y Acad Sci 1994; 734: 414–429
- Romero R, Gomez R, Mazor M, Ghezzi F, Yoon B H. The preterm labor syndrome. Preterm labor, M G Elder, R Romero, R F Lamont. Churchill Livingstone, New York, NY 1997; 29–49
- Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol 1988; 31: 553–584
- Romero R, Espinoza J, Mazor M, Chaiworapongsa T. The preterm parturition syndrome. Preterm birth, H Critchely, P Bennett, S Thornton. RCOG press, London 2004; 28–60
- Mazaki-Tovi S, Romero R, Kusanovic J P, Erez O, Pineles B L, Gotsch F, et al. Recurrent preterm birth. Semin Perinatol 2007; 31: 142–158
- Joseph K S, Demissie K, Kramer M S. Obstetric intervention, stillbirth, and preterm birth. Semin Perinatol 2002; 26: 250–259
- Moutquin J M. Classification and heterogeneity of preterm birth. BJOG 2003; 110(Suppl 20)30–33
- Ananth C V, Vintzileos A M. Medically indicated preterm birth: recognizing the importance of the problem. Clin Perinatol 2008; 35: 53–67, viii
- Ananth C V, Vintzileos A M. Epidemiology of preterm birth and its clinical subtypes. J Matern Fetal Neonatal Med 2006; 19: 773–782
- Ananth C V, Vintzileos A M. Maternal-fetal conditions necessitating a medical intervention resulting in preterm birth. Am J Obstet Gynecol 2006; 195: 1557–1563
- Ananth C V, Getahun D, Peltier M R, Salihu H M, Vintzileos A M. Recurrence of spontaneous versus medically indicated preterm birth. Am J Obstet Gynecol 2006; 195: 643–650
- Joseph K S, Kramer M S, Marcoux S, Ohlsson A, Wen S W, Allen A, Platt R. Determinants of preterm birth rates in Canada from 1981 through 1983 and from 1992 through 1994. N Engl J Med 1998; 339: 1434–1439
- Kramer M S, Platt R, Yang H, Joseph K S, Wen S W, Morin L, Usher R H. Secular trends in preterm birth: a hospital-based cohort study. JAMA 1998; 280: 1849–1854
- Ananth C V, Joseph K S, Oyelese Y, Demissie K, Vintzileos A M. Trends in preterm birth and perinatal mortality among singletons: United States, 1989 through 2000. Obstet Gynecol 2005; 105: 1084–1091
- Raju T N, Higgins R D, Stark A R, Leveno K J. Optimizing care and outcome for late-preterm (near-term) infants: a summary of the workshop sponsored by the National Institute of Child Health and Human Development. Pediatrics 2006; 118: 1207–1214
- Mathews T J, MacDorman M F. Infant mortality statistics from the 2005 period linked birth/infant death data set. Natl Vital Stat Rep 2008; 57: 1–32
- Kosloske A M. Epidemiology of necrotizing enterocolitis. Acta Paediatr Suppl 1994; 396: 2–7
- Holman R C, Stoll B J, Clarke M J, Glass R I. The epidemiology of necrotizing enterocolitis infant mortality in the United States. Am J Public Health 1997; 87: 2026–2031
- Caplan M S, Jilling T. New concepts in necrotizing enterocolitis. Curr Opin Pediatr 2001; 13: 111–115
- O'Connor A R, Stephenson T, Johnson A, Tobin M J, Moseley M J, Ratib S, Ng Y, Fielder A R. Long-term ophthalmic outcome of low birth weight children with and without retinopathy of prematurity. Pediatrics 2002; 109: 12–18
- Lee J S, Polin R A. Treatment and prevention of necrotizing enterocolitis. Semin Neonatol 2003; 8: 449–459
- Chow L C, Wright K W, Sola A. Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in very low birth weight infants. Pediatrics 2003; 111: 339–345
- Marlow N, Wolke D, Bracewell M A, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med 2005; 352: 9–19
- Tasman W, Patz A, McNamara J A, Kaiser R S, Trese M T, Smith B T. Retinopathy of prematurity: the life of a lifetime disease. Am J Ophthalmol 2006; 141: 167–174
- Harrell S N, Brandon D H. Retinopathy of prematurity: the disease process, classifications, screening, treatment, and outcomes. Neonatal Netw 2007; 26: 371–378
- Chen J, Smith L E. Retinopathy of prematurity. Angiogenesis 2007; 10: 133–140
- Fleck B W, McIntosh N. Pathogenesis of retinopathy of prematurity and possible preventive strategies. Early Hum Dev 2008; 84: 83–88
- Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev 2008; 84: 77–82
- Bastek J A, Sammel M D, Pare E, Srinivas S K, Posencheg M A, Elovitz M A. Adverse neonatal outcomes: examining the risks between preterm, late preterm, and term infants. Am J Obstet Gynecol 2008; 199: 367–368
- Moster D, Lie R T, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med 2008; 359: 262–273
- Swamy G K, Ostbye T, Skjaerven R. Association of preterm birth with long-term survival, reproduction, and next-generation preterm birth. JAMA 2008; 299: 1429–1436
- Redline R W, Wilson-Costello D, Borawski E, Fanaroff A A, Hack M. Placental lesions associated with neurologic impairment and cerebral palsy in very low-birth-weight infants. Arch Pathol Lab Med 1998; 122: 1091–1098
- Moon J B, Kim J C, Yoon B H, Romero R, Kim G, Oh S Y, Kim M, Shim S S. Amniotic fluid matrix metalloproteinase-8 and the development of cerebral palsy. J Perinat Med 2002; 30: 301–306
- Bashiri A, Burstein E, Mazor M. Cerebral palsy and fetal inflammatory response syndrome: a review. J Perinat Med 2006; 34: 5–12
- Platt M J, Cans C, Johnson A, Surman G, Topp M, Torrioli M G, Krageloh-Mann I. Trends in cerebral palsy among infants of very low birthweight (<1500 g) or born prematurely (<32 weeks) in 16 European centres: a database study. Lancet 2007; 369: 43–50
- O'Shea M. Cerebral palsy. Semin Perinatol 2008; 32: 35–41
- Wood N S, Marlow N, Costeloe K, Gibson A T, Wilkinson A R. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. N Engl J Med 2000; 343: 378–384
- Litt J, Taylor H G, Klein N, Hack M. Learning disabilities in children with very low birthweight: prevalence, neuropsychological correlates, and educational interventions. J Learn Disabil 2005; 38: 130–141
- Robinson G C, Jan J E, Kinnis C. Congenital ocular blindness in children, 1945 to 1984. Am J Dis Child 1987; 141: 1321–1324
- Gilbert C, Awan H. Blindness in children. BMJ 2003; 327: 760–761
- Van Marter L J, Dammann O, Allred E N, Leviton A, Pagano M, Moore M, Martin C, Developmental Epidemiology Network Investigators. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr 2002; 140: 171–176
- Askie L M, Henderson-Smart D J, Irwig L, Simpson J M. Oxygen-saturation targets and outcomes in extremely preterm infants. N Engl J Med 2003; 349: 959–967
- Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 2007; 357: 1946–1955
- Eichenwald E C, Stark A R. Management and outcomes of very low birth weight. N Engl J Med 2008; 358: 1700–1711
- Tyson J E, Parikh N A, Langer J, Green C, Higgins R D. Intensive care for extreme prematurity–moving beyond gestational age. N Engl J Med 2008; 358: 1672–1681
- Kiren V, Barbi E, Ventura A. Chronic lung disease after premature birth. N Engl J Med 2008; 358: 745–746
- Cutz E, Chiasson D. Chronic lung disease after premature birth. N Engl J Med 2008; 358: 743–745
- Morley C J, Davis P G, Doyle L W, Brion L P, Hascoet J M, Carlin J B. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med 2008; 358: 700–708
- Barker D J, Eriksson J G, Forsen T, Osmond C, Cutfield W S. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 2002; 31: 1235–1239
- Hofman P L, Regan F, Jackson W E, Jefferies C, Knight D B, Robinson E M, Cutfield W S. Premature birth and later insulin resistance. N Engl J Med 2004; 351: 2179–2186
- Fernandez-Twinn D S, Ozanne S E. Mechanisms by which poor early growth programs type-2 diabetes, obesity and the metabolic syndrome. Physiol Behav 2006; 88: 234–243
- Hovi P, Andersson S, Eriksson J G, Jarvenpaa A L, Strang-Karlsson S, Makitie O, Kajantie E. Glucose regulation in young adults with very low birth weight. N Engl J Med 2007; 356: 2053–2063
- Alexander G R. Measurement of fetal and infant maturity. Preterm birth: Causes, Consequences and Prevention, R E Behrman, A S Butler. Institute of Medicine. 2007; 72
- Allen M C, Alexander G R, Tompkins M E, Hulsey T C. Racial differences in temporal changes in newborn viability and survival by gestational age. Paediatr Perinat Epidemiol 2000; 14: 152–158
- Costeloe K, Hennessy E, Gibson A T, Marlow N, Wilkinson A R. The EPICure study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics 2000; 106: 659–671
- Lemons J A, Bauer C R, Oh W, Korones S B, Papile L A, Stoll B J, Verter J, Temprosa M, Wright L L, Ehrenkranz R A, et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics 2001; 107: E1
- Doyle L W. Neonatal intensive care at borderline viability–is it worth it. Early Hum Dev 2004; 80: 103–113
- Vanhaesebrouck P, Allegaert K, Bottu J, Debauche C, Devlieger H, Docx M, Francois A, Haumont D, Lombet J, Rigo J, et al. The EPIBEL study: outcomes to discharge from hospital for extremely preterm infants in Belgium. Pediatrics 2004; 114: 663–675
- Markestad T, Kaaresen P I, Ronnestad A, Reigstad H, Lossius K, Medbo S, Zanussi G, Engelund I E, Skjaerven R, Irgens L M, , Norwegian Extreme Prematurity Study Group, et al. Early death, morbidity, and need of treatment among extremely premature infants. Pediatrics 2005; 115: 1289–1298
- Bartels D B, Wypij D, Wenzlaff P, Dammann O, Poets C F. Hospital volume and neonatal mortality among very low birth weight infants. Pediatrics 2006; 117: 2206–2214
- Fanaroff A A, Stoll B J, Wright L L, Carlo W A, Ehrenkranz R A, Stark A R, Bauer C R, Donovan E F, Korones S B, Laptook A R, , NICHD Neonatal Research Network, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol 2007; 196: 147–148
- Doyle L W. Cardiopulmonary outcomes of extreme prematurity. Semin Perinatol 2008; 32: 28–34
- Anderson P J, Doyle L W. Cognitive and educational deficits in children born extremely preterm. Semin Perinatol 2008; 32: 51–58
- Saigal S, Doyle L W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008; 371: 261–269
- Escobar G J, Clark R H, Greene J D. Short-term outcomes of infants born at 35 and 36 weeks gestation: we need to ask more questions. Semin Perinatol 2006; 30: 28–33
- Shapiro-Mendoza C K, Tomashek K M, Kotelchuck M, Barfield W, Weiss J, Evans S. Risk factors for neonatal morbidity and mortality among “healthy,” late preterm newborns. Semin Perinatol 2006; 30: 54–60
- Jain L. Morbidity and mortality in late-preterm infants: more than just transient tachypnea!. J Pediatr 2007; 151: 445–446
- Tomashek K M, Shapiro-Mendoza C K, Davidoff M J, Petrini J R. Differences in mortality between late-preterm and term singleton infants in the United States, 1995–2002. J Pediatr 2007; 151: 450–456
