?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aims
In order to mitigate early hypoglycemia in preterm infants, some clinicians have recently explored interventions such as delivery room commencement of dextrose infusions or delivery room administration of buccal dextrose gel. This review aimed to systematically investigate the literature regarding the provision of delivery room (prior to admission) parenteral glucose as a method to reduce the risk of initial hypoglycemia (measured at the time of NICU admission blood testing) in preterm infants.
Materials and methods
Using PRISMA guidelines a literature search (May 2022) was conducted using PubMed, Embase, Scopus, Cochrane Library, OpenGrey, and Prospero databases. The clinicaltrials.gov database was searched for possible completed/ongoing clinical trials. Studies that included moderate preterm (33+6 weeks) or younger birth gestations or very low birth weight (or smaller) infants, and that administered parenteral glucose in the delivery room were included. The literature was appraised via data extraction, narrative synthesis, and critical review of the study data.
Results
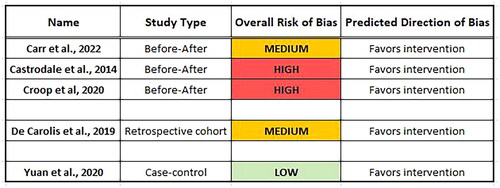
A total of five studies (published 2014–2022) were eligible for inclusion (three before-after “quasi-experimental” studies, one retrospective cohort study, and one case-control study). Most included studies used intravenous dextrose as the intervention. Individual study effects (odds ratios) favored the intervention in all included studies. It was felt that the low number of studies, the variability in study design, and the nonadjustment for confounding co-interventions (co-exposures) precluded a meta-analysis. Quality assessment of the studies revealed a spectrum of bias from low to high risk, however, most studies had moderate to high risk of bias, and their direction of bias favored the intervention.
Conclusions
This extensive search and systematic appraisal of the literature indicates that there exists few studies (these are low grade and at moderate to high risk of bias) for the interventions of either intravenous or buccal dextrose given in the delivery room. It is not clear if these interventions impact on rates of early (NICU admission) hypoglycemia in these preterm infants. Obtaining intravenous access in the delivery room is not guaranteed and can be difficult in these small infants. Future research should consider various routes for commencing delivery room glucose in these preterm infants and should take the form of randomized controlled trials.
Introduction
Is neonatal hypoglycemia associated with adverse neurodevelopmental outcomes? Literature shows that both discrete episodes [Citation1] and severe episodes (repeated/prolonged) [Citation2,Citation3] of neonatal hypoglycemia have been associated with pathological findings on brain magnetic resonance imaging (MRI). However, the literature examining the association between neonatal hypoglycemia and abnormal neurodevelopmental outcomes is divided. Some literature has associated neonatal hypoglycemia with abnormal neurodevelopmental outcomes at both early (infant/toddler) [Citation4,Citation5] and later [Citation6–9] childhood ages. Equally, other literature has not shown this association, neither at early [Citation10] nor later [Citation11–13] childhood ages.
The management of very preterm and very low birth weight infants in the NICU is complex and perinatal and postnatal morbidity is often inevitable, with the end result being adverse neurodevelopmental outcome. When assessed at preschool ages, very preterm infants (10,293 infants, 36 studies) have been seen to have significant cognitive 17% (95% CI 10–26%) and motor 21% (95% CI 14–29%) impairments, with a trend for higher rates with decreasing gestational age and birth weight [Citation14]. When assessed at adult age, very preterm infants (1,068 infants, 8 studies) have been shown to have a mean intelligence quotient (IQ) score 12 points lower than an equivalent size (and aged) cohort of term controls from the same time period [Citation15]. Preterm infants are known to be at greater risk of hypoglycemic episodes and are monitored regularly for this in the NICU. Again, in the preterm population specifically, the literature is conflicting whether hypoglycemia is associated [Citation8,Citation9] with neurodevelopmental impairment or not [Citation10–13].
