Abstract
Introduction
Prenatal exposure to polycyclic aromatic hydrocarbons (PAHs) is a risk factor for the occurrence of congenital heart diseases (CHDs). Genetic susceptibility to PAHs metabolism may modify the exposure–risk relationship. The role of uridine diphosphoglucuronosyl transferase 1A1 (UGT1A1) genetic polymorphisms for modulating the impacts of prenatal PAHs exposure on the risk of CHDs remains to be discovered.
Objective
The aim of this study was to investigate whether maternal UGT1A1 genetic polymorphisms are associated with fetal susceptibility to CHDs and to assess whether the risk is modified by maternal PAHs exposure.
Methods
Maternal urinary biomarker of PAHs exposure was determined in 357 pregnant women with CHDs fetuses and 270 controls (pregnant women carrying fetuses without major congenital malformations). Urinary 1-hydroxypyrene-glucuronide (1-OHPG) concentration, a sensitive biomarker for PAHs exposure, was measured using ultra-high-performance liquid chromatography coupled with tandem mass spectrometry. Maternal single nucleotide polymorphisms (SNPs) in UGT1A1, including rs3755319, rs887829, rs4148323, rs6742078, and rs6717546, were genotyped using an improved multiplex ligation detection reaction (iMLDR) technique. Unconditional logistic regression was performed to determine the impacts of UGT1A1 polymorphisms on the risks of CHDs and their subtypes. Generalized multifactor dimensionality reduction (GMDR) was used to analyze the gene–gene and gene–PAHs exposure interactions.
Results
None of the selected UGT1A1 polymorphisms was independently associated with the risk of CHDs. The interaction between SNP rs4148323 and PAHs exposure was observed to be associated with CHDs (p< .05). Pregnant women with high-level PAHs exposure and rs4148323 had an increased risk of carrying CHDs fetuses (GA-AA vs. GG: aOR = 2.00, 95% CI = 1.06–3.79). Moreover, the joint effect of rs4148323 and PAHs exposure was found to be significantly associated with risks of septal defects, conotruncal heart defects, and right-sided obstructive malformations.
Conclusions
Maternal genetic variations of UGT1A1 rs4148323 may modify the association between prenatal PAHs exposure and CHDs risk. This finding needs to be further confirmed in a larger-scale study.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental contaminants that are derived mostly from the incomplete combustion of tobacco, coal, and other organic substances [Citation1]. Exposure to PAHs is widespread for human beings through multiple routes, such as inhalation of cigarette smoke or polluted air and ingestion of foods containing PAHs [Citation2]. Concern about fetal hazards (e.g. congenital defects and intrauterine growth restriction) after maternal exposure to PAHs has been raised [Citation3–5].
Congenital heart diseases (CHDs) are among the most common forms of all human congenital defects. Growing evidence has indicated that PAHs can increase the risk of CHDs. Animal studies have shown that prenatal exposure to PAHs increases the occurrence of CHDs [Citation6,Citation7]. Some epidemiological studies have also found that women with prenatal exposure to PAHs are more likely to have offspring with CHDs, such as conotruncal heart defects and septal defects [Citation3], but a population-based study has shown a null association [Citation8]. Thus, we hypothesize that genetic variations such as metabolic enzyme gene polymorphisms influencing PAHs may partly be accountable for the inconsistent conclusions.
Some metabolic enzyme genes such as cytochrome P450 1A1 (CYP1A1) and uridine diphosphoglucuronosyl transferase 1A1 (UGT1A1) have been reported to modulate the metabolism and activation of PAHs [Citation9,Citation10]. Human studies have also observed that CYP1A1 and UGT1A1 gene polymorphisms significantly affect the concentrations of urinary 1-hydroxypyrene (1-OHP) (a biomarker of PAHs exposure) [Citation11,Citation12]. Moreover, maternal genotypes such as CYP1B1 polymorphisms have been shown to modify the association between maternal exposure to PAHs and CHDs risk [Citation13]. However, the role of UGT1A1 genetic polymorphisms for modulating the impacts of prenatal PAHs exposure on the risk of CHDs remains to be discovered.
