Abstract
Objective. To examine whether the intra-amniotic inflammation is a risk factor for the development of atypical chronic lung disease (CLD).
Study design. A retrospective cohort study was undertaken in 72 patients who delivered preterm neonates (gestational age: 24–32 weeks) within 5 days of amniocentesis and whose neonates subsequently developed CLD. Atypical CLD was defined as CLD without respiratory distress syndrome (RDS). Intra-amniotic inflammation was defined as an elevated amniotic fluid (AF) concentration of matrix metalloproteinase-8 (MMP-8) (>23 ng/ml).
Results. (1) Atypical CLD was identified in 54.2% (39/72) of cases with CLD; (2) there were no significant differences in the median gestational age at birth and the rate of antenatal corticosteroid use between infants with atypical CLD and CLD with RDS; (3) preterm newborns with atypical CLD had a significantly higher median AF MMP-8 concentration (median 373.1 ng/ml vs. 8.6 ng/ml, p = 0.003) and median AF white blood cell count (median 450.0/mm3vs. 5.5/mm3, p = 0.009), and a higher rate of intra-amniotic inflammation (74.4%vs. 45.5%, p = 0.012) than those with CLD with RDS.
Conclusion. Intra-amniotic inflammation confers a greater risk for atypical CLD than for typical CLD with initial RDS. This novel observation strengthens the importance of prenatal inflammation as a mechanism of lung injury.
Introduction
Chronic lung disease (CLD) is the major cause of long-term morbidities in preterm newborns. It develops in 15–47% of neonates with birth weights <1500 g and affected infants are at an increased risk for death, cerebral palsy, recurrent pneumonia, reactive airway disease, cardiovascular disease and delayed growth Citation[1-3].
CLD was originally described as a lung injury caused by oxygen therapy and mechanical ventilation in preterm neonates with respiratory distress syndrome (RDS) Citation[4]. However, the clinical presentation and pathogenesis of the disease has changed in the last 20 years. The term ‘atypical CLD’ or ‘new CLD’ has been coined to refer to CLD, which develops without RDS or only after mild RDS Citation[5],Citation[6].
An inflammatory mechanism has been implicated in the genesis of CLD Citation[3],Citation[7]. Previous studies including ours have shown that the presence of histological chorioamnionitis in the placenta and elevated concentrations of inflammatory markers in the amniotic fluid (AF) are associated with CLD Citation[8-10]. However, it is unclear if intra-amniotic inflammation plays a role in the atypical form. Some investigators have demonstrated that histological chorioamnionitis is a risk factor for CLD without preceding RDS Citation[11],Citation[12]. But other investigators have not confirmed such findings Citation[13]. In addition, the relationship between the intra-amniotic inflammation reflecting the antenatal inflammatory status and atypical CLD has not been demonstrated so far. This study was conducted to address this question.
Material and methods
Study design
The relationship between the presence of intra-amniotic inflammation and the occurrence of atypical CLD was examined in patients who delivered preterm singleton newborns (gestational age: 24–32 weeks) within 5 days of amniocentesis and whose neonates subsequently developed CLD. The neonates were born at the Seoul National University Hospital between December 1992 and March 2008. Intra-amniotic inflammation was defined as an elevated AF matrix metalloproteinase-8 (MMP-8) concentration (>23 ng/ml), as previously reported Citation[14]. Amniocentesis for the retrieval of AF was routinely offered to all patients admitted to our institution with the diagnosis of preterm labor or preterm premature rupture of membranes (PROM). AF was analysed for microbiological and fetal lung maturity studies, if indicated. Amniocentesis was also performed to assess fetal lung maturity in patients with hypertensive disorders in pregnancy. The institutional review board of our institution (Seoul National University Hospital, Seoul, Korea) has approved the collection and utilisation of the biological materials and clinical data for research purposes. The Seoul National University has a Federal Wide Assurance with the Office for Human Research Protections (OHRP) of the Department of Health and Human Services of the United States.
