Abstract
Objective
Opioid use in pregnant women is a growing public health concern and is shown to be associated with lower infant birth weights. Placental volume changes in prior studies correlated with various maternal and fetal conditions. We aimed to identify differences between placental volumes in pregnant women with opioid use, and control pregnant women without drug use.
Methods
We prospectively recruited 27 healthy pregnant women and 17 pregnant women with opioid use disorder who were on medication-assisted treatment (MAT). All women underwent placenta/fetal MRI at 27–39 weeks gestation on a 3 Tesla MR scanner. Placental volumes were measured in a blinded fashion using a previously validated technique. Multiple linear regression was used to identify associations of placental volume with multiple maternal and fetal clinical factors. The significance threshold was set at p < .05.
Results
Placental volume was significantly associated with gestational age at MRI (p < .0001), fetal sex (p = .027), MAT with smoking (p = .0008), MAT with polysubstance use (p = .01), and maternal BMI (p = .032). Placental volume was not associated with opioid MAT alone in our cohort.
Conclusion
For pregnant women on medication-assisted treatment for opioid use disorder, there was no significant difference in placental volume compared to healthy pregnant women. However, concomitant smoking and polysubstance use in the setting of medication-assisted treatment may be detrimental to placental health. To our knowledge, this is the first study assessing placental volume in opioid use on prenatal MRI. These results support the benefit of medication-assisted treatment during pregnancy; however additional studies are needed to further elucidate the impact of opioid use on placental and fetal development and postnatal outcomes.
Introduction
Opioid use in the United States has increased significantly over the past few decades, and there has been a parallel increase in opioid use in pregnant women [Citation1,Citation2]. Opioid use in pregnancy is known to increase the risk of maternal and fetal/neonatal morbidity and mortality, including increased risk of maternal delivery complications, intrauterine growth restriction, and neonatal abstinence syndrome [Citation1,Citation3]. Many adverse outcomes in pregnancy have been associated with abnormal placental function [Citation4]. In particular, placental insufficiency is shown to be associated with intrauterine growth restriction, and numerous studies have demonstrated significant relationships between placental size and birth weight [Citation5–7]. Opioids are known to cross the blood-placenta barrier, exposing the developing fetus to their effects. In humans and some other mammals, placental trophoblast cells—the cells directly exposed to maternal blood—express opioid receptors. Some evidence suggests that these receptors are involved in important developmental processes by promoting or preventing the production of several different cytokines and hormones [Citation8]. However, little is known about the direct effects of exogenous opioid use on the placenta. One study in mice showed sex-dependent changes in placental genetic expression in opioid use [Citation9], while multiple retrospective studies in human women showed increases in delayed villous maturation in placentas exposed to buprenorphine or methadone [Citation10,Citation11]. A study on neonates with Neonatal Opioid Withdrawal Syndrome (NOWS) evaluated the entire placental transcriptome of these infants and found changes in several gene expression pathways [Citation12]. One histologic study demonstrated larger placental volumes after delivery, and lower birth weights in the setting of medication-assisted treatment during pregnancy [Citation11]. However, there have so far been no studies evaluating the opioid exposure-related gestational alterations in the human placenta.
Though ultrasound remains an efficient and ubiquitous method of evaluating the placenta earlier in pregnancy, MRI is valuable in the consistent evaluation of placental volume and other characteristics, especially later in gestation. New, faster MR sequences allow for easier acquisition of quality images despite the fetal movement, and the larger field of view allows for easier visualization of the entire placenta as well as maternal pelvic organs. Historically, placental MRI has been used clinically in the evaluation of known or suspected placenta accreta spectrum. More recently, placental MR imaging is being used to evaluate the placenta more quantitatively, including in the assessment of normal placental development as well as the evaluation of perfusion and oxygenation status [Citation13–16]. Further, ventures into 2D time-of-flight imaging have allowed for the evaluation of placental vasculature via MR angiography [Citation17], while MR spectroscopy is being explored in the assessment of placental metabolism [Citation13]. With these advantages and continued advancements in image resolution and functional assessment, prenatal MRI continues to add value as a complementary modality in placental evaluation [Citation18].
