Abstract
Purpose
To determine the association between the occurrence of sporadic and periodic fetal heart rate accelerations during labor and acidemia at birth.
Materials and methods
This is a case–control study of fetal heart rate patterns from 364 neonates with acidemia at birth (cord blood pH <7.05 at vaginal birth, or pH <7.10 at birth after first stage cesarean delivery) and 731 controls with pH ≥7.15. The last 30–60 min of the cardiotocographic traces before birth from the neonates born with acidemia and from the corresponding stage in labor for the controls were scrutinized. Odds ratios (OR) with 95% confidence interval for acidemia at birth were determined.
Results
During the first stage, ≥2 sporadic accelerations were present in 16% of cases and 78% of controls; OR for acidemia (compared to 0–1 accelerations) 0.05 (0.02–0.10). In the second stage, the corresponding rates were 13% and 60%, OR 0.09 (0.06–0.14). Isolated periodic accelerations were infrequent. A weak negative association between ≥2 periodic accelerations and acidemia (compared with 0–1 accelerations) was found in the second stage, OR 0.51 (0.30–0.86), but was not significant in the first stage, OR 0.24 (0.04–1.4). Even among fetuses with normal fetal heart rate variability (5–25 beats per minute) the occurrence of less than two sporadic accelerations was associated with an increased risk of acidemia, OR 10.3 (7.2–14.8).
Conclusions
Sporadic accelerations indicate a very low probability of acidosis but are absent in 40% of fetuses with normal pH during a 30–60 min second-stage recording.
Introduction
In term pregnancy, an acceleration is defined as a transient elevation of the fetal heart rate of at least 15 beats per minute, bpm, lasting for at least 15 s [Citation1–3]. During labor, accelerations may be categorized as sporadic, having no relation in time to uterine contractions, and periodic, occurring during uterine contractions [Citation4–6].
In the late sixties, accelerations were demonstrated to occur simultaneously with fetal movements [Citation7] but the significance of accelerations was unknown [Citation6]. Later, fetuses having accelerations in the first stage of labor were found to have pH > 7.3 at the time of fetal blood sampling [Citation8].
Krebs stated that fetuses exhibiting three or more sporadic accelerations in 30 min had a favorable outcome, whereas periodic accelerations during contractions may progress into variable decelerations [Citation4]. A periodic acceleration has been explained as a sympathetic response to diminished venous return [Citation9], mediated by pressor receptor reflex due to intermittently disturbed umbilico-placental perfusion [Citation10]. Thus, periodic accelerations may be an early sign of cord compression [Citation9].
Lack of accelerations has been associated with acidemia [Citation4,Citation11]. Guidelines indicate that the presence of accelerations is reassuring [Citation1–3], but accelerations are not specified as sporadic and periodic. Al-Fahdi et al. alerted that not all transient increases in fetal heart rate are true accelerations [Citation12].
Assessment of accelerations is not part of present FIGO [Citation1], NICE [Citation2], or Swedish [Citation13] guidelines for the classification of intrapartum CTG, since a lack of accelerations is unspecific [Citation1] and common even without hypoxia [Citation13]. In previous studies, we found that the sensitivity of the FIGO guideline from 2015, as well as the modified Swedish version from 2017, had lower sensitivity to identify acidosis with a pathological pattern compared to a previous Swedish template [Citation14,Citation15]. One of the differences between these templates is that the older template [Citation16] stipulated at least two accelerations per hour for the classification “normal.”
The primary aim of this study was to assess the association between the presence of sporadic and periodic accelerations and acidemia at birth. We also intended to assess if the association between variability and acidemia was changed by the presence of accelerations. Finally, we intended to evaluate how frequently accelerations were present in nonacidemic fetuses during the different stages of labor, and if other factors than acidemia influenced the presence of accelerations.
Materials and methods
This is a case–control study including cases with acidemia at birth as an indicator for exposure to hypoxia during labor, and controls with normal pH at birth.