- Shapiro-Mendoza C K, Tomashek K M, Kotelchuck M, Barfield W, Nannini A, Weiss J, Declercq E. Effect of late-preterm birth and maternal medical conditions on newborn morbidity risk. Pediatrics 2008; 121: e223–e232
- McIntire D D, Leveno K J. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol 2008; 111: 35–41
- Engle W A, Kominiarek M A. Late preterm infants, early term infants, and timing of elective deliveries. Clin Perinatol 2008; 35: 325–341, vi
- Ananth C V, Gyamfi C, Jain L. Characterizing risk profiles of infants who are delivered at late preterm gestations: does it matter. Am J Obstet Gynecol 2008; 199: 329–331
- Lubow J M, How H Y, Habli M, Maxwell R, Sibai B M. Indications for delivery and short-term neonatal outcomes in late preterm as compared with term births. Am J Obstet Gynecol 2009, [epub ahead of print]
- Khashu M, Narayanan M, Bhargava S, Osiovich H. Perinatal outcomes associated with preterm birth at 33 to 36 weeks' gestation: a population-based cohort study. Pediatrics 2009; 123: 109–113
- Steer C M. Electrohysterography. Ann N Y Acad Sci 1959; 75: 809–812
- Devedeux D, Marque C, Mansour S, Germain G, Duchene J. Uterine electromyography: a critical review. Am J Obstet Gynecol 1993; 169: 1636–1653
- Hofmeister J F, Slocumb J C, Kottmann L M, Picchiottino J B, Ellis D G. A noninvasive method for recording the electrical activity of the human uterus in vivo. Biomed Instrum Technol 1994; 28: 391–404
- Euliano T Y, Marossero D, Nguyen M T, Euliano N R, Principe J, Edwards R K. Spatiotemporal electrohysterography patterns in normal and arrested labor. Am J Obstet Gynecol 2009; 200: 54–57
- Romero R. The child is the father of the man. Prenat Neonat Med 1996; 1: 8–11
- Ferrand P E, Parry S, Sammel M, Macones G A, Kuivaniemi H, Romero R, Strauss J F, 3rd. A polymorphism in the matrix metalloproteinase-9 promoter is associated with increased risk of preterm premature rupture of membranes in African Americans. Mol Hum Reprod 2002; 8: 494–501
- Witkin S S, Gerber S, Ledger W J. Influence of interleukin-1 receptor antagonist gene polymorphism on disease. Clin Infect Dis 2002; 34: 204–209
- Fujimoto T, Parry S, Urbanek M, Sammel M, Macones G, Kuivaniemi H, Romero R, Strauss J F, 3rd. A single nucleotide polymorphism in the matrix metalloproteinase-1 (MMP-1) promoter influences amnion cell MMP-1 expression and risk for preterm premature rupture of the fetal membranes. J Biol Chem 2002; 277: 6296–6302
- Genc M R, Gerber S, Nesin M, Witkin S S. Polymorphism in the interleukin-1 gene complex and spontaneous preterm delivery. Am J Obstet Gynecol 2002; 187: 157–163
- Wang H, Parry S, Macones G, Sammel M D, Ferrand P E, Kuivaniemi H, Tromp G, Halder J, Shriver M D, Romero R, et al. Functionally significant SNP MMP8 promoter haplotypes and preterm premature rupture of membranes (PPROM). Hum Mol Genet 2004; 13: 2659–2669
- Genc M R, Onderdonk A B, Vardhana S, Delaney M L, Norwitz E R, Tuomala R E, Paraskevas L R, Witkin S S, MAP Study Group. Polymorphism in intron 2 of the interleukin-1 receptor antagonist gene, local midtrimester cytokine response to vaginal flora, and subsequent preterm birth. Am J Obstet Gynecol 2004; 191: 1324–1330
- Genc M R, Vardhana S, Delaney M L, Onderdonk A, Tuomala R, Norwitz E, Witkin S S, MAP Study Group. Relationship between a toll-like receptor-4 gene polymorphism, bacterial vaginosis-related flora and vaginal cytokine responses in pregnant women. Eur J Obstet Gynecol Reprod Biol 2004; 116: 152–156
- Varner M W, Esplin M S. Current understanding of genetic factors in preterm birth. BJOG 2005; 112((Suppl 1))28–31
- Esplin M S, Varner M W. Genetic factors in preterm birth–the future. BJOG 2005; 112((Suppl 1))97–102
- Menon R, Fortunato S J, Thorsen P, Williams S. Genetic associations in preterm birth: a primer of marker selection, study design, and data analysis. J Soc Gynecol Investig 2006; 13: 531–541
- Esplin M S. Preterm birth: a review of genetic factors and future directions for genetic study. Obstet Gynecol Surv 2006; 61: 800–806
- Menon R, Merialdi M, Betran A P, Dolan S, Jiang L, Fortunato S J, Williams S. Analysis of association between maternal tumor necrosis factor-alpha promoter polymorphism (-308), tumor necrosis factor concentration, and preterm birth. Am J Obstet Gynecol 2006; 195: 1240–1248
- Wang H, Parry S, Macones G, Sammel M D, Kuivaniemi H, Tromp G, Argyropoulos G, Halder I, Shriver M D, Romero R, et al. A functional SNP in the promoter of the SERPINH1 gene increases risk of preterm premature rupture of membranes in African Americans. Proc Natl Acad Sci USA 2006; 103: 13463–13467
- DeFranco E, Teramo K, Muglia L. Genetic influences on preterm birth. Semin Reprod Med 2007; 25: 40–51
- Nesin M. Genetic basis of preterm birth. Front Biosci 2007; 12: 115–124
- Hollegaard M V, Grove J, Thorsen P, Wang X, Mandrup S, Christiansen M, Norgaard-Pedersen B, Wojdemann K R, Tabor A, Attermann J, et al. Polymorphisms in the tumor necrosis factor alpha and interleukin 1-beta promoters with possible gene regulatory functions increase the risk of preterm birth. Acta Obstet Gynecol Scand 2008; 87: 1285–1290
- Plunkett J, Muglia L J. Genetic contributions to preterm birth: implications from epidemiological and genetic association studies. Ann Med 2008; 40: 167–195
- Velez D R, Fortunato S, Thorsen P, Lombardi S J, Williams S M, Menon R. Spontaneous preterm birth in African Americans is associated with infection and inflammatory response gene variants. Am J Obstet Gynecol 2009; 200: e1–e27
- Velez D R, Fortunato S J, Thorsen P, Lombardi S J, Williams S M, Menon R. Preterm birth in Caucasians is associated with coagulation and inflammation pathway gene variants. PLoS ONE 2008; 3: e3283
- Menon R, Velez D R, Morgan N, Lombardi S J, Fortunato S J, Williams S M. Genetic regulation of amniotic fluid TNF-alpha and soluble TNF receptor concentrations affected by race and preterm birth. Hum Genet 2008; 124: 243–253
- Anum E A, Springel E H, Shriver M D, Strauss J F, III. Genetic contributions to disparities in preterm birth. Pediatr Res 2009; 65: 1–9
- Holst D, Garnier Y. Preterm birth and inflammation-The role of genetic polymorphisms. Eur J Obstet Gynecol Reprod Biol 2008; 141: 3–9
- Uma R, Forsyth J S, Struthers A D, Fraser C G, Godfrey V, Murphy D J. Correlation of angiotensin converting enzyme activity and the genotypes of the I/D polymorphism in the ACE gene with preterm birth and birth weight. Eur J Obstet Gynecol Reprod Biol 2008; 141: 27–30
- Wang H, Sammel M D, Tromp G, Gotsch F, Halder I, Shriver M D, Romero R, Strauss J F, 3rd. A 12-bp deletion in the 5′-flanking region of the SERPINH1 gene affects promoter activity and protects against preterm premature rupture of membranes in African Americans. Hum Mutat 2008; 29: 332
- Simhan H N, Ryckman K K, Williams S M, Krohn M A. Genetic regulation of cervical anti-inflammatory cytokine concentrations during pregnancy. Am J Obstet Gynecol 2008; 199: 163
- Suh Y J, Ha E H, Park H, Kim Y J, Kim H, Hong Y C. GSTM1 polymorphism along with PM10 exposure contributes to the risk of preterm delivery. Mutat Res 2008; 656: 62–67
- Himes K P, Simhan H N. Genetic susceptibility to infection-mediated preterm birth. Infect Dis Clin North Am 2008; 22: 741–753, vii
- Reiman M, Kujari H, Ekholm E, Lapinleimu H, Lehtonen L, Haataja L. Interleukin-6 polymorphism is associated with chorioamnionitis and neonatal infections in preterm infants. J Pediatr 2008; 153: 19–24
- Romero R, Espinoza J, Gotsch F, Kusanovic J P, Friel L A, Erez O, Mazaki-Tovi S, Than N G, Hassan S, Tromp G. The use of high-dimensional biology (genomics, transcriptomics, proteomics, and metabolomics) to understand the preterm parturition syndrome. BJOG 2006; 113((Suppl 3))118–135
- Romero R, Espinoza J, Kusanovic J P, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG 2006; 113((Suppl 3))17–42
- Andrews W W, Hauth J C, Goldenberg R L, Gomez R, Romero R, Cassell G H. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol 1995; 173: 606–612
- Arntzen K J, Kjollesdal A M, Halgunset J, Vatten L, Austgulen R. TNF, IL-1, IL-6, IL-8 and soluble TNF receptors in relation to chorioamnionitis and premature labor. J Perinat Med 1998; 26: 17–26
- Athayde N, Edwin S S, Romero R, Gomez R, Maymon E, Pacora P, Menon R. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am J Obstet Gynecol 1998; 179: 1248–1253
- Athayde N, Romero R, Gomez R, Maymon E, Pacora P, Mazor M, Yoon B H, Fortunato S, Menon R, Ghezzi F, et al. Matrix metalloproteinases-9 in preterm and term human parturition. J Matern Fetal Med 1999; 8: 213–219
- Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Araneda H, Yoon B H. A role for the novel cytokine RANTES in pregnancy and parturition. Am J Obstet Gynecol 1999; 181: 989–994
- Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Yoon B H, Edwin S S. Interleukin 16 in pregnancy, parturition, rupture of fetal membranes, and microbial invasion of the amniotic cavity. Am J Obstet Gynecol 2000; 182: 135–141
- Baggia S, Gravett M G, Witkin S S, Haluska G J, Novy M J. Interleukin-1 beta intra-amniotic infusion induces tumor necrosis factor-alpha, prostaglandin production, and preterm contractions in pregnant rhesus monkeys. J Soc Gynecol Investig 1996; 3: 121–126
- Bashiri A, Horowitz S, Huleihel M, Hackmon R, Dukler D, Mazor M. Elevated concentrations of interleukin-6 in intra-amniotic infection with Ureaplasma urealyticum in asymptomatic women during genetic amniocentesis. Acta Obstet Gynecol Scand 1999; 78: 379–382
- Chaiworapongsa T, Romero R, Kim J C, Kim Y M, Blackwell S C, Yoon B H, Gomez R. Evidence for fetal involvement in the pathologic process of clinical chorioamnionitis. Am J Obstet Gynecol 2002; 186: 1178–1182
- Chaiworapongsa T, Romero R, Tolosa J E, Yoshimatsu J, Espinoza J, Kim Y M, Kim J C, Bujold E, Kalache K, Edwin S. Elevated monocyte chemotactic protein-1 in amniotic fluid is a risk factor for pregnancy loss. J Matern Fetal Neonatal Med 2002; 12: 159–164
- Chaiworapongsa T, Espinoza J, Kim Y M, Edwin S, Bujold E, Berman S, et al. A novel mediator of septic shock, macrophage migration inhibitory factor, is increased in intra-amniotic infection. Am J Obstet Gynecol 2002; 187: S73
- Chaiworapongsa T, Romero R, Espinoza J, Kim Y M, Edwin S, Bujold E, Gomez R, Kuivaniemi H. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med 2005; 18: 405–416