So, what defines hypoglycemia in a preterm infant? Recommendations from the Pediatric Endocrine Society (2015) are that for high-risk neonates (without a suspected congenital hypoglycemia disorder), the goal of treatment is to maintain a plasma glucose concentration >50 mg/dL for those aged <48 h (grade 2+ evidence) [Citation16]. The American Academy of Pediatrics (AAP) Committee on Fetus and Newborn advise that if asymptomatic, intravenous treatment of hypoglycemia is not needed until glucose concentrations are <25 mg/dL (within 4 h after birth) or <36 mg/dL (from 4 to 24 h after birth) [Citation17]. However, if “symptomatic” and blood glucose <40 mg/dL they recommend treating immediately [Citation17]. For the clinician, knowing when a very/extreme preterm infant is (or is not) “symptomatic” is challenging. A recent study has shown that clinical observation of signs of hypoglycemia in term infants is neither sensitive nor specific to detect neonatal hypoglycemia, with large interobserver differences [Citation18]. In practice this means that applying the AAP guideline is difficult, especially if attempting to apply it to preterm infants. As a result, it is routine practice to check blood glucose levels while admitting a preterm infant to the neonatal unit, regardless of presence of signs/symptoms.
Preterm, small for gestational age (GA) and intra-uterine growth restricted neonates are especially vulnerable to hypoglycemia due to their lack of metabolic reserves and associated co morbidities. It is recommended to achieve central venous access within 1 h of life in these infants [Citation19]. Despite the adoption of many of the suggested measures of the golden hour [Citation20,Citation21] including improved thermoregulation, nearly 30–60% of these high-risk infants are hypoglycemic at admission and require immediate intervention [Citation22].
While term infants can mobilize energy stores to maintain normoglycemia, very/extremely preterm infants have limited glycogen stores prior to the third trimester [Citation23]. Gluconeogenesis is then the major pathway of glucose production in these premature neonates. The time necessary to induce the enzymes involved in gluconeogenesis and the absence of stored glycogen make hypoglycemia almost unavoidable in the first few hours after birth if exogenous glucose is not administrated [Citation23]. Ketogenesis is also limited as fat stores make up less than 2% of preterm infants total body weight. It is also known that steady-state glucose utilization rates in term neonates are 4 to 6 mg/min/kg, however, at earlier gestational ages (both fetal and preterm infants) these values are higher 8–9 mg/min/kg [Citation24] indicating that preterm infants need an early and reliable glucose source at birth.
Should the clinician accept hypoglycemia as normal or inevitable in these infants? For non-asphyxiated and appropriate for gestational age grown preterm infants, born below 33 weeks gestation, it has been shown that the glycemic nadir is at approximately 60–70 min of life [Citation25]. At the same time as this nadir, these preterm infants are often subject to other physiological stressors in parallel (such as asphyxia, hypoxia, sepsis, and hypothermia) [Citation23]. It is known that when hypoglycemia occurs in parallel to another pathology there can be resulting worse outcome. For example, in a study of infants with hypoxic-ischemic encephalopathy (all received cooling), infants with hypoglycemia had 9 points lower IQs (p = 0.023) and higher odds of adverse outcomes at preschool age (3.6; 95% CI, 1.4–9.0) (after adjustment for HIE severity) compared to those without hypoglycemia [Citation26]. It can be argued that this glycemic nadir is not beneficial and not physiological in very/extremely preterm infants who have little energy stores, are unable to enterally feed, and have a high risk of co-morbidities. It may best be prevented completely.
Clinicians continue attempts to prevent early (admission) hypoglycemia in smaller preterm infants. Recently, some clinicians are attempting to site an intravenous line immediately in the delivery room, however, obtaining intravenous access in the delivery room can be difficult. Other clinicians are recently considering other routes of administration of early dextrose including the buccal route. Already dextrose gel is used in newborns of older gestations (late preterm/term) and bigger newborns with risks factors for hypoglycemia in order to prevent NICU admission for hypoglycemia [Citation27–30]. A recent large randomized controlled trial has shown that giving prophylactic dextrose gel to at risk infants (late preterm/term) decreases hypoglycemia [Citation31] (although not NICU admission). Recently, some neonatal units have adopted protocols which include administration of early buccal dextrose gel to the younger/smaller preterm population in the delivery room. We as authors feel this an example of therapeutic creep and we questioned whether there already existed any evidence basis for this practice. This systematic review aimed to investigate whether there is evidence for any delivery room intervention (intravenous route or buccal route) and to highlight the quantity and quality (bias) of any current literature.
