Abstract
Objective
Rates of neonatal abstinence syndrome/neonatal opioid withdrawal syndrome (NAS/NOWS), a withdrawal syndrome from opioids and other substances resulting from intrauterine exposure, have been increasing exponentially in the U.S. To improve health outcomes, it is important to understand population health risks, including rehospitalization and related diagnoses, using current data. This study will compare and describe the rates of rehospitalization, the demographic characteristics and the rehospitalization diagnoses and age at diagnosis between the infants affected by NAS/NOWS to those sampled who were unaffected. This study will also describe the frequency of NAS/NOWS births per year along with a yearly comparison of readmissions in those affected by NAS/NOWS to those who were not (2016–2020).
Methods
Health claims data were used to conduct a case/control study. Diagnosis codes for neonatal withdrawal syndrome/NAS/NOWS (P04.49 or P96.1 and P96.1 alone) from 1 October 2015 to 1 June 2021 were extracted, and controls were case-matched based on month/year of birth. Rehospitalizations following birth and the related diagnoses were described and grouped using the Agency of Healthcare Research Quality Clinical Classifications Software Refined Frequency distribution. The chi-square test of association and generalized estimating equation modeling were used for data analysis.
Results
Infants affected by NAS/NOWS are 2.7 times more likely to have a rehospitalization. White, non-Hispanic neonates (OR = 1.5; p = .007) and those infants residing in rural areas (OR = 1.9; p < .001) were disproportionately affected. We identified a host of admission diagnoses with increased prevalence in infants affected by NAS/NOWS when compared to those who were not affected (e.g. infectious diseases, feeding disorders).
Conclusions
Infants with NAS/NOWS are at increased risk of rehospitalization with a host of diagnoses, and specific demographic groups (White, rural) are more highly affected.
Introduction
Neonatal abstinence syndrome (NAS) is “a withdrawal syndrome that can occur in newborns exposed to certain substances, including opioids (neonatal opioid withdrawal syndrome [NOWS]), during pregnancy” [Citation1]. NAS/NOWS is characterized by gastrointestinal, neurologic, and autonomic signs (e.g. poor feeding, tachypnea, tremors, and high-pitched cry) [Citation2]. From 2010 to 2018, rates of NAS in the U.S. have increased from 4/1000 to 6.8/1000 newborn hospitalizations [Citation3]. Some U.S. states have drastically higher NAS-related inpatient rates; specifically, Kentucky (23.1/1000 newborn hospitalizations), Maine (27.7/1000), and West Virginia (49.6/1000) rates are 3.9, 4.1, and 7.3 times higher than the U.S. average (6.8/1000), respectively [Citation3]. In the U.S. $573 million was spent in 2016 to provide inpatient care to infants affected by NAS [Citation4].
Evidence suggests that some neonates are at greater risk of NAS; specifically, neonates born to low income families and those residing in rural areas are disproportionally affected (9.3/1000 births among low income families vs. 5.7/1000 among other births; 10.6/1000 births in rural areas vs. 6.2/1000 births in metropolitan locations) [Citation4,Citation5]. Contributing to this disparity is the lack of access to clinical and social services in rural areas highly affected by the opioid crisis, including facilities that provide screening and treatment services [Citation5–7]. Racial and ethnic groups are also disproportionately affected; specifically Whites and Native American/Alaska Natives (10.5/1000 births – Whites; 15.9/1000 births – Native American/Alaska Natives) [Citation4,Citation8]. Those receiving Medicaid insurance coverage are also highly affected by NAS (nearly 82% of NAS births received Medicaid insurance in 2014) with increasing incidence rates (2.8/1000 in 2004 to 14.4/1000 in 2014) [Citation9].
NAS/NOWS related hospitalizations and rehospitalizations are a significant healthcare concern [Citation3]. Infants diagnosed with NAS were hospitalized on average 15.9 days [Citation4]. Hospitalization rates and related diagnoses for infants with NAS are similar to that of high-risk late pre-term infants (respiratory diagnoses (36.0% vs. 39.6% for infants with NAS and high risk late pre-term infants, respectively), possible sepsis (13.8% vs. 11.1%), and feeding problems (11.1% vs. 10.7%)) [Citation2]. Prior studies suggest infants affected by NAS are 1.5–2.5 times more likely to be rehospitalized [Citation2,Citation10–12]. The gaps identified in published literature include lack of recent data on hospital readmission rates among infants affected by NAS/NOWS who reside in regions of the U.S. highly affected by the opioid crisis, specifically the East South-Central Division of the U.S., according to the U.S. Census Region [Citation5,Citation7,Citation10].
Therefore, this study purpose was to utilize a healthcare claims database to compare hospital readmissions for infants affected by NAS/NOWS compared to controls in a geographic area highly affected by the opioid epidemic [Citation13]. This study is based on encounters seen at one university healthcare system and children’s hospital serving Central, Eastern, and Southern Kentucky, which is located in the East South-Central region of the U.S. [Citation14]. This study will compare and describe the rates of rehospitalization, the demographic characteristics and the rehospitalization diagnoses and age at diagnosis between the infants affected by NAS/NOWS to those sampled who were unaffected. This study will also describe the frequency of NAS/NOWS births per year along with a yearly comparison of readmissions in those affected by NAS/NOWS to those who were not (2016–2020).
Methods
Procedure
This project utilized the University of Kentucky’s Clinical and Translational Science bioinformatics services as honest brokers to obtain deidentified clinical/claims data following IRB approved protocol number 45668. We extracted billed codes from UK Healthcare for neonatal withdrawal syndrome/neonatal abstinence syndrome/neonatal opioid withdrawal syndrome (NWS/NAS/NOWS; P04.49 or P96.1 and P96.1 alone) from 1 October 2015 to 1 June 2021 [Citation15]. UK Healthcare is one of two Level 1 Trauma Centers in the state of Kentucky. It serves the Eastern, Southern, and Central regions of the state. Neonates with encounters for NWS/NAS/NOWS were identified as cases and matched to control neonates born during the same month and year in a 1:1 ratio. Control neonates were matched at random if there was more than one potential match based on month and year of birth. For each cohort (cases and controls), date of birth, sex, race/ethnicity, and county of residence of each neonate was extracted. Using rural urban continuum codes (RUCC), county of residence was classified as urban (codes 1–3) or non-urban (4–8).
Hospital readmission and diagnosis codes
For each neonate, date of hospital readmission and related diagnoses codes (ICD-10 codes) occurring following birth were extracted. For this analysis, any readmission after the date of birth was considered a hospital readmission. Diagnoses were collapsed using the Agency of Healthcare Research Quality Clinical Classifications Software Refined [Citation16]. Those diagnoses groupings that were present in at least 10% of the hospital readmissions for the combined group of case and control infants were compared between the NAS/NOWS affected and unaffected groups.
Data analysis
Frequency distributions were used to summarize demographic and clinical characteristics. The chi-square test of association was used to evaluate differences between cases and controls in demographic characteristics and diagnoses among all infants readmitted during the follow-up period. Generalized estimating equation (GEE) modeling was used to estimate the relative risk of hospital readmission among cases and controls, adjusting for demographics, and preterm status and accounting for individual matching of case and control infants. The diagnosis of low birth weight was considered as a covariate in the GEE modeling; yet, there was a 92% overlap between preterm status and low birth weight. Therefore, preterm status was used in the GEE modeling reported in our results. All data analysis was conducted using SAS, version 9.4 (SAS Inc., Cary, NC) with an alpha of .05 throughout.
Results
In comparing demographics between cases (n = 2248) and controls (n = 2248), a higher proportion of cases were White, non-Hispanic (92.4% vs. 81.7%, p < .001; see ) and residing in a county designated as non-urban (67.1% vs. 51.6%, p < .001). Cases and controls did not differ by sex. A higher proportion of cases were born prematurely (27.1% vs. 5.6%, p < .001) and were readmitted following birth, compared to controls (41.8% vs. 13.8%, p < .001).
Table 1. Comparison of infant demographic and clinical characteristics between infants diagnosed with NAS and controls.
Adjusting for sex, race/ethnicity, county of residence designation and preterm status, neonates diagnosed with NAS/NOWS had 2.74 times the rate of hospital readmission relative to control neonates (RR = 2.74, 95% CI = 2.44–3.07, p < .001; see ). White, non-Hispanic neonates were also at increased risk of readmission compared to those who were Black, non-Hispanic (RR = 1.50, 95% CI = 1.12–2.01, p = .007), as were those residing in a county designated as non-urban (RR = 1.85, 95% CI = 1.64–2.08, p < .001). Rehospitalization risk did not differ by neonate sex, and there was no difference in risk between Black/non-Hispanic and infants in the Other race/ethnicity category, or by prematurity status.
Table 2. Generalized estimated equations modeling hospital readmission (n = 4241).
Hospitalization was more likely among infants with NAS/NOWS diagnosis compared with control infants (41.8% vs. 13.8%, respectively). Of the 34 diagnoses represented at least 10% of hospital readmissions in the combined group of cases and controls, there were 22 which affected the case and control groups at significantly different rates (). There was also significant difference in the time frame or age at readmission. Specifically, children affected by NAS/NOWS were significantly (p < .001) younger when rehospitalized with a median of three days (range: 1–13 days; mean: 54 days) compared with unaffected children with a rehospitalization median age of 68 days (range: 20–321; mean 219). Most of the diagnoses were more prevalent among cases than control infants. Specifically, the most prevalent diagnoses include newborns affected by maternal conditions or complications of labor/delivery (95.5% vs. 17.4%, respectively; p < .001); other specified and unspecified perinatal conditions (75% vs. 61.2%, respectively; p < .001); and neonatal digestive and feeding disorders (51.1% vs. 25.1%, respectively; p < .001; ).
Table 3. Diagnoses among case and control infants readmitted to the hospital.
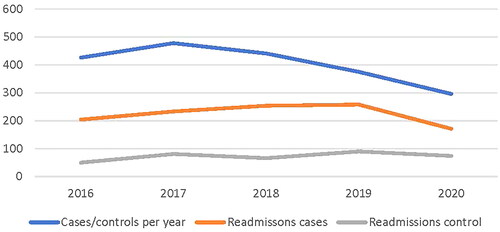
Following an increase in NAS/NOWS between 2016 and 2017, there has been a decreasing trend in infants affected by NAS/NOWS (). In contrast, there was a slight increase in readmissions in the infants affected by NAS/NOWS from 2016 to 2019, and control group readmissions were relatively consistent over time. Both NAS/NOWS births and rehospitalizations decreased in 2020 relative to 2019.
Figure 1. Cases, controls, and readmission per cohort yearly (year 2015 and 2021 were excluded due to incomplete yearly data. Given the average monthly estimates based on the 5 months of available data the projected 2021 total cases would be 276. The projected number of rehospitalizations for this same time period would be 151 for cases and 88 for controls).
Discussion
Findings from this study indicate that infants affected by NAS/NOWS are 2.7 times more likely to have a subsequent hospital admission. Our findings demonstrated that White, non-Hispanic neonates (RR = 1.5; p=.006) and those infants residing in rural areas (RR = 1.9; p < .001) are disproportionately affected. We identified that infants affected by NAS/NOWS are rehospitalized at a significantly younger age for a host of diagnoses, with some general categories including infectious diseases, feeding disorders, infant growth retardation, and socioeconomic/psychosocial factors. We have described the trends in total cases of infants affected by NAS/NOWS per year and then readmission per year in cases and controls. We have identified an interesting decrease in the number of NAS/NOWS rehospitalizations between 2019 and 2020, which follows the consistent decline in NAS/NOWS births between 2017 and 2020. Findings from this study not only support prior studies, which have identified that infants with NAS/NOWS are at higher risk for health conditions which result in hospitalization, but these results also provide insight into the medical problems for which they are being treated [Citation10].
Published studies reporting relative risk or odds ratios for rates of hospital readmissions using case-control studies suggest that infants affected with NAS are 1.5–2.5 times more likely to be readmitted to the hospital when compared to unaffected infants [Citation2,Citation10,Citation11,Citation17]. An Australian population-based study (2000–2011) found that infants with NAS were 3.3 times more likely to die during hospitalization when compared to infant without NAS [Citation10]. Increased relative risk of death (RR = 1.9), generally, have been reported in this population [Citation11]. Infants affected by NAS were more likely to hospitalized for a variety of diagnoses including but not limited to mental and behavioral disorder (OR = 2.1), maltreatment (OR = 5.1), assault (OR = 4.3), and poisoning from opioids (OR = 7.1) [Citation10]. Additional medical conditions have been identified including infectious diseases (adjusted relative risk (aRR)=1.7); disease of the nervous system (aRR = 2.1), respiratory system (aRR = 1.6), digestive system (aRR = 2.1), genitourinary system (aRR = 2.3), the skin (aRR = 3.0); injury and poisoning (aRR = 1.6); asphyxia (aRR = 1.6); and child abuse (aRR = 4.5) [Citation11]. Puls et al. evaluated the risk of physical abuse hospitalizations in infants affected by NAS compared to those unaffected using a national database and ICD-9 codes from 2013 to 2014 [Citation18]. Authors reported that the NAS group was more likely to be admitted for physical abuse within 6 months of birth (aRR = 3.8) [Citation18]. Findings from these studies support the findings of our study, which indicate that infants with NAS/NOWS are at increased risk for hospital readmission and these readmissions are for a wide variety of medical and health-related conditions. Of interest, many of these studies were conducted or used data from decades ago, and the issue of NAS/NOWS is now more pervasive [Citation9–11].
In parallel to the increased rates of NAS/NOWS identified in rural areas, our study found that those infants affected by NAS/NOWS that reside in rural areas are at increased risk of hospitalization [Citation4]. Similar results were reported by Puls et al., who studied the outcome of physical abuse specifically and identified lower populated regions to be at higher risk for hospitalization in infants with NAS/NOWS (aRR = 1.5; p=.01) [Citation18]. Increased rates could result from the need for additional care and at-home support post-discharge which is limited by lack of access in rural areas [Citation19,Citation20]. Similarly, rural communities were most affected by the prescription opioid crisis of the 1990s, and they continue to have staggeringly high addiction and overdose rates when compared to the rest of the nation [Citation6]. It is critically important that innovative approaches proposing structural changes in health delivery that support the complexity of care for infants affected by NAS and their families are developed and evaluated.
Since 2014, there has been an effort to recognize that NAS/NOWS management should not only focus on supporting medical care issues but also on maternal dyads using comprehensive models; however, few standardized approaches to support families are described in published literature [Citation21]. Outpatient infant weaning programs, which are multidisciplinary team approaches to addressing the complex problems of families affected by NAS/NOWS, have been one tactic described [Citation17,Citation22–25]. The evidence supporting this approach is dated and lacking with observational, review, or retrospective studies conducted in small samples. In addition, many of these limited studies were conducted outside of the U.S., a significant constraint for comparison to evaluations of programs for U.S. NAS/NOWS populations considering the different health care systems internationally [Citation22–24,Citation26–29]. Similarly, outpatient weaning programs require adequate interdisciplinary staffing and could be a challenge for small rural hospitals. These programs also rely largely on parental participation to administer the accurate prescribed medication dose and in that present a larger risk for medical error [Citation10].
Since 2003 child welfare legislation has required the use of the Plan of Safe Care (POSC), which is a section of the Child Abuse and Prevention Treatment Act that guides the comprehensive service management of infants affected by NAS/NOWS [Citation30]. Although the National Center on Substance Abuse and Child Welfare provides some “best practices”, and general guidelines policies are stipulated by state-level processes [Citation30], many state-level procedures are highly reliant on social services which have limited resources in relation to the populations in which they serve [Citation31]. Often the state-level process involves multiple community entities such the medical professionals, law enforcement, social services, and the judicial system. Communication between key stakeholders is critical to optimal functioning, yet each system uses different software platforms and jargon, and privacy issues often affect communication. Interestingly, an mHealth platform to facilitate learning and care coordination is currently under investigation [Citation32]. Our study findings suggest that rehospitalization rates in infants affected by NAS/NWOS remain a significant problem despite POSC legislation suggesting innovative, feasible, and pragmatic approaches to POSC needs to be investigated using large-scale clinical trial design. Similarly, quality improvement and implementation science projects are needed to successfully apply these programs into the highly affected populations which are impacted by social determinants of health.
Our findings report the trend in infants affected NAS/NOWS at one university healthcare system serving the central region of Kentucky. We identified that the number of NAS/NOWS cases in this system started trending down in 2017 (relative to 2016) and this continued into 2020. In contrast, the number of rehospitalizations among NAS/NOWS infants continued to trend upward between 2016 and 2019, only declining in 2020. This trend is similar to that identified in state data outside of the uptrend identified in 2019 in the state [Citation33]. Of great concern, the overdose rate in Kentucky increased 48% from 2019 to 2020 [Citation34].
Limitations
Limitations of the study include the retrospective nature of the data used in the analysis. Our control group was based on neonates born in the same month and year of the infants born with NAS/NOWS or cases as a control for seasonality of illnesses that may spur rehospitalization. We recognize that different matching approaches could have been used, such as gestational age. We controlled for the diagnoses of prematurity in our analysis of relative risk. An alternate approach could be controlling for birthweight or gestational age. However, we were unable to include birthweight in the analysis due to poor data entry on this variable, with some weights apparently reported in pounds rather than kilograms. This concern is somewhat mitigated by the observation that 92% of included infants had agreement between preterm birth status (yes/no) and diagnosed low birthweight (yes/no), indicating these variables were nearly coincident. We extracted neonate with diagnoses codes for NWS/NAS/NOWS and then identified a rehospitalization following that admission. As such, it is recognized that those with a more recent birth date would be followed for a shorter duration of time. We also recognize that dates of inclusion were affected by an international pandemic which may have impacted study findings. The magnitude of this potential effect is outside of the scope of this project, but it worthy of future consideration. An additional limitation, given the use of a billed dataset, is that we are limited by billed codes which may not have fully reflected the underlying medical conditions (e.g. other aftercare encounter).
Conclusions
Infants affected by NAS/NOWS are 2.7 times more likely to be rehospitalized after birth at significantly younger ages for conditions affecting physical health (i.e. involving most organ systems) and related to psychosocial factors. In addition to NAS/NOWS status, we identified White, non-Hispanic, and rural residing infants to be at significantly higher risk of rehospitalization. It is critically important that innovative approaches to care are evaluated to determine best practices. It is equally critical that dissemination and implementation of evidence-based approaches are prioritized to improve the care provided to these vulnerable populations.
Ethics statement
This study complies with the Declaration of Helsinki, that the locally appointed ethics committee has approved the research protocol and that informed consent has been obtained from the subjects (or their legally authorized representative). This project utilized the University of Kentucky’s Clinical and Translational Science bioinformatics services as honest brokers to obtain deidentified clinical/claims data following IRB approved protocol number 45668.
Acknowledgements
Authors would like to acknowledge the bioinformatics staff at the CCTS for their work on this project.
Disclosure statement
There are no significant financial disclosures relevant to this project.
Data availability statement
Data is available upon request.
Additional information
Funding
References
- Center for Disease Control and Prevention. Key findings: public health reporting of NAS offers opportunities for treatment and prevention 2021; 2021 [cited 2021 Jan 27]. Available from: https://www.cdc.gov/pregnancy/features/public-health-reporting-of-NAS.html
- Patrick SW, Burke JF, Biel TJ, et al. Risk of hospital readmission among infants with neonatal abstinence syndrome. Hosp Pediatr. 2015;5(10):513–519.
- Agency for Healthcare Research and Quality. Neonatal abstinence syndrome (NAS) among newborn hospitalizations 2021; 2021 [cited 2021 Feb 7]. Available from: https://www.hcup-us.ahrq.gov/faststats/NASMap
- Strahan AE, Guy GP Jr., Bohm M, et al. Neonatal abstinence syndrome incidence and health care costs in the United States, 2016. JAMA Pediatr. 2020;174(2):200–202.
- Brown JD, Goodin AJ, Talbert JC. Rural and Appalachian disparities in neonatal abstinence syndrome incidence and access to opioid abuse treatment. J Rural Health. 2018;34(1):6–13.
- Kroelinger CD, Addison D, Rodriguez M, et al. Implementing a learning collaborative framework for states working to improve outcomes for vulnerable populations: the opioid use disorder, maternal outcomes, and neonatal abstinence syndrome initiative learning community. J Womens Health. 2020;29(4):475–486.
- Center for Disease Control and Prevention. U.S. opioid dispensing rate maps; 2021 [cited 2022 Jan 27]. Available from: https://www.cdc.gov/drugoverdose/rxrate-maps/index.html
- Pourcyrous M, Elabiad MT, Rana D, et al. Racial differences in opioid withdrawal syndrome among neonates with intrauterine opioid exposure. Pediatr Res. 2021;90(2):459–463.
- Winkelman TNA, Villapiano N, Kozhimannil KB, et al. Incidence and costs of neonatal abstinence syndrome among infants with Medicaid: 2004–2014. Pediatrics. 2018;141(4):e20173520.
- Uebel H, Wright IM, Burns L, et al. Reasons for rehospitalization in children who had neonatal abstinence syndrome. Pediatrics. 2015;136(4):e811–e820.
- Witt CE, Rudd KE, Bhatraju P, et al. Neonatal abstinence syndrome and early childhood morbidity and mortality in Washington State: a retrospective cohort study. J Perinatol. 2017;37(10):1124–1129.
- Milliren CE, Melvin P, Ozonoff A. Pediatric hospital readmissions for infants with neonatal opioid withdrawal syndrome, 2016–2019. Hosp Pediatr. 2021;11(9):979–988.
- Center for Disease Control and Prevention. Drug overdose mortality by state 2021; 2021 [cited 2021 Aug 25]. Available from: https://www.cdc.gov/nchs/pressroom/sosmap/drug_poisoning_mortality/drug_poisoning.htm
- U.S. Census Bureau; 2021 [cited 2021 Jan 27]. Available from: https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf
- Goyal S, Saunders KC, Moore CS, et al. Identification of substance-exposed newborns and neonatal abstinence syndrome using ICD-10-CM – 15 hospitals, Massachusetts, 2017. MMWR Morb Mortal Wkly Rep. 2020;69(29):951–955.
- Agency of Healthcare Research and Quality. Clinical Classifications Software Refined (CCSR); 2021 [cited 2022 Jan 27]. Available from: https://www.hcup-us.ahrq.gov/toolssoftware/ccsr/ccs_refined.jsp
- Van Horn A, Powell W, Wicker A, et al. Outpatient healthcare access and utilization for neonatal abstinence syndrome children: a systematic review. J Clin Transl Sci. 2019;4(5):389–397.
- Puls HT, Anderst JD, Farst K, et al. Intrauterine substance exposure and the risk for subsequent physical abuse hospitalizations. Acad Pediatr. 2020;20(4):468–474.
- Health Resources and Service Administration. Medical underserved populations find; 2021 [cited 2022 Feb 1]. Available from: https://data.hrsa.gov/tools/shortage-area/mua-find
- Jackson A, Shannon L. Barriers to receiving substance abuse treatment among rural pregnant women in Kentucky. Matern Child Health J. 2012;16(9):1762–1770.
- Wiles JR, Isemann B, Ward LP, et al. Current management of neonatal abstinence syndrome secondary to intrauterine opioid exposure. J Pediatr. 2014;165(3):440–446.
- Chau KT, Nguyen J, Miladinovic B, et al. Outpatient management of neonatal abstinence syndrome: a quality improvement project. Jt Comm J Qual Patient Saf. 2016;42(11):506–515.
- Lai A, Philpot P, Boucher J, et al. An outpatient methadone weaning program by a neonatal intensive care unit for neonatal abstinence syndrome. Popul Health Manag. 2017;20(5):397–401.
- Backes CH, Backes CR, Gardner D, et al. Neonatal abstinence syndrome: transitioning methadone-treated infants from an inpatient to an outpatient setting. J Perinatol. 2012;32(6):425–430.
- Murphy-Oikonen J, McQueen K. Outpatient pharmacologic weaning for neonatal abstinence syndrome: a systematic review. Prim Health Care Res Dev. 2018;20:e76.
- Smirk CL, Bowman E, Doyle LW, et al. Home-based detoxification for neonatal abstinence syndrome reduces length of hospital admission without prolonging treatment. Acta Paediatr. 2014;103(6):601–604.
- Oei J, Feller JM, Lui K. Coordinated outpatient care of the narcotic-dependent infant. J Paediatr Child Health. 2001;37(3):266–270.
- Kelly LE, Knoppert D, Roukema H, et al. Oral morphine weaning for neonatal abstinence syndrome at home compared with in-hospital: an observational cohort study. Paediatr Drugs. 2015;17(2):151–157.
- Dickes L, Summey J, Mayo R, et al. Potential for Medicaid savings: a state and national comparison of an innovative neonatal abstinence syndrome treatment model. Popul Health Manag. 2017;20(6):458–464.
- National Center on Substance Abuse and Child Welfare. Plan of Safe Care; 2021 [cited 2022 Feb 3]. Available from: https://reporter.nih.gov/search/ha8K0fKpKEmXcJ-BvgDVrQ/project-details/10379584
- Schroeder M, editor. Kentucky’s comprehensive system of care model for pregnant and parenting women with SUD. Atlanta (GA): National Drug Abuse and Heroin Summit; 2018.
- Ma TX. SAFE4BOTH integration of mobile technologies with case management (CM) systems provide education, work flow among multiple stakeholders to implement Plan of Safe Care (POSC) for SUD mom and infants; 2021 [cited 2022 Feb 3]. Available from: https://reporter.nih.gov/search/-6OTOoEVw0yA9rrj58_0Ww/project-details/9908839
- Kentucky Department of Public Health Maternal and Child Health. Annual report 2019 public health neonatal abstinence syndrome reporting registry; 2020 [cited 2022 Mar 1]. Available from: https://chfs.ky.gov/agencies/dph/dmch/Documents/NASReport.pdf
- Commonwealth of Kentucky Justice & Public Safety Cabinet. 2020 overdose fatality report Kentucky office of drug control policy; 2021. Available from: https://odcp.ky.gov/Documents/2020%20KY%20ODCP%20Fatality%20Report%20.(final).pdf