Amniotic fluid
AF was retrieved by transabdominal amniocentesis and cultured for aerobic and anaerobic bacteria and for genital mycoplasma (Ureaplasma urealyticum and Mycoplasma hominis). Fluid that was not used for diagnostic studies was centrifuged and stored at −70°C until assayed. MMP-8 concentrations were measured with the use of a commercially available enzyme-linked immunosorbent assay (Amersham Pharmacia Biotech, Little Chalfont, Bucks, UK) with a sensitivity of 0.3 ng/ml. Intra- and inter-assay coefficients of variation were <10%.
Diagnosis of CLD, neonatal morbidity, histological chorioamnionitis and funisitis
CLD was defined using the criteria of the National Institute of Child Health Workshop definition for bronchopulmonary dysplasia (BPD), i.e. the treatment with oxygen >21% for at least 28 days Citation[15] and also diagnosed in the presence of typical findings at autopsy. The total CLD group was divided into two groups: (1) the CLD with RDS and (2) atypical CLD (CLD without RDS). The diagnosis of CLD with RDS was made when there was continuous oxygen supplementation for at least 28 days with initial RDS and atypical CLD was diagnosed when the neonate developed CLD in the absence of RDS. A diagnosis of RDS required the presence of respiratory distress, increased oxygen requirement (FiO2 > 0.4) and diagnostic radiological and laboratory findings in the absence of evidence of any other causes of respiratory distress. Neonatal morbidities were diagnosed according to the definition previously described in detail Citation[16]. Patent ductus arteriosus (PDA) was diagnosed by echocardiography and only cases treated with indomethacin or surgical ligation were included.
Histological chorioamnionitis was defined in the presence of acute inflammatory changes on examination of a membrane roll and chorionic plate of the placenta, and funisitis was diagnosed in the presence of neutrophil infiltration into the umbilical vessel walls or Wharton's jelly using criteria previously published and used in our studies Citation[14],Citation[16].
Statistical analysis
Univariate analysis was conducted using χ2 test, Fisher's exact test or Mann–Whitney U test as appropriate.
Results
During the study period, CLD was diagnosed in 142 singleton preterm newborns (gestational age: 24–32 weeks). Among these, AF was available in 89 cases, and AF within 5 days before delivery was available in 72 cases. Atypical CLD was identified in 39 of 72 cases. The prevalence of intra-amniotic inflammation was 61.1% (44/72) in the newborns with CLD.
compares the clinical characteristics of infants with atypical CLD and those with CLD with RDS. There were no significant differences in the median gestational age at birth and birth weight, and the rate of antenatal steroid administration between the two groups. No significant differences were found between the group with atypical CLD and the group with CLD with RDS in the significant neonatal complications, including oxygen duration, ventilation duration, congenital pneumonia, necrotizing enterocolitis, congenital sepsis and PDA.
Table I. Comparison of the clinical characteristics of the study population between cases with atypical CLD and those with CLD with RDS.
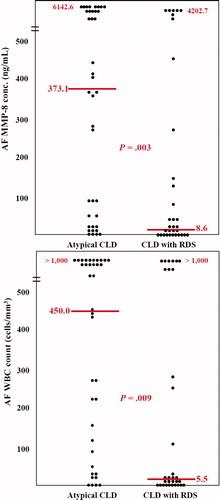
illustrates the AF concentration of MMP-8 and AF white blood cell count in the two study groups. Mothers whose preterm babies had atypical CLD had a significantly higher median AF MMP-8 concentration and AF white blood cell count than those whose preterm babies had CLD with RDS (AF MMP-8: median 373.1, range <0.3 to 6142.6 ng/ml vs. median 8.6, range <0.3 to 4202.7 ng/ml; p = 0.003; AF WBC count: median 450.0, range 0 to >1000/mm3vs. median 5.5, range 0 to >1000/mm3; p = 0.009).