In a previous case-control study, we found that PAHs exposure was associated with an increased risk of CHDs [Citation13]. The present study aimed to explore whether maternal UGT1A1 (rs3755319, rs887829, rs4148323, rs6742078, and rs6717546) polymorphisms modified the association of CHDs risk with PAHs exposure.
Materials and methods
Study participants and data collection
All pregnant women were selected from an established case-control study examining maternal PAHs exposure’s impacts on the risk of CHDs [Citation13]. In brief, cases were pregnant women who had fetuses diagnosed with CHDs and without any extracardiac abnormalities, while controls in the same hospital were pregnant women carrying fetuses without major congenital malformations. Pregnancies with multiple births and fetuses diagnosed with chromosomal aberrations and syndromic diseases were excluded. All CHDs cases were confirmed by echocardiography, cardiac catheterization, surgery, or autopsy. CHDs cases based on the anatomic lesions were also classified into six subgroups: septal defects, conotruncal heart defects, left-sided obstructive malformations, right-sided obstructive malformations, anomalous pulmonary venous return, and other heart abnormalities [Citation14]. The well-trained investigators administered a face-to-face interview with each pregnant woman at enrollment. The trained investigators collected information on demographic characteristics, living environment, lifestyle habits, maternal reproductive history, maternal illness and drug use history, maternal diet and nutrition.
The study was conducted under the approval of the Ethics Committee of Sichuan University (no. 2010004) and followed the tenets of the Declaration of Helsinki. Informed consent was obtained from each participant.
PAH exposure assessment
Urine samples from each pregnant woman were collected and stored at −70 °C until analysis. The 1-hydroxypyrene-glucuronide (1-OHPG) in urine is a sensitive exposure biomarker for low-level PAHs exposure [Citation15]. As previously described [Citation13], urinary 1-OHPG concentrations were measured using an ultra-high-performance liquid chromatograph system (Nexera X2 LC-30AD, Shimadzu, Kyoto, Japan) coupled with a TSQ Vantage tandem mass spectrometer (Thermo Fisher Scientific, Waltham, MA) with an electrospray ionization (ESI) interface operated in a negative ion mode. We used urine creatinine (Cr) contents to adjust the concentrations of 1-OHPG [Citation16]. Moreover, the levels of PAHs exposure were categorized into two groups (high and low exposure groups) according to the established optimal cutoff value of 1-OHPG (0.03 μg/g Cr) in our previous study [Citation13].
Polymorphism selection and genotyping
Blood samples from each pregnant woman were collected and stored at −70 °C. Genomic DNA was extracted using a QIAamp® DNA Blood Mini Kit (Qiagen, Hilden, Germany, Cat. No. 51106). DNA samples were then stored at −80 °C before further analysis. In our analysis, the selection of single nucleotide polymorphisms (SNPs) in UGT1A1 gene was in accordance with the following criteria: (1) previously reported that they were significantly associated with some diseases or PAHs metabolism [Citation17–21]. (2) Minor allele frequency (MAF) > 0.05 in Chinese Han population. Five SNPs (rs3755319, rs887829, rs4148323, rs6742078, and rs6717546) were selected in UGT1A1 gene.
SNP genotyping was further performed using an improved multiplex ligation detection reaction (iMLDR) technique (Genesky Biotechnologies Inc., Shanghai, China). For quality control, we randomly selected 10% of samples to monitor the reproducibility of the assays, and the concordance was 100%. Five cases and one control failed to be genotyped for UGT1A1 rs6717546. More detailed information about the studied UGT1A1 polymorphisms is provided in Additional file (Table S1).