Materials and methods
This literature review was conducted following the PRISMA guidelines and checklist [Citation32].
Literature search
To frame the research question, the PICO format was used [Citation33].
Population
Moderately preterm (33+6 weeks gestational age), very preterm (<32 weeks gestational age), extremely preterm (<28 weeks gestation age), OR very (VLBW), OR extremely (ELBW) low birth weight infants, admitted to the NICU.
Intervention
Parenteral glucose administration in the delivery room (prior to NICU admission).
Comparison
Those not receiving the intervention.
Outcomes
Hypoglycemia (dichotomous “present/absent,” defined by local study) at the time of blood glucose testing as part of NICU admission protocol.
Statement of research question:
Does delivery room parenteral glucose administration reduce the risk of NICU admission hypoglycemia in preterm infants?
Study selection
Inclusion criteria consisted of:
1. VLBW or ELBW infants or infants
33+6 weeks birth gestation (moderately premature or younger) born in-house, 2. Parenteral glucose administration prior to NICU admission (note: the “buccal” route was included as this glucose enters the systemic circulation and is not subject to enteric absorption and first pass metabolism), 3. Blood glucose measurement at time of admission to NICU, 4. Any of: interventional studies (RCT/NRCT), quality improvement studies (quasi-experimental studies), cohort studies, case-control studies, pilot studies.
Exclusion criteria consisted of:
1. Enteral glucose prior to blood glucose measurement, 2. Non-human studies, 3. Non-glucose (dextrose) containing fluids only (prior to NICU admission), 4. Intramuscular glucagon in delivery room, 5. Abstract not available in electronic format.
The literature was appraised via data extraction, narrative synthesis, and critical review (quality assessment) of the study data.
Data extraction
Data were extracted using a headed table/template as included in the Data Extraction Table in Supplementary Materials. The headings of the data extraction table were chosen based on the common headings in the literature and based on the pertinent data specific to the research question. Two authors (GK and KT) independently extracted data from the original research articles and compared findings; any discrepancies were resolved by consensus.
Quality assessment
The Joanna Briggs Institute provides quality assessment tools for a large variety of study types including all the study types included in this review (before-after, cohort, and case-control) [Citation34, Citation35]. These simple checklists were used initially to aid the critical review of the included studies in this review. The included studies were then further critically reviewed using the following headings: internal validity; publication bias; selection bias; performance bias; detection bias; measurement bias; bias due to deviations from intervention; bias due to missing data; statistical quality; quality of reporting.
Results
Literature search
Screening of search results was carried out by assessing the title/abstract and adhering to the inclusion/exclusion criteria. Where titles only (but not their abstracts) were only available in electronic format these articles did not pass the initial screening (these were primarily titles from prior to the 1980s).
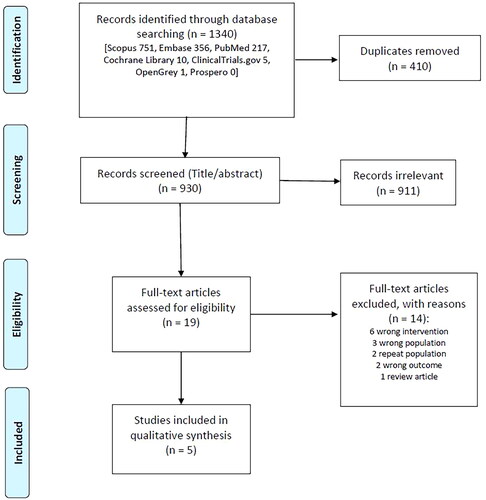
Nineteen studies were assessed as full text. Based on the assessment of the full text, 14 studies were excluded. Reasons for exclusion are given in . As a result, only five studies were included in the final analysis.