Figure 1. AF concentration of matrix metalloproteinase-8 (MMP-8) and white blood cell (WBC) count according to the type of CLD. Mothers whose preterm babies had atypical CLD had a significantly higher median AF MMP-8 concentration and AF WBC count than those whose preterm babies had CLD with RDS (AF MMP-8: median 373.1, range <0.3 to 6142.6 ng/ml vs. median 8.6, range <0.3 to 4202.7 ng/ml; p = 0.003; AF WBC count: median 450.0, range 0 to >1000/mm3vs. median 5.5, range 0 to >1000/mm3; p = 0.009).
compares the rates of intra-amniotic infection and intra-amniotic and placental inflammation between cases with atypical CLD and those with CLD with RDS. There were no significant differences in the rates of positive AF culture, histologic chorioamnionitis and funisitis between the two groups of cases. However, patients whose newborns developed atypical CLD had significantly higher rates of intra-amniotic inflammation and intra-amniotic infection and/or inflammation than those whose newborns developed CLD with RDS (p < 0.05).
Table II. Comparison of several inflammatory characteristics of the study population between cases with atypical CLD and those with CLD with RDS.
Discussion
Principal findings of the study
Intra-amniotic inflammation was present in 74.4% of newborns with atypical CLD and intra-amniotic infection and/or inflammation were present in 79.5%. However, 45.5% of babies with CLD with RDS had intra-amniotic inflammation.
Intra-amniotic infection, intra-amniotic inflammation and CLD
Microorganisms present in the amniotic cavity in patients with preterm labor, preterm PROM or in cases of chronic intrauterine infection can gain access to the fetus through several ports of entry, including the fetal airway. They may also elicit an intra-amniotic inflammatory response with increased concentrations of many inflammatory mediators including pro-inflammatory cytokines Citation[17-23], chemokines Citation[24-26], arachidonate metabolites Citation[27], catalase Citation[28], adipokines Citation[29] and multiple MMPs Citation[21],Citation[30-33]. An elevated AF MMP-8 concentration is an index of intra-amniotic inflammation Citation[14],Citation[30],Citation[33],Citation[34]. Microorganisms and AF inflammatory mediators may elicit a local inflammatory response in the airways and lungs. The higher concentrations of pro-inflammatory cytokines are detected in the lungs of preterm neonates with histological chorioamnionitis than in those without chorioamnionitis Citation[9],Citation[35]. Moreover, we have previously reported that these newborns are at an increased risk for developing CLD Citation[10],Citation[36],Citation[37].
Intra-amniotic infection, intra-amniotic inflammation and RDS
Several investigators have noted that newborns exposed to intra-amniotic inflammation/infection appear to have a reduced rate of RDS Citation[38-40]. Experimental evidence that explains these observations includes the findings that administration of bacterial endotoxin into the amniotic cavity of a pregnant animal elicits a local inflammatory response in the fetal airways, and also an increase in the mRNA and protein for surfactant proteins Citation[41-45]. Importantly, these changes were associated with an improved lung function comparable to that induced by prenatal administration of steroids. Similar findings have been reported in response to IL-1 Citation[46],Citation[47]. One interpretation of these findings is that intrauterine infection/inflammation not only leads to the induction of preterm labor and preterm PROM by mechanisms which involve inflammatory mediators, but also induces the production of surfactant proteins perhaps in preparation for preterm birth Citation[48]. This increased surfactant protein production may explain the reduced rate of RDS in infants born at a very early gestational age when intra-amniotic infection is present.
Intra-amniotic infection, intra-amniotic inflammation and atypical CLD
We found that the rate of intra-amniotic inflammation was 61.1% in all cases of CLD and higher in neonates with atypical CLD than in those with CLD with RDS. This suggests that inflamed AF may be more important in the pathogenesis of atypical CLD than in the development of CLD with RDS. Exposure to bacterial products and inflammatory markers reduces the rate of RDS, while it also induces apoptosis and proliferation of epithelial cells lining the fetal lung and alters lung development Citation[45]. These changes may be the basis for a developmental disorder characterised by arrest of alveolar development, which is considered central to the pathophysiology of CLD. It would seem likely that a local inflammatory response in the preterm fetal lung might improve surfactant production and even lung function. However, in some cases, this is achieved at a high price: interference with normal lung development. This is probably the explanation for why intra-amniotic inflammation may be a risk factor for atypical CLD (CLD without RDS).