The present study was performed on compiled CTG recordings and obstetric data from two previous studies [Citation14,Citation15] at Skåne University Hospital and Helsingborg Hospital 2012–2017. The present study includes all neonates with acidosis (as defined below) born during the study period, fulfilling the inclusion criteria.
Acidemia at birth was defined as cord arterial or venous pH <7.05 at vaginal birth or at second stage cesarean delivery (CD), or pH <7.10 at birth by first stage CD. Fetal pH declines during the second stage of labor [Citation17,Citation18], and therefore a higher pH for inclusion in the first stage of labor was chosen.
Inclusion criteria for both cases and controls were singleton pregnancy, labor after ≥34 + 0 gestational weeks, and available CTG trace for ≥30 min. A maximum interval of 30 min from the end of the CTG to delivery was allowed for CD. For vaginal births, no interval was allowed. Both high and low risk pregnancies were included.
As controls were included the two consecutive neonates born at the same hospital after each case and meeting all inclusion criteria, including umbilical cord arterial and venous pH ≥7.15 and at least 0.02 apart, to reassure samples from both vessels [Citation19], and Apgar scores 9 or 10 at five and 10 min. Since the inclusion criteria for controls in the previous two studies were not identical, 33 controls were replaced by new controls full filling these uniform criteria.
Careful consideration was made to exclude erroneously monitored maternal pulse as fetal accelerations. A visual examination of all included CTGs for patterns considered as high risk for maternal pulse [Citation12,Citation20,Citation21] were done by two of the authors. In 11 cases and 3 controls, apparent sequences of maternal heart rate were found. In 13 of these, the main part of the tracing was found to be correct registration of fetal heart rate where fetal accelerations could be distinguished with certainty, whereas one recording, from a case, was judged to be mostly maternal pulse and was excluded.
The total number of analyzed CTGs was therefore 1095, i.e. 364 from acidotic neonates and 731 from controls. Of the cases, 70 were delivered by CD in the first stage of labor, and 295 were born vaginally (n = 271) or by CD (n = 24) in the second stage.
For acidemic neonates the fetal heart rate trace from the last 30–60 min before vaginal birth or before termination prior to emergency CD, was analyzed. For controls matched to cases delivered vaginally or by CD in the second stage, the last 30–60 min of CTG before second stage birth were analyzed. For controls matched to cases delivered by CD in the first stage, 30–60 min of CTG recording at the same cervical dilation as the case were analyzed. Thus, cases and controls were not matched for the delivery mode.
As described previously, each CTG trace has been systematically assessed by three clinicians blinded to group and outcome [Citation14,Citation15]. In the present analysis of variability, the categorization of variability by the majority of observers was used. Normal variability was defined as 5–25 bpm. We sub-categorized reduced variability into “reduced” as visually assessed to be 2–4 bpm, and “absent” when visually assessed to be 0–1 bpm, in accordance with the CTG-classification used together with ST-analysis of the fetal ECG [Citation22] and with the national Swedish CTG template used during 2009–2017 [16]. For the classification of reduced variability (2–4 bpm), a duration of 50 min was required, consistent with the FIGO-15 classification [Citation1]. For absent variability, there was no time limit for classification according to the CTG-classifications above [Citation16,Citation22]. For the classification, increased variability (more than 25 bpm) and a duration of 30 min was needed, consistent with the FIGO-15 classification [Citation1].
All CTG traces were reassessed by the first author (FE) to differentiate between sporadic and periodic accelerations. An acceleration was defined as a transient elevation of the fetal heart rate of at least 15 bpm for at least 15 s. The baseline fetal heart rate before and after the acceleration had to be identified. Accelerations were classified as sporadic when occurring without association to contractions, and as periodic, occurring simultaneously with contractions. If there were any doubts about classification, another author (AH) also assessed the trace, and type of accelerations was agreed upon. It was not possible to keep the first author blind to outcome, whereas the second author was blinded to outcome.
A paper speed of 1 cm/min was used in all cardiotocograms. This is the standard for all interpreters in the study.
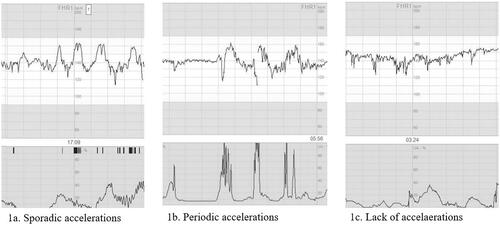
We categorized the occurrence of accelerations during the studied 30–60 min into three groups: (1) at least two sporadic accelerations; (2) less than two sporadic accelerations, but at least two periodic accelerations; (3) less than two sporadic and less than two periodic accelerations ().
Figure 1. Accelerations as defined in the study. (a) Sporadic accelerations, with a raise in basal heart line of at least 15 beats per minute, lasting for at least 15 s and occurring without relation to contractions. (b) Periodic accelerations, with a raise in basal heart line of at least 15 beats per minute, lasting for at least 15 s and occurring simultaneously with contractions. (c) No transient raises in basal heart line fulfilling the definition of accelerations.
In order to assess whether other variables than acidemia had influence on the presence of sporadic accelerations, the study included a logistic regression analysis between the presence of accelerations and other intrapartum factors in the control group.
OR for acidemia with the presence of ≥2 accelerations during the analyzed 30–60 min of CTG tracing was analyzed separately for the first and second stage of labor, and separately for sporadic and only periodic accelerations. Sub-analyses were made excluding cases and controls with less than 60 min of recording.
OR was also calculated for acidemia with reduced variability, and for increased variability, with and without accelerations.
Unadjusted and adjusted OR for the absence of accelerations for the following intrapartum factors was analyzed: stage of labor (cervical dilation 3–6 cm, 6–9 cm, and ≥10 cm), analgesia (opioids, N2O gas, epidural), oxytocin augmentation, method of monitoring (internal vs. external), and membrane rupture.
Statistical analyses
Stat View® computer software (SAS Institute, version 5.0.1; Cary, NC, USA) was used to gather the data. SPSS statistical software (version 25.0; SPSS, Chicago, IL, USA) was used to analyze the data. OR with 95% confidence intervals were calculated for the association of different patterns to acidosis.
To analyze the independent associations between different labor characteristics and the presence of sporadic accelerations, a logistic regression analysis was performed, adjusted for the studied intrapartum variables as well as for cord blood pH as a continuous variable.
A p-value <.05, and a 95% confidence interval not including 1 was considered statistically significant.
Details of ethics approval
Ethical approval was obtained from the Regional Ethical Review Board in Lund, Dnr 2016/371, 24 May 2016.
Results
This case–control study encompassed 1096 births, including all 365 neonates born with acidosis (as defined above) during the study period and 731 controls. One case was excluded due to erroneously registered maternal pulse, leaving 1095 CTG.
During the first stage, sporadic accelerations were present in 16% of cases and 78% of controls; OR for acidemia 0.05 (0.02–0.10) (). In the second stage, the corresponding rates were 13% and 60%; OR 0.09 (0.06–0.14). The estimates were similar when only tracings of 60 min duration were included.
Table 1. Occurrence of accelerations during the last CTG recording before birth in cases, and for controls at the same cervical dilation. Odds ratio for acidemia with 95% CI. Accelerations are defined as a transient raise in basal heart frequency of at least 15 bpm for at least 15 s.
Isolated periodic accelerations were more infrequent and showed a weaker negative association with acidemia in the second stage; OR 0.51 (0.30–0.86), and not significantly in the first stage; OR 0.24 (0.04–1.4), or when only tracings of 60 min were included.
Associations between fetal heart rate variability, sporadic accelerations and acidosis are shown in . Reduced, but not absent, variability (2–4 bpm) with sporadic accelerations was not seen in any neonate with acidosis but was strongly associated with acidemia if accelerations were absent (OR 36). Absent variability (<2 bpm) was highly associated with acidemia regardless of if accelerations occurred in the trace (OR 43) or not (OR 478). Absent variability occurred in only 3 of 731, all in the second stage.
Table 2a. Association between variability and acidemia. All traces (duration 30–30 min) included.
Table 2b. Association between acidemia at birth, variability in bpm and the presence of sporadic accelerations.
Even with a normal variability the absence of sporadic accelerations was associated with a higher risk of acidosis (OR 10), although this pattern occurred in 35% of controls without acidemia. In contrast, decreased variability (2–4 bpm) with absent accelerations was only seen in 1% of controls (OR for acidemia 36).
Among controls, 37% of tracings did not show sporadic accelerations. Intrapartum factors associated with absent accelerations were internal monitoring with scalp electrode, second stage of labor, ruptured membranes, opioid administration, epidural, and oxytocin augmentation. In regression analysis, only the second stage of labor and monitoring with a scalp electrode were significantly associated with absence of accelerations (). Sporadic accelerations among controls were identified in 39% with scalp electrode and in 67% with external monitoring; adjusted OR 0.49 (0.30–0.79). Within the normal range (>7.15), cord artery pH was not correlated to the presence of accelerations.
Table 3. Associations between the occurrence of at least two sporadic accelerations or not, and mode of monitoring, cervical dilatation, rupture of membranes, analgesia, and oxytocin administration in fetuses with umbilical cord arterial or venous pH ≥7.15 and Apgar scores 9–10 at 5 min. CTG traces of 30–60 min duration.
Discussion
The presence of sporadic accelerations in the first stage as well as in the second stage of labor strongly lowered the risk for low pH at birth. This is in concordance with Ogunyemi et al. reporting that accelerations during the last hour of labor were negatively associated with umbilical artery pH <7 at birth (OR 0.2), base excess >12 (OR 0.58), NICU admission (OR 0.6), and neonatal hypoxia (OR 0.4) [Citation23]. Marti Gamboa et al. found that the absence of accelerations during the second stage of labor had a higher validity for neonatal acidemia than a category II tracing [Citation24]. Spencer et al. found that the absence of accelerations and low fetal heart rate variability was associated with neonatal encephalopathy, whereas late decelerations were not [Citation25]. In contrast, Holzmann et al. reported no correlation between the duration since the last acceleration and increased lactate concentration at fetal scalp blood sampling on indication [Citation26].
In our study, reduced variability (2–4 bpm) was only associated with acidemia in the absence of sporadic accelerations. Sporadic accelerations together with reduced variability (2–4 bpm) was only seen in five neonates, all with normal pH. Due to the rarity of such patterns, we may not confidently conclude that decreased variability together with sporadic accelerations always is an innocuous pattern.
Isolated periodic accelerations compared with no accelerations did not imply a lower risk for acidemia during the first stage of labor, but it did slightly in the second stage. Periodic accelerations were more common in the second stage, supporting the theory that they are caused by pressure of the umbilical cord [Citation10]. Periodic accelerations are probably rather a precursor to variable decelerations and not a sign of fetal well-being. Since only sporadic accelerations had a clear negative association with acidosis, we consider it important to differ between sporadic and periodic accelerations.
Since controls lacked accelerations in 22% in the first stage and 40% in the second stage of labor, it was of interest to identify factors associated with the absence of sporadic accelerations in controls. The only variables independently associated with sporadic accelerations were the stage of labor and mode of monitoring. The incidence of accelerations coinciding with uterine contractions in the second stage of labor has previously been reported to occur less than half as often with a scalp electrode as with external monitoring [Citation20]. Accelerated heart rate during contractions may in some cases indicate erroneously monitored maternal pulse [Citation27,Citation28], and such errors might explain a higher rate of accelerations with external monitoring. However, we scrutinized the traces for maternal sequences, and a more likely explanation is that scalp electrode is more often used when the heart rate pattern is suspicious or pathological. Thus, the use of a scalp electrode may be an effect of the lack of accelerations rather than the opposite.