The objective of this study was to evaluate for changes in placental volume in pregnant women with opioid use disorder (OUD) on medication-assisted treatment (MAT) with buprenorphine or methadone. As much is still unknown about placental function and pathology at this time, evaluation of placental size remains the first step in assessing overall placental function. Prior studies have evaluated the opioid-exposed placenta after delivery, but to our knowledge, there are no studies that have assessed the effects of opioids on the placenta before birth. In this study, we used semi-automated segmentation of the placenta from placental MRI to assess placental volume in pregnant women with OUD and medication-assisted treatment, as well as other comorbidities that are associated with this condition, especially smoking and polysubstance use.
Methods
Study participants
This prospective study was performed with Indiana University Institutional Review Board approval as a part of an ongoing study in mothers and infants with prenatal opioid exposure (IRB #1807356299) [Citation19]. To assess placental development in the setting of prenatal opioid exposure, healthy pregnant women and pregnant women with opioid use disorder were recruited to participate in this study. All pregnant women using opioids were recruited from antenatal medication-assisted treatment clinics at Indiana University Health hospital and were on buprenorphine or methadone at the time of enrollment. All control group pregnant women were recruited from healthy antenatal clinics in the same health system. Written informed consent was obtained from all pregnant women. Maternal clinical information was obtained by chart review and subject self-report and included age, race, parity, body mass index (BMI), history of tobacco use, and self-reported use of other substances during and before the current pregnancy. Chart review was also used to assess for a history of gestational diabetes and hypertensive disorders (hypertension, pre-eclampsia, or eclampsia). For the subjects with opioid use, dose of opioid replacement therapy before and at the time of MRI was determined via chart review. Chart review was also used to obtain neonatal clinical information, such as infant birth weight, neonate gestational age at MRI and at birth, and infant sex.
Image acquisition
Placental-fetal MRI was performed on a 3 Tesla (3 T) Skyra MR scanner (Siemens Healthineers, Erlangen, Germany). Women were positioned supine and/or lateral decubitus. Dedicated imaging through the placenta included Half-Fourier Acquired Single-shot Turbo spin Echo (HASTE) images in the axial, sagittal, and coronal planes, with TE 100 ms and TR 1000 ms. Images were obtained with a slice thickness of 5 mm with no gap between slices. The intraslice resolution was ∼2 × 2.5 mm. is a representative image from the sagittal HASTE sequence through the placenta. Additional evaluation of the fetus was performed using the following sequences: True fast imaging with steady-state free precession (TRUFISP), T1 volume interpolated breath-hold examination (VIBE), diffusion-weighted, and gradient echo (GRE) images.
Imaging review
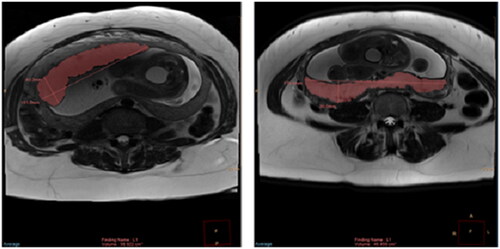
We reviewed magnetic resonance images of each subject to identify the plane that allowed for the clearest visualization of the entire placenta including placental margins; for the majority of study subjects, this was the axial plane. For a few subjects, the use of the sagittal or coronal plane was required due to suboptimal axial image quality or incomplete visualization of the placenta on axial due to placental position. One trained observer (R.L.W., physician in residency), who was blinded to clinical data, performed semi-automated placental segmentation () using Phillips Intellispace Tumor Tracking Tool (Koninklijke Philips N.V., Amsterdam, The Netherlands), as previously validated at our institution [Citation16]. A sample of placental volumes was verified by a board-certified pediatric radiologist (B.P.B.) with experience in placental imaging. demonstrates the calculation of cross-sectional placental area on MR images from two different subjects. Phillips Intellispace software was then used to sum the placental area on each slice and multiply by the image slice thickness to yield the total placental volume. Placental volume was recorded for each subject.
