?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
This study analyzed the levels of peripheral blood tumor necrosis factor-α (TNF-α), Decorin (DCN) and Mitogen-activated protein kinase 1 (MAPK1) mRNA in neutrophils of patients with preeclampsia and their correlations, in order to provide more thoughts on the diagnosis and treatment of clinical patients.
Methods
81 patients with preeclampsia who had regular prenatal checkups and delivered in our hospital from June 2020 to April 2022 were analyzed, including 26 patients with early-onset and 55 patients with late-onset, and 50 pregnant women with normal pregnancy who had prenatal checkups and delivered in our hospital during the same period were selected as the control group. Record the clinical data of patients, record the expression of peripheral blood TNF-α, DCN and neutrophils MAPK1 mRNA of patients with early-onset, late-onset and the control group, and record the correlation between DCN level, MAPK1 mRNA expression and TNF-α level of patients with preeclampsia.
Results
The diastolic and systolic blood pressures were significantly higher in early-onset and late-onset patients, and the gestational week of delivery was significantly lower in early-onset and late-onset patients, respectively (p < .05); there was no statistically significant difference in the average age, BMI, average pregnancy time, and average births between the three groups (p > .05). The expressions of peripheral blood TNF-α, DCN, and neutrophils MAPK1 mRNA of the early-onset and late-onset groups were all higher than those in the control group (p < .05); and the expressions of TNF-α, DCN, and MAPK1 mRNA in the peripheral blood of the early-onset group were all higher than those in the late-onset group (p < .05); Pearson correlation analysis showed that DCN level and TNF-α level in patients with preeclampsia were positively correlated (r = 0.98639, p < .05); Neutrophils MAPK1 mRNA expression and TNF-α level were positively correlated (r = 0.9611, p < .05).
Conclusion
TNF-α, DCN and neutrophils MAPK1 mRNA were all highly expressed in the peripheral blood of patients with pre-eclampsia and were more significantly elevated in patients with early-onset preeclampsia, and the expression levels of DCN and MAPK1 mRNA were positively correlated with TNF-α levels. It is possible that all three factors are involved in the pathogenesis of preeclampsia, and are expected to be used as indicators for early prediction and diagnosis.
Preeclampsia is one of the more severe forms of hypertensive disorders in pregnancy, with edema, proteinuria, and hypertension as the main clinical manifestations after 20 weeks of gestation, which have serious effects on the pregnancy outcome, physical and mental health, and quality of life of patients [Citation1]. According to relevant epidemiological research, preeclampsia is a condition that affects between 3% and 5% of pregnant women globally [Citation2], while the prevalence in China is about 10% [Citation3]. Depending on the timing of the onset of the disease, preeclampsia is typically classed as early (before 34 weeks of gestation) and late (after 34 weeks of gestation). The pathogenesis of the two conditions may be distinct, with early-onset patients experiencing rapid disease progression and a higher rate of preterm birth, low birth weight infants, and neonatal asphyxia, whereas late-onset patients experience relatively normal placental development but have abnormalities in the maternal cardiovascular system and metabolism [Citation4]. Patients with preeclampsia have atypical clinical signs and rapid progression during the onset of the disease, and are often misdiagnosed or missed in clinical practice [Citation5]. The pathogenesis of preeclampsia is not fully understood, and endothelial damage, aseptic systemic inflammation, and placental “superficial placental” are thought to play a role in the pathogenesis of Pre-eclampsia (PE) [Citation6,Citation7]. Tumor necrosis factor-α (TNF-α), a pro-inflammatory cytokine, is a broadly bioactive central mediator, especially in inflammatory and immune-related diseases, where the severity of the patient’s condition may be the result of an imbalance of TNF-α [Citation8]. Plasma levels of TNF-α have been found to be considerably greater in individuals with pre-eclampsia than in normal pregnant women [Citation9]. The Decorin (DCN) is a small leucine-containing proteoglycan secreted by stromal cells (including endometrial metaplastic cells and chorionic villous interstitial cells), which has an important impact on the regulation of angiogenesis in the body [Citation10]. DCN can bind to vascular endothelial growth factor receptor-2 (VEGFR-2) on extravillous trophoblast (EVT), cross-activating its function, inhibiting cell proliferation and antagonizing angiogenesis, and it is hypothesized that DCN may play a factor in the pathophysiological process of PE [Citation11]. Mitogen-activated protein kinase (MAPK) is a serine/threonine protein kinase system capable of signaling from the cell surface to the nucleus, which is involved in tumor cell invasion, and placental implantation has a similar process to tumor cell growth and invasion [Citation12,Citation13]. The Mitogen-activated protein kinase 1 (MAPK1) signaling pathway may be involved in the placental “superficial placental” process and play a role in the pathogenesis of preeclampsia. This study attempts to investigate the pathogenesis of preeclampsia by analyzing TNF-α, DCN and MAPK1 mRNA levels and correlation in peripheral blood of patients with preeclampsia.