Statistical analysis
Descriptive statistics for maternal and fetal characteristics of the study participants were conducted. Parametric and nonparametric methods were respectively used to test the statistical significance for differences in categorical or continuous variables. Deviation from the Hardy–Weinberg equilibrium (HWE) expectation in controls was analyzed by Chi-square test, and p< .05 indicated a deviation from equilibrium. We first utilized unconditional logistic regression analysis to investigate the associations of maternal UGT1A1 polymorphisms with CHDs risk. The associations of UGT1A1 genotypes with CHDs risk were also stratified by specific CHD subtypes. For genotype comparisons, homozygous wild-type served as the reference group to which heterozygotes and variant homozygotes were compared. Then, testing for gene–gene and gene–PAHs exposure interactions associated with CHDs was performed using generalized multifactor dimensionality reduction (GMDR, version 0.7, University of Virginia, Charlottesville, VA). The test detects and characterizes non-linear interactions among discrete genetic and environmental attributes [Citation22]. Meanwhile, the identified effects of gene–PAHs exposure on the risks of CHDs and their subtypes were further analyzed by logistic regression to obtain the adjusted odds ratio (aOR) and 95% confidence interval (CI). All logistic regression analyses were adjusted for maternal age (years), gestational week (weeks), housing renovation, proximity to a factory or landfill (<1000 m), cooking at home (≥4 times/week), parental smoking or environmental tobacco smoke (ETS) exposure, maternal alcohol consumption (≥1 time(s)/week), and use of folic acid supplements. Two-sided p< .05 was considered statistically significant. Statistical analyses were calculated using Stata version 16.0 (Stata Corp LP, College Station, TX).
Results
Characteristics of participants
A total of 627 pregnant women (357 with CHDs fetuses and 270 controls) were enrolled in our study. summarizes the maternal and fetal characteristics of the study participants. Data on the environmental characteristics of the cases and controls revealed no significant differences, with the exception of cooking at home and folic acid supplement. Meanwhile, urinary 1-OHPG concentrations were significantly higher in cases (p < .001). In fetuses with CHDs, septal defects were the most frequent malformations (65.83%), followed by conotruncal heart defects (44.82%) and right-sided obstructive malformations (31.93%).
Table 1. Maternal and fetal characteristics among the study subjects.
Association of UGT1A1 polymorphisms with CHDs
For all five polymorphisms (rs3755319, rs887829, rs4148323, rs6742078, and rs6717546) in UGT1A1, the distribution of the genotypes conformed well to the HWE (see Additional file Table S2). No significant association of UGT1A1 polymorphisms with CHDs was found (). Although rs4148323 was associated with an increased risk of right-sided obstructive malformations (see Additional file Table S3), the association was of borderline significance (aOR = 1.58, 95% CI = 0.95–2.63).
Table 2. Association between maternal UGT1A1 polymorphisms and the risk of CHDs.
Gene–gene and gene–environment interaction
We used GMDR to screen for the best interaction combination among five polymorphisms of UGT1A1 gene (). We found that the best model to represent the multi-loci interaction analyses consisted of rs4148323 and PAHs exposure. The model had the greatest cross-validation consistency (10/10) and the highest testing balanced accuracy (0.5698) (p<.05), indicating that there was a potential interaction between rs4148323 and PAHs exposure influencing CHDs risk. shows the score distributions in the best model, from which we could see that women carrying with GG or GA genotypes for rs4148323 under the high-level PAHs exposure condition, and AA genotypes under the low-level PAHs exposure condition, had higher risks of having CHDs fetuses. Although rs4148323 was not independently associated with CHDs (), our analysis using an additional model after adjusting for confounders showed that pregnant women with the A allele of rs4148323 had a significantly increased risk of having CHDs fetuses under the high-level PAHs exposure condition (aOR = 2.00, 95% CI = 1.06–3.79) (). Moreover, women with high-level PAHs exposure and rs4148323 had increased risks of carrying fetuses with septal defects, conotruncal heart defects and right-sided obstructive malformations (see Additional file Table S4).
Figure 1. The best GMDR model of interaction among UGT1A1 rs4148323 and PAHs. Note: The graph was generated by the GMDR software. In each cell, the left bar represents a positive score, and the right bar a negative score. The cell with dark shading represents an interaction resulting in a high risk of having congenital heart diseases fetuses (the absolute magnitude of the positive score > the absolute magnitude of the negative score). The cell with light shading represents an interaction resulting in a low risk of having congenital heart diseases fetuses (the absolute magnitude of the positive score < the absolute magnitude of the negative score). The genotypes for UGT1A1 rs4148323 include GG, GA, and AA. The levels of PAHs exposure were categorized into two groups (high and low exposure groups).