Figure 2. Axial HASTE image through the placenta, prior to (left image) and after (right image) semiautomated segmentation.
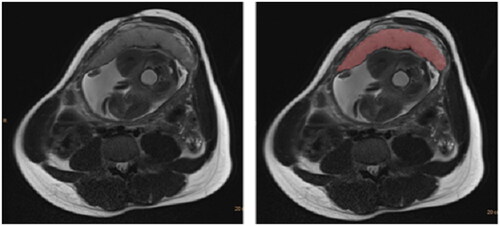
Figure 3. Axial HASTE images through the placentas of two different subjects demonstrating the semiautomated segmentation for calculation of cross-sectional area of placenta on each slice. The placental area on each slice was then summed up and multiplied by the image slice thickness to yield total placental volume. Linear calipers are automatically generated by the software.
Statistical analysis
Descriptive statistics were performed. Multiple linear regression was performed using placental volumes as the dependent variable and independent variables chosen based on existing literature and those of interest, including medication treatment for opioid use disorder, gestational age at the time of MRI, maternal age, maternal body mass index (BMI) closest to time of MRI, presence of smoking, polysubstance use, maternal parity, maternal gestational diabetes, hypertensive disorders of pregnancy, and fetal sex. All statistical analysis was performed using R statistical software [Citation20]. p-Value of <.05 was considered statistically significant.
Results
Demographics
A total of 44 pregnancies carried out by 43 study participants underwent fetal/placental MRI. One woman participated in the study during two separate pregnancies, which for the purposes of this study were considered to be separate events. The control group was made up of 27 women, 20 of whom self-identified as white non-Hispanic and one of whom identified as white Hispanic. The remaining six women in the control group identified as African-American or multiple races. The opioid-using group was made up of 17 women, 15 of whom self-identified as white non-Hispanic and one of whom identified as white Hispanic. One woman in this group reported multiple races. Twenty-three of the imaged fetuses were male (12 in the opioid-exposed group and 11 in the control group), and 21 were female. There were 17 fetuses (12 male) with in-utero opioid exposure and 27 fetuses (11 male) without in-utero opioid exposure. Seventeen of the pregnant women were on medication-assisted treatment for opioid use disorder during their pregnancy. Fifteen pregnant women were prescribed buprenorphine throughout gestation (with a dosage ranging from 6 mg daily to 24 mg daily), while one woman was prescribed methadone (44 mg daily). The dosage for each woman was titrated according to her experienced symptoms and managed by the clinical team. One subject was prescribed buprenorphine through most of her pregnancy (up to 12 mg daily at the time of MRI) but switched to methadone during her third trimester (after the time of MRI). Fourteen of the 17 women in the opioid using group also reported smoking. None of the women in the control group reported smoking. Of the 17 fetuses with in-utero opioid exposure, 10 had variable exposure to multiple other drugs during pregnancy including cannabinoids, benzodiazepines, cocaine, methamphetamine, and alcohol. One of the non-opioid-exposed fetuses had exposure to another substance (cannabinoids). details the demographics of the study group. There were no significant differences in average maternal age, maternal BMI, gestational age at MRI or birth, or birth weight between the two study groups.
Table 1. Study population characteristics.
Placental volume
shows the results of the assessment for the association between placental volume and maternal and fetal clinical factors. Placental volume was significantly associated with gestational age at MRI (p < .0001), MAT and smoking (p = .0008), MAT and polysubstance use (p = .01), maternal BMI (p = .032), and male fetal sex (p = .027). Larger placental volumes were associated with greater gestational age at the time of MRI, higher maternal BMI, and in the setting of maternal smoking with MAT. Placental volumes were significantly smaller in the setting of MAT with concomitant polysubstance use, and male fetal sex. Placental volume was not significantly associated with MAT alone, gestational diabetes, hypertensive disorders, maternal age, or parity. Additionally, placental volumes were significantly correlated with birth weight (p = .049) when corrected for gestational age at MRI, gestational age at birth, and fetal sex ().