- Chaiworapongsa T, Hong J S, Hull W M, Kim C J, Gomez R, Mazor M, Romero R, Whitsett J A. The concentration of surfactant protein-A in amniotic fluid decreases in spontaneous human parturition at term. J Matern Fetal Neonatal Med 2008; 21: 652–659
- Chaiworapongsa T, Erez O, Kusanovic J P, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Than N G, Mittal P, Kim Y M, Camacho N, et al. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med 2008; 21: 449–461
- Chaiworapongsa T, Hong J S, Hull W M, Romero R, Whitsett J A. Amniotic fluid concentration of surfactant proteins in intra-amniotic infection. J Matern Fetal Neonatal Med 2008; 21: 663–670
- Fidel P I, Jr, Romero R, Maymon E, Hertelendy F. Bacteria-induced or bacterial product-induced preterm parturition in mice and rabbits is preceded by a significant fall in serum progesterone concentrations. J Matern Fetal Med 1998; 7: 222–226
- Fidel P L, Jr, Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, Cotton D B. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol 1994; 170: 1467–1475
- Fidel P L, Jr, Romero R, Cutright J, Wolf N, Gomez R, Araneda H, Ramirez M, Yoon B H. Treatment with the interleukin-I receptor antagonist and soluble tumor necrosis factor receptor Fc fusion protein does not prevent endotoxin-induced preterm parturition in mice. J Soc Gynecol Investig 1997; 4: 22–26
- Ghezzi F, Gomez R, Romero R, Yoon B H, Edwin S S, David C, Janisse J, Mazor M. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol 1998; 78: 5–10
- Ghezzi F, Franchi M, Raio L, Di Naro E, Bossi G, D'Eril G V, Bolis P. Elevated amniotic fluid C-reactive protein at the time of genetic amniocentesis is a marker for preterm delivery. Am J Obstet Gynecol 2002; 186: 268–273
- Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton D B. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol 1994; 32: 200–210
- Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa J E, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol 1995; 22: 281–342
- Gomez R, Ghezzi F, Romero R, et al. Two thirds of human fetuses with microbial invasion of the amniotic cavity have a detectable systemic cytokine response before birth. Am J Obstet Gynecol 1997; 176: S14
- Goncalves L F, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev 2002; 8: 3–13
- Gotsch F, Romero R, Kusanovic J P, Erez O, Espinoza J, Kim C J, Vaisbuch E, Than N G, Mazaki-Tovi S, Chaiworapongsa T, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med 2008; 21: 529–547
- Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, Kusanovic J P, Mittal P, Mazaki-Tovi S, Kim C J, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med 2008; 21: 605–616
- Gravett M G, Sadowsky D, Witkin M, Novy M. Immunomodulators plus antibiotics to prevent preterm delivery in experimental intra-amniotic infection (IAI). Am J Obstet Gynecol 2003; 189: S56
- Gravett M G, Hummel D, Eschenbach D A, Holmes K K. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol 1986; 67: 229–237
- Gravett M G, Witkin S S, Haluska G J, Edwards J L, Cook M J, Novy M J. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol 1994; 171: 1660–1667
- Gravett M G, Haluska G J, Cook M J, Novy M J. Fetal and maternal endocrine responses to experimental intrauterine infection in rhesus monkeys. Am J Obstet Gynecol 1996; 174: 1725–1731
- Gravett M G, Hitti J, Hess D L, Eschenbach D A. Intrauterine infection and preterm delivery: evidence for activation of the fetal hypothalamic-pituitary-adrenal axis. Am J Obstet Gynecol 2000; 182: 1404–1413
- Gray D J, Robinson H B, Malone J, Thomson R B, Jr. Adverse outcome in pregnancy following amniotic fluid isolation of Ureaplasma urealyticum. Prenat Diagn 1992; 12: 111–117
- Grigsby P L, Hirst J J, Scheerlinck J P, Phillips D J, Jenkin G. Fetal responses to maternal and intra-amniotic lipopolysaccharide administration in sheep. Biol Reprod 2003; 68: 1695–1702
- Gross G, Imamura T, Vogt S K, Wozniak D F, Nelson D M, Sadovsky Y, Muglia L J. Inhibition of cyclooxygenase-2 prevents inflammation-mediated preterm labor in the mouse. Am J Physiol Regul Integr Comp Physiol 2000; 278: R1415–R1423
- Hanna N, Bonifacio L, Weinberger B, Reddy P, Murphy S, Romero R, Sharma S. Evidence for interleukin-10-mediated inhibition of cyclo-oxygenase-2 expression and prostaglandin production in preterm human placenta. Am J Reprod Immunol 2006; 55: 19–27
- Heine R P, Wiesenfeld H, Mortimer L, Greig P C. Amniotic fluid defensins: potential markers of subclinical intrauterine infection. Clin Infect Dis 1998; 27: 513–518
- Helmig B R, Romero R, Espinoza J, Chaiworapongsa T, Bujold E, Gomez R, Ohlsson K, Uldbjerg N. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med 2002; 12: 237–246
- Hertelendy F, Rastogi P, Molnar M, Romero R. Interleukin-1beta-induced prostaglandin E2 production in human myometrial cells: role of a pertussis toxin-sensitive component. Am J Reprod Immunol 2001; 45: 142–147
- Hillier S L, Krohn M A, Kiviat N B, Watts D H, Eschenbach D A. Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am J Obstet Gynecol 1991; 165: 955–961
- Hillier S L, Witkin S S, Krohn M A, Watts D H, Kiviat N B, Eschenbach D A. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol 1993; 81: 941–948
- Hirsch E, Saotome I, Hirsh D. A model of intrauterine infection and preterm delivery in mice. Am J Obstet Gynecol 1995; 172: 1598–1603
- Hirsch E, Blanchard R, Mehta S P. Differential fetal and maternal contributions to the cytokine milieu in a murine model of infection-induced preterm birth. Am J Obstet Gynecol 1999; 180: 429–434
- Hirsch E, Muhle R A, Mussalli G M, Blanchard R. Bacterially induced preterm labor in the mouse does not require maternal interleukin-1 signaling. Am J Obstet Gynecol 2002; 186: 523–530
- Hirsch E, Muhle R. Intrauterine bacterial inoculation induces labor in the mouse by mechanisms other than progesterone withdrawal. Biol Reprod 2002; 67: 1337–1341
- Hirsch E, Muhle R A, Mussalli G M, Blanchard R. Bacterially induced preterm labor in the mouse does not require maternal interleukin-1 signaling. Am J Obstet Gynecol 2002; 186: 523–530
- Hirsch E, Filipovich Y, Mahendroo M. Signalling via the type I IL-1 and TNF receptors is necessary for bacterially induced preterm labor in a murine model. Am J Obstet Gynecol 2006; 194(5)1334–1340
- Hirsch E, Wang H. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc Gynecol Investig 2005; 12: 145–155
- Jeffcoat M K, Geurs N C, Reddy M S, Cliver S P, Goldenberg R L, Hauth J C. Periodontal infection and preterm birth: results of a prospective study. J Am Dent Assoc 2001; 132: 875–880
- Kim Y M, Romero R, Chaiworapongsa T, Kim G A, Kim M R, Kuivaniemi H, Tromp G, Espinoza J, Bujold E, Abrahams V M, et al. Toll-like receptor-2 and-4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol 2004; 191: 1346–1355
- Krueger M, Nauck M S, Sang S, Hentschel R, Wieland H, Berner R. Cord blood levels of interleukin-6 and interleukin-8 for the immediate diagnosis of early-onset infection in premature infants. Biol Neonate 2001; 80: 118–123
- Kusanovic J P, Romero R, Mazaki-Tovi S, Chaiworapongsa T, Mittal P, Gotsch F, Erez O, Vaisbuch E, Edwin S S, Than N G, et al. Resistin in amniotic fluid and its association with intra-amniotic infection and inflammation. J Matern Fetal Neonatal Med 2008; 21: 902–916
- Lee S E, Romero R, Kim C J, Shim S S, Yoon B H. Funisitis in term pregnancy is associated with microbial invasion of the amniotic cavity and intra-amniotic inflammation. J Matern Fetal Neonatal Med 2006; 19: 693–697
- Lee S E, Romero R, Park I S, Seong H S, Park C W, Yoon B H. Amniotic fluid prostaglandin concentrations increase before the onset of spontaneous labor at term. J Matern Fetal Neonatal Med 2008; 21: 89–94
- Maymon E, Ghezzi F, Edwin S S, Mazor M, Yoon B H, Gomez R, Romero R. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am J Obstet Gynecol 1999; 181: 1142–1148
- Maymon E, Romero R, Pacora P, Gervasi M T, Edwin S S, Gomez R, Seubert D E. Matrilysin (matrix metalloproteinase 7) in parturition, premature rupture of membranes, and intrauterine infection. Am J Obstet Gynecol 2000; 182: 1545–1553
- Maymon E, Romero R, Pacora P, Gomez R, Athayde N, Edwin S, Yoon B H. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol 2000; 183: 94–99
- Maymon E, Romero R, Pacora P, Gervasi M T, Gomez R, Edwin S S, Yoon B H. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intra-amniotic infection. Am J Obstet Gynecol 2000; 183: 887–894
- Blank V, Hirsch E, Challis J R, Romero R, Lye S J. Cytokine signalling, inflammation, innate immunity and preterm labour – a workshop report. Placenta 2008; 29(Suppl A)S102–S104
- Maymon E, Romero R, Pacora P, Gervasi M T, Bianco K, Ghezzi F, Yoon B H. Evidence for the participation of interstitial collagenase (matrix metalloproteinase 1) in preterm premature rupture of membranes. Am J Obstet Gynecol 2000; 183: 914–920
- Maymon E, Romero R, Chaiworapongsa T, Berman S, Conoscenti G, Gomez R, Edwin S. Amniotic fluid matrix metalloproteinase-8 in preterm labor with intact membranes. Am J Obstet Gynecol 2001; 185: 1149–1155
- Maymon E, Romero R, Pacora P, Gomez R, Mazor M, Edwin S, Chaiworapongsa T, Kim J C, Yoon B H, Menon R, et al. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J Perinat Med 2001; 29: 308–316
- Maymon E, Romero R, Chaiworapongsa T, Kim J C, Berman S, Gomez R, Edwin S. Value of amniotic fluid neutrophil collagenase concentrations in preterm premature rupture of membranes. Am J Obstet Gynecol 2001; 185: 1143–1148
- Simhan H N, MacPherson T, Caritis S N, Krohn M A. Maternal and fetal Toll-like receptor 4 genotype and chorionic plate inflammatory lesions. Am J Obstet Gynecol 2008; 199(4)400e1–400e4
- Mazor M, Hershkovitz R, Ghezzi F, Maymon E, Horowitz S, Leiberman J R. Intraamniotic infection in patients with preterm labor and twin pregnancies. Acta Obstet Gynecol Scand 1996; 75: 624–627
- McDuffie R S, Jr, Blanton S J, Shikes R H, Gibbs R S, et al. A rabbit model for bacterially induced preterm pregnancy loss: intervention studies with ampicillin-sulbactam. Am J Obstet Gynecol 1991; 165: 1568–1574
- McDuffie R S, Jr, Sherman M P, Gibbs R S. Amniotic fluid tumor necrosis factor-alpha and interleukin-1 in a rabbit model of bacterially induced preterm pregnancy loss. Am J Obstet Gynecol 1992; 167: 1583–1588