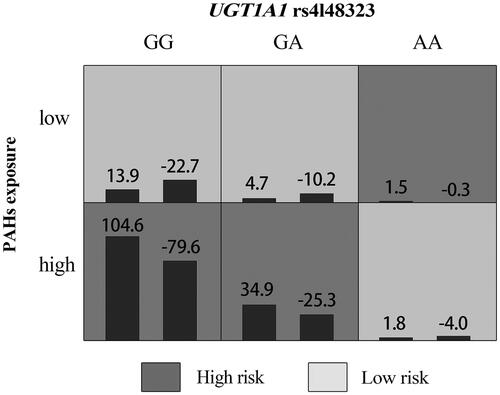
Table 3. Gene–gene and gene–PAHs exposure interaction models in CHDs obtained using the GMDR method.
Table 4. The interaction between maternal PAHs exposure and UGT1A1 rs4148323 influencing CHDs based on additive model.
Discussion
Congenital heart diseases rank as the leading cause of birth defect-related mortality [Citation23]. To date, the etiology of CHDs has not been completely understood. It is widely believed that most CHDs are caused by a complex combination of genetic and environmental factors [Citation24]. Our study investigated an underlying role of UGT1A1 gene in modulating CHDs risk, on the basis of a previously identified association between PAHs exposure and CHDs risk [Citation13]. In the current study, we found that the association of PAHs exposure with CHDs risk was modified by rs4148323 polymorphism in UGT1A1 gene.
The uridine diphosphoglucuronosyl transferases (UGTs) belong to a superfamily of metabolizing enzymes participating in detoxifying endogenous and exogenous compounds such as steroid hormones, xenobiotics, and drugs [Citation25,Citation26]. Several polymorphisms in UGT1A1 gene can affect the expression and the activity of encoded enzymes [Citation26,Citation27]. In recent years, several studies have reported the associations of UGT1A1 gene variations such as rs4148323 and rs887829 with disease risks, including neonatal hyperbilirubinemia and cancer risks. Two meta-analysis studies have shown that rs4148323 polymorphism is a risk factor for developing neonatal hyperbilirubinemia in the Asian population but not in the Caucasian population [Citation20,Citation28]. For rs887829 polymorphism, a case-control study showed that it was associated with a reduced risk of neonatal hyperbilirubinemia [Citation17], but it was not associated with risks of endometrial cancer and gallstone [Citation18,Citation29].
So far, there is limited research evaluating the impact of other UGT1A1 polymorphisms (rs3755319, rs6742078, and rs6717546) on disease risks. No significant association between rs3755319 polymorphism and progression-free survival or overall survival of irinotecan-treated colorectal cancer patients was observed [Citation30]. As for rs6742078 polymorphism, it has been shown to be associated with a reduced risk of new-onset type 2 diabetes in a Dutch population [Citation19] and with increased gallstone risks in German and Indian populations [Citation29,Citation31]. Regarding rs6717546 polymorphism, a retrospective case-control study found that it was likely a protective factor against neonatal hyperbilirubinemia [Citation17]. However, none of the UGT1A1 polymorphisms was detected to be independently associated with the risk of CHDs in our study. We speculated that the heterogeneity observed among various diseases may be related to different levels of UGT1A1 expression in various organ sites and different gene–environmental interactions.
The interaction of gene–environment has been investigated to explore the etiology of various diseases in previous studies. A meta-analysis has shown that UGT1A1 rs4148323 polymorphism is associated with an increased risk of irinotecan-induced severe neutropenia (GA + AA genotypes vs. GG genotype: OR = 2.66, 95% CI = 1.10–6.45, p=.03) [Citation32]. Another study in Japan has found that rs4148323 polymorphism is a risk factor for neonatal hyperbilirubinemia in infants with inadequate breastfeeding [Citation33]. However, a population-based study failed to find a joint effect of UGT1A1 rs887829 polymorphism and soy food intake on the risk of endometrial cancer [Citation18]. In our study, we demonstrated that maternal UGT1A1 rs4148323 polymorphism was associated with an increased CHDs risk under the circumstance of high-level PAHs exposure. Considering that there are inconsistent conclusions regarding the association between PAHs exposure and CHDs [Citation3,Citation8], we speculate that the inconsistency might partly be due to the role of rs4148323 in UGT1A1 gene. Given the relatively limited sample size, our finding needs to be further confirmed in studies with larger sample sizes.
