Abstract
Objective
To describe the prevalence and predictors of postpartum sleep disorders.
Design
A retrospective cohort study.
Setting
Postpartum.
Population
Commercially insured women delivering in California (USA) between 2011 and 2014.
Methods
Using the Optum Clinformatics Datamart Database.
Main outcome measures
Prevalence of a postpartum sleep disorder diagnosis with and without a depression diagnosis up to 12 months following hospital discharge for inpatient delivery. We also identified predictors of a postpartum sleep disorder diagnosis using multivariable logistic regression.
Results
We identified 3535 (1.9%) women with a postpartum sleep disorder diagnosis. The prevalence of sleep disorder diagnoses was insomnia (1.3%), sleep apnea (0.25%), and other sleep disorder (0.25%). The odds of a postpartum sleep disorder were highest among women with a history of drug abuse (adjusted odds ratio (aOR): 2.70, 95% confidence interval (CI): 1.79–4.09); a stillbirth delivery (aOR: 2.15, 95% CI: 1.53–3.01); and chronic hypertension (aOR: 1.82; 95% CI: 1.57–2.11). A comorbid diagnosis of a postpartum sleep disorder and depression occurred in 1182 women (0.6%). These women accounted for 33.4% of all women with a postpartum sleep disorder. The strongest predictors of a comorbid diagnosis were a history of drug abuse (aOR: 4.13; 95% CI: 2.37–7.21) and a stillbirth delivery (aOR: 2.93; 95% CI: 1.74–4.92).
Conclusions
Postpartum sleep disorders are underdiagnosed conditions, with only 2% of postpartum women in this cohort receiving a sleep diagnosis using International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes. Insomnia was the most common disorder and one-third of women diagnosed with a postpartum sleep disorder had a co-morbid diagnosis of depression. Future studies are needed to improve the screening and diagnostic accuracy of postpartum sleep disorders.
Introduction
Sleep disorders, which include insomnia, obstructive sleep apnea, circadian rhythm disorder, hypersomnolence disorder, and restless legs syndrome, are common, affecting at least 21% of adult women [Citation1,Citation2]. These conditions are associated with major economic costs to affected individuals and society [Citation3] and major detrimental health effects for women after delivery. For example, insomnia can result in impaired maternal well-being [Citation4], cognition [Citation5], physical activity [Citation6], social relationships [Citation7], maternal–neonatal bonding [Citation8], and fatigue [Citation9].
Dysfunctional sleep also contributes toward the development of mood disorders, including depression [Citation10]. Based on non-obstetric data, insomnia is associated with a co-morbid diagnosis of depression; sleep disturbance is also a known diagnostic criterion for depression [Citation11,Citation12]. However, there are scant epidemiological data of postpartum sleep disorders and comorbid diagnoses of a postpartum sleep disorder and postpartum depression. Population-level prevalence estimates would assist in informing decisions about the prevention, protocols surrounding optimal screening, and specialist referral for treatment of these disorders to promote maternal mental health and well-being.
Therefore, the primary aims of this study were to report the prevalence for postpartum sleep disorder diagnoses in the first year following childbirth among a large cohort of commercially insured US women and identify potential predictors for these disorders. Our secondary aims were to report the prevalence and predictors for comorbid diagnoses of sleep disorders and postpartum depression within the first year postpartum.
Methods
Study sample
We analyzed data obtained from the Optum Clinformatics Datamart, Database (OptumInsight, Inc., Eden Prairie, MN). The database comprises health care claims for members of large commercial and Medicare Advantage health plans and accounts for >15 million annual covered lives. The data are derived from claims for inpatient, outpatient, and pharmacy health care services. The population covered is geographically diverse, spanning all 50 US states. The study protocol was reviewed and exempted by the Institutional Review Board at Stanford University School of Medicine since it only used de-identified data.