- McDuffie R S, Jr, Davies J K, Leslie K K, Lee S, Sherman M P, Gibbs R S. A randomized controlled trial of interleukin-1 receptor antagonist in a rabbit model of ascending infection in pregnancy. Infect Dis Obstet Gynecol 2001; 9: 233–237
- Menon R, Fortunato S J. Fetal membrane inflammatory cytokines: a switching mechanism between the preterm premature rupture of the membranes and preterm labor pathways. J Perinat Med 2004; 32: 391–399
- Minkoff H. Prematurity: infection as an etiologic factor. Obstet Gynecol 1983; 62: 137–144
- Minkoff H, Grunebaum A N, Schwarz R H, Feldman J, Cummings M, Crombleholme W, Clark L, Pringle G, McCormack W M. Risk factors for prematurity and premature rupture of membranes: a prospective study of the vaginal flora in pregnancy. Am J Obstet Gynecol 1984; 150: 965–972
- Moss T J, Nitsos I, Ikegami M, Jobe A H, Newnham J P. Experimental intrauterine Ureaplasma infection in sheep. Am J Obstet Gynecol 2005; 192: 1179–1186
- Nhan-Chang C L, Romero R, Kusanovic J P, Gotsch F, Edwin S S, Erez O, Mittal P, Kim C J, Kim M J, Espinoza J, et al. A role for CXCL13 (BCA-1) in pregnancy and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2008; 21: 763–775
- Offenbacher S, Beck J D, Lieff S, Slade G. Role of periodontitis in systemic health: spontaneous preterm birth. J Dent Educ 1998; 62: 852–858
- Offenbacher S, Lieff S, Boggess K A, Murtha A P, Madianos P N, Champagne C M, McKaig R G, Jared H L, Mauriello S M, Auten R L, Jr, et al. Maternal periodontitis and prematurity. Part I: Obstetric outcome of prematurity and growth restriction. Ann Periodontol 2001; 6: 164–174
- Offenbacher S, Boggess K A, Murtha A P, Jared H L, Lieff S, McKaig R G, Mauriello S M, Moss K L, Beck J D. Progressive periodontal disease and risk of very preterm delivery. Obstet Gynecol 2006; 107: 29–36
- Okawa T, Suzuki H, Yaanagida K, Sato A, Vedernikov Y, Saade G, Garfield R. Effect of lipopolysaccharide on uterine contractions and prostaglandin production in pregnant rats. Am J Obstet Gynecol 2001; 184: 84–89
- Pacora P, Maymon E, Gervasi M T, Gomez R, Edwin S S, Yoon B H, Romero R. Lactoferrin in intrauterine infection, human parturition, and rupture of fetal membranes. Am J Obstet Gynecol 2000; 183: 904–910
- Pacora P, Chaiworapongsa T, Maymon E, Kim Y M, Gomez R, Yoon B H, Ghezzi F, Berry S M, Qureshi F, Jacques S M, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Med 2002; 11: 18–25
- Park K H, Chaiworapongsa T, Kim Y M, Espinoza J, Yoshimatsu J, Edwin S, Gomez R, Yoon B H, Romero R. Matrix metalloproteinase 3 in parturition, premature rupture of the membranes, and microbial invasion of the amniotic cavity. J Perinat Med 2003; 31: 12–22
- Holt D, Garnier Y. Preterm birth and inflammation. The role of genetic polymorphisms. Eur J Obstet Gynecol Reprod Biol 2008; 141(141)3–9
- Romero R, Emamian M, Quintero R, Wan M, Hobbins J C, Mitchell M D. Amniotic fluid prostaglandin levels and intra-amniotic infections. Lancet 1986; 1: 1380
- Romero R, Durum S K, Dinarello C A, et al. Interleukin-1: A signal for the initiation of labor in chorioamnionitis. 33rd Annual Meeting for the Society for Gynecologic Investigation, Toronto, OntarioCanada, 1986
- Romero R, Kadar N, Hobbins J C, Duff G W. Infection and labor: the detection of endotoxin in amniotic fluid. Am J Obstet Gynecol 1987; 157: 815–819
- Romero R, Emamian M, Wan M, Quintero R, Hobbins J C, Mitchell M D. Prostaglandin concentrations in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol 1987; 157: 1461–1467
- Romero R, Quintero R, Emamian M, Wan M, Grzyboski C, Hobbins J C, Mitchell M D. Arachidonate lipoxygenase metabolites in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol 1987; 157: 1454–1460
- Romero R, Mazor M, Wu Y K, Sirtori M, Oyarzun E, Mitchell M D, Hobbins J C. Infection in the pathogenesis of preterm labor. Semin Perinatol 1988; 12: 262–279
- Romero R, Hobbins J C, Mitchell M D. Endotoxin stimulates prostaglandin E2 production by human amnion. Obstet Gynecol 1988; 71: 227–228
- Romero R, Quintero R, Oyarzun E, Wu Y K, Sabo V, Mazor M, Hobbins J C. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol 1988; 159: 661–666
- Romero R, Manogue K R, Mitchell M D, Wu Y K, Oyarzun E, Hobbins J C, Cerami A. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol 1989; 161: 336–341
- Romero R, Mazor M, Wu Y K, Avila C, Oyarzun E, Mitchell M D. Bacterial endotoxin and tumor necrosis factor stimulate prostaglandin production by human decidua. Prostaglandins Leukot Essent Fatty Acids 1989; 37: 183–186
- Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis A P, Hobbins J C. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol 1989; 161: 817–824
- Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbins J C, Bracken M. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol 1989; 73: 576–582
- Romero R, Brody D T, Oyarzun E, Mazor M, Wu Y K, Hobbins J C, Durum S K. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol 1989; 160: 1117–1123
- McElrath T F, Hecht J L, Dammann O, Boggess K, Onderdonk A, Markenson G, Harper M, Delpapa E, Allred E N, Leviton A, ELGAN Study Investigators. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol 2008; 168(9)980–989
- Romero R, Wu Y K, Oyarzun E, Hobbins J C, Mitchell M D. A potential role for epidermal growth factor/alpha-transforming growth factor in human parturition. Eur J Obstet Gynecol Reprod Biol 1989; 33: 55–60
- Romero R, Durum S, Dinarello C A, Oyarzun E, Hobbins J C, Mitchell M D. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins 1989; 37: 13–22
- Romero R, Wu Y K, Brody D T, Oyarzun E, Duff G W, Durum S K. Human decidua: a source of interleukin-1. Obstet Gynecol 1989; 73: 31–34
- Romero R, Avila C, Santhanam U, Sehgal P B. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest 1990; 85: 1392–1400
- Romero R, Parvizi S T, Oyarzun E, Mazor M, Wu Y K, Avila C, Athanassiadis A P, Mitchell M D. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med 1990; 35: 235–238
- Zahl P A, Bjerknes C. Induction of decidua-placental hemorrhage in mice by the endotoxins of certain gram-negative bacteria. Proc Soc Exper Biol Med 1943; 54: 329–332
- Takeda Y, Tsuchiya I. Studies on the pathological changes caused by the injection of the Shwartzman filtrate and the endotoxin into pregnant rabbits. Jap J Exper Med 1953; 21: 9–16
- McKay D G, Wong T C. The effect of bacterial endotoxin on the placenta of the rat. Am J Pathol 1963; 42: 357–377
- Himes K P, Simhan H N. Risk of recurrent preterm birth and placental pathology. Obstet Gynecol 2008; 112(1)121–126
- Romero R, Shamma F, Avila C, Jimenez C, Callahan R, Nores J, et al. Infection and labor. VI. Prevalence, microbiology, and clinical significance of intraamniotic infection in twin gestations with preterm labor. Am J Obstet Gynecol 1990; 163: 757–761
- Romero R, Salafia C M, Athanassiadis A P, Hanaoka S, Mazor M, Sepulveda W, Bracken M D. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am J Obstet Gynecol 1992; 166: 1382–1388
- Romero R, Gonzalez R, Sepulveda W, Brandt F, Ramirez M, Sorokin Y, Mazor M, Treadwell M C, Cotton D B. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol 1992; 167: 1086–1091
- Romero R, Munoz H, Gomez R, Ramirez M, Araneda H, Cutright J, Wolf N, Cotton D, Fidel P L. Antibiotic therapy reduces the rate of infection-induced preterm delivery and perinatal mortality. Am J Obstet Gynecol 1994; 170: 390
- Romero R, Munoz H, Gomez R, Sherer D M, Ghezzi F, Gibbs R S, et al. Two thirds of spontaneous abortion/fetal deaths after genetic amniocentesis are the result of a pre-existing sub-clinical inflammatory process of the amniotic cavity. Am J Obstet Gynecol 1995; 172: S261
- Wenstrom K D, Andrews W W, Tamura T, DuBard M B, Johnston K E, Hemstreet G P. Elevated amniotic fluid interleukin-6 levels at genetic amniocentesis predict subsequent pregnancy loss. Am J Obstet Gynecol 1996; 175: 830–833
- Kaul A K, Khan S, Martens M G, Crosson J T, Lupo V R, Kaul R. Experimental gestational pyelonephritis induces preterm births and low birth weights in C3H/HeJ mice. Infect Immun 1999; 67: 5958–5966
- Jeffcoat M K, Geurs N C, Reddy M S, Goldenberg R L, Hauth J C. Current evidence regarding periodontal disease as a risk factor in preterm birth. Ann Periodontol 2001; 6: 183–188
- Yoon B H, Oh S Y, Romero R, Shim S S, Han S Y, Park J S, Jun J K. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol 2001; 185: 1162–1167
- Wang H, Hirsch E. Bacterially-induced preterm labor and regulation of prostaglandin-metabolizing enzyme expression in mice: the role of toll-like receptor 4. Biol Reprod 2003; 69: 1957–1963
- Fidel P, Ghezzi F, Romero R, Chaiworapongsa T, Espinoza J, Cutright J, Wolf N, Gomez R. The effect of antibiotic therapy on intrauterine infection-induced preterm parturition in rabbits. J Matern Fetal Neonatal Med 2003; 14: 57–64
- Elovitz M A, Mrinalini C. Animal models of preterm birth. Trends Endocrinol Metab 2004; 15: 479–487
- Gibbs R S, McDuffie R S, Jr, Kunze M, Barr J M, Wolf D M, Sze C I, Shikes R, Sherman M P. Experimental intrauterine infection with Prevotella bivia in New Zealand White rabbits. Am J Obstet Gynecol 2004; 190: 1082–1086
- Goepfert A R, Jeffcoat M K, Andrews W W, Faye-Petersen O, Cliver S P, Goldenberg R L, Hauth J C. Periodontal disease and upper genital tract inflammation in early spontaneous preterm birth. Obstet Gynecol 2004; 104: 777–783
- Xiong X, Buekens P, Fraser W D, Beck J, Offenbacher S. Periodontal disease and adverse pregnancy outcomes: a systematic review. BJOG 2006; 113: 135–143
- Romero R, Avila C, Brekus C A, Morotti R. The role of systemic and intrauterine infection in preterm parturition. Ann N Y Acad Sci 1991; 622: 355–375
- Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol 1991; 165: 813–820
- Romero R, Mazor M, Manogue K, Oyarzun E, Cerami A. Human decidua: a source of cachectin-tumor necrosis factor. Eur J Obstet Gynecol Reprod Biol 1991; 41: 123–127
- Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol 1991; 165: 969–971
- Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol 1992; 166: 1576–1587
- Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton D B, Dinarello C A. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol 1992; 27: 117–123
- Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol 1992; 167: 1041–1045
- Romero R, Sepulveda W, Kenney J S, Archer L E, Allison A C, Sehgal P B. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp 1992; 167: 205–220
- Romero R, Sepulveda W, Mazor M, Brandt F, Cotton D B, Dinarello C A, Mitchell M D. The natural interleukin-1 receptor antagonist in term and preterm parturition. Am J Obstet Gynecol 1992; 167: 863–872
- Romero R, Mazor M, Morrotti R, Avila C, Oyarzun E, Insunza A, Parra M, Behnke E, Montiel F, Cassell G H. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol 1992; 166: 129–133
- Romero R, Sepulveda W, Baumann P, Yoon B H, Brandt F, Gomez R, Mazor M, Sorokin Y, Cotton D. The preterm labor syndrome: Biochemical, cytologic, immunologic, pathologic, microbiologic, and clinical evidence that preterm labor is a heterogeneous disease. Am J Obstet Gynecol 1993; 168: 288
- Romero R, Nores J, Mazor M, Sepulveda W, Oyarzun E, Parra M, Insunza A, Montiel F, Behnke E, Cassell G H. Microbial invasion of the amniotic cavity during term labor. Prevalence and clinical significance. J Reprod Med 1993; 38: 543–548
- Romero R, Yoon B H, Kenney J S, Gomez R, Allison A C, Sehgal P B. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol 1993; 30: 167–183
- Romero R, Yoon B H, Gonzalez R, Brandt F, Sepulveda W, Gomez R, et al. The clinical significance of microbial invasion of the amniotic cavity with mycoplasmas in patients with preterm PROM. Presented at the 40th Annual Meeting of the Society for Gynecologic Investigation, 31 March–3 April, 1993, Toronto, Ontario, Canada, p70, Abstract S4
- Romero R, Baumann P, Gomez R, Salafia C, Rittenhouse L, Barberio D, Behnke E, Cotton D B, Mitchell M D. The relationship between spontaneous rupture of membranes, labor, and microbial invasion of the amniotic cavity and amniotic fluid concentrations of prostaglandins and thromboxane B2 in term pregnancy. Am J Obstet Gynecol 1993; 168: 1654–1664
- Romero R, Sibai B, Caritis S, Paul R, Depp R, Rosen M, Klebanoff M, Sabo V, Evans J, Thom E, et al. Antibiotic treatment of preterm labor with intact membranes: a multicenter, randomized, double-blinded, placebo-controlled trial. Am J Obstet Gynecol 1993; 169: 764–774
- Romero R, Avila C, Sepulveda W, . The role of systemic and intrauterine infection in preterm labor. Preterm Birth: Causes, Prevention, and Management, A Fuchs, F Fuchs, P Stubblefield, et al. McGraw-Hill, New York 1993
- Romero R, Gomez R, Galasso M, Munoz H, Acosta L, Yoon B H, Svinarich D, Cotton D B. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol 1994; 32: 108–113
- Romero R, Gomez R, Galasso M, Mazor M, Berry S M, Quintero R A, Cotton D B. The natural interleukin-1 receptor antagonist in the fetal, maternal, and amniotic fluid compartments: the effect of gestational age, fetal gender, and intrauterine infection. Am J Obstet Gynecol 1994; 171: 912–921
- Romero R, Baumann P, Gonzalez R, Gomez R, Rittenhouse L, Behnke E, Mitchell M D. Amniotic fluid prostanoid concentrations increase early during the course of spontaneous labor at term. Am J Obstet Gynecol 1994; 171: 1613–1620
- Romero R, Munoz H, Gomez R, Parra M, Polanco M, Valverde V, Hasbun J, Garrido J, Ghezzi F, Mazor M, et al. Increase in prostaglandin bioavailability precedes the onset of human parturition. Prostaglandins Leukot Essent Fatty Acids 1996; 54: 187–191
- Romero R, Gomez R, Ghezzi F, Yoon B H, Mazor M, Edwin S S, Berry S M. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol 1998; 179: 186–193
- Romero R, Athayde N, Gomez R, Mazor M, Yoon B H, Edwin S, et al. The fetal inflammatory response syndrome is characterized by the outpouring of a potent extracellular matrix degrading enzyme into the fetal circulation. Am J Obstet Gynecol 1998; 178: S3
- Romero R, Athayde N, Maymon E, Pacora P, Bahado-Singh R. Premature rupture of the membranes. Medicine of the Fetus and Mother, A Reece, J Hobbins. JB Lippincott, Philadelphia 1998; 1581–1625
- Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol 2002; 7: 259–274
- Romero R, Chaiworapongsa T, Espinoza J, Gomez R, Yoon B H, Edwin S, Mazor M, Maymon E, Berry S. Fetal plasma MMP-9 concentrations are elevated in preterm premature rupture of the membranes. Am J Obstet Gynecol 2002; 187: 1125–1130
- Romero R, Kuivaniemi H, Tromp G. Functional genomics and proteomics in term and preterm parturition. J Clin Endocrinol Metab 2002; 87: 2431–2434
- Romero R, Chaiworapongsa T, Kuivaniemi H, Tromp G. Bacterial vaginosis, the inflammatory response and the risk of preterm birth: a role for genetic epidemiology in the prevention of preterm birth. Am J Obstet Gynecol 2004; 190: 1509–1519
- Romero R, Espinoza J, Rogers W T, Moser A, Nien J K, Kusanovic J P, Gotsch F, Erez O, Gomez R, Edwin S, et al. Proteomic analysis of amniotic fluid to identify women with preterm labor and intra-amniotic inflammation/infection: the use of a novel computational method to analyze mass spectrometric profiling. J Matern Fetal Neonatal Med 2008; 21: 367–388
- Silver R M, Schwinzer B, McGregor J A. Interleukin-6 levels in amniotic fluid in normal and abnormal pregnancies: preeclampsia, small-for-gestational-age fetus, and premature labor. Am J Obstet Gynecol 1993; 169: 1101–1105
- Smulian J C, Bhandari V, Campbell W A, Rodis J F, Vintzileos A M. Value of umbilical artery and vein levels of interleukin-6 and soluble intracellular adhesion molecule-1 as predictors of neonatal hematologic indices and suspected early sepsis. J Matern Fetal Med 1997; 6: 254–259
- Soto E, Espinoza J, Nien J K, Kusanovic J P, Erez O, Richani K, Santolaya-Forgas J, Romero R. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med 2007; 20: 15–22
- Steel J H, O'donoghue K, Kennea N L, Sullivan M H, Edwards A D. Maternal origin of inflammatory leukocytes in preterm fetal membranes, shown by fluorescence in situ hybridisation. Placenta 2005; 26: 672–677
- Svinarich D M, Wolf N A, Gomez R, Gonik B, Romero R. Detection of human defensin 5 in reproductive tissues. Am J Obstet Gynecol 1997; 176: 470–475
- Tauscher M K, Berg D, Brockmann M, Seidenspinner S, Speer C P, Groneck P. Association of histologic chorioamnionitis, increased levels of cord blood cytokines, and intracerebral hemorrhage in preterm neonates. Biol Neonate 2003; 83: 166–170
- Terrone D A, Rinehart B K, Granger J P, Barrilleaux P S, Martin J N, Jr, Bennett W A. Interleukin-10 administration and bacterial endotoxin-induced preterm birth in a rat model. Obstet Gynecol 2001; 98: 476–480
- Vadillo-Ortega F, Hernandez A, Gonzalez-Avila G, Bermejo L, Iwata K, Strauss J F, III. Increased matrix metalloproteinase activity and reduced tissue inhibitor of metalloproteinases-1 levels in amniotic fluids from pregnancies complicated by premature rupture of membranes. Am J Obstet Gynecol 1996; 174: 1371–1376
- Vadillo-Ortega F, Sadowsky D W, Haluska G J, Hernandez-Guerrero C, Guevara-Silva R, Gravett M G, Novy M J. Identification of matrix metalloproteinase-9 in amniotic fluid and amniochorion in spontaneous labor and after experimental intrauterine infection or interleukin-1 beta infusion in pregnant rhesus monkeys. Am J Obstet Gynecol 2002; 186: 128–138
- Vogel I, Thorsen P, Curry A, Sandager P, Uldbjerg N. Biomarkers for the prediction of preterm delivery. Acta Obstet Gynecol Scand 2005; 84: 516–525
- Watari M, Watari H, DiSanto M E, Chacko S, Shi G P, Strauss J F, III. Pro-inflammatory cytokines induce expression of matrix-metabolizing enzymes in human cervical smooth muscle cells. Am J Pathol 1999; 154: 1755–1762
- Wenstrom K D, Andrews W W, Hauth J C, Goldenberg R L, DuBard M B, Cliver S P. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol 1998; 178: 546–550
- Romero R, Espinoza J, Goncalves L F, Kusanovic J P, Gomez R. The fetal inflammatory response syndrome. Inflammation and pregnancy, 1st edition, D Peebles, L Myatt. Informa Healthcare. 2006; 149–172
- Yoon B H, Romero R, Kim C J, Jun J K, Gomez R, Choi J H, Syn H C. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol 1995; 172: 960–970
- Yoon B H, Romero R, Yang S H, Jun J K, Kim I O, Choi J H. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol 1996; 174: 1433–1440
- Mattar R, de Souza E, Daher S. Preterm delivery and cytokine gene polymorphisms. J Reprod Med 2006; 51(4)317–320
- Yoon B H, Kim C J, Romero R, Jun J K, Park K H, Choi S T, Chi J G. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol 1997; 177: 797–802
- Yoon B H, Romero R, Park J S, Chang J W, Kim Y A, Kim J C, Kim K S. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol 1998; 179: 1254–1260
- Yoon B H, Romero R, Park J S, Kim M, Oh S Y, Kim C J, Jun J K. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol 2000; 183: 1124–1129
- Yoon B H, Romero R, Shim J Y, Shim S S, Kim C J, Jun J K. C-reactive protein in umbilical cord blood: a simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J Matern Fetal Neonatal Med 2003; 14: 85–90
- Yoon B H, Romero R, Moon J, Chaiworapongsa T, Espinoza J, Kim Y M, Edwin S, Kim J C, Camacho N, Bujold E, et al. Differences in the fetal interleukin-6 response to microbial invasion of the amniotic cavity between term and preterm gestation. J Matern Fetal Neonatal Med 2003; 13: 32–38
- Espinoza J, Chaiworapongsa T, Romero R, Gomez R, Kim J C, Yoshimatsu J, Edwin S, Rathnasabapathy C, Yoon B H. Evidence of participation of soluble CD14 in the host response to microbial invasion of the amniotic cavity and intra-amniotic inflammation in term and preterm gestations. J Matern Fetal Neonatal Med 2002; 12: 304–312
- Espinoza J, Romero R, Chaiworapongsa T, Kim J C, Yoshimatsu J, Edwin S, Rathnasabapathy C, Tolosa J, Donnenfeld A, Craparo F, et al. Lipopolysaccharide-binding protein in microbial invasion of the amniotic cavity and human parturition. J Matern Fetal Neonatal Med 2002; 12: 313–321
- Shim S S, Romero R, Jun J K, Moon K C, Kim G, Yoon B H. C-reactive protein concentration in vaginal fluid as a marker for intra-amniotic inflammation/infection in preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2005; 18: 417–422
- Ovalle A, Romero R, Gomez R, Martinez M A, Nien J K, Ferrand P, Aspillaga C, Figueroa J. Antibiotic administration to patients with preterm labor and intact membranes: is there a beneficial effect in patients with endocervical inflammation. J Matern Fetal Neonatal Med 2006; 19: 453–464