1. Materials and methods
1.1. Case data
Between June 2020 and April 2022, 81 patients with preeclampsia who received routine prenatal care and delivered at our hospital were studied, including 26 patients with early-onset and 55 patients with late-onset. The control group consisted of 50 pregnant women with normal pregnancies who received routine prenatal care and delivered in our hospital over the same time period. Inclusion criteria: (1) Meet the diagnostic criteria for preeclampsia in the 9th edition of Obstetrics and Gynecology of China, with systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg with urinary protein ≥ 300 mg/24h or random urinary protein (+) after 20 weeks of gestation, or with any of the following: thrombocytopenia, hepatic impairment, renal impairment, pulmonary edema, new central nervous system abnormalities or visual impairment. (2) singleton pregnancy, ≥28 weeks’ gestation; (3) no other pregnancy comorbidities; (4) no serious tissue and organ diseases; (5) no symptoms of vital organ involvement; (6) informed and signed consent form by the patient or family. Exclusion criteria: (1) smokers and alcoholics; (2) existence of primary hypertension before pregnancy; (3) combination of malignant tumors; (4) combination of renal diseases, diabetes mellitus, autoimmune diseases; (5) small for gestational age (SGA) or fetal growth restriction (FGR). This clinical trial was approved by the ethics committee of the hospital, and all pregnant women enrolled were informed and signed the consent form. The subject recruitment flow chart for the clinical study of peripheral blood TNF-α, DCN and neutrophils MAPK1 mRNA levels from patients with pre-eclampsia is shown in .
1.2. Research methods
Baseline data including age, blood pressure, body mass index, gestational week of delivery, gestation and delivery were recorded for the three groups.
Collection of specimens: For the first definite diagnosis of pre-eclampsia without medication and in the control group at the corresponding gestational age, 5 mL of blood was collected from each elbow vein in the early morning on an empty stomach for at least 8 h and injected into anticoagulated and non-anticoagulated centrifuge tubes, respectively. The non-anticoagulated specimens were left for 30 min at 2000 r/min and centrifuged for 15 min, and the supernatant was removed and stored at −80 °C for use. Extraction was done by 5 mL of blood from anticoagulant-containing blood collection tubes, diluted in PBS. A 15 mL centrifuge tube was selected and 7 mL of lymphocyte separation solution was placed, the diluted blood was spread flat on the separation solution and the cells were divided into four layers after centrifugation in the centrifuge, while layer 2 cells were quickly aspirated and placed in a PE tube for use. Layers 1 and 3 were discarded and layer 4 was left in pre-cooled PBS, mixed and centrifuged for 20 min. The supernatant was discarded and the erythrocyte lysate was added proportionally and mixed well. Lysate mix on ice for 8 min, mix upside down and centrifuge for 5 min. The supernatant was discarded and the milky suspension in the EP tube was placed in a centrifuge and the erythrocyte lysate was added, centrifuged and the supernatant discarded. The reaction was terminated by adding 1 mL of sterile PBS to the centrifuge tube and then centrifuged for 5 min in a new EP tube, the supernatant was discarded and stored at −80 °C in the refrigerator.
Real-time PCR was performed to detect neutrophils MAPK1 mRNA levels in peripheral blood, containing anticoagulant to harvest 5 mL of blood, diluted in phosphate buffer (PBS), Trizol method to extract total cellular RNA to amplify MAPK1 upstream primer 5′-GAGTGTAGCTGTACTGCTG-3′, downstream primer 5′-GTGCTAGCATCATGATAGT-3′; internal reference β-actin upstream primer 5′-GCTACGATCGACTACGTAT-3′ PCR reaction conditions: 94 °C 45s, 55 °C 50s, 72 °C 75s, △△Ct (△△Ct = Ct target gene –△Ct internal reference gene) analysis was used to calculate the 2−△△Ct target gene relative expression.