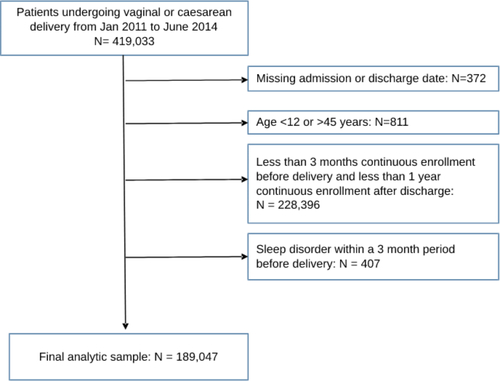
Our analytic sample comprised women aged 12–45 years who underwent vaginal or cesarean delivery between 1 January 2011 and 30 June 2014. All codes used to define a delivery hospitalization are presented in Table S1. We selected patients with at least 3 months of continuous health plan enrollment prior to delivery and at least 12 months of continuous enrollment after hospital discharge following delivery. We excluded women with a sleep diagnosis code identified within a 3-month period before delivery. If a woman had multiple deliveries during the study period, only the first birth was included in our analysis.
Table 1. Maternal characteristics among women with and without a sleep disorder.
Outcomes
The primary outcome of interest was a sleep disorder diagnosis, defined by the presence of at ≥1 sleep disorder diagnosis International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) code associated with an inpatient or outpatient health care encounter up to 1 year after hospital discharge for inpatient delivery. We used an approach described by Felder et al. for classifying women with a sleep disorder [Citation13]. Sleep disorders were classified into seven categories: insomnia, sleep apnea, sleep-related movement disorders, hypersomnia, circadian rhythm disorder, parasomnia, and narcolepsy. Descriptions of each type of sleep disorder and the ICD-9-CM codes used to classify each disorder are presented in Table S1.
Our secondary outcome was a comorbid diagnosis of a sleep disorder and depression up to 12 months after discharge. Depression was classified by the presence of any of the following ICD-9-CM codes: 296.2, 296.82, 300.4, 309.0, 309.1, 309.28, and 311.
Statistical analysis
We examined the overall prevalence of any sleep disorder diagnosis up to 12 months post-hospital discharge. We also determined the prevalence of any sleep disorder diagnosis by completed month up to 12 months post-hospital discharge. We separately examined the overall and monthly prevalence of each category of sleep disorder. To assess factors associated with a postpartum sleep disorder diagnosis, we identified a set of candidate variables based on literature review [Citation14–18], their potential relevance to a postpartum sleep disorder, and availability of codes in the Optum database. Sociodemographic variables included maternal age, race, highest educational level, and annual household income. Obstetric variables included obesity, chronic hypertension, diabetes (pre-existing or gestational), alcohol abuse, tobacco use disorder, multiple gestation, hypertensive disorder of pregnancy (gestational hypertension, pre-eclampsia, or eclampsia), mode of delivery (vaginal, cesarean without labor, and cesarean with labor), preterm delivery, stillbirth, and year of delivery. Variables identified using ICD-9-CM codes are presented in Table S2. Race, educational level, and household income data were included in this analysis because the majority of US prospective postpartum sleep studies have thus far been limited to small cohorts of white, middle class, married, and highly educated women [Citation10,Citation19–26].
Table 2. Maternal predictors of a sleep disorder.
We compared the frequency of each maternal characteristic for women with versus without a sleep disorder diagnosis using χ2 tests. Univariate odds ratios (ORs) and 95% confidence intervals (CIs) were used to describe the association of each candidate variable with a sleep disorder diagnosis. Multivariable logistic regression analysis was performed to identify variables independently associated with a sleep disorder diagnosis. We included all candidate variables in a full model without selection. Due to the small number of women with alcohol abuse and a sleep disorder diagnosis (n = 2), alcohol abuse was not included in the multivariable model.