- Gomez R, Romero R, Nien J K, Medina L, Carstens M, Kim Y M, Espinoza J, Chaiworapongsa T, Gonzalez R, Iams J D, et al. Antibiotic administration to patients with preterm premature rupture of membranes does not eradicate intra-amniotic infection. J Matern Fetal Neonatal Med 2007; 20: 167–173
- Bujold E, Romero R, Kusanovic J P, Erez O, Gotsch F, Chaiworapongsa T, Gomez R, Espinoza J, Vaisbuch E, Mee Kim T, et al. Proteomic profiling of amniotic fluid in preterm labor using two-dimensional liquid separation and mass spectrometry. J Matern Fetal Neonatal Med 2008; 21: 697–713
- Soto E, Romero R, Richani K, Espinoza J, Nien J K, Chaiworapongsa T, Santolaya-Forgas J, Edwin S, Mazor M. Anaphylatoxins in preterm and term labor. J Perinat Med 2005; 33: 306–313
- Friel L A, Romero R, Edwin S, Nien J K, Gomez R, Chaiworapongsa T, Kusanovic J P, Tolosa J E, Hassan S S, Espinoza J. The calcium binding protein, S100B, is increased in the amniotic fluid of women with intra-amniotic infection/inflammation and preterm labor with intact or ruptured membranes. J Perinat Med 2007; 35: 385–393
- Hamill N, Romero R, Gotsch F, Pedro K J, Edwin S, Erez O, Than N G, Mittal P, Espinoza J, Friel L A, et al. Exodus-1 (CCL20): evidence for the participation of this chemokine in spontaneous labor at term, preterm labor, and intrauterine infection. J Perinat Med 2008; 36: 217–227
- Romero R, Espinoza J, Hassan S, Gotsch F, Kusanovic J P, Avila C, Erez O, Edwin S, Schmidt A M. Soluble receptor for advanced glycation end products (sRAGE) and endogenous secretory RAGE (esRAGE) in amniotic fluid: modulation by infection and inflammation. J Perinat Med 2008; 36: 388–398
- Mazaki-Tovi S, Romero R, Kusanovic J P, Erez O, Gotsch F, Mittal P, Than N G, Nhan-Chang C L, Hamill N, Vaisbuch E, et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat Med 2008; 36: 485–496
- Park C W, Lee S M, Park J S, Jun J K, Romero R, Yoon B H. The antenatal identification of funisitis with a rapid MMP-8 bedside test. J Perinat Med 2008; 36: 497–502
- Nien J K, Yoon B H, Espinoza J, Kusanovic J P, Erez O, Soto E, Richani K, Gomez R, Hassan S, Mazor M, et al. A rapid MMP-8 bedside test for the detection of intra-amniotic inflammation identifies patients at risk for imminent preterm deolivery. Am J Obstet Gynecol 2006; 195: 1025–1030
- Kim K W, Romero R, Park H S, Park C W, Shim S S, Jun J K, Yoon B H. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with premature rupture of membranes. Am J Obstet Gynecol 2007; 197: e1–e5, 292
- Romero R, Schaudinn C, Kusanovic J P, Gorur A, Gotsch F, Webster P, Nhan-Chang C L, Erez O, Kim C J, Espinoza J, et al. Detection of a microbial biofilm in intraamniotic infection. Am J Obstet Gynecol 2008; 198: 135
- Funderburk S J, Guthrie D, Meldrum D. Outcome of pregnancies complicated by early vaginal bleeding. Br J Obstet Gynaecol 1980; 87: 100–105
- Vintzileos A M, Campbell W A, Nochimson D J, Weinbaum P J. Preterm premature rupture of the membranes: a risk factor for the development of abruptio placentae. Am J Obstet Gynecol 1987; 156: 1235–1238
- Moretti M, Sibai B M. Maternal and perinatal outcome of expectant management of premature rupture of membranes in the midtrimester. Am J Obstet Gynecol 1988; 159: 390–396
- Brar H S, Medearis A L, DeVore G R, Platt L D. Maternal and fetal blood flow velocity waveforms in patients with preterm labor: prediction of successful tocolysis. Am J Obstet Gynecol 1988; 159: 947–950
- Brar H S, Medearis A L, De Vore G R, Platt L D. Maternal and fetal blood flow velocity waveforms in patients with preterm labor: relationship to outcome. Am J Obstet Gynecol 1989; 161: 1519–1522
- Arias F. Placental insufficiency: an important cause of preterm labor and preterm premature ruptured membranes. Presented at the 10th Annual Meeting of the Society of Perinatal Obstetricians, Houston, TexasUSA, January, 23–271990
- Major C, Nageotte M, Lewis D. Preterm premature rupture of membranes and placental abruption: is there an association between these pregnancy complications. Am J Obstet Gynecol 1991; 164: 381
- Williams M A, Mittendorf R, Lieberman E, Monson R R. Adverse infant outcomes associated with first-trimester vaginal bleeding. Obstet Gynecol 1991; 78: 14–18
- Arias F, Rodriquez L, Rayne S C, Kraus F T. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol 1993; 168: 585–591
- Strigini F A, Lencioni G, De Luca G, Lombardo M, Bianchi F, Genazzani A R. Uterine artery velocimetry and spontaneous preterm delivery. Obstet Gynecol 1995; 85: 374–377
- Signore C C, Sood A K, Richards D S. Second-trimester vaginal bleeding: correlation of ultrasonographic findings with perinatal outcome. Am J Obstet Gynecol 1998; 178: 336–340
- Kim Y M, Chaiworapongsa T, Gomez R, Bujold E, Yoon B H, Rotmensch S, Thaler H T, Romero R. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol 2002; 187: 1137–1142
- Nagy S, Bush M, Stone J, Lapinski R H, Gardo S. Clinical significance of subchorionic and retroplacental hematomas detected in the first trimester of pregnancy. Obstet Gynecol 2003; 102: 94–100
- Kim Y M, Bujold E, Chaiworapongsa T, Gomez R, Yoon B H, Thaler H T, Rotmensch S, Romero R. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2003; 189: 1063–1069
- Gomez R, Romero R, Nien J K, Medina L, Carstens M, Kim Y M, Chaiworapongsa T, Espinoza J, Gonzalez R. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med 2005; 18: 31–37
- Kloeck F K, Jung H. In vitro release of prostaglandins from the human myometrium under the influence of stretching. Am J Obstet Gynecol 1973; 115: 1066–1069
- Laudanski T, Rocki W. The effects on stretching and prostaglandin F2alpha on the contractile and bioelectric activity of the uterus in rat. Acta Physiol Pol 1975; 26: 385–393
- Hill L M, Breckle R, Thomas M L, Fries J K. Polyhydramnios: ultrasonically detected prevalence and neonatal outcome. Obstet Gynecol 1987; 69: 21–25
- Kanayama N, Fukamizu H. Mechanical stretching increases prostaglandin E2 in cultured human amnion cells. Gynecol Obstet Invest 1989; 28: 123–126
- Ludmir J, Samuels P, Brooks S, Mennuti M T. Pregnancy outcome of patients with uncorrected uterine anomalies managed in a high-risk obstetric setting. Obstet Gynecol 1990; 75: 906–910
- Phelan J P, Park Y W, Ahn M O, Rutherford S E. Polyhydramnios and perinatal outcome. J Perinatol 1990; 10: 347–350
- Chow L, Lye S J. Expression of the gap junction protein connexin-43 is increased in the human myometrium toward term and with the onset of labor. Am J Obstet Gynecol 1994; 170: 788–795
- Maehara K, Kanayama N, Maradny E E, Uezato T, Fujita M, Terao T. Mechanical stretching induces interleukin-8 gene expression in fetal membranes: a possible role for the initiation of human parturition. Eur J Obstet Gynecol Reprod Biol 1996; 70: 191–196
- Maradny E E, Kanayama N, Halim A, Maehara K, Terao T. Stretching of fetal membranes increases the concentration of interleukin-8 and collagenase activity. Am J Obstet Gynecol 1996; 174: 843–849
- Ou C W, Orsino A, Lye S J. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology 1997; 138: 5398–5407
- Ou C W, Chen Z Q, Qi S, Lye S J. Increased expression of the rat myometrial oxytocin receptor messenger ribonucleic acid during labor requires both mechanical and hormonal signals. Biol Reprod 1998; 59: 1055–1061
- Wu W X, Ma X H, Yoshizato T, Shinozuka N, Nathanielsz P W. Differential expression of myometrial oxytocin receptor and prostaglandin H synthase 2, but not estrogen receptor alpha and heat shock protein 90 messenger ribonucleic acid in the gravid horn and nongravid horn in sheep during betamethasone-induced labor. Endocrinology 1999; 140: 5712–5718
- Challis J RG, Matthews S G, Gibb W, Lye S J. Endocrine and paracrine regulation of birth at term and preterm. Endocrine Reviews 2000; 21: 514–550
- Millar L K, Stollberg J, DeBuque L, Bryant-Greenwood G. Fetal membrane distention: determination of the intrauterine surface area and distention of the fetal membranes preterm and at term. Am J Obstet Gynecol 2000; 182: 128–134
- Nemeth E, Millar L K, Bryant-Greenwood G. Fetal membrane distention: II. Differentially expressed genes regulated by acute distention in vitro. Am J Obstet Gynecol 2000; 182: 60–67
- Nemeth E, Tashima L S, Yu Z, Bryant-Greenwood G D. Fetal membrane distention: I. Differentially expressed genes regulated by acute distention in amniotic epithelial (WISH) cells. Am J Obstet Gynecol 2000; 182: 50–59
- Oldenhof A D, Shynlova O P, Liu M, Langille B L, Lye S J. Mitogen-activated protein kinases mediate stretch-induced c-fos mRNA expression in myometrial smooth muscle cells. Am J Physiol Cell Physiol 2002; 283: C1530–C1539
- Shynlova O P, Oldenhof A D, Liu M, Langille L, Lye S J. Regulation of c-fos expression by static stretch in rat myometrial smooth muscle cells. Am J Obstet Gynecol 2002; 186: 1358–1365
- Shynlova O, Williams S J, Draper H, White B G, Macphee D J, Lye S J. Uterine stretch regulates temporal and spatial expression of fibronectin protein and its alpha 5 integrin receptor in myometrium of unilaterally pregnant rats. Biol Reprod 2007; 77: 880–888
- Shynlova O, Tsui P, Dorogin A, Langille B L, Lye S J. The expression of transforming growth factor beta in pregnant rat myometrium is hormone and stretch dependent. Reproduction 2007; 134: 503–511
- Holmes C H, Simpson K L, Okada H, Okada N, Wainwright S D, Purcell D F, Houlihan J M. Complement regulatory proteins at the feto-maternal interface during human placental development: distribution of CD59 by comparison with membrane cofactor protein (CD46) and decay accelerating factor (CD55). Eur J Immunol 1992; 22: 1579–1585
- Holmes C H, Simpson K L. Complement and pregnancy: new insights into the immunobiology of the fetomaternal relationship. Baillieres Clin Obstet Gynaecol 1992; 6: 439–460
- Vanderpuye O A, Labarrere C A, McIntyre J A. The complement system in human reproduction. Am J Reprod Immunol 1992; 27: 145–155
- Benirschke K, Kaufmann P. Villitis of unknown etiology. Pathology of the Human Placenta, K Benirschke, P Kaufmann. Springer-Verlag, New York 1995; 596
- Cunningham D S, Tichenor J R, Jr. Decay-accelerating factor protects human trophoblast from complement-mediated attack. Clin Immunol Immunopathol 1995; 74: 156–161
- Gonzalez N C, Chairez J A, Cueto S M. [Immunology of the fetal-maternal relationship]. Rev Alerg Mex 1996; 43: 18–22