The levels of serum DNC and TNF-α were measured by enzyme-linked immunosorbent assay (ELISA) from Wuhan Biotech Company, China, and the assays were performed by the same person and in the same laboratory in strict accordance with the kit instructions.
1.3. Observation indexes
(1) Record the clinical data of patients; (2) Record the peripheral blood TNF-α, and MAPK1 mRNA and DCN levels in early-onset, late-onset and control groups; (3) Record the correlation between DCN, MAPK1 mRNA levels and TNF-α levels in patients with pre-eclampsia.
1.4. Statistical analysis
The data analysis software SPSS 21.0 was utilized, and the measurement data were expressed as one-way variance or t-test, the count data rate (%) was expressed as χ2 test. The correlation analysis of DCN, MAPK1 mRNA and TNF-α levels was Pearson function, and p < .05 difference means statistically significant.
2. Results
2.1. Comparison of baseline data and blood TNF-α, DCN and MAPK1 mRNA among the three groups of subjects
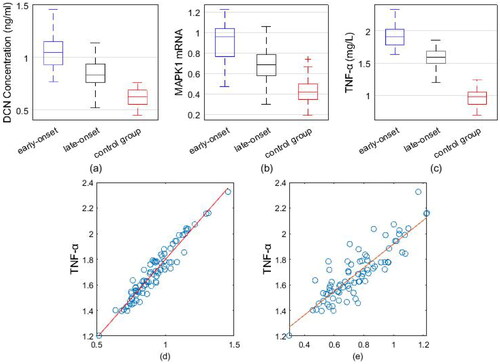
Blood samples taken 2–4 weeks prior to enrollment were retrospectively tested in the identified pregnant women. The results showed a significant difference between the early-onset PE group (TNF-α:1.63 ± 0.12;DCN:0.72 ± 0.21; MAPK1 mRNA:0.84 ± 0.11) and the control group (TNF-α:1.01 ± 0.19; DCN:0.39 ± 0.16; MAPK1 mRNA:0.56 ± 0.10); there was no significant difference between the late-onset PE group (TNF-α: 1.08 ± 0.18; DCN:0.42 ± 0.18; MAPK1 mRNA:0.60 ± 0.14) and the control group (TNF-α:1.01 ± 0.19; DCN:0.39 ± 0.16; MAPK1 mRNA:0.56 ± 0.10). The first test after enrollment revealed that early and late-onset patients had greater diastolic and systolic blood pressures than the control group, while the control group had a lower gestational week of birth (p < .05). No statistically significant differences were found in the comparison of mean age, admission BMI, mean gestation and mean number of deliveries among the three groups of subjects (p > .05). The expression of peripheral blood TNF-α, DCN and neutrophils MAPK1 mRNA was higher in the early and late-onset groups than in the control group (p < .05). The peripheral blood TNF-α, DCN and neutrophils MAPK1 mRNA expressions of patients in the early haircut group were higher than those in the late-onset group (p < .05), as shown in .
Table 1. Comparison of baseline data and blood TNF-α, DCN and MAPK1 mRNA in pregnant women.
2.2. Correlation of peripheral blood DCN, MAPK1 mRNA and TNF-α levels in patients with pre-eclampsia
Pearson correlation analysis showed a positive correlation between DCN and TNF-α levels in the peripheral blood circulation of patients with preeclampsia (r = 0.8639, p < .05), and a positive correlation between MAPK1 mRNA and TNF-α levels (r = 0.9611, p < .05) as well ().
3. Discussion
PE is a pregnancy-specific complication of unknown etiology characterized by new onset hypertension during pregnancy and complications affecting maternal organs and the fetal-placental system. PE often has an acute onset, with sudden placental abruption, eclampsia, and HELLP syndrome seriously threatening the health of the mother and child, while recent studies have shown that PE also leads to poor maternal and child health in later life [Citation14]. Early prediction and prevention in the occurrence of PE is a widespread concern in the clinical and scientific fields at home and abroad. There are many current theories on the etiology of preeclampsia, such as insulin resistance, placental ischemia and hypoxia theory, nutritional deficiency, inflammatory immune hyperactivation theory, genetic factors, and vascular endothelial cell damage theory. The mechanisms proposed by PE suggest that superficial invasion of trophoblast cells leads to helical artery remodeling and reduction of placental vascularity. Insufficient trophoblast invasion causes placental ischemia, producing an immune imbalance and leading to chronic inflammation [Citation15]. The role of the inflammatory response in the pathogenesis and progression of preeclampsia is currently receiving much attention. Successful pregnancy requires adequate tolerance of the maternal immune system to the fetus, but in the organism of patients with preeclampsia, there is an over-activation of the immune response and inflammation, both systemically and locally [Citation16].