Matrix metalloproteinase-8 in AF and fetal lung injury
MMP-8, also known as a neutrophil collagenase, is an enzyme detected in the sites of inflammation and is produced by several cell types including neutrophils, synovial fibroblast, endothelial cells, chondrocytes and odontoblasts Citation[34]. It is also present in AF during microbial invasion and inflammation and is considered as a powerful predictor of intra-amniotic infection, preterm delivery and poor neonatal outcomes Citation[30],Citation[33],Citation[34]. But the presence of MMP-8 in AF may not only mean one kind of several inflammatory markers in AF, but may also be considered as the direct and local injury of the fetal lung.
MMP-8 predominantly degrades type I collagen, which is a major structural protein of the lung's extracellular matrix Citation[49]. Excessive MMP-8 activity has been implicated in adult lung diseases, such as emphysema, bronchiectasis and chronic obstructive pulmonary disease Citation[50],Citation[51]. Some authors have demonstrated that higher levels of MMP-8 in bronchoalveolar lavage are found in preterm infants from pregnancies complicated by chorioamnionitis and in those who later develop CLD, and that MMP-8 may play an important role in lung injury as a prelude to CLD Citation[49],Citation[52]. Cederqvist et al. Citation[53] have reported that an imbalance between pulmonary MMP-8 and TIMP-2 (tissue inhibitor of metalloproteinase-2) may contribute to the development of chronic lung injury by examining MMP‐8 in tracheal aspirates from preterm infants.
We found that the AF MMP-8 concentration was related to the likelihood of the development of atypical CLD. It is possible that AF MMP-8 had a deleterious effect on the fetal lung.
Strength and weakness of the study
Strengths include (1) the large number of neonates studied to investigate atypical CLD; and (2) this study is the first to establish a relationship between AF inflammatory status and atypical CLD.
It could be argued that a weakness of this study is that it was conducted only in the cases that amniocentesis had been performed. In the process of enrollment, we had to exclude 53 preterm singleton newborns with CLD because of the lack of AF for study and 17 infants with CLD were excluded because they had been born more than 5 days after the amniocentesis had been performed. We believe, however, that this is unavoidable in clinical research. Oligohydramnios in preterm PROM limits the amount of fluid available for research and the 5 day cut off was used to preserve a meaningful temporal relationship between the assessment of intra-amniotic inflammation and neonatal outcome.
We divided the study population into two groups: CLD with RDS and atypical CLD. This classification is somewhat arbitrary because some authors have proposed that atypical CLD includes not only CLD without RDS, but also CLD after RDS resolved (initial RDS that resolved by day 10 and no oxygen supplementation for at least 72 h after the resolution of RDS) Citation[5]. But consensus has not been reached on the definition of ‘atypical CLD’ or ‘new CLD’.
As patients and their babies without CLD were not included in this study, the risk for the development of atypical CLD or CLD with RDS in preterm singleton newborns as a function of the presence of intra-amniotic inflammation could not be evaluated. However, our group previously demonstrated that intra-amniotic inflammation is a risk factor for the development of CLD Citation[10],Citation[37] and this study showed that the rate of intra-amniotic inflammation in babies with atypical CLD is higher than in babies with CLD with RDS.
Unanswered questions and proposals for future research
Clinical strategies to prevent the development of atypical CLD in the preterm newborns with intra-amniotic inflammation, especially elevated concentration of AF MMP-8 may need to begin in utero. Further studies are required to determine whether antimicrobials, anticytokine agents or inhibitors of MMP-8 may be effective in reducing the incidence of atypical CLD.