Study characteristics
All the main information of the included studies was extracted as per the Data Extraction Table in Supplementary Materials. The included studies were before-after (quasi-experimental), retrospective cohort, and case-control studies all published between 2014 and 2022. A summary of the pertinent data from all included studies is listed in .
Table 1. Summary of data from included studies.
Early dextrose and admission hypoglycemia
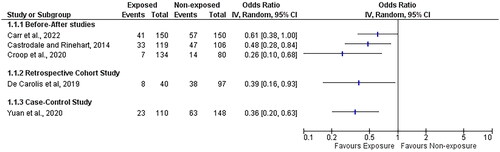
The studies were heterogenous in their design. In one before-after (quality improvement) study by Carr et al. [Citation36] prophylactic 40% dextrose gel (buccal) was given prospectively to all infants born less than 34 weeks gestation (median 28+5, IQR 25+4–31+6 gestation) during the quality improvement cycles. Two doses of gel were given to each infant, first as soon as ventilation established, and second after birthday cuddles and prior to transfer from the delivery room. The dose was based on estimated fetal weight [weight <500 g, 0.25 ml per dose/500–1000 g, 0.5 ml per dose/>1000 g, 1 ml per dose]. The other two before-after studies adopted interventions based on the Golden Hour protocol and these included peripheral intravenous (PIV) or umbilical venous catheter (UVC) insertion either in the delivery room [Citation37,Citation38] or immediately upon arrival to the NICU (prior to definitive UVC line insertion) [Citation38]. Both these two studies included infants of a similar gestational age at either <27 [Citation38] or <28 [Citation37] weeks gestation. All three studies had a different threshold for hypoglycemia (36 mg/dL [Citation36], 50 mg/dL [Citation37], and 45 mg/dL [Citation38], respectively). All three before-after studies showed significantly less admission hypoglycemia favoring their respective exposures. However, two of these three studies did not correct for an important parallel exposure (“temperature control” methods) in their results or in their statistical analysis [Citation37,Citation38] while in the third study, delivery room glucose was the single new recognized intervention/exposure [Citation36]. In all three studies, the comparison group was a non-contemporaneous cohort of infants from a time prior to the delivery room intervention being adopted in the respective neonatal unit.
In the retrospective cohort study [Citation39], a UVC was inserted in the delivery room and dextrose infusion commenced. Only extremely low birth weight infants were included and so participant numbers were small (n = 137) and the definition of hypoglycemia was much lower (≤ 29 mg/dL) [Citation39] than the other four studies. Although in univariate analysis, there was a significant relationship between exposure and outcome, the multivariate analysis showed this relationship diminished so that it was weakly associated but no longer significant (p < 0.07) [Citation39]. The fifth study was a case-control study and it included the gestationally most mature population (most participants were 29–32 weeks gestation at birth) [Citation40]. In this study, the intervention consisted of peripheral intravenous insertion and commencement of intravenous glucose infusion before admission to NICU (and before admission blood glucose). This study allowed for multiple confounding variables in its multi-variate regression analysis showing significant relationship between groups (cases/controls) with regards the exposure [Citation40].
Consideration was given to the possibility of combining the various studies data, however It is known that combining results is not appropriate unless the focus or design of the studies are similar [Citation41]. It was felt that the low number of studies, the variability in study design, the variability in outcome definition (hypoglycemia), and the presence of confounding co-interventions (co-exposures) in some of these studies precluded a meta-analysis of same.
However, to visually indicate the direction of the effect of each study a forest plot was used (omitting the forest plot summary value, i.e. the study effects are not pooled). The effect measure used was the odds ratio for the outcome of NICU admission hypoglycemia (). It can be seen, at the individual study level, that the odds ratios favor the exposure (delivery room intervention). However, it is important to note that the risk of bias was considered “moderate” or “high” for 4 out of 5 of these studies (see Quality Assessment, below).