Table 2. Linear regression model of placental size and multiple independent covariates.
Table 3. Linear regression model of birth weight and multiple independent covariates including the placental volume on placental MRI.
Discussion
Opioid use disorder (OUD) during pregnancy increases the risk of poor perinatal outcomes for both the pregnant mother and her fetus. Medication-assisted treatment is the standard of care for pregnant women with OUD, leading to overall better adherence to prenatal care, lower risk of maternal withdrawal, and decreased risk of obstetric complications [Citation3]. Ours is the first study showing placental volume alterations on MRI in pregnant women with OUD and other high risk conditions of smoking and polysubstance use.
The placenta has historically been considered a passive organ, allowing the transport of nutrients and waste between mother and fetus. However, recent consideration has been given to the role of the placenta as an active mediator of fetal exposure and a direct target of substances and medications. Opioids and other substances may be metabolized by the placenta and may also impact placental function. Placental aromatase is known to be involved in the metabolism of methadone and buprenorphine as well as some other medications, and methadone exposure has in turn been shown to down-regulate placental aromatase activity [Citation8,Citation21], impacting downstream pathways including those responsible for the biosynthesis of estrogens.
Our study found the expected association of increased placental volume with increasing gestational age at the time of MRI, which is concordant with the known trend in normal placental development. In our evaluation of placental volumes in women with opioid use disorder, we found no significant difference between placental volume in pregnant women on medication-assisted treatment and placental volume in pregnancies without opioid exposure. These findings are in contradiction to those described in a histologic study by Staszewski et al. which found greater placental at delivery in subjects undergoing medication-assisted treatment compared to healthy pregnancies [Citation11]. This is likely because we considered other covariates in our model including maternal age, BMI, parity, use of other substances, and smoking. While our model is more complex, it also is more representative of the population of pregnant women with opioid use disorder and of the multiple variables that may influence placental growth and function in pregnancy. As placental size may be one indicator of placental and fetal health, and we showed placental volume correlated with infant birth weight, the lack of significant changes in placental volume in our opioid-exposed group may represent a beneficial effect of medication-assisted treatment on placental development. These results parallel the protective effects of medication-assisted treatment on neonatal brain development described in a separate study by Liu et al. [Citation22]. However, the presence of concomitant smoking or other substance use in those with medication-assisted treatment for OUD was associated with significantly altered placental volume. Our results suggest that medication-assisted treatment may “protect” against alterations in placental volume that may occur in the setting of untreated OUD; however, efforts must be directed at decreasing concomitant smoking and polysubstance use in this population.
We found significantly smaller placental volume in pregnant women on medication-assisted treatment with concurrent polysubstance use. Other substances used by some pregnant women in our study included cannabinoids, benzodiazepines, cocaine, methamphetamine, and alcohol. Alcohol, cocaine, and marijuana are known to have varying detrimental effects on birth weight and neonatal outcomes [Citation23,Citation24] and therefore might be expected to also negatively impact placental development. Methamphetamine use during pregnancy has also been shown to be associated with decreased birth weight [Citation25], and similar effects have been shown in pregnancies complicated by benzodiazepine use [Citation26]. Our study is in line with existing literature regarding the detrimental effects of polysubstance use on placental-fetal health.