Our study had several strengths. The study was the first to evaluate the effect of maternal UGT1A1 genetic polymorphisms on the risks of CHDs and their subtypes. Second, we used 1-OHPG in urine as a quantitative biomarker for estimating prenatal exposure to PAHs. Third, we examined the interactions of gene–gene and gene–PAHs exposure in CHDs using the method of GMDR. However, several limitations should be noticed. First, limited sample size and multiple comparisons reduced the statistical power in the evaluation of the association between the risks of CHDs subtypes and UGT1A1 genetic polymorphisms and their combination with PAHs exposure. Second, a single spot urine measurement cannot precisely estimate the mother’s long-term exposure level. Thus, future studies are needed to collect multiple urine samples. Third, fetal genotypes were not considered, and future studies are needed to investigate the effects of maternal and fetal genotypes and gene–exposure interaction on the risk of CHDs.
Our study indicates that maternal genetic variation of UGT1A1 rs4148323 modified the association between PAHs exposure and CHDs. A more comprehensive, larger-scale study is needed to further detect other genetic polymorphisms and gene–exposure interactions with respect to the risk of CHDs.
Conclusions
In the present work, we investigated the impacts of UGT1A1 polymorphisms on the risks of CHDs and their subtypes and analyzed the gene–gene and gene–PAHs exposure interactions. The maternal genetic variation of UGT1A1 rs4148323 may modify the association between prenatal PAHs exposure and the risk of CHDs.
Supplemental Material
Download MS Word (24.4 KB)Acknowledgements
The authors are grateful to the obstetricians, pediatricians, geneticists, experimental technicians, and other participants involved in the project for recruiting the cases and control participants and collecting the data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zhang Y, Tao S. Global atmospheric emission inventory of polycyclic aromatic hydrocarbons (PAHs) for 2004. Atmos Environ. 2009;43:812–819.
- Suzuki K, Yoshinaga J. Inhalation and dietary exposure to polycyclic aromatic hydrocarbons and urinary 1-hydroxypyrene in non-smoking university students. Int Arch Occup Environ Health. 2007;81(1):115–121.
- Patel J, Nembhard WN, Politis MD, et al. Maternal occupational exposure to polycyclic aromatic hydrocarbons and the risk of isolated congenital heart defects among offspring. Environ Res. 2020;186:109550.
- Lupo PJ, Langlois PH, Reefhuis J, et al. Maternal occupational exposure to polycyclic aromatic hydrocarbons: effects on gastroschisis among offspring in the national birth defects prevention study. Environ Health Perspect. 2012;120(6):910–915.
- Choi H, Rauh V, Garfinkel R, et al. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and risk of intrauterine growth restriction. Environ Health Perspect. 2008;116(5):658–665.
- Farwell A, Nero V, Croft M, et al. Modified Japanese medaka embryo-larval bioassay for rapid determination of developmental abnormalities. Arch Environ Contam Toxicol. 2006;51(4):600–607.
- Incardona JP, Collier TK, Scholz NL. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol. 2004;196(2):191–205.
- Lupo PJ, Symanski E, Langlois PH, et al. Maternal occupational exposure to polycyclic aromatic hydrocarbons and congenital heart defects among offspring in the national birth defects prevention study. Birth Defects Res A Clin Mol Teratol. 2012;94(11):875–881.
- Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 2004;95(1):1–6.
- Yang H, Shi Z, Wang X-X, et al. Phenanthrene, but not its isomer anthracene, effectively activates both human and mouse nuclear receptor constitutive androstane receptor (CAR) and induces hepatotoxicity in mice. Toxicol Appl Pharmacol. 2019;378:114618.
- Chen B, Hu Y, Jin T, et al. The influence of metabolic gene polymorphisms on urinary 1-hydroxypyrene concentrations in Chinese coke oven workers. Sci Total Environ. 2007;381(1–3):38–46.
- Nerurkar PV, Okinaka L, Aoki C, et al. CYP1A1, GSTM1, and GSTP1 genetic polymorphisms and urinary 1-hydroxypyrene excretion in non-occupationally exposed individuals. Cancer Epidemiol Biomarkers Prev. 2000;9:1119–1122.