Quality assessment
Internal validity
Internal validity undermined by large number of confounders is a big issue in observational and quasi-experimental studies such as those included in this review. In the before-after studies [Citation36–38], it is unclear what specifically is the cause versus effect of the outcome (hypoglycemia). Is it due to early dextrose? Or thermoregulation? Or other nonmeasured variables including other new standards of care during the post-exposure era (years)? In the before-after study by Castrodale et al., only the bare minimum of confounds are considered (gestation, birth weight, admission temperature) [Citation37]. Other studies considered more confounds and only two studies accounted for these confounds (using statistical methods) [Citation39,Citation40]. Only one study considered maternal medications (impacting on glycemic control) [Citation36] and no study considered infant hematocrit (which can impact on glucometer measurement).
Publication bias
The studies in this review were of the observational study type and it is known that far fewer observational studies publish protocols in advance, so often these types of studies are subject to publication bias (more than RCTs for example). This review attempted to somewhat address publication bias by including results from databases such as Prospero, OpenGrey, and Clinicaltrials.gov.
Selection/allocation bias
In the before-after studies [Citation36–38], the main variable regarding allocation of participants to the exposure was their date of birth and the institutional adoption of a new protocol (golden hour protocol or dextrose gel protocol) for all babies born after a specific date. Due to this the exposure/nonexposure groups generally contained a similar mix of baseline characteristics. However, an important confounder in these cases is the general clinical care between the time lapse (a year or two). In the retrospective cohort study [Citation39], allocation was mainly decided based on time of birth (24 h clock) and the availability of extra personnel (larger resuscitation team) during daytime hours to allow placement of umbilical venous catheters in the delivery suite. As a result, these daytime deliveries may have selected more clinically stable infants with a more stable metabolic status (compared to babies born out of hours). In the case-control study [Citation40], the control group was selected by a random algorithm from infants inborn during the same period. Although there were no specific details on matching, the groups appeared similar except for some identified differences which were allowed for in the multivariate regression analysis (small for gestational age, maternal hypertension, antenatal steroids) [Citation40].
Performance bias
This is a considerable factor in the before-after studies [Citation36–38] as the treating clinicians knew that exposed participants were in the intervention group and so their management of the infants with regard other factors (temperature regulation, handling, speed of procedures) may have been influenced.
Detection bias
All five studies had a different threshold for the definition of hypoglycemia (range: 29–50 mg/dL). This may have impacted on rate of the dichotomous outcome in this review (present/absence of admission hypoglycemia). Timing of admission blood glucose is also an important factor in the primary outcome. These preterm infants experience a nadir (“physiological” or “pathological” depending on opinion) in blood glucose between 60 and 70 min of life [Citation25]. The level of detail whereby the exact time of the outcome in the individual participant is not known in 3 out of the 5 included studies [Citation37–39]. Whether blood glucose measurement was taken close to this nadir or not is a variable that is not adjusted/accounted for in these three studies.
Measurement bias
Type of sample and method of measurement of the outcome (hypoglycemia) was specified in 4 out of the 5 studies. One study used blood gas machine only [Citation36], one study used either blood gas machine or glucometer [Citation39], two studies used glucometers only [Citation38, Citation40], and one study did not specify. Glucometers are known to have variable accuracy with compared to a gold standard laboratory result and are also impacted by neonatal factors such as hematocrit [Citation42]. This has impact on the outcome validity of this study.
Bias due to deviations from intended interventions
All infants born in the post-intervention stage of the before-after studies [Citation36–38] were supposed to receive the exposure. However, in 2 of these 3 studies, not all eligible infants received the exposure for reasons such as unavailability or difficulty applying the intervention (difficulty with umbilical/peripheral line insertion in the delivery room) [Citation37,Citation38]. This bias is similarly seen for some infants not receiving the intervention in the retrospective cohort study [Citation39]. It could be argued that these same infants would have a higher baseline risk of the outcome.
Bias due to missing data
In the retrospective cohort study, 29 participants had incomplete data [Citation39] which was not an insignificant number compared to overall cohort numbers.