There is not much literature on the effects of smoking on placental development. While it is well-known that smoking during pregnancy increases the risk of low birth weight, the direct impact of tobacco use on the placenta remains undefined. Our study found larger placental volumes in pregnancies in which the mother was on MAT and also smoked cigarettes. This is similar to the results of a study performed by Mitsuda et al. which examined placental weight in the setting of smoking [Citation27] but is inconsistent with some other studies [Citation28]. One study found that smoking during pregnancy was associated with overall lower placental weight, but also demonstrated an association with higher relative placental weights when accounting for the resulting smaller infant birthweight after exposure to smoking [Citation29]. While published data is inconclusive regarding the effects of smoking on placental volume, our results suggest a possible compensatory hypertrophy of the placenta in the setting of MAT and tobacco exposure in our cohort. Further studies examining subjects with tobacco or opioid exposure alone are needed to clarify this issue.
We found a positive association between maternal BMI and placental volume in this cohort. Because body mass index can be associated with several metabolic and physiologic factors, and because BMI is often a fluid metric in pregnancy given the necessary gestational weight gain, it is likely there is a multifactorial relationship between maternal body mass index, placental volume, and infant birthweight [Citation6].
In our cohort, male fetal sex was associated with smaller placental volumes. Recent studies have shown differing placental characteristics and functions with male or female fetal sex [Citation30,Citation31]. Our results are consistent with prior findings of sex-dependent placental volumes and could suggest more robust compensatory mechanisms in the placenta in female fetuses.
Several limitations of this study are discussed in the preceding paragraphs. Overall, this study is limited most by the small size of this cohort. Future studies with larger numbers of pregnant women are needed to better evaluate for the placental impact of opioid use disorder in pregnancy. Additionally, we acknowledge the fact that not all opioid use is equal, and it may be difficult to generalize our results in a population on medication-assisted treatment to a larger population which includes untreated opioid use disorder. Despite the contributions to prenatal care that could result from studying untreated opioid use disorder in pregnancy, ethical considerations make research on this population challenging.
Conclusion
Opioid use disorder is increasing in the general population, with a parallel increase in pregnant women. We used fetal MRI to evaluate placental volumes in women with and without opioid use disorder and found no significant association between medication-assisted treatment and placental volume. However, MAT with polysubstance use or smoking significantly impacted placental volumes, suggesting that comprehensive management of the opioid disorder in pregnancy must also address these additional comorbidities. The ability to assess these opioid and other comorbid effects on placental and consequently fetal health is crucial to help mitigate adverse perinatal and infant outcomes.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Maeda A, Bateman BT, Clancy CR, et al. Opioid abuse and dependence during pregnancy: temporal trends and obstetrical outcomes. Anesthesiology. 2014;121(6):1158–1165.
- Hirai AH, Ko JY, Owens PL, et al. Neonatal abstinence syndrome and maternal opioid-related diagnoses in the US, 2010–2017. JAMA. 2021;325(2):146–155.
- Committee opinion no. 711: opioid use and opioid use disorder in pregnancy. Obstet Gynecol. 2017;130(2):e81–e94.
- Smith GC. First-trimester determination of complications of late pregnancy. JAMA. 2010;303(6):561–562.
- Sun C, Groom KM, Oyston C, et al. The placenta in fetal growth restriction: what is going wrong? Placenta. 2020;96:10–18.
- Brett KE, Ferraro ZM, Yockell-Lelievre J, et al. Maternal-fetal nutrient transport in pregnancy pathologies: the role of the placenta. Int J Mol Sci. 2014;15(9):16153–16185.
- Effendi M, Demers S, Giguère Y, et al. Association between first-trimester placental volume and birth weight. Placenta. 2014;35(2):99–102.
- Rosenfeld CS. The placenta as a target of opioid drugs. Biol Reprod. 2022;106(4):676–686.
- Green MT, Martin RE, Kinkade JA, et al. Maternal oxycodone treatment causes pathophysiological changes in the mouse placenta. Placenta. 2020;100:96–110.
- Serra AE, Lemon LS, Mokhtari NB, et al. Delayed villous maturation in term placentas exposed to opioid maintenance therapy: a retrospective cohort study. Am J Obstet Gynecol. 2017;216(4):418.e1–418.e5.