In our secondary analyses, we examined the overall prevalence of a comorbid diagnosis of a postpartum sleep disorder with depression up to 12 months postpartum. We compared the maternal characteristics of women with a comorbid diagnosis to women without a sleep disorder diagnosis and depression. We performed exploratory analysis to identify potential predictors for a comorbid diagnosis using the aforementioned set of candidate variables with univariable and multivariable logistic regression. Given that a sleep disorder and depression may be diagnosed at different timepoints in the postpartum period, we calculated the median lag time (in days) between each diagnosis stratified according to whether a sleep diagnosis or a depression diagnosis occurred first.
As an antepartum sleep disorder diagnosis more than 3 months before delivery may have been captured as a sleep disorder ICD-9-CM code after delivery, we performed a sensitivity analysis excluding women with a sleep disorder diagnosis code at or up to 12 months before delivery. The denominator for this sensitivity analysis comprised women with at least 12 months of continuous insurance enrollment before and after delivery.
Data are presented as percentages with 95% CIs, unadjusted ORs, and adjusted odds ratios (aORs) with 95% CIs. All statistical analyses were performed using STATA vsn 14 (StataCorp, College Station, TX). Statistical significance was set at a p value <.05 for two-sided tests.
Results
The analytic sample comprised 189,047 women who underwent delivery between January 2011 and June 2014. presents a flowchart of the analytic sample selection. Among all deliveries, 3535 women (1.9%; 95% CI: 1.8–1.9%) had a sleep disorder diagnosis within 1 year of hospital discharge following childbirth. The monthly prevalence of a sleep disorder diagnosis increased slightly over the 12-month period after discharge, from 0.11% (95% CI: 0.10–0.13%) in month 1 to 0.18% (95% CI: 0.16–0.20%) in month 12. Insomnia was the most common sleep disorder diagnosis (n = 2392; 1.3%; 95% CI: 1.2–1.3%). The prevalence was substantially lower for other sleep disorder diagnoses: sleep apnea (n = 482; 0.25%; 95% CI: 0.23–0.28%), other sleep disorder (n = 481; 0.25%; 95% CI: 0.23–0.28%), sleep-related movement disorder (n = 221; 0.12%; 95% CI: 0.10–0.13%), hypersomnia (n = 158; 0.08%; 95% CI: 0.07–0.10%), narcolepsy (n = 72; 0.04%; 95% CI: 0.03–0.05%), circadian rhythm disorder (n = 57; 0.03%; 95% CI: 0.02–0.04%), and parasomnia (n = 20; 0.01%; 95% CI: 0.01–0.02%).
Characteristics of women with and without a postpartum sleep disorder diagnosis are presented in . Compared to women without a sleep disorder diagnosis, women with a sleep disorder diagnosis were more likely to: be older than 34 years old, have a degree less than a bachelor or no education beyond a high school diploma, have a household income between $75,000 and $99,999 or less than $59,000, be white or have unknown race, and have obesity, chronic hypertension, diabetes, a history of drug abuse or tobacco use disorder, multiple gestation, hypertensive disorder of pregnancy, or a diagnosis of stillbirth. A sleep disorder diagnosis was more common after a cesarean delivery and less common after a vaginal delivery.
In the multivariable model (), the strongest independent predictors of a postpartum sleep disorder diagnosis were: drug abuse (aOR: 2.70, 95% CI: 1.79–4.09); stillbirth (aOR: 2.15, 95% CI: 1.53–3.01); and chronic hypertension (aOR: 1.82; 95% CI: 1.57–2.11). Compared to women with an annual household income of less than $40,000, those with an income ≥$100,000 had 20% lower odds of a sleep disorder diagnosis (aOR: 0.80; 95% CI: 0.71–0.91). The odds of a sleep disorder diagnosis were 25% lower for African-American women (aOR: 0.75; 95% CI: 0.65–0.87) and Hispanic women (aOR: 0.75; 95% CI: 0.66–0.86), and were 54% lower for Asian women (aOR: 0.46; 95% CI: 0.38–0.56) compared to white women.