Statistical quality
As these studies were not combined in a meta-analysis, the individual study statistical quality is important. Two of the before-after studies did not incorporate statistical methods to try and account for an important confound (improved thermoregulation practices) threatening their internal validity [Citation37, Citation38].
A summary of the overall risk of bias for each study was presented as low/medium/high risk and whether the direction of bias favored the intervention or controls. These are presented in .
Discussion
Metabolic adaption of preterm infants after birth is an important part of the fetal to neonatal transition. Immediately after birth a preterm infant has relatively high energy consumption and already poor energy reserves. It is a focus of debate whether a nadir in blood glucose values is a good or bad part of this metabolic adaption (“physiological” or “pathological”?) in these infants. Further, the literature regarding neurodevelopmental outcomes following hypoglycemia is also conflicting. In this setting of uncertainty clinicians continue to attempt to avoid early hypoglycemia in these preterm infants.
This systematic review investigated the literature regarding the provision of early (prior to NICU admission) parenteral glucose, as soon as possible after birth, to these infants and whether this early glucose helped reduce the risk of admission hypoglycemia. The “buccal” route was included as this glucose enters the systemic circulation (and is not subject to enteric absorption and first pass metabolism). Intramuscular glucagon was excluded as a method of parenteral glucose, as glucagon alone does not provide exogenous energy (glucose) but instead attempts to mobilize endogenous energy stores. There is concern that glucagon’s effect in preterm infants is reduced due to diminished glycogen stores and higher/dysregulated insulin secretion in these infants [Citation43].
The review was unable to show that the exposure impacted on the outcome as the studies were few, heterogenous in study design, of lower level in the levels of evidence grades, and at high risk of bias. Only one study reported on potential harms (hyperglycemia, late hypoglycemia, necrotizing enterocolitis, and spontaneous intestinal perforation) which were insignificant [Citation36]. It is recommended to achieve central venous access within 1 h of life in these preterm infants [Citation19], however, focusing on commencing early parenteral glucose in the delivery room is not supported or refuted by the evidence from this literature review. In the absence of sound evidence, clinicians should continue current practices of perinatal care in these infants to try and mitigate early hypoglycemia. These best current practices include [Citation19,Citation22]: prevention of hypothermia, obtaining intravenous access early, commencing an initial glucose infusion rate (GIR) of 6–8 mg/kg/min, measuring the blood glucose within the first hour, targeting a blood glucose range of 50–110 mg/dL, and adjusting the GIR as needed.
The limitations of this review included the small number of studies meeting the inclusion criteria and the limitations to study type. The critical review of before-after (quasi-experimental) studies proved difficult as there is a lack of dedicated guidelines for reviewing these study types. There were no randomized controlled trials included.
This review followed the PRISMA Checklist [Citation32] (See Supplementary Materials). The strengths of this review include the extensive searching of many databases including ongoing trials and grey literature. Broad search criteria (relative to the specific research question) were used in attempt to discover any studies. In addition, the studies included were appraised using detailed quality assessment.
Conclusion
The important message in this systematic literature review is that the literature is not sound (not without bias) and low grade for the interventions of either dextrose gel or intravenous dextrose given in the delivery room. Obtaining intravenous access immediately in the delivery room is difficult in these small preterm infants. Some clinical units feel that giving buccal dextrose gel may be a more feasible route of administration of dextrose. However, the lack of current literature and the poor quality (bias) of the literature is very important for clinicians to realize while attempting to mitigate the ongoing problem of neonatal hypoglycemia at admission in this preterm population. In the absence of sound evidence clinicians should continue current best practices of perinatal care in these infants to try and mitigate early hypoglycemia. Future research is needed and should ideally take the form of well-designed randomized placebo-controlled trials.
Supplemental Material
Download Zip (93.8 KB)Acknowledgments
This work was initially conducted by GK as part of a thesis dissertation (MSc Neonatology, University of Southampton). In addition, all authors contributed to the work as follows: GK: developed the protocol, searched literature, assessed literature for eligibility, extracted data, analyzed and critically reviewed the literature, and wrote the manuscript. KT: assessed literature for eligibility, extracted data, reviewed and approved the manuscript. MH: contributed to protocol development, reviewed and approved the manuscript. JK: reviewed and approved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Nivins S, Kennedy E, Thompson B, et al. Associations between neonatal hypoglycaemia and brain volumes, cortical thickness and white matter microstructure in mid-childhood: an MRI study. NeuroImage Clin. 2022;33:102943.
- Burns CM, Rutherford MA, Boardman JP, et al. Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics. 2008;122:65–74.
- Filan PM, Inder TE, Cameron FJ, et al. Neonatal hypoglycemia and occipital cerebral injury. J Pediatr. 2006;148:552–555.
- Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ. 1988;297(6659):1304–1308.
- Edwards T, Alsweiler JM, Gamble GD, et al. Neurocognitive outcomes at age 2 years after neonatal hypoglycemia in a cohort of participants from the hPOD randomized trial. JAMA Netw Open. 2022;5:e2235989.
- Duvanel CB, Fawer CL, Cotting J, et al. Long-term effects of neonatal hypoglycemia on brain growth and psychomotor development in small-for-gestational-age preterm infants. J Pediatr. 1999;134:492–498.
- McKinlay CJD, Alsweiler JM, Anstice NS, et al. Association of neonatal glycemia with neurodevelopmental outcomes at 4.5 years. JAMA Pediatr. 2017;171:972–983.
- Kaiser JR, Bai S, Gibson N, et al. Association between transient newborn hypoglycemia and fourth-grade achievement test proficiency. JAMA Pediatr. 2015;169:913.
- Kerstjens JM, Bocca-Tjeertes IF, Winter AF, et al. Neonatal morbidities and developmental delay in moderately preterm-born children. Pediatrics. 2012;130:e265–e272.
- Tottman AC, Alsweiler JM, Bloomfield FH, et al. Relationship between measures of neonatal glycemia, neonatal illness, and 2-year outcomes in very preterm infants. J Pediatr. 2017;188:115–121.
- Shah R, Dai DWT, Alsweiler JM, et al. Association of neonatal hypoglycemia with academic performance in mid-childhood. JAMA. 2022;327(12):1158–1170.
- Goode RH, Rettiganti M, Li J, et al. Developmental outcomes of preterm infants with neonatal hypoglycemia. Pediatrics. 2016;138(6):e20161424.
- Tin W, Brunskill G, Kelly T, et al. 15-year follow-up of recurrent “hypoglycemia” in preterm infants. Pediatrics. 2012;130(6):e1497–e1503.
- Pascal A, Govaert P, Oostra A, et al. Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: a meta-analytic review. Dev Med Child Neurol. 2018;60(4):342–355.
- Eves R, Mendonça M, Baumann N, et al. Association of very preterm birth or very low birth weight with intelligence in adulthood: an individual participant data meta-analysis. JAMA Pediatr. 2021;175(8):e211058.
- Thornton PS, Stanley CA, De Leon DD, et al. Recommendations from the Pediatric Endocrine Society for Evaluation and Management of Persistent Hypoglycemia in Neonates, Infants, and Children. J Pediatr. 2015;167(2):238–245.
- Adamkin DH. Neonatal hypoglycemia. Semin Fetal Neonatal Med. 2017;22(1):36–41.
- Hoermann H, Mokwa A, Roeper M, et al. Reliability and observer dependence of signs of neonatal hypoglycemia. J Pediatr. 2022;245:22–29.e2.
- Sharma A, Davis A, Shekhawat PS. Hypoglycemia in the preterm neonate: etiopathogenesis, diagnosis, management and long-term outcomes. Transl Pediatr. 2017;6(4):335–348.
- Reynolds RD, Pilcher J, Ring A, et al. The golden hour: care of the LBW infant during the first hour of life one unit’s experience. Neonatal Netw. 2009;28(4):211–219. quiz 255–258.
- Vento M, Cheung P-Y, Aguar M. The first golden minutes of the extremely-low-gestational-age neonate: a gentle approach. Neonatology. 2009;95(4):286–298.