- Hagmann M. Embryos attacked by mom's natural defenses. Science 2000; 287: 408
- Xu C, Mao D, Holers V M, Palanca B, Cheng A M, Molina H. A critical role for murine complement regulator crry in fetomaternal tolerance. Science 2000; 287: 498–501
- Romero R, Mazor M, Avila C, et al. Uterine “allergy”: A novel mechanism for preterm labor. Am J Obstet Gynecol 1991; 164: 375
- Bytautiene E, Vedernikov Y P, Saade G R, Romero R, Garfield R E. Endogenous mast cell degranulation modulates cervical contractility in the guinea pig. Am J Obstet Gynecol 2002; 186: 438–445
- Garfield R E, Bytautiene E, Vedernikov Y P, Marshall J S, Romero R. Modulation of rat uterine contractility by mast cells and their mediators. Am J Obstet Gynecol 2000; 183: 118–125
- Bytautiene E, Romero R, Vedernikov Y, Saade G, Garfield R. Induction of preterm labor and delivery by allergic reaction and prevention by histamine H1 receptor antagonist. Am J Obstet Gynecol 2004; 191: 1356–1361
- Garfield R E, Irani A M, Schwartz L B, Bytautiene E, Romero R. Structural and functional comparison of mast cells in the pregnant versus nonpregnant human uterus. Am J Obstet Gynecol 2006; 194: 261–267
- Jolley J A, Wing D A. Pregnancy management after cervical surgery. Curr Opin Obstet Gynecol 2008; 20(6)528–533
- Iams J D, Johnson F F, Sonek J, Sachs L, Gebauer C, Samuels P. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol 1995; 172: 1097–1103
- Milewich L, Gant N F, Schwarz B E, Chen G T, MacDonald P C. Initiation of human parturition. VIII. Metabolism of progesterone by fetal membranes of early and late human gestation. Obstet Gynecol 1977; 50: 45–48
- Mitchell B F, Wong S. Changes in 17 beta,20 alpha-hydroxysteroid dehydrogenase activity supporting an increase in the estrogen/progesterone ratio of human fetal membranes at parturition. Am J Obstet Gynecol 1993; 168: 1377–1385
- How H, Huang Z H, Zuo J, Lei Z M, Spinnato J A, Rao C V. Myometrial estradiol and progesterone receptor changes in preterm and term pregnancies. Obstet Gynecol 1995; 86: 936–940
- Karalis K, Goodwin G, Majzoub J A. Cortisol blockade of progesterone: a possible molecular mechanism involved in the initiation of human labor. Nat Med 1996; 2: 556–560
- Rezapour M, Backstrom T, Lindblom B, Ulmsten U. Sex steroid receptors and human parturition. Obstet Gynecol 1997; 89: 918–924
- Smith R. Alterations in the hypothalamic pituitary adrenal axis during pregnancy and the placental clock that determines the length of parturition. J Reprod Immunol 1998; 39: 215–220
- Smith R. The timing of birth. Sci Am 1999; 280: 68–75
- Allport V C, Pieber D, Slater D M, Newton R, White J O, Bennett P R. Human labour is associated with nuclear factor-kappaB activity which mediates cyclo-oxygenase-2 expression and is involved with the ‘functional progesterone withdrawal’. Mol Hum Reprod 2001; 7: 581–586
- Henderson D, Wilson T. Reduced binding of progesterone receptor to its nuclear response element after human labor onset. Am J Obstet Gynecol 2001; 185: 579–585
- Pieber D, Allport V C, Hills F, Johnson M, Bennett P R. Interactions between progesterone receptor isoforms in myometrial cells in human labour. Mol Hum Reprod 2001; 7: 875–879
- Young I R. The comparative physiology of parturition in mammals. The enodocrinology of parturition, R Smith. Reinhardt Druck, Basel 2001; 10–30
- Smith R, Mesiano S, McGrath S. Hormone trajectories leading to human birth. Regul Pept 2002; 108: 159–164
- Mesiano S, Chan E C, Fitter J T, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab 2002; 87: 2924–2930
- Blanks A M, Vatish M, Allen M J, Ladds G, de Wit N C, Slater D M, Thornton S. Paracrine oxytocin and estradiol demonstrate a spatial increase in human intrauterine tissues with labor. J Clin Endocrinol Metab 2003; 88: 3392–3400
- Condon J C, Hardy D B, Kovaric K, Mendelson C R. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol 2006; 20: 764–775
- Smith R. Parturition. N Engl J Med 2007; 356: 271–283
- Zakar T, Hertelendy F. Progesterone withdrawal: key to parturition. Am J Obstet Gynecol 2007; 196: 289–296
- Valenzuela G J, Sanchez-Ramos L, Romero R, Silver H M, Koltun W D, Millar L, Hobbins J, Rayburn W, Shangold G, Wang J, et al. Maintenance treatment of preterm labor with the oxytocin antagonist atosiban. The Atosiban PTL-098 Study Group. Am J Obstet Gynecol 2000; 182: 1184–1190
- Romero R, Sibai B M, Sanchez-Ramos L, Valenzuela G J, Veille J C, Tabor B, Perry K G, Varner M, Goodwin T M, Lane R, et al. An oxytocin receptor antagonist (atosiban) in the treatment of preterm labor: a randomized, double-blind, placebo-controlled trial with tocolytic rescue. Am J Obstet Gynecol 2000; 182: 1173–1183
- King J F, Flenady V J, Papatsonis D N, Dekker G A, Carbonne B. Calcium channel blockers for inhibiting preterm labour. Cochrane Database Syst Rev 2003, CD002255
- Anotayanonth S, Subhedar N V, Garner P, Neilson J P, Harigopal S. Betamimetics for inhibiting preterm labour. Cochrane Database Syst Rev 2004, CD004352
- Dodd J M, Crowther C A, Dare M R, Middleton P. Oral betamimetics for maintenance therapy after threatened preterm labour. Cochrane Database Syst Rev 2006, CD003927
- Lamont R F. The pathophysiology of pulmonary oedema with the use of beta-agonists. BJOG 2000; 107: 439–444
- Lockwood C J, Senyei A E, Dische M R, Casal D, Shah K D, Thung S N, Jones L, Deligdisch L, Garite T J. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med 1991; 325: 669–674
- Nageotte M P, Casal D, Senyei A E. Fetal fibronectin in patients at increased risk for premature birth. Am J Obstet Gynecol 1994; 170: 20–25
- Inglis S R, Jeremias J, Kuno K, Lescale K, Peeper Q, Chervenak F A, Witkin S S. Detection of tumor necrosis factor-alpha, interleukin-6, and fetal fibronectin in the lower genital tract during pregnancy: relation to outcome. Am J Obstet Gynecol 1994; 171: 5–10
- Iams J D, Casal D, McGregor J A, Goodwin T M, Kreaden U S, Lowensohn R, Lockitch G. Fetal fibronectin improves the accuracy of diagnosis of preterm labor. Am J Obstet Gynecol 1995; 173: 141–145
- Goldenberg R L, Thom E, Moawad A H, Johnson F, Roberts J, Caritis S N. The preterm prediction study: fetal fibronectin, bacterial vaginosis, and peripartum infection. Obstet Gynecol 1996; 87: 656–660
- Goldenberg R L, Mercer B M, Meis P J, Copper R L, Das A, McNellis D. The preterm prediction study: fetal fibronectin testing and spontaneous preterm birth. NICHD Maternal Fetal Medicine Units Network. Obstet Gynecol 1996; 87: 643–648
- Berghella V, Hayes E, Visintine J, Baxter J K. Fetal fibronectin testing for reducing the risk of preterm birth. Cochrane Database Syst Rev 2008, CD006843
- Andersen H F, Nugent C E, Wanty S D, Hayashi R H. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol 1990; 163: 859–867
- Iams J D, Goldenberg R L, Meis P J, Mercer B M, Moawad A, Das A, Thom E, McNellis D, Copper R L, Johnson F, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med 1996; 334: 567–572
- Heath V C, Southall T R, Souka A P, Elisseou A, Nicolaides K H. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol 1998; 12: 312–317
- Visintine J, Berghella V, Henning D, Baxter J. Cervical length for prediction of preterm birth in women with multiple prior induced abortions. Ultrasound Obstet Gynecol 2008; 31: 198–200
- Hassan S S, Romero R, Berry S M, Dang K, Blackwell S C, Treadwell M C, Wolfe H M. Patients with an ultrasonographic cervical length <or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol 2000; 182: 1458–1467
- Berghella V, Ness A, Bega G, Berghella M. Cervical sonography in women with symptoms of preterm labor. Obstet Gynecol Clin North Am 2005; 32: 383–396
- Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, Nien J K, Berry S M, Bujold E, Camacho N, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med 2006; 34: 13–19
- Grimes-Dennis J, Berghella V. Cervical length and prediction of preterm delivery. Curr Opin Obstet Gynecol 2007; 19: 191–195
- Crane J M, Hutchens D. Transvaginal sonographic measurement of cervical length to predict preterm birth in asymptomatic women at increased risk: a systematic review. Ultrasound Obstet Gynecol 2008; 31: 579–587
- Keeler S M, Roman A S, Coletta J M, Kiefer D G, Feuerman M, Rust O A. Fetal fibronectin testing in patients with short cervix in the midtrimester: can it identify optimal candidates for ultrasound-indicated cerclage?. Am J Obstet Gynecol 2009; 200(2)158.e1–e6
- Goldenberg R L, Iams J D, Mercer B M, Meis P, Moawad A, Das A, Copper R, Johnson F, National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. What we have learned about the predictors of preterm birth. Semin Perinatol 2003; 27: 185–193
- Gomez R, Romero R, Medina L, Nien J K, Chaiworapongsa T, Carstens M, Gonzalez R, Espinoza J, Iams J D, Edwin S, et al. Cervicovaginal fibronectin improves the prediction of preterm delivery based on sonographic cervical length in patients with preterm uterine contractions and intact membranes. Am J Obstet Gynecol 2005; 192: 350–359
- Smith V, Devane D, Begley C M, Clarke M, Higgins S. A systematic review and quality assessment of systematic reviews of fetal fibronectin and transvaginal length for predicting preterm birth. Eur J Obstet Gynecol Reprod Biol 2007; 133: 134–142
- Goldenberg R L, Iams J D, Das A, Mercer B M, Meis P J, Moawad A H, Miodovnik M, Van Dorsten J P, Caritis S N, Thurnau G R, et al. The Preterm Prediction Study: sequential cervical length and fetal fibronectin testing for the prediction of spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol 2000; 182: 636–643
- Berghella V, Odibo A O, To M S, Rust O A, Althuisius S M. Cerclage for short cervix on ultrasonography: meta-analysis of trials using individual patient-level data. Obstet Gynecol 2005; 106: 181–189
- Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified. Am J Obstet Gynecol 2006; 194: 1–9
- To M S, Alfirevic Z, Heath V C, Cicero S, Cacho A M, Williamson P R, Nicolaides K, Fetal Medicine Foundation Second Trimester Screening Group. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet 2004; 363: 1849–1853
- Eschenbach D A, Nugent R P, Rao A V, Cotch M F, Gibbs R S, Lipscomb K A, Martin D H, Pastorek J G, Rettig P J, Carey J C, et al. A randomized placebo-controlled trial of erythromycin for the treatment of Ureaplasma urealyticum to prevent premature delivery. The Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol 1991; 164: 734–742