In our secondary analysis, 1182 women (0.6%; 95% CI: 0.6–0.7%) had a comorbid diagnosis of a sleep disorder and depression. These women accounted for 33.4% of all women with a postpartum sleep disorder diagnosis. By comparison, among women without a sleep disorder diagnosis, 7.1% had depression. The characteristics of women with a comorbid diagnosis of sleep disorder and depression are presented in . Results of the univariable and multivariable analyses examining predictors of a comorbid diagnosis are presented in . Similar to the results of our main analysis, the strongest predictors of a comorbid diagnosis were women with a history of drug abuse (aOR: 4.13; 95% CI: 2.37–7.21) and a stillbirth delivery (aOR: 2.93; 95% CI: 1.74–4.92). Variables associated with a reduced risk of a comorbid diagnosis included a non-White race or ethnicity and women with a household income >$100,000.
Table 3. Maternal characteristics among women with and without a comorbid diagnosis of sleep disorder and postnatal depression.
Table 4. Maternal predictors of a comorbid diagnosis of a sleep disorder and postpartum depression.
Among the 1182 women with a comorbid sleep disorder and depression diagnosis, 289 (24.4%; 95% CI: 22.0–26.9%) had ICD-9-CM codes for a sleep disorder diagnosis and depression in the same healthcare encounter, 344 (29.1%; 95% CI: 26.5–31.7%) had a sleep disorder diagnosis that preceded depression, and 549 (46.5%; 95% CI: 43.6–49.3%) had depression that preceded a sleep disorder diagnosis. The median lag time was 66 days (interquartile range: 29.5–146 days; range 1–336 days) in those who had a sleep disorder diagnosis before a depression diagnosis and 101 days (interquartile range: 39–168 days; range 1–352 days) in those who had a sleep disorder diagnosis after a depression diagnosis.
In our sensitivity analysis, we estimated the prevalence of a postpartum sleep disorder diagnosis among women without a sleep disorder ICD-9-CM code up to 12 months before delivery. The sub-cohort for this analysis comprised 121,662 women with at least 12 months of continuous enrollment. In this analysis, 1933 women (1.6%; 95% CI: 1.5–1.7%) had a postpartum sleep disorder diagnosis within 12 months of hospital discharge. Consistent with our main findings, the most common sleep disorder diagnosis was insomnia (n = 1312; 1.1%; 95% CI: 1.0–1.1%).
Discussion
Among this cohort of commercially insured women, we observed a low prevalence of sleep disorder diagnoses (1.9%). Insomnia was the most prevalent sleep disorder diagnosis. Approximately, one-third of all women with a postpartum sleep disorder diagnosis had a co-existing depression diagnosis up to 12 months postpartum. The strongest predictors for a postpartum sleep disorder diagnosis were a history of drug abuse and stillbirth. Similar predictors were identified for those with a comorbid diagnosis of postpartum sleep disorder and depression.
Patient-reported outcome measures are widely regarded as gold standard measures of postpartum recovery [Citation1,Citation27,Citation28]. Previously reported prevalence estimates using patient-reported outcome measures are substantially higher than the prevalence of sleep disorders reported in our analytic cohort. For example, in a Norwegian study of 1480 women, the prevalence of insomnia at 8 weeks and 2 years postpartum was 60% and 40%, respectively [Citation29]. In an observational study of 2427 US women [Citation30], 57% of pregnant women had insomnia. Our reliance on administrative data for capturing sleep diagnoses may explain why the prevalence was lower in our study than in the aforementioned prospective studies. Possible explanations may be poor coding accuracy or misclassification because sleep disturbance can be a symptom of depression. Future studies are needed to examine the accuracy of administrative data compared to gold standard methods (sleep studies and structured clinical interviews which require expertise and time away from the infant) or patient-reported outcome measures, which can be used to screen for sleep disorders, to determine the true prevalence of sleep disorders in large patient cohort studies involving patient assessment at different postpartum time points.