- Staszewski C, Herrera KM, Kertowidjojo E, et al. Histological changes observed in placentas exposed to medication-assisted treatment. J Pregnancy. 2021;2021:2175026.
- Radhakrishna U, Nath SK, Vishweswaraiah S, et al. Maternal opioid use disorder: placental transcriptome analysis for neonatal opioid withdrawal syndrome. Genomics. 2021;113(6):3610–3617.
- Andescavage NN, Du Plessis A, Limperopoulos C. Advanced MR imaging of the placenta: exploring the in utero placenta-brain connection. Semin Perinatol. 2015;39(2):113–123.
- Andescavage N, Kapse K, Lu YC, et al. Normative placental structure in pregnancy using quantitative magnetic resonance imaging. Placenta. 2021;112:172–179.
- Capuani S, Guerreri M, Antonelli A, et al. Diffusion and perfusion quantified by magnetic resonance imaging are markers of human placenta development in normal pregnancy. Placenta. 2017;58:33–39.
- León RL, Li KT, Brown BP. A retrospective segmentation analysis of placental volume by magnetic resonance imaging from first trimester to term gestation. Pediatr Radiol. 2018;48(13):1936–1944.
- Neelavalli J, Krishnamurthy U, Jella PK, et al. Magnetic resonance angiography of fetal vasculature at 3.0 T. Eur Radiol. 2016;26(12):4570–4576.
- Zaghal AA, Hussain HK, Berjawi GA. MRI evaluation of the placenta from normal variants to abnormalities of implantation and malignancies. J Magn Reson Imaging. 2019;50(6):1702–1717.
- Radhakrishnan R, Brown BP, Haas DM, et al. Pilot study of fetal brain development and morphometry in prenatal opioid exposure and smoking on fetal MRI. J Neuroradiol. 2022;49(1):53–58.
- R Core Team. R: a language and environment for statistical computing. Vienna: R Core Team; 2016. Available from: https://www.R-project.org/
- Rubinchik-Stern M, Eyal S. Drug interactions at the human placenta: what is the evidence? Front Pharmacol. 2012;3:126.
- Liu J, Grewen K, Gao W. Evidence for the normalization effects of medication for opioid use disorder on functional connectivity in neonates with prenatal opioid exposure. J Neurosci. 2022;42(22):4555–4566.
- Janisse JJ, Bailey BA, Ager J, et al. Alcohol, tobacco, cocaine, and marijuana use: relative contributions to preterm delivery and fetal growth restriction. Subst Abus. 2014;35(1):60–67.
- Bada HS, Das A, Bauer CR, et al. Low birth weight and preterm births: etiologic fraction attributable to prenatal drug exposure. J Perinatol. 2005;25(10):631–637.
- Smith LM, LaGasse LL, Derauf C, et al. The infant development, environment, and lifestyle study: effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics. 2006;118(3):1149–1156.
- Wikner BN, Stiller CO, Bergman U, et al. Use of benzodiazepines and benzodiazepine receptor agonists during pregnancy: neonatal outcome and congenital malformations. Pharmacoepidemiol Drug Saf. 2007;16(11):1203–1210.
- Mitsuda N, N Awn JP, Eitoku M, et al. Association between maternal active smoking during pregnancy and placental weight: the Japan environment and children’s study. Placenta. 2020;94:48–53.
- Wang N, Tikellis G, Sun C, et al. The effect of maternal prenatal smoking and alcohol consumption on the placenta-to-birth weight ratio. Placenta. 2014;35(7):437–441.
- McNamara H, Hutcheon JA, Platt RW, et al. Risk factors for high and low placental weight. Paediatr Perinat Epidemiol. 2014;28(2):97–105.
- Er P, Ijr NA. Fetal gender-specific difference for placental volume assessed with 3D-ultrasonography. Perinatal Journal. 2016;24(3):156–161.
- Clifton VL. Review: sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31 Suppl:S33–S39.