Clinical implication of this study
Our findings suggest that atypical CLD is strongly associated with intra-amniotic inflammation and that intra-amniotic inflammation plays a more important role in the pathogenesis of atypical CLD than in the pathogenesis of CLD with RDS.
Acknowledgements
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) (No. R01-2006-000-10607-0), and in part by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Heath and Human Development, NIH and DHHS.
References
- Dusick A M. Medical outcomes in preterm infants. Semin Perinatol 1997; 21: 164–177
- Majnemer A, Riley P, Shevell M, Birnbaum R, Greenstone H, Coates A L. Severe bronchopulmonary dysplasia increases risk for later neurological and motor sequelae in preterm survivors. Dev Med Child Neurol 2000; 42: 53–60
- Bhandari A, Bhandari V. Bronchopulmonary dysplasia: an update. Indian J Pediatr 2007; 74: 73–77
- Northway W H, Jr, Rosan R C, Porter D Y. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 1967; 276: 357–368
- Charafeddine L, D'Angio C T, Phelps D L. Atypical chronic lung disease patterns in neonates. Pediatrics 1999; 103: 759–765
- Jobe A J. The new BPD: an arrest of lung development. Pediatr Res 1999; 46: 641–643
- Speer C P. Inflammation and bronchopulmonary dysplasia. Semin Neonatol 2003; 8: 29–38
- Speer C P. New insights into the pathogenesis of pulmonary inflammation in preterm infants. Biol Neonate 2001; 79: 205–209
- Watterberg K L, Demers L M, Scott S M, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 1996; 97: 210–215
- Yoon B H, Romero R, Jun J K, Park K H, Park J D, Ghezzi F, Kim B I. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-α, interleukin-1β, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1997; 177: 825–830
- Choi C W, Kim B I, Koh Y Y, Choi J H, Choi J Y. Clinical characteristics of chronic lung disease without preceding respiratory distress syndrome in preterm infants. Pediatr Int 2005; 47: 72–79
- Choi C W, Kim B I, Park J D, Koh Y Y, Choi J H, Choi J Y. Risk factors for the different types of chronic lung diseases of prematurity according to the preceding respiratory distress syndrome. Pediatr Int 2005; 47: 417–423
- Panickar J, Scholefield H, Kumar Y, Pilling D W, Subhedar N V. Atypical chronic lung disease in preterm infants. J Perinat Med 2004; 32: 162–167
- Shim S S, Romero R, Hong J S, Park C W, Jun J K, Kim B I, Yoon B H. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 2004; 191: 1339–1345
- Jobe A H, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001; 163: 1723–1729
- Yoon B H, Romero R, Kim C J, Jun J K, Gomez R, Choi J H, Syn H C. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol 1995; 172: 960–970
- Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Yoon B H, Edwin S S. Interleukin 16 in pregnancy, parturition, rupture of fetal membranes, and microbial invasion of the amniotic cavity. Am J Obstet Gynecol 2000; 182: 135–141
- Andrews W W, Hauth J C, Goldenberg R L, Gomez R, Romero R, Cassell G H. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol 1995; 173: 606–612
- Greig P C, Ernest J M, Teot L, Erikson M, Talley R. Amniotic fluid interleukin-6 levels correlate with histologic chorioamnionitis and amniotic fluid cultures in patients in premature labor with intact membranes. Am J Obstet Gynecol 1993; 169: 1035–1044