Based on general data from the primary care population, 50% of patients with insomnia have co-morbid conditions such as anxiety and depression [Citation31]. In our study cohort, one-third of women with a sleep disorder also had a diagnosis of depression. Among patients with a comorbid diagnosis, the timing of each diagnosis after delivery was highly variable. Although we could not explain this variability, prior obstetric and non-obstetric data suggest that a bidirectional relationship may exist between sleep disorders and depression [Citation32–34]. As the causal relationship between postpartum sleep disorders and depression is underexplored, longitudinal prospective studies are needed to examine the long-term impact of sleep disturbance or a sleep disorder diagnosis on the risk of postpartum depression and other major mental health disorders, such as anxiety.
In our secondary analysis, the strongest independent predictors of a postpartum sleep disorder were drug abuse and stillbirth. Nearly, one in five pregnant individuals have used nicotine, alcohol, or an illicit substance in the preceding month [Citation35]. After stillbirth delivery, women are at risk of mental health problems such as depression and anxiety [Citation36,Citation37]. Further research is needed to examine potential mechanisms that may explain why a history of drug abuse and stillbirth impacts on the risk of postpartum sleep disturbance. We also observed differences in the risk of a sleep disorder by race and ethnicity. The lower likelihood of a sleep disorder diagnosis for Asian, Hispanic, and African-American women compared with White women should be interpreted with caution as we did not account for structural and institutional factors, such as barriers to mental health services or a sleep clinics [Citation38]. Further research is needed to examine potential reasons for these disparities, such as shame (internalized behavior), stigma, language or cultural barriers, institutional racism, and poverty, and how factors contribute toward screening, diagnosis, and treatment [Citation39].
While the data used in this analysis are from 2011 to 2014, to our knowledge, this is the largest data set that has been used to determine postpartum sleep disorder prevalence to date. The accuracy of sleep disorder diagnostic codes in the postpartum period has not previously been examined. However, a cohort study of Canadian non-obstetric patients reported high sensitivity and specificity (79% and 89%) for ICD-9-CM codes [Citation40]. We were unable to ascertain whether diagnoses were made by sleep specialists versus non-specialists and the accuracy of these diagnoses. While sleep disorders are more prevalent during the 3rd trimester of pregnancy [Citation41] and patients frequently see a physician in the 3 months prior to delivery (including during hospitalization for childbirth), we acknowledge that there may be patients in our cohort with pre-existing sleep disorder diagnoses that did not receive an ICD-9 code in the last 3 months of their pregnancy. Due to the limited granularity of data available, we could not examine the potential influence of factors on the risk of postpartum sleep disorders, such as infant sleep patterns, breastfeeding success, co-sleeping, social support structures, paternity leave, pain following childbirth, and surgical complications.
Our findings are only generalizable to women with commercial insurance. Therefore, studies are needed to examine the epidemiology of postpartum sleep disorders among women covered by Medicaid. We adopted an approach for classifying sleep disorders previously described by Felder et al. for sleep disorders during pregnancy [Citation13]. We acknowledge that this classification may not be applicable to a postpartum cohort. In addition, we used ICD-9-CM codes to identify women with postpartum depression. However, because there are no specific ICD-9-CM codes for postpartum depression, the prevalence estimates for a co-morbid diagnosis may be biased.
Conclusions
Approximately, two out of every 100 US women with commercial insurance receive a diagnosis of a sleep disorder within the first 12 months postpartum, which is significantly lower than rates of sleep disorder reported in prospective studies. The strongest predictors for a postpartum sleep disorder diagnosis are stillbirth delivery and a history of drug abuse. One-third of women with a postpartum sleep disorder diagnosis also have a co-morbid diagnosis of depression. Future studies are needed to improve clinical screening and diagnosis of postpartum sleep disorders and to determine the accuracy of claims data compared to clinical data at different postpartum time points.
Ethical approval
The study was exempted from review by the institutional review board at Stanford University School of Medicine since it only used de-identified data.
Author contributions
PS and BC were involved in study conception, planning, study design, data interpretation, and manuscript writing. NG and MAK were involved in planning, data analysis, and interpretation. MK, FHB, SM, MAK, and CE were involved in data interpretation and manuscript writing. AJB was involved in study conception, planning, study design, data analysis, data interpretation, and manuscript writing.
Supplemental Material
Download Zip (29.7 KB)Acknowledgements
Pervez Sultan is an Arline and Pete Harman Endowed Faculty Scholar of the Stanford Maternal and Child Health Research Institute. Data for this project were accessed using the Stanford Center for Population Health Sciences Data Core.
Disclosure statement
None of the authors have any conflicts of interest to declare.
Data availability statement
Study data available upon reasonable request.
Additional information
Funding
References
- Sultan P, Ando K, Sultan E, et al. A systematic review of patient-reported outcome measures used to assess sleep in postpartum women using Consensus Based Standards for the Selection of Health Measurement Instruments (COSMIN) guidelines. Sleep. 2021;44(10):zsab128.
- Ferrie J, Kumari M, Salo P, et al. Sleep epidemiology – a rapidly growing field. Int J Epidemiol. 2011;40(6):1431–1437.
- Streatfeild J, Smith J, Mansfield D, et al. The social and economic cost of sleep disorders. Sleep. 2021;44(11):zsab132.
- Hiscock H, Wake M. Infant sleep problems and postnatal depression: a community-based study. Pediatrics. 2001;107(6):1317–1322.
- Swain AM, O'Hara MW, Starr KR, et al. A prospective study of sleep, mood, and cognitive function in postpartum and nonpostpartum women. Obstet Gynecol. 1997;90(3):381–386.
- Vladutiu C, Evenson K, Borodulin K, et al. The association between physical activity and maternal sleep during the postpartum period. Matern Child Health J. 2014;18(9):2106–2114.
- Piteo A, Roberts R, Nettelbeck T, et al. Postnatal depression mediates the relationship between infant and maternal sleep disruption and family dysfunction. Early Hum Dev. 2013;89(2):69–74.
- Tikotzky L. Postpartum maternal sleep, maternal depressive symptoms and self-perceived mother–infant emotional relationship. Behav Sleep Med. 2016;14(1):5–22.
- Insana S, Williams K, Montgomery-Downs H. Sleep disturbance and neurobehavioral performance among postpartum women. Sleep. 2013;36(1):73–81.
- Bhati S, Richards K. A systematic review of the relationship between postpartum sleep disturbance and postpartum depression. J Obstet Gynecol Neonatal Nurs. 2015;44(3):350–357.
- Daghlas I, Lane J, Saxena R, et al. Genetically proxied diurnal preference, sleep timing, and risk of major depressive disorder. JAMA Psychiatry. 2021;78(8):903–910.
- American Psychiatric Association. DSM-5 Taskforce. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington (VA): American Psychiatric Association; 2013.
- Felder J, Baer R, Rand L, et al. Sleep disorder diagnosis during pregnancy and risk of preterm birth. Obstet Gynecol. 2017;130(3):573–581.
- Li C, Ford E, Zhao G, et al. Prevalence of self-reported clinically diagnosed sleep apnea according to obesity status in men and women: National Health and Nutrition Examination Survey, 2005–2006. Prev Med. 2010;51(1):18–23.
- Buxton O, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010;71(5):1027–1036.
- Lu Q, Zhang X, Wang Y, et al. Sleep disturbances during pregnancy and adverse maternal and fetal outcomes: a systematic review and meta-analysis. Sleep Med Rev. 2021;58:101436.
- Rowshan R, Bengtsson C, Lissner L, et al. Thirty-six-year secular trends in sleep duration and sleep satisfaction, and associations with mental stress and socioeconomic factors—results of the population study of women in Gothenburg, Sweden. J Sleep Res. 2010;19(3):496–503.
- Al-Qattan H, Al-Omairah H, Al-Hashash K, et al. Prevalence, risk factors, and comorbidities of obstructive sleep apnea risk among a working population in Kuwait: a cross-sectional study. Front Neurol. 2021;12:620799.