TNF-α is a potent tumor-killing factor and also a cytokine that participates in the regulation of the body’s immunity. TNF-α is a peptide pro-inflammatory cytokine formed by mononuclear macrophages, and is formed by Helper T cells 1 (Th1), which has a wide range of biological functions and plays an important role in the body’s inflammatory response and immune system as an initiator of inflammation. TNF-α is a vascular growth factor that accelerates the formation of neovascularization. The level of TNF-α is low in normal pregnancy, which regulates the normal growth of the placenta and the stability of the endometrial tissue environment. As a pro-inflammatory cytokine, TNF-α is able to stimulate a series of cascade responses at the subcellular level and can then induce upregulation of the expression of various inflammatory factors, including TNF-α. In addition, TNF-α can act on vascular endothelial cells, thereby increasing capillary permeability and inducing thrombosis, leading to local tissue ischemia and hypoxia. Studies have shown [Citation17] that TNF-α levels are elevated in the serum of clinical preeclamptic patients compared with normal pregnant women, while TNF-α expression in placental tissues is also elevated along with the further increase of their condition, thus suggesting that TNF-α plays a role in the pathological development of preeclampsia. Hung et al. [Citation18] reported that preeclampsia patients’ serum included a large number of inflammatory mediators activated by leukocytes, and that there was a substantial association between preeclampsia and the presence of inflammatory reactions in the organism. In this study, we showed that TNF-α levels in peripheral blood were elevated in both early and late-onset patients relative to healthy controls, suggesting that immune inflammatory mechanisms are already disturbed in pre-eclamptic patients, and that TNF-α levels were higher in early-onset patients than in late ones. This is presumed to be due to the relative hypoxia of trophoblast cells during pregnancy, which increases the expression of the pro-inflammatory cytokine TNF-α. As TNF-α acts on vascular endothelial cells and trophoblast cells in the placental area, endothelial cells are damaged, capillary permeability increases, and thrombosis is stimulated, leading to local tissue ischemia and hypoxia, superficial placental implantation, and preeclampsia.
DCN is a leucine-rich extracellular matrix composed of metaplastic cells, dermal fibroblasts, chorionic villous mesenchymal cells and chondrocytes in the endometrium of pregnancy. Its regulation of endothelial cell function and angiogenesis is mainly mediated by binding to extracellular matrix molecules or by antagonizing tyrosine kinase receptors such as Vascular Endothelial Growth Factor 2 (VEGFR-2), insulin-like growth factor receptor 1 (IGFR-1) and epidermal growth factor receptor. Iacob et al. [Citation19] reported that abnormal release of some ecdysteroid-derived DCN peptides or overexpression of intra-eclamptic DCN may play a significant role in the pathogenesis of preeclampsia patients, and that DCN can compete with VEGFR-2 to block other activating endothelial activity peptides and delay vascular responses or angiogenesis. In addition, DCN mediates a dual impairment of angiogenesis and intravascular differentiation of extravillous trophoblasts [Citation20]. The inhibitory or promotional effect of DCN on the vasculature of the organism is determined by the angiogenic microenvironment. Within the normal organogenic and vascular microenvironment, DCN actively promotes angiogenesis [Citation21], but its effects are reversed in the gestational state or in tumors [Citation22]. Numerous basic studies have demonstrated that DCN interacts directly with a variety of active mediators found in the extracellular matrix, acting as an anti-adhesive factor and regulating cell migration and proliferation, as well as binding to activate, store, and degrade basic fibroblast growth factor (bGFG), matrix metalloproteinases (MMPs), TNF-α, and other cytokines. In recent years, it has been reported [Citation23] that high expression of DCN can lead to an increase in the levels of TNF-α, interleukin-4 (IL-4), etc., and a decrease in the levels of interleukin-1β (IL-1β), etc., then conversely cytokines can also exert a negative feedback effect on DCN. This also suggests that DCN may be involved in the inflammatory response of the body through indirect or direct pathways. DCN, as a negative regulator, inhibits the proliferation, migration and invasion of EVT cells after binding to VEGFR-2 and promotes apoptosis, thus affecting the development of the maternal uteroplacental vascular bed and the recasting process of spiral arteries, leading to endothelial damage [Citation24]. In this study, we showed that peripheral blood DCN levels were elevