Abstract
Objectives
The relationship between prenatal physical activity (PA) and adverse birth outcomes is still inconclusive. We aimed to investigate the association between PA during pregnancy and adverse birth outcomes by using data from the Guangxi Zhuang birth cohort (GZBC) in China.
Study Design
A total of 11,292 mother-infant pairs were included from GZBC in China. The information on PA status, intensity, adequacy, and volume and birth outcomes were collected. Multivariable linear and logistic regression models were applied to analyze the effects of PA during pregnancy on birth weight z-scores (BW z-scores) and gestational age and risk of small-for-gestational age (SGA) and preterm birth (PTB), respectively. Cubic spline analysis was conducted to detect a nonlinear dose-response of total weekly activity metabolic equivalents (MET) and birth outcomes.
Results
Compared to no regular PA during pregnancy, moderate and high-intensity PA (MVPA) was associated with increase BW z-scores (β = 0.08, 95%CI: 0.002, 0.15, p = .044) and associated with a marginal significant decrease in risk of PTB (OR = 0.73, 95%CI: 0.51, 1.05, p = .093). However, PA had no relationship with gestational age and risk of SGA, and Nonlinear relationships were not observed between total weekly activity MET and risk of SGA and PTB.
Conclusion
These finding shows that PA during pregnancy may increase the BW z-score and reduce risk of PTB, supporting the guidelines that pregnant women should be encouraged to engage in appropriate physical activity during pregnancy in China.
Introduction
Preterm birth (PTB) and small-for-gestational-age (SGA) are the two most common adverse pregnancy outcomes, with a global incidence of 11% and 9.7% [Citation1,Citation2], respectively. Previous studies have shown that PTB and SGA are not only the leading causes of neonatal death, but also high-risk factors for neonatal dysplasia, such as bronchopulmonary dysplasia, neurodevelopment impairment, and extrauterine growth restriction [Citation3–5]. In addition, according to the theory of Developmental Origins of Health and Disease (DOHaD), PTB and SGA also increase the risk of chronic diseases in adulthood, including chronic kidney disease [Citation6,Citation7], diabetes [Citation8], and cognitive impairment [Citation9]. These evidence suggests the short- and long-tern health risk of PTB and SGA to the children. In China, the estimated incidence rate of PTB and SGA is about 6%∼10% and 5.7%∼6.6% respectively in recent years [Citation10,Citation11], lower than most of the countries worldwide. However, a recent study shows that PTB has ranked the first in the disease burden of children under 5 years old in China, much higher than that of Western Europe and North America [Citation12], showing the urgency of identifying the factors that may contribute to PTB and SGA.
PA during pregnancy is one of the most widely discussed interventions in recent years. The American College of Obstetricians and Gynecologists (ACOG) recommends that pregnant women without contraindications should participate in at least 20–30 min of moderate-intensity aerobic exercise per day or 150 min per week [Citation13]. The World Health Organization and health departments in other countries also make similar recommendations [Citation14]. And most studies in China have used similar standard to guide physical activity during pregnancy [Citation15,Citation16]. However, studies show that only 11.1% [Citation17] to 22.6% [Citation18] of pregnant women in China meet the standard of exercise during pregnancy, which is lower than that reported in the United Kingdom and the United States [Citation19]. Moreover, data regarding the impact of PA on fetus birth outcomes are still limited and inconsistent. Previous meta-analyses reported that PA (active vs inactive and high intensity vs low intensity) during pregnancy reduced the risks of SGA [Citation20,Citation21] and PTB [Citation22–25], while other meta-analyses showed that PA (active vs inactive) has no effect on SGA and PTB [Citation26–28]. In addition, most of the current evidence comes from Western European countries, while only a few are from China [Citation22,Citation23,Citation29]. Furthermore, most of the subjects of previous studies are from urban population, and there are few studies on rural pregnant women, although the number is much large, especially in less developed regions or countries. Moreover, most of the studies are intervention studies among high-risk individuals or case-control studies with recall bias [Citation23,Citation30,Citation31]. Evidence from prospective cohort studies are still very lacking.
To address the research gaps, we conducted a prospective cohort study to assess the relationship between PA during pregnancy and birth outcomes based on the Guangxi Zhuang Birth Cohort (GZBC), a large and well-characterized cohort from China. We investigated the PA status, types, frequencies per-week and duration per-time using a questionnaire modified from the International Physical Activity Scale and assessed the PA intensity, adequacy, and volume, which will provide a comprehensive understanding of the effects on birth outcomes.
Method
Study population
The participants in this study were derived from a prospective birth cohort, Guangxi Zhuang Birth Cohort (GZBC). This study was an ongoing birth cohort which was carried out in Guangxi Zhuang Autonomous Region of China from June 2015. Baseline survey of GZBC were performed in 11 hospitals in 7 counties of Guangxi, which has been described in previous studies [Citation32,Citation33]. Up to September 2018, GZBC has completed a baseline survey of 17,315 individuals, of which 12,064 have been followed up to obtain delivery outcome information. In the present study, individuals who delivered a single child and had complete maternal and infant information were selected as study participants. Those with no delivery data (n = 5,251), multiple births (n = 565), stillbirths (n = 46), or birth defects (n = 157) were excluded Four mother-infants were excluded because of total weekly activity MET and birth weight z-scores (BW z-scores) were outliers. Finally, a total of 11,292 pregnant women were included in the present study (Figure S1, Supplementary material). The study protocol has been approved by the Ethics Committee of Guangxi Medical University (No.20140305-001), and each participant has signed an informed consent form.
Data collection
The baseline data was collected by a face-to-face questionnaire survey, which was performed by rigorously trained investigators. The content of the questionnaire included general demographic characteristics (age, ethnicity, occupation, and household address), behaviors (smoking or passive smoking during pregnancy, alcohol consumption, PA during pregnancy, and whether to take folic acid), and gestational history. Smoking was defined as smoking more than one cigarette per day for three months, including former or current smokers, which means ever. We extracted information on each examination during pregnancy and birth outcomes from the Guangxi Maternal and Child Health Information Management System, which included gravidity and parity, pre-pregnancy BMI, pregnancy complications, birth weight(g), and gestational age (weeks) of the baby, and adverse birth outcomes (stillbirth and birth defect). Since birth weight was influenced by gestational age, we calculated the BW z-scores according to the Chinese standard [Citation34]. PTB was defined as birth before 37 completed weeks of gestation. SGA is defined as a birth weight of less than 10th percentile for gestational age. According to the Chinese standards, the participants were classified as underweight, normal weight, and overweight when their BMI was <18.5 kg/m2, 18.5–23.9 kg/m2 and ≥24 kg/m2 respectively.
PA measurement
The PA during pregnancy was measured by using a form of questionnaire in the baseline survey. We asked the participants whether they participated in PA regularly at ordinary times, which was defined as exercising 3 to 5 times a week for 20 to 60 min per time. If they participated in PA regularly, they need to be asked clearly about the types, frequencies per week, and duration (minutes) per time. The types of PA included aerobic exercise (walking, brisk walking, jogging, going up and downstairs, and swimming), ball sports (badminton, basketball, tennis, and billiards), and others such as yoga, hula hoop, skipping rope, fishing, and so on. We quantified the energy expenditure of each activity as metabolic equivalents (MET) according to the intensities scale developed by Aadahl [Citation35] and calculated the total weekly activity MET (Total MET·minutes/week) of each woman by multiplying MET of each activity by the weekly frequency and total duration (minutes) as the total volume per-week. Furthermore, we divided PA intensity into three levels, i.e. low-intensity, moderate-intensity, and high-intensity exercise, by total weekly activity MET and activity intensity type based on the International Physical Activity Scale [Citation36]. High-intensity was defined as participating in various high-intensity PAs more than 3 days per week with a total MET ≥ 1,500 MET·min/week, or participating in more than 3 types of PA for the whole week with a total MET ≥ 3,000 MET·min/week. Moderate-intensity was defined as participating in various high-intensity PAs more than 3 days per week and at least 20 min per day, or various moderate-intensity and/or walking activities more than 5 days per week and at least 30 min per day, or three different intensities activity more than 5 days with a total MET ≥ 600 MET·min/week. Low-intensity was defined as participating in some kinds of activities but did not meet the above criteria. Because of the small number of people who performed high-intensity levels of exercise during pregnancy, we combined moderate- and high-intensity levels of exercise as moderate to vigorous PA (MVPA)) for subsequent analyses. In addition, in order to assess the applicability of recommendations of the U.S. Department of Health and Human Services (DHHS) in 2018, we also defined adequate PA as a woman who met the recommendations that individuals should participate in at least 150 min of moderate intensity aerobic activity per week during pregnancy and the postpartum period [Citation37]. Otherwise, we considered the individuals had inadequate PA during pregnancy.
Statistical analysis
Descriptive statistics were performed on characteristics of the mother-infant pairs. Multivariable linear and logistic regressions were used to evaluate the relationship between PA during pregnancy and birth outcomes. In crude regression model, we did not adjust any variables. In the adjusted models, covariates were selected based on the directed acyclic graph (DAG), including age, pre-pregnancy BMI (continuous variable), occupation, passive smoking during pregnancy, folic acid supplementation, gravidity, and parity (). Only forty of the pregnant women smoked before pregnancy; thus, this variable was not included in the adjustment model. In addition, we applied cubic spline analysis to detect a nonlinear dose-response of total weekly activity MET and birth outcomes. Knots were located at the 50th, 75th, and 95th percentiles, and the reference value was set to the median. The adjusted variables of the spline curve model were consistent with those of the fully adjusted model in logistic regression. As a high proportion of imputed data on pre-pregnancy BMI and folic acid supplementation during pregnancy for our primary analyses, we performed a sensitivity analysis by excluding the missing data to test the robustness of the results in our main effects models, respectively, and then refitted models among cases with complete baseline data. All above analyses were carried out using SPSS 19.0 software and R software (version3.6.3). All P values were double-tailed and p < .05 was considered statistically significant.
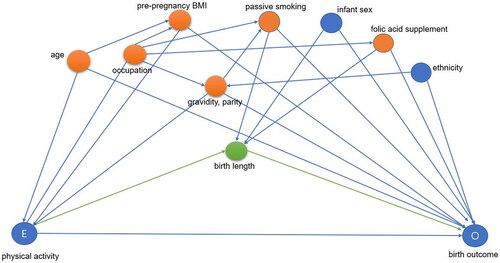
Figure 1. Directed acyclic graph for the association between physical activity and birth weight z-scores and gestational age. Minimally sufficient adjustment sets: age, occupation, pre-pregnancy BMI, folic acid supplementation during pregnancy, passive smoking during pregnancy, gravidity, and parity. Potential mediator was birth length.
Results
Characteristics of the included and overall participants
A total of 11,292 individuals were included in the present study (), The age of the individuals was 25 ∼ 34 years old (62.5%). Most participants in this study were Zhuang ethnicity (91.6%) and from rural areas (91.7%), but only a few smoked (0.4%) or consumed alcohol (0.5%) during pregnancy. Approximately half of them (42.7%) were exposed to secondhand smoke during pregnancy. Most of them were multiparous (59.6%), had a normal BMI before pregnancy (64.4%), and gestational week was in the first trimester (66.0%) at enrollment. Among infants, 52.9% of them were male. The distribution of demographic characteristics of the present study was like that of the overall study population.
Table 1. Characteristics of the included and overall participants.
Associations between PA during pregnancy and birth weight z-scores and gestational age
We first analyzed the impact of participating in PA during pregnancy on birth weight z-scores and gestational age by using multivariable linear regression model, which are shown in . We only found those individuals who participated in MVPA during pregnancy had a significantly increased BW z-scores (β = 0.077, 95%CI: 0.002, 0.152, p = .044) in Model1, when compared with those who had no regular activity during pregnancy. While in Model 2, we observed the same direction of the effect, although there was no statistically significant difference (β = 0.041, 95%CI: −0.032, 0.114, p = .270). We observed no significant relationship between PA and BW z-scores in different models when based on whether there was regular or adequate PA during pregnancy. When regarding to gestational age of the fetuses, we did not observe any significant associations neither.
Table 2. Adjusted association of maternal exercise during pregnancy and birth outcomes.
The relationship between PA during pregnancy and risk of SGA and PTB
By using multivariable logistic regression model, we assessed the relationship between PA during pregnancy and risk of SGA and PTB according to different PA evaluation methods, which are shown in . We found MVPA during pregnancy was associated with a decreased PTB risk in both crude model (OR = 0.72, 95%CI: 0.50, 1.02, p = .067) and adjusted model (OR = 0.73, 95%CI: 0.51, 1.05, p = .093) when compared without regular exercise. We also observed the same pattern of decreased risk of PTB when regarding to whether there was regular (p = .602) or adequate PA during pregnancy (p = .131), although there were no statistically significant differences. Although regular exercise can also reduce the risk of SGA, we did not observe any statistically significant difference.
Nonlinear relationship analysis
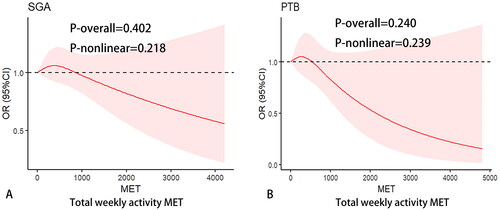
In addition, we applied cubic spline analysis to detect a nonlinear dose-response of PA volume (total weekly activity MET) with risk of SGA and PTB by using the rms package in R software, as shown in . We found there were no non-linear relationships between PA volume with risk of SGA and PTB (p = .218 and .239, respectively).
Figure 2. Restricted cubic spline with 3 knots was performed to determine the relationship of total weekly activity MET with risk of small-for-gestational age (SGA, A) and preterm birth (PTB, B). Adjusted factors were variables, including mother age, occupation, pre-pregnancy BMI, folic acid supplementation during pregnancy, passive smoking during pregnancy, gravidity, parity.
Sensitivity analysis
In comparison with the main analyses, the results were similar when we excluded those without data on pre-pregnancy BMI (Table S1, Supplementary material). In addition, when we repeated the analyses by excluding the individuals without information on folic acid supplementation, the association of MVPA and risk of PTB (OR = 0.68, 95%CI: 0.45, 1.01, p = .059) was consistent with the main analysis and became stronger. It is worth noting that we even observed an association between adequate PA and decreased risk of PTB (OR = 0.32, 95%CI: 0.10, 1.02, p = .054) (Table S2, Supplementary material).
Discussion
The present study evaluated the relationship between PA during pregnancy and birth outcomes based on the Guangxi Zhuang Birth Cohort in China. We found that MVPA were associated with increase in BW z-scores and associated with a decrease in risk of PTB. However, PA during pregnancy had no association with gestational age and risk of SGA. The findings suggest that PA during pregnancy may benefit the fetus’s development.
We found that moderate and high-intensity PA were associated with a marginal significant increase in BW z-scores. Inconsistently, a cohort study in Ethiopia showed that those who performed high intensity physical activity before mid-pregnancy significantly reduced fetal birth weight [Citation38]. Another meta-analysis also showed that high levels of physical activity during pregnancy were associated with a decreased birth weight [Citation39]. Furthermore, another previous study indicated that PA during pregnancy had no significant association with neonatal birth weight [Citation40]. These discrepant results may be attributed to the population characteristics and sample sizes. For example, the individuals in above studies were mainly in their second and third trimesters. However, most individuals in our study were enrolled in the first trimester (66.0%). In support of our result, Clapp et al. [Citation41] found moderate-intensity physical activity in the first trimester but not in the third trimester were associated with increased fetal birth weight. Previous studies have also showed that continuing to run regularly throughout pregnancy increases both absolute and relative villous vascular volume and cell proliferation at term [Citation42]. This exercise may improve feta-placental growth by enhancing placental transfer of oxygen and diffusible substrate, suggesting the underlying mechanism of effects of PA on fetal growth. Moreover, we found that exercise during pregnancy was associated with decreased risk of SGA, which was consistent with the effects of increasing BW z-scores although without statistically significant differences. In addition, a cohort study in Puerto Rico showed that physical activity in early pregnancy was not associated with delivery of SGA infants, while individuals with high total activity in the second trimester had a decreased risk of SGA [Citation43]. These findings suggest that PA during pregnancy may benefit fetal growth and recommendations of PA during pregnancy may require a higher upper limit to decrease the risk of SGA.
We found moderate and high intensity of PA during pregnancy was associated with decreased risk of PTB. Inconsistently, a birth cohort study from Japan reported a significant increase of PTB risk in the low intensity exercise group [Citation44]. Similarly, a previous study has found that physical and mental factors such as fatigue, standing, and repetitive physical activity during exercise can increase levels of catecholamine, stimulate uterine muscle activity, and reduce placental function, which increased risk of PTB [Citation45]. However, several previous studies also reported null associations [Citation24,Citation26,Citation46]. Consistent with our results, recent studies have shown that moderate PA was associated with decreased risk of PTB, but no significant effects on gestational age [Citation22,Citation24]. It is worth noting that an earlier meta-analysis also reported that individuals with vigorous exercise had a decreased risk of PTB [Citation21]. One possible mechanism for reducing PTB risk was that PA during pregnancy may be beneficial for controlling maternal weight, such as adiposity and excess gestational weight gain [Citation24], which has been associated with increased risk of PTB. Additionally, inflammation is also considered a risk factor for PTB, and exercise during pregnancy can improve insulin sensitivity and reduce inflammation [Citation47], which may reduce the risk of PTB.
The main advantage of the present study is that it comprised a large sample size and made a comprehensive measurement of PA during pregnancy, which will help understanding of the effects on birth outcomes. Nevertheless, some limitations should be also taken into consideration. First, the present study mainly recorded the exercises of individuals during the first trimester, which hampers our ability to accurately evaluate the influence of exercise during the entire pregnancy on the birth outcomes. Second, exercise during pregnancy relied on a self-report by the research subject, which may be subject to recall bias and reporting bias. Third, the subjects of this study were mainly from the Zhuang ethnicity of Guangxi in China, which may hinder the generalizability of our findings to individuals from other regions or ethnicities.
Conclusion
The present study shows that PA during pregnancy may increase the BW z-scores and reduce risk of PTB, but is not associated with gestational age and risk of SGA. These findings suggest beneficial effect of PA during pregnancy on fetal growth, supporting the guidelines that pregnant women should be encouraged to engage in appropriate physical activity during pregnancy.
Supplemental Material
Download MS Word (62.7 KB)Acknowledgments
This study is the result of the joint efforts of many people and institutions. We acknowledge the participants for their support and the study staff for their contributions in the study. We are very grateful to the following institutions for their strong support and cooperation: Tiandong People’s Hospital, Tiandong Maternity and Child Health Care Hospital, Jingxi People’s Hospital, Jingxi Maternity and Child Health Care Hospital, Pingguo People’s Hospital, Pingguo Maternity and Child Health Care Hospital, Debao People’s Hospital, Debao Maternity and Child Health Care Hospital, Longan People’s Hospital, Wuming People’s Hospital, Wuming Maternity and Child Health Care Hospital, and Clinical Epidemiology Research Center for Complex Diseases of Guangxi Medical University.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Walani SR. Global burden of preterm birth. Int J Gynaecol Obstet. 2020;150(1):31–33.
- Ding G, Tian Y, Zhang Y, et al. Application of a global reference for fetal-weight and birthweight percentiles in predicting infant mortality. BJOG. 2013;120(13):1613–1621.
- Morrow CB, McGrath-Morrow SA, Collaco JM. Predictors of length of stay for initial hospitalization in infants with bronchopulmonary dysplasia. J Perinatol. 2018;38(9):1258–1265.
- You J, Shamsi BH, Hao MC, et al. A study on the neurodevelopment outcomes of late preterm infants. BMC Neurol. 2019;19(1):108.
- Peila C, Spada E, Giuliani F, et al. Extrauterine growth restriction: definitions and predictability of outcomes in a cohort of very low birth weight infants or preterm neonates. Nutrients. 2020;12(5):1224.
- Crump C, Sundquist J, Winkleby MA, et al. Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: national cohort study. BMJ. 2019;365:l1346.
- Gjerde A, Lillås BS, Marti H-P, et al. Intrauterine growth restriction, preterm birth and risk of end-stage renal disease during the first 50 years of life. Nephrol Dial Transplant. 2020;35(7):1157–1163.
- Xia Q, Cai H, Xiang Y-B, et al. Prospective cohort studies of birth weight and risk of obesity, diabetes and hypertension in adulthood among Chinese population. J Diabetes. 2019;11(1):55–64.
- Lohaugen GC, Ostgard HF, Andreassen S, et al. Small for gestational age and intrauterine growth restriction decreases cognitive function in young adults. J Pediatr. 2013;163(2):447–453.
- Zou L, Wang X, Ruan Y, et al. Preterm birth and neonatal mortality in China in 2011. Int J Gynaecol Obstet. 2014;127(3):243–247.
- Zhongzhou S, Yawen W, Shuai M. Risk factors for preterm birth, low birth weight and small for gestational age: a prospective cohort study. Chin J Epidemiol. 2019;40(9):1125–1129.
- Li FM, Xie SY, Jiang ZX, et al. Burden of disease and risk factors among children under 5 years in China from 1990 to 2019: a perspective of international comparison. Chin J Prev Med. 2022;56(5):619–625.
- Birsner LM, Gyamfi-Bannerman C. Physical activity and exercise during pregnancy and the postpartum period. Obstet Gynecol. 2020;135:4.
- Licence: CC BY-NC-SA 3.0 IGO. Global status report on physical activity 2022. Geneva: World Health Organization; 2022.
- Yun-Li C, Hai-Hui M, Ying-Jie S, et al. The status and influencing factors of physical activity among Chinese pregnant women during different pregnancies. Chin J Dis Control Prev. 2021;25(2):149–154.
- Yan Z, Yue Z, Sheng-Wen D, et al. Reliability and validity of the Chinese version of the Pregnancy Physical Activity Questionnaire (PPAQ). Chin J Nurs. 2013;48(9):825–827.
- Zhang Y, Dong S, Zuo J, et al. Physical activity level of urban pregnant women in Tianjin, China: a cross-sectional study. PLoS One. 2014;9(10):e109624.
- Hongmei Y, Yongfang D. Lingling G. Current status and infuencing factors of physical activity in pregnant women during pregnancy in Guangzhou. Chin Evid-Base Nurs. 2017;3(3):238–243.
- Liu J, Blair SN, Teng Y, et al. Physical activity during pregnancy in a prospective cohort of British women: results from the Avon longitudinal study of parents and children. Eur J Epidemiol. 2010;26(3):237–247.
- Teede HJ, Bailey C, Moran LJ, et al. Association of antenatal diet and physical activity-based interventions with gestational weight gain and pregnancy outcomes: a systematic review and meta-analysis. JAMA Intern Med. 2022;182(2):106–114.
- Beetham KS, Giles C, Noetel M, et al. The effects of vigorous intensity exercise in the third trimester of pregnancy: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2019;19(1):281.
- Cai M, Zhang B, Yang R, et al. Association between maternal outdoor physical exercise and the risk of preterm birth: a case-control study in Wuhan, China. BMC Pregnancy Childbirth. 2021;21(1):206.
- Huang L, Fan L, Ding P, et al. Maternal exercise during pregnancy reduces the risk of preterm birth through the mediating role of placenta. J Matern Fetal Neonatal Med. 2019;32(1):109–116.
- Aune D, Schlesinger S, Henriksen T, et al. Physical activity and risk of preterm birth: a systematic review of epidemiological studies and meta-analysis. Int J Obstet Gy. 2017;124(12):1816–1826.
- Magro-Malosso ER, Saccone G, Di Mascio D, et al. Exercise during pregnancy and risk of preterm birth in overweight and obese women: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2017;96(3):263–273.
- Di Mascio D, Magro-Malosso ER, Saccone G, et al. Exercise during pregnancy in normal-weight women and risk of preterm birth: a systematic review and meta-analysis of randomized controlled trials. Am J Obstet Gynecol. 2016;215(5):561–571.
- da Silva SG, Ricardo LI, Evenson KR, et al. Leisure-time physical activity in pregnancy and maternal-child health: a systematic review and meta-analysis of randomized controlled trials and cohort studies. Sports Med. 2017;47(2):295–317.
- Chen Y, Ma G, Hu Y, et al. Effects of maternal exercise during pregnancy on perinatal growth and childhood obesity outcomes: a meta-analysis and meta-regression. Sports Med. 2021;51(11):2329–2347.
- Wang C, Wei Y, Zhang X, et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am J Obstet Gynecol. 2017;216(4):340–351.
- Xi C, Luo M, Wang T, et al. Association between maternal lifestyle factors and low birth weight in preterm and term births: a case-control study. Reprod Health. 2020;17(1):93.
- Louise J, Poprzeczny AJ, Deussen AR, et al. The effects of dietary and lifestyle interventions among pregnant women with overweight or obesity on early childhood outcomes: an individual participant data meta-analysis from randomised trials. BMC Med. 2021;19(1):128.
- Feng BY, Peng Y, Liang J, et al. Risk factors for adverse pregnancy outcomes among Zhuang ethnic pregnant women: a cohort study in Guangxi, China. Curr Med Sci. 2021;41(2):219–227.
- Liang J, Liu S, Liu T, et al. Association of prenatal exposure to bisphenols and birth size in zhuang ethnic newborns. Chemosphere. 2020;252:126422.
- Pediatrics C I o, Children C S G o N C o t P G a D o. Growth standard curves of birth weight, length and head circumference of Chinese newborns of different gestation. Chin J Pediatr. 2020;58(9):738–746.
- Aadahl M, Jorgensen T. Validation of a new self-report instrument for measuring physical activity. Med Sci Sports Exerc. 2003;35(7):1196–1202.
- Mengyu F, Jun L, Pingping H. Chinese guidelines for data processing and analysis concerning the international physical activity questionnaire. Chin J Epidemiol. 2014;35(8):961–964.
- ACOG. Physical activity and exercise during pregnancy and the postpartum period. Obstet Gynecol. 2020;135(4):e178–e188.
- Bisson M, Tremblay F, St-Onge O, et al. Influence of maternal physical activity on infant’s body composition. Pediatr Obes. 2017;12(Suppl 1):38–46.
- Bisson M, Lavoie-Guenette J, Tremblay A, et al. Physical activity volumes during pregnancy: a systematic review and meta-analysis of observational studies assessing the association with Infant’s birth weight. AJP Rep. 2016;6(2):e170–e197.
- Pathirathna ML, Sekijima K, Sadakata M, et al. Effects of physical activity during pregnancy on neonatal birth weight. Sci Rep. 2019;9(1):6000.
- Clapp JF, 3rd, Kim H, Burciu B, et al. Continuing regular exercise during pregnancy: effect of exercise volume on fetoplacental growth. Am J Obstet Gynecol. 2002;186(1):142–147.
- Bergmann A, Zygmunt M, Clapp JF. Running throughout pregnancy: effect on placental villous vascular volume and cell proliferation. Placenta. 2004;25(8–9):694–698.
- Gollenberg AL, Pekow P, Bertone-Johnson ER, et al. Physical activity and risk of small-for-gestational-age birth among predominantly Puerto Rican women. Matern Child Health J. 2011;15(1):49–59.
- Takami M, Tsuchida A, Takamori A, et al. Effects of physical activity during pregnancy on preterm delivery and mode of delivery: the Japan environment and children’s study, birth cohort study. PLoS One. 2018;13(10):e0206160.
- Mozurkewich EL, Luke B, Avni M, et al. Working conditions and adverse pregnancy outcome-a meta-analysis. Obstet Gynecol. 2000;95(4):623–635.
- Juhl M, Andersen PK, Olsen J, et al. Physical exercise during pregnancy and the risk of preterm birth: a study within the Danish National birth cohort. Am J Epidemiol. 2008;167(7):859–866.
- Retnakaran R, Qi Y, Sermer M, et al. Pre-gravid physical activity and reduced risk of glucose intolerance in pregnancy: the role of insulin sensitivity. Clin Endocrinol. 2009;70(4):615–622.