- Doering J, Dogan S. A postpartum sleep and fatigue intervention feasibility pilot study. Behav Sleep Med. 2018;16(2):185–201.
- Manber R, Bei B, Simpson N, et al. Cognitive behavioral therapy for prenatal insomnia: a randomized controlled trial. Obstet Gynecol. 2019;133(5):911–919.
- Bei B, Pinnington D, Quin N, et al. Improving perinatal sleep via a scalable cognitive behavioural intervention: findings from a randomised controlled trial from pregnancy to 2 years postpartum. Psychol Med. 2021;2021:1–11.
- Symon B, Bammann M, Crichton G, et al. Reducing postnatal depression, anxiety and stress using an infant sleep intervention. BMJ Open. 2012;2(5):e001662.
- Stremler R, Hodnett E, Lee K, et al. A behavioral–educational intervention to promote maternal and infant sleep: a pilot randomized, controlled trial. Sleep. 2006;29(12):1609–1615.
- Hiscock H, Bayer J, Gold L, et al. Improving infant sleep and maternal mental health: a cluster randomised trial. Arch Dis Child. 2007;92(11):952–958.
- Hiscock H, Wake M. Randomised controlled trial of behavioural infant sleep intervention to improve infant sleep and maternal mood. BMJ. 2002;324(7345):1062–1065.
- O'Connor E, Senger CA, Henninger ML, et al. Interventions to prevent perinatal depression: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321(6):588–601.
- Sultan P, Sadana N, Sharawi N, et al. Evaluation of domains of patient-reported outcome measures for recovery after childbirth: a scoping and systematic review. JAMA Netw Open. 2020;3(5):e205540.
- Sultan P, Sharawi N, Blake L, et al. Use of patient-reported outcome measures to assess outpatient postpartum recovery: a systematic review. JAMA Netw Open. 2021;4(5):e2111600.
- Sivertsen B, Hysing M, Dørheim S, et al. Trajectories of maternal sleep problems before and after childbirth: a longitudinal population-based study. BMC Pregnancy Childbirth. 2015;15:129.
- Mindell J, Cook R, Nikolovski J. Sleep patterns and sleep disturbances across pregnancy. Sleep Med. 2015;16(4):483–488.
- Ford D, Kamerow D. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–1484.
- Saxbe D, Schetter C, Guardino C, et al. Sleep quality predicts persistence of parental postpartum depressive symptoms and transmission of depressive symptoms from mothers to fathers. Ann Behav Med. 2016;50(6):862–875.
- Jansson-Frojmark M, Lindblom K. A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. J Psychosom Res. 2008;64(4):443–449.
- Ettensohn M, Soto Y, Bassi B, et al. Sleep problems and disorders in patients with depression. Psychiatr Ann. 2016;46(7):390–395.
- Smid M, Terplan M. What obstetrician-gynecologists should know about substance use disorders in the perinatal period. Obstet Gynecol. 2022;139(2):317–337.
- Burden C, Bradley S, Storey C, et al. From grief, guilt pain and stigma to hope and pride – a systematic review and meta-analysis of mixed-method research of the psychosocial impact of stillbirth. BMC Pregnancy Childbirth. 2016;16:9.
- Hogue C, Parker C, Willinger M, et al. The association of stillbirth with depressive symptoms 6–36 months post-delivery. Paediatr Perinat Epidemiol. 2015;29(2):131–143.
- Jackson C. Determinants of racial/ethnic disparities in disordered sleep and obesity. Sleep Health. 2017;3(5):401–415.
- Guo N, Robakis T, Miller C, et al. Prevalence of depression among women of reproductive age in the United States. Obstet Gynecol. 2018;131(4):671–679.
- Jolley R, Liang Z, Peng M, et al. Identifying cases of sleep disorders through International Classification of Diseases (ICD) codes in administrative data. Int J Popul Data Sci. 2018;3(1):448.
- Facco F, Kramer J, Ho K, et al. Sleep disturbances in pregnancy. Obstet Gynecol. 2010;115(1):77–83.