- Sharma D. Golden 60 minutes of newborn’s life: part 1: preterm neonate. J Matern Fetal Neonatal Med. 2017;30:2716–2727.
- Mitanchez D. Glucose regulation in preterm newborn infants. Horm Res. 2007;68(6):265–271.
- Hay WW. Recent observations on the regulation of fetal metabolism by glucose. J Physiol. 2006;572(Pt 1):17–24.
- Kaiser JR, Bai S, Rozance PJ. Newborn plasma glucose concentration nadirs by gestational-age group. Neonatology. 2018;113(4):353–359.
- Parmentier CEJ, de Vries LS, van der Aa NE, et al. Hypoglycemia in infants with hypoxic-ischemic encephalopathy is associated with additional brain injury and worse neurodevelopmental outcome. J Pediatr. 2022;245:30–38.e1.
- Harris DL, Weston PJ, Signal M, et al. Dextrose gel for neonatal hypoglycaemia (the sugar babies study): a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382(9910):2077–2083.
- Gupta K, Amboiram P, Balakrishnan U, et al. Dextrose gel for neonates at risk with asymptomatic hypoglycemia: a randomized clinical trial. Pediatrics. 2022;149:e2021050733.
- Meneghin F, Manzalini M, Acunzo M, et al. Management of asymptomatic hypoglycemia with 40% oral dextrose gel in near term at-risk infants to reduce intensive care need and promote breastfeeding. Ital J Pediatr. 2021;47(1):201.
- Makker K, Alissa R, Dudek C, et al. Glucose gel in infants at risk for transitional neonatal hypoglycemia. Am J Perinatol. 2018;35(11):1050–1056.
- Harding JE, Hegarty JE, Crowther CA, et al. Evaluation of oral dextrose gel for prevention of neonatal hypoglycemia (hPOD): a multicenter, double-blind randomized controlled trial. PLoS Med. 2021;18(1):e1003411.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Purssell E, McCrae N. How to perform a systematic literature review: a guide for healthcare researchers, practitioners and students [Internet]. Switzerland: Springer International Publishing; 2020. [cited 2020 Nov 17]. Available from: https://www.springer.com/gp/book/9783030496715.
- Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L. Chapter 3: Systematic reviews of effectiveness. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. Joanna Briggs Institute; 2020. https://synthesismanual.jbi.global/
- Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, Lisy K, Mu P-F. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. Joanna Briggs Institute. https://synthesismanual.jbi.global
- Carr CP, MacMillan H, Reynolds PR. Routine buccal glucose reduces admission hypoglycaemia in premature newborns: a quality improvement project Acta Paediatr. 2022;111(9):1709–1711.
- Castrodale V, Rinehart S. The golden hour: improving the stabilization of the very low birth-weight infant. Adv Neonatal Care. 2014;14(1):9–14.
- Croop SEW, Thoyre SM, Aliaga S, et al. The golden hour: a quality improvement initiative for extremely premature infants in the neonatal intensive care unit. J Perinatol. 2020;40:530–539.
- De Carolis MP, Casella G, Serafino E, et al. Delivery room interventions to improve the stabilization of extremely-low-birth-weight infants. J Matern-Fetal Neonatal Med. 2019;34:1925–1931.
- Yuan Z-X, Gao H, Duan C-C, et al. Risk factors for hypoglycemia in preterm infants with a gestational age of ≤32 weeks. Chin J Contemp Pediatr. 2020;22:1154–1158.
- Aveyard H. Doing a literature review in health and social care: a practical guide [Internet]. Maidenhead, UK: McGraw-Hill Education; 2018. [cited 2021 Sep 24]. Available from: http://ebookcentral.proquest.com/lib/soton-ebooks/detail.action?docID=6212137.
- Movalia MK, Ogino MT. Point of care glucose testing in neonatal hypoglycemia. Point Care. 2006;5:95–99.
- Walsh EPG, Alsweiler JM, Ardern J, et al. Glucagon for neonatal hypoglycaemia: systematic review and meta-analysis. Neonatology. 2022;119(3):285–294.