- Li N, Mu Y, Liu Z, et al. Assessment of interaction between maternal polycyclic aromatic hydrocarbons exposure and genetic polymorphisms on the risk of congenital heart diseases. Sci Rep. 2018;8(1):3075.
- Deng K, Liu Z, Lin Y, et al. Periconceptional paternal smoking and the risk of congenital heart defects: a case-control study. Birth Defects Res A Clin Mol Teratol. 2013;97(4):210–216.
- Strickland P, Kang D. Urinary 1-hydroxypyrene and other PAH metabolites as biomarkers of exposure to environmental PAH in air particulate matter. Toxicol Lett. 1999;108(2–3):191–199.
- Li M, Wang Q, Zhu J, et al. A simple analytical method of determining 1-hydroxypyrene glucuronide in human urine by isotope dilution with ultra performance liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem. 2017;409(6):1513–1518.
- Zhou Y, Wang S, Li H, et al. Association of UGT1A1 variants and hyperbilirubinemia in breast-fed full-term Chinese infants. PLOS One. 2014;9(8):e104251.
- Deming SL, Zheng W, Xu W-H, et al. UGT1A1 genetic polymorphisms, endogenous estrogen exposure, soy food intake, and endometrial cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17(3):563–570.
- Abbasi A, Deetman PE, Corpeleijn E, et al. Bilirubin as a potential causal factor in type 2 diabetes risk: a Mendelian randomization study. Diabetes. 2015;64(4):1459–1469.
- Long J, Zhang S, Fang X, et al. Association of neonatal hyperbilirubinemia with uridine diphosphate-glucuronosyltransferase 1A1 gene polymorphisms: meta-analysis. Pediatr Int. 2011;53(4):530–540.
- Shin HJ, Kim JY, Cheong HS, et al. Functional study of haplotypes in UGT1A1 promoter to find a novel genetic variant leading to reduced gene expression. Ther Drug Monit. 2015;37(3):369–374.
- Xu H-M, Xu L-F, Hou T-T, et al. GMDR: versatile software for detecting gene–gene and gene–environment interactions underlying complex traits. Curr Genomics. 2016;17(5):396–402.
- Mensah GA, Brown DW. An overview of cardiovascular disease burden in the United States. Health Aff. 2007;26(1):38–48.
- Krauss RS, Hong M. Gene–environment interactions and the etiology of birth defects. Curr Top Dev Biol. 2016;116:569–580.
- Riedmaier S, Klein K, Hofmann U, et al. UDP-glucuronosyltransferase (UGT) polymorphisms affect atorvastatin lactonization in vitro and in vivo. Clin Pharmacol Ther. 2010;87(1):65–73.
- Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol. 2000;40:581–616.
- Miners JO, McKinnon RA, Mackenzie PI. Genetic polymorphisms of UDP-glucuronosyltransferases and their functional significance. Toxicology. 2002;181–182:453–456.
- Mehrad-Majd H, Haerian MS, Akhtari J, et al. Effects of Gly71Arg mutation in UGT1A1 gene on neonatal hyperbilirubinemia: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2019;32:1575–1585.
- Ravikanth VV, Rao GV, Govardhan B, et al. Polymorphisms in UGT1A1 gene predispose South Indians to pigmentous gallstones. J Clin Exp Hepatol. 2016;6(3):216–223.
- Yu Q, Zhang T, Xie C, et al. UGT1A polymorphisms associated with worse outcome in colorectal cancer patients treated with irinotecan-based chemotherapy. Cancer Chemother Pharmacol. 2018;82(1):87–98.
- Buch S, Schafmayer C, Völzke H, et al. Loci from a genome-wide analysis of bilirubin levels are associated with gallstone risk and composition. Gastroenterology. 2010;139(6):1942–1951.e2.
- Zhang X, Yin J-F, Zhang J, et al. UGT1A1*6 polymorphisms are correlated with irinotecan-induced neutropenia: a systematic review and meta-analysis. Cancer Chemother Pharmacol. 2017;80(1):135–149.
- Sato H, Uchida T, Toyota K, et al. Association of breast-fed neonatal hyperbilirubinemia with UGT1A1 polymorphisms: 211G > A (G71R) mutation becomes a risk factor under inadequate feeding. J Hum Genet. 2013;58(1):7–10.