Accelerations in controls were less common in the second than in the first stage of labor, also after controlling for other factors. Putatively, the increased uterine forces in the second stage of labor [Citation29,Citation30] may inhibit fetal movements. It may also reflect the fetus conservation of energy at a time when contractions limit respiration through the feto-placental unit.
The administration of opioids or epidural analgesia was not associated with absence of accelerations, in concordance with previous studies [Citation31–34]. Previous studies have reported that accelerations were less frequent [Citation35] or unaltered with oxytocin augmentation [Citation26]. We did not find an independent association between oxytocin augmentation and absence of accelerations.
To our knowledge, our study is the first to assess the associations between acidemia at birth and the presence of sporadic and periodic accelerations separately.
A strength of the study is that fetal heart rate patterns were assessed by clinicians using CTG in their daily work, being blinded for group and outcome. A limitation is that the categorization of accelerations had to be done by the re-assessment of all traces by the first author, who was not blinded for group.
The duration of the CTGs were limited to 30–60 min. With the high number of traces, we did not find it feasible to assess the complete traces. We studied the last 60 min of CTG, with a minimum of 30 min if the registration was shorter. The similar results of the total analysis and the sub-analysis including only traces of 60 min indicates that including traces less than one hour had minor influence on the results.
In the first stage of labor a time span of 30 min between termination of the CTG and birth was allowed. Most (54 of 73) had a span of less than 15 min. The fetal heart rate pattern may improve or worsen during these minutes. A shorter minimum span, however, would have introduced a bias by only including cases clinically recognized as most emergent.
In this study, cord blood pH below <7.05, or <7.1 at first stage CD, was used as an indicator of exposure to intrapartum hypoxia. This definition may include cases with respiratory acidosis, which may be a limitation. Still, a cord artery pH <7.1 has been associated with a small but significantly increased risk of encephalopathy or death [Citation36], and in another study, pH <7.1 had a similar association to low 5-min Apgar scores as lactate >10 mmol/L, or pH <7.05 together with base excess >12 mmol/L [Citation37]. We therefore consider our inclusion criteria to be pertinent.
Even with a high number of examined traces, cases and controls with infrequent patterns such as increased variability were few, resulting in wide confidence intervals for some examined associations. This is a limitation of the study.
Despite a compelling association between the lack of accelerations and acidemia at birth, the positive predictive value of absent accelerations must be expected to be low, since acidosis is rare, and as many as 22% of controls did not show sporadic accelerations in the first stage and 40% in the second. In our population, 1.0% of newborns had cord artery pH <7.05 [14]. Thus, we may roughly estimate the positive predictive value of absent sporadic accelerations for such a degree of acidosis to be about 2.2%, and the negative predictive value to about 99.7%. Thus, isolated lack of accelerations is too unspecific to be graded as a pathological pattern.
This study focused on fetal heart rate accelerations. Of 364 cases with acidemia, only 50 expressed accelerations. Fifteen of these had an acute bradycardia. It is evident that in a clinical context other fetal heart rate variables must be assessed as well. Probably, the presence of sporadic accelerations may be of value in interpretation templates, to rule out asphyxia.
Conclusion
Sporadic fetal heart rate accelerations during labor are associated with normal cord artery pH and should be considered as a sign of fetal well-being. Periodic accelerations may be regarded as a more indifferent pattern.
We consider that the assessment of the presence and type of accelerations should be part of the interpretation of fetal heart rate patterns during labor. Further studies are merited to evaluate how the inclusion of sporadic accelerations in classification templates may improve the specificity to identify intrapartum hypoxia.
Preprint available
Sporadic fetal heart rate accelerations during labor practically rules out acidaemia; a case–control study. Authorea 13 May 2022, DOI: 10.22541/au.165244502.28410525/v1
Acknowledgments
All midwives and physicians who classified all the CTG tracings in the original studies are gratefully acknowledged. PhD Mats Pihlsgård, statistician, for help with the statistics.
Disclosure statement
The authors report that there are no competing interests to declare.
Additional information
Funding
References
- Ayres-de-Campos D, Spong CY, Chandraharan E, et al.. FIGO consensus guidelines on intrapartum fetal monitoring: cardiotocography. Int J Gynaecol Obstet. 2015;131(1):13–24.
- NICE Guideline. Intrapartum care for healthy women and babies. 2014. Available from: https://www.nice.org.uk/guidance/cg190/chapter/Recommendations#monitoring-during-labour.
- ACOG Practice Bulletin No 106. Intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles. Obstet Gynecol. 2009;114(1):192–202.
- Krebs HB, Petres RE, Dunn LJ, et al. Intrapartum fetal heart rate monitoring. VI. Prognostic significance of accelerations. Am J Obstet Gynecol. 1982;142(3):297–305.
- Murphy KW, Turnbull A. Fetal heart rate accelerations in second-stage labour; two case reports. Eur J Obstet Gynecol Reprod Biol. 1989;32(2):163–168.
- Kubli FW, Hon EH, Khazin AF, et al. Observations on heart rate and pH in the human fetus during labor. Am J Obstet Gynecol. 1969;104(8):1190–1206.
- John AH. Effect of foetal movements on foetal heart rate. J Obstet Gynaecol Br Commonw. 1967;74(1):60–63.
- Beard RW, Filshie GM, Knight CA, et al. The significance of the changes in the continuous fetal heart rate in the first stage of labour. J Obstet Gynaecol Br Commonw. 1971;78(10):865–881.
- James LS, Yeh MN, Morishima HO, et al. Umbilical vein occlusion and transient acceleration of the fetal heart rate. Experimental observations in subhuman primates. Am J Obstet Gynecol. 1976;126(2):276–283.
- Eichhorn KH, Gross W, Seewald HJ, et al. Fetal heart rate accelerations and fetal oxygen pressure Sub partu. Zentralbl Gynakol. 1988;110(21):1377–1384.
- Milsom I, Ladfors L, Thiringer K, et al. Influence of maternal, obstetric and fetal risk factors on the prevalence of birth asphyxia at term in a Swedish urban population. Acta Obstet Gynecol Scand. 2002;81(10):909–917.
- Al Fahdi B, Chandraharan E. True vs spurious intrapartum fetal heart rate accelerations on the cardiotocograph (CTG): an urgent need for caution. Global J Reprod Med. 2020;7(5):96–104.
- SFOG. CTG och fosterövervakning Sweden: CTG-utbildning. 2017. Available from: http://ctgutbildning.se.
- Ekengård F, Cardell M, Herbst A. Low sensitivity of the new FIGO classification system for electronic fetal monitoring to identify fetal acidosis in the second stage of labor. Eur J Obstet Gynecol Reprod Biol X. 2021;9:100120.
- Ekengård F, Cardell M, Herbst A. Impaired validity of the new FIGO and Swedish CTG classification templates to identify fetal acidosis in the first stage of labor. J Matern-Fetal Neonatal Med. 2022;35(25):4853–4860.
- SFOG. CTG kort slutversion Sweden: SFOG. 2009. Available from: https://www.sfog.se/media/17094/ctg_kort__slutversion.pdf
- Wiberg N, Kallen K. Fetal scalp blood lactate during second stage of labor: determination of reference values and impact of obstetrical interventions. J Matern Fetal Neonatal Med. 2017;30(5):612–617.
- Bretscher J, Saling E. pH values in the human fetus during labor. Am J Obstet Gynecol. 1967;97(7):906–911.
- Mokarami P, Wiberg N, Källén K, et al. Arterio-venous blood gas Δvalues for validation of umbilical cord blood samples at birth are not only biased by sample mix ups but also affected by clinical factors. Acta Obstet Gynecol Scand. 2019;98(2):167–175.