- Greig P C, Herbert W N, Robinette B L, Teot L A. Amniotic fluid interleukin-10 concentrations increase through pregnancy and are elevated in patients with preterm labor associated with intrauterine infection. Am J Obstet Gynecol 1995; 173: 1223–1227
- Harirah H, Donia S E, Hsu C D. Amniotic fluid matrix metalloproteinase-9 and interleukin-6 in predicting intra-amniotic infection. Obstet Gynecol 2002; 99: 80–84
- Hillier S L, Witkin S S, Krohn M A, Watts D H, Kiviat N B, Eschenbach D A. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol 1993; 81: 941–948
- Hsu C D, Meaddough E, Aversa K, Hong S F, Lu L C, Jones D C, Copel J A. Elevated amniotic fluid levels of leukemia inhibitory factor, interleukin 6, and interleukin 8 in intra-amniotic infection. Am J Obstet Gynecol 1998; 179: 1267–1270
- Jacobsson B, Holst R M, Wennerholm U B, Andersson B, Lilja H, Hagberg H. Monocyte chemotactic protein-1 in cervical and amniotic fluid: relationship to microbial invasion of the amniotic cavity, intra-amniotic inflammation, and preterm delivery. Am J Obstet Gynecol 2003; 189: 1161–1167
- Nhan-Chang C L, Romero R, Kusanovic J P, Gotsch F, Edwin S S, Erez O, Mittal P, Kim C J, Kim M J, Espinoza J, et al. A role for CXCL13 (BCA-1) in pregnancy and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2008; 21: 763–775
- Hamill N, Romero R, Gotsch F, Pedro Kusanovic J, Edwin S, Erez O, Than N G, Mittal P, Espinoza J, Friel L A, et al. Exodus-1 (CCL20): evidence for the participation of this chemokine in spontaneous labor at term, preterm labor, and intrauterine infection. J Perinat Med 2008; 36: 217–227
- Romero R, Quintero R, Emamian M, Wan M, Grzyboski C, Hobbins J C, Mitchell M D. Arachidonate lipoxygenase metabolites in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol 1987; 157: 1454–1460
- Font G E, Gauthier D W, Meyer W J, Myles T D, Janda W, Bieniarz A. Catalase activity as a predictor of amniotic fluid culture results in preterm labor or premature rupture of membranes. Obstet Gynecol 1995; 85: 656–658
- Mazaki-Tovi S, Romero R, Kusanovic J P, Erez O, Gotsch F, Mittal P, Than N G, Nhan-Chang C L, Hamill N, Vaisbuch E, et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat Med 2008; 36: 485–496
- Angus S R, Segel S Y, Hsu C D, Locksmith G J, Clark P, Sammel M D, Macones G A, Strauss J F, III, Parry S. Amniotic fluid matrix metalloproteinase-8 indicates intra-amniotic infection. Am J Obstet Gynecol 2001; 185: 1232–1238
- Maymon E, Romero R, Chaiworapongsa T, Kim J C, Berman S, Gomez R, Edwin S. Value of amniotic fluid neutrophil collagenase concentrations in preterm premature rupture of membranes. Am J Obstet Gynecol 2001; 185: 1143–1148
- Locksmith G J, Clark P, Duff P, Schultz G S. Amniotic fluid matrix metalloproteinase-9 levels in women with preterm labor and suspected intra-amniotic infection. Obstet Gynecol 1999; 94: 1–6
- Maymon E, Romero R, Chaiworapongsa T, Berman S, Conoscenti G, Gomez R, Edwin S. Amniotic fluid matrix metalloproteinase-8 in preterm labor with intact membranes. Am J Obstet Gynecol 2001; 185: 1149–1155
- Yoon B H, Oh S Y, Romero R, Shim S S, Han S Y, Park J S, Jun J K. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol 2001; 185: 1162–1167
- Schmidt B, Cao L, Mackensen-Haen S, Kendziorra H, Klingel K, Speer C P. Chorioamnionitis and inflammation of the fetal lung. Am J Obstet Gynecol 2001; 185: 173–177
- Yoon B H, Romero R, Kim K S, Park J S, Ki S H, Kim B I, Jun J K. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1999; 181: 773–779