- Okun N, Gronau K A, Hannah M E. Antibiotics for bacterial vaginosis or Trichomonas vaginalis in pregnancy: a systematic review. Obstet Gynecol 2005; 105: 857–868
- Meis P J, Goldenberg R L, Mercer B, Moawad A, Das A, McNellis D, Johnson F, Iams J D, Thom E, Andrews W W. The preterm prediction study: significance of vaginal infections. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol 1995; 173: 1231–1235
- Roberts A K, Monzon-Bordonaba F, Van Deerlin P G, Holder J, Macones G A, Morgan M A, Strauss J F, III, Parry S. Association of polymorphism within the promoter of the tumor necrosis factor alpha gene with increased risk of preterm premature rupture of the fetal membranes. Am J Obstet Gynecol 1999; 180: 1297–1302
- Macones G, Parry S, Elkousy M, Clothier B, Ural S H, Strauss J F, III. A polymorphism in the promoter region of TNF and bacterial vaginosis: preliminary evidence of gene-environment interaction in the etiology of spontaneous preterm birth. Am J Obstet Gynecol 2004; 190: 1504–1508
- McDonald H M, Brocklehurst P, Gordon A. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev 2007, CD000262
- Lye S J, Ou C W, Tech T G, Erb G, Stevens Y, Casper R, et al. The molecular basis of labour and tocolysis. Fetal Maternal Med Rev 1998; 10: 121–136
- Ou C W, Chen Z Q, Qi S, Lye S J. Expression and regulation of the messenger ribonucleic acid encoding the prostaglandin F(2alpha) receptor in the rat myometrium during pregnancy and labor. Am J Obstet Gynecol 2000; 182: 919–925
- Myatt L, Lye S J. Expression, localization and function of prostaglandin receptors in myometrium. Prostaglandins Leukot Essent Fatty Acids 2004; 70: 137–148
- Shynlova O, Oldenhof A, Dorogin A, Xu Q, Mu J, Nashman N, Lye S J. Myometrial apoptosis: activation of the caspase cascade in the pregnant rat myometrium at midgestation. Biol Reprod 2006; 74: 839–849
- Shynlova O, Tsui P, Dorogin A, Langille B L, Lye S J. Insulin-like growth factors and their binding proteins define specific phases of myometrial differentiation during pregnancy in the rat. Biol Reprod 2007; 76: 571–578
- Liggins G. Cervical ripening as an inflammatory reaction. The Cervix in Pregnancy and Labour: Clinical and Biochemical Investigations, D Ellwood, A Anderson. Churchill Livingstone, Edinburgh 1981
- Chan E C, Fraser S, Yin S, Yeo G, Kwek K, Fairclough R J, Smith R. Human myometrial genes are differentially expressed in labor: a suppression subtractive hybridization study. J Clin Endocrinol Metab 2002; 87: 2435–2441
- Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim Y M, Romero R. Spontaneous labor at term is characterized by a genomic signature of acute inflammation in the chorioamniotic membranes but not in the systemic circulation. Am J Obstet Gynecol 2004; 191: S138
- Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim Y M, Mazor M, Romero R. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol 2006; 195: 394–324
- Hassan S S, Romero R, Haddad R, Hendler I, Khalek N, Tromp G, Diamond M P, Sorokin Y, Malone J, Jr. The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol 2006; 195: 778–786
- Romero R, Espinoza J, Goncalves L F, Kusanovic J P, Friel L A, Nien J K. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med 2006; 11: 317–326
- Romero R, Espinoza J, Goncalves L F, Kusanovic J P, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med 2007; 25: 21–39
- Christiaens I, Zaragoza D B, Guilbert L, Robertson S A, Mitchell B F, Olson D M. Inflammatory processes in preterm and term parturition. J Reprod Immunol 2008; 79: 50–57
- Bollopragada S, Youssef R, Jordan F, Greer I, Norman J, Nelson S. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol 2009; 200: 104–111
- Keelan J A, Blumenstein M, Helliwell R J, Sato T A, Marvin K W, Mitchell M D. Cytokines, prostaglandins and parturition–a review. Placenta 2003; 24((Suppl A))S33–S46
- Marvin K W, Keelan J A, Eykholt R L, Sato T A, Mitchell M D. Expression of angiogenic and neurotrophic factors in the human amnion and choriodecidua. Am J Obstet Gynecol 2002; 187: 728–734
- Simpson K L, Keelan J A, Mitchell M D. Labor-associated changes in interleukin-10 production and its regulation by immunomodulators in human choriodecidua. Am J Obstet Gynecol 1998; 83: 4332–4337
- Keelan J A, Marvin K W, Sato T A, Coleman M, McCowan L M, Mitchell M D. Cytokine abundance in placental tissue: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J Obstet Gynecol 1999; 181: 1530–1536
- Marvin K W, Keelan J A, Eykholt R L, Sato T A, Mitchell M D. Use of cDNA arrays to generate differential expression profiles for inflammatory genes in human gestational membranes delivered at term and preterm. Mol Hum Reprod 2002; 8: 399–408
- Carroll S G, Papaioannou S, Ntumazah I L, Philpott-Howard J, Nicolaides K H. Lower genital tract swabs in the prediction of intrauterine infection in preterm prelabour rupture of the membranes. Br J Obstet Gynaecol 1996; 103: 54–59
- Gomez R, Romero R, Ghezzi F, Yoon B H, Mazor M, Berry S M. The fetal inflammatory response syndrome. Am J Obstet Gynecol 1998; 179: 194–202
- Romero R, Maymon E, Pacora P, Gomez R, Mazor M, Yoon B H, Berry S M. Further observations on the fetal inflammatory response syndrome: a potential homeostatic role for the soluble receptors of tumor necrosis factor alpha. Am J Obstet Gynecol 2000; 183: 1070–1077
- Gotsch F, Romero R, Kusanovic J P, Mazaki-Tovi S, Pineles B L, Erez O, Espinoza J, Hassan S S. The fetal inflammatory response syndrome. Clin Obstet Gynecol 2007; 50: 652–683
- Yanowitz T D, Jordan J A, Gilmour C H, Towbin R, Bowen A, Roberts J M, Brozankis B S. Hemodynamic disturbances in premature infants born after chorioamnionitis: association with cord blood cytokine concentrations. Pediatr Res 2002; 51: 310–316
- Romero R, Espinoza J, Goncalves L F, Gomez R, Medina L, Silva M, Chaiworapongsa T, Yoon B H, Ghezzi F, Lee W, et al. Fetal cardiac dysfunction in preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2004; 16: 146–157
- Yoon B H, Romero R, Jun J K, Maymon E, Gomez R, Mazor M, Park J S. An increase in fetal plasma cortisol but not dehydroepiandrosterone sulfate is followed by the onset of preterm labor in patients with preterm premature rupture of the membranes. Am J Obstet Gynecol 1998; 179: 1107–1114
- Eastman N J, DeLeon M. The etiology of cerebral palsy. Am J Obstet Gynecol 1955; 69: 950–961
- Bejar R, Wozniak P, Allard M, Benirschke K, Vaucher Y, Coen R, Berry C, Schragg P, Villegas I, Resnik R. Antenatal origin of neurologic damage in newborn infants. I. Preterm infants. Am J Obstet Gynecol 1988; 159: 357–363
- Alexander J M, Gilstrap L C, Cox S M, McIntire D M, Leveno K J. Clinical chorioamnionitis and the prognosis for very low birth weight infants. Obstet Gynecol 1998; 91: 725–729
- Murphy D J, Sellers S, MacKenzie I Z, Yudkin P L, Johnson A M. Case-control study of antenatal and intrapartum risk factors for cerebral palsy in very preterm singleton babies. Lancet 1995; 346: 1449–1454
- Grether J K, Nelson K B. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA 1997; 278: 207–211
- Yoon B H, Kim C J, Romero R, Jun J K, Park K H, Choi S T, Chi J G. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol 1997; 177: 797–802
- Verma U, Tejani N, Klein S, Reale M R, Beneck D, Figueroa R, Visintainer P. Obstetric antecedents of intraventricular hemorrhage and periventricular leukomalacia in the low-birth-weight neonate. Am J Obstet Gynecol 1997; 176: 275–281
- Dammann O, Leviton A. Infection remote from the brain, neonatal white matter damage, and cerebral palsy in the preterm infant. Semin Pediatr Neurol 1998; 5: 190–201
- O'Shea T M, Klinepeter K L, Dillard R G. Prenatal events and the risk of cerebral palsy in very low birth weight infants. Am J Epidemiol 1998; 147: 362–369
- Nelson K B, Dambrosia J M, Grether J K, Phillips T M. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol 1998; 44: 665–675
- Leviton A, Paneth N, Reuss M L, Susser M, Allred E N, Dammann O, Kuban K, Van Marter L J, Pagano M, Hegyi T, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators. Pediatr Res 1999; 46: 566–575
- Dammann O, Leviton A. Role of the fetus in perinatal infection and neonatal brain damage. Curr Opin Pediatr 2000; 12: 99–104
- Wu Y W, Colford J M, Jr. Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA 2000; 284: 1417–1424
- Dammann O, Kuban K C, Leviton A. Perinatal infection, fetal inflammatory response, white matter damage, and cognitive limitations in children born preterm. Ment Retard Dev Disabil Res Rev 2002; 8: 46–50
- Nelson K B. The epidemiology of cerebral palsy in term infants. Ment Retard Dev Disabil Res Rev 2002; 8: 146–150
- Wu Y W, Escobar G J, Grether J K, Croen L A, Greene J D, Newman T B. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA 2003; 290: 2677–2684
- Kaukola T, Herva R, Perhomaa M, Paakko E, Kingsmore S, Vainionpaa L, Hallman M. Population cohort associating chorioamnionitis, cord inflammatory cytokines and neurologic outcome in very preterm, extremely low birth weight infants. Pediatr Res 2006; 59: 478–483
- Watterberg K L, Demers L M, Scott S M, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 1996; 97: 210–215
- Yoon B H, Romero R, Jun J K, Park K H, Park J D, Ghezzi F, Kim B I. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1997; 177: 825–830
- Speer C P. Inflammatory mechanisms in neonatal chronic lung disease. Eur J Pediatr 1999; 158((Suppl 1))S18–S22
- Speer C P. New insights into the pathogenesis of pulmonary inflammation in preterm infants. Biol Neonate 2001; 79: 205–209
- Speer C P. Inflammation and bronchopulmonary dysplasia. Semin Neonatol 2003; 8: 29–38
- Jobe A H. Antenatal associations with lung maturation and infection. J Perinatol 2005; 25((Suppl 2))S31–S35
- Speer C P. Inflammation and bronchopulmonary dysplasia: a continuing story. Semin Fetal Neonatal Med 2006; 11: 354–362
- Watts D H, Krohn M A, Hillier S L, Eschenbach D A. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol 1992; 79: 351–357
- Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim J C, Kim Y M. The role of infection in preterm labour and delivery. Pediatr Perinat Epidemiol 2001; 15((Suppl 2))41–56