- Nurani R, Chandraharan E, Lowe V, et al. Misidentification of maternal heart rate as fetal on cardiotocography during the second stage of labor: the role of the fetal electrocardiograph. Acta Obstet Gynecol Scand. 2012;91(12):1428–1432.
- Pinto P, Costa-Santos C, Gonçalves H, et al. Improvements in fetal heart rate analysis by the removal of maternal-fetal heart rate ambiguities. BMC Pregnancy Childbirth. 2015;15(1):301.
- Amer-Wahlin I, Arulkumaran S, Hagberg H, et al. Fetal electrocardiogram: ST waveform analysis in intrapartum surveillance. BJOG. 2007;114(10):1191–1193.
- Ogunyemi D, Jovanovski A, Friedman P, et al. Temporal and quantitative associations of electronic fetal heart rate monitoring patterns and neonatal outcomes(†).J Matern Fetal Neonatal Med. 2019;32(18):3115–3124.
- Martí-Gamboa S, Rodríguez-Lázaro L, Redrado-Giménez O, et al. Second stage of labor: do accelerations matter? Ginecol Obstet Mex. 2016;84(5):287–293.
- Spencer JA, Badawi N, Burton P, et al. The intrapartum CTG prior to neonatal encephalopathy at term: a case-control study. Br J Obstet Gynaecol. 1997;104(1):25–28.
- Holzmann M, Wretler S, Nordström L. Absence of accelerations during labor is of little value in interpreting fetal heart rate patterns. Acta Obstet Gynecol Scand. 2016;95(10):1097–1103.
- Sherman DJ, Frenkel E, Kurzweil Y, et al. Characteristics of maternal heart rate patterns during labor and delivery. Obstet Gynecol. 2002;99(4):542–547.
- Ramadan MK, Fasih R, Itani S, et al. Characteristics of fetal and maternal heart rate tracings during labor: a prospective observational study. J Neonatal Perinatal Med. 2019;12(4):405–410.
- Pinas A, Chandraharan E. Continuous cardiotocography during labour: Analysis, classification and management. Best Pract Res Clin Obstet Gynaecol. 2016;30:33–47.
- Ugwumadu A. Understanding cardiotocographic patterns associated with intrapartum fetal hypoxia and neurologic injury. Best Pract Res Clin Obstet Gynaecol. 2013;27(4):509–536.
- Poehlmann S, Pinette M, Stubblefield P. Effect of labor analgesia with nalbuphine hydrochloride on fetal response to vibroacoustic stimulation. J Reprod Med. 1995;40(10):707–710.
- Giannina G, Guzman ER, Lai YL, et al. Comparison of the effects of meperidine and nalbuphine on intrapartum fetal heart rate tracings. Obstet Gynecol. 1995;86(3):441–445.
- Hoffman CT, 3rd, Guzman ER, Richardson MJ, et al. Effects of narcotic and non-narcotic continuous epidural anesthesia on intrapartum fetal heart rate tracings as measured by computer analysis. J Matern Fetal Neonatal Med. 1997;6(4):200–205.
- Lavin JP. The effects of epidural anesthesia on electronic fetal heart rate monitoring. Clin Perinatol. 1982;9(1):55–62.
- Shakouri F, Iorizzo L, Edwards HMK, et al. Effectiveness of fetal scalp stimulation test in assessing fetal wellbeing during labor, a retrospective cohort study. BMC Pregnancy Childbirth. 2020;20(1):347.
- Yeh P, Emary K, Impey L. The relationship between umbilical cord arterial pH and serious adverse neonatal outcome: analysis of 51,519 consecutive validated samples. BJOG. 2012;119(7):824–831.
- Wiberg N, Källén K, Herbst A, et al. Relation between umbilical cord blood pH, base deficit, lactate, 5-minute Apgar score and development of hypoxic ischemic encephalopathy. Acta Obstet Gynecol Scand. 2010;89(10):1263–1269.