- Ghezzi F, Gomez R, Romero R, Yoon B H, Edwin S S, David C, Janisse J, Mazor M. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol 1998; 78: 5–10
- Shimoya K, Taniquchi T, Matsuzaki N, Moriyama A, Murata Y, Kitajima H, Fujimura M, Nakayama M. Chorioamnionitis decreased incidence of respiratory distress syndrome by elevating fetal interleukin-6 serum concentration. Hum Reprod 2000; 15: 2234–2240
- Moss T J, Nitsos I, Kramer B W, Ikegami M, Newnham J P, Jobe A H. Intra-amniotic endotoxin induces lung maturation by direct effects on the developing respiratory tract in preterm sheep. Am J Obstet Gynecol 2002; 187: 1059–1065
- Hannaford K, Todd D A, Jeffery H, John E, Blyth K, Gilbert G L. Role of ureaplasma urealyticum in lung disease of prematurity. Arch Dis Child Fetal Neonatal Ed 1999; 81: F162–F167
- Bachurski C J, Ross G F, Ikegami M, Kramer B W, Jobe A H. Intra-amniotic endotoxin increases pulmonary surfactant proteins and induces SP-B processing in fetal sheep. Am J Physiol Lung Cell Mol Physiol 2001; 280: L279–L285
- Kramer B W, Moss T J, Willet K E, Newnham J P, Sly P D, Kallapur S G, Ikegami M, Jobe A H. Dose and time response after intraamniotic endotoxin in preterm lambs. Am J Respir Crit Care Med 2001; 164: 982–988
- Bry K, Lappalainen U. Intra-amniotic endotoxin accelerates lung maturation in fetal rabbits. Acta Paediatr 2001; 90: 74–80
- Moss T J, Newnham J P, Willett K E, Kramer B W, Jobe A H, Ikegami M. Early gestational intra-amniotic endotoxin: lung function, surfactant, and morphometry. Am J Respir Crit Care Med 2002; 165: 805–811
- Kramer B W, Kramer S, Ikegami M, Jobe A H. Injury, inflammation, and remodeling in fetal sheep lung after intra-amniotic endotoxin. Am J Physiol Lung Cell Mol Physiol 2002; 283: L452–L459
- Vayrynen O, Glumoff V, Hallman M. Regulation of surfactant proteins by LPS and proinflammatory cytokines in fetal and newborn lung. Am J Physiol Lung Cell Mol Physiol 2002; 282: L803–L810
- Bry K, Lappalainen U, Hallman M. Intraamniotic interleukin-1 accelerates surfactant protein synthesis in fetal rabbits and improves lung stability after premature birth. J Clin Invest 1997; 99: 2992–2999
- Chaiworapongsa T, Hong J S, Hull W M, Romero R, Whitsett J A. Amniotic fluid concentration of surfactant proteins in intra-amniotic infection. J Matern Fetal Neonatal Med 2008; 21: 663–670
- Curley A E, Sweet D G, MacMahon K J, O'Connor C M, Halliday H L. Chorioamnionitis increases matrix metalloproteinase-8 concentrations in bronchoalveolar lavage fluid from preterm babies. Arch Dis Child Fetal Neonatal Ed 2004; 89: F61–F64
- Sepper R, Konttinen Y T, Ding Y, Takagi M, Sorsa T. Human neutrophil collagenase (MMP-8), identified in bronchiectasis BAL fluid, correlates with severity of disease. Chest 1995; 107: 1641–1647
- Betsuyaku T, Nishimura M, Takeyabu K, Tanino M, Venqe P, Xu S, Kawakami Y. Neutrophil granule proteins in bronchoalveolar lavage fluid from subjects with subclinical emphysema. Am J Respir Crit Care Med 1999; 159: 1985–1991
- Sweet D G, McMahon K J, Curley A E, O'Connor C M, Halliday H L. Type I collagenases in bronchoalveolar lavage fluid from preterm babies at risk of developing chronic lung disease. Arch Dis Child Fetal Neonatal Ed 2001; 84: F168–F171
- Cederqvist K, Sorsa T, Tervahartiala T, Maisi P, Reunanen K, Lassus P, Andersson S. Matrix metalloproteinases-2, -8, and -9 and TIMP-2 in tracheal aspirates from preterm infants with respiratory distress. Pediatrics 2001; 108: 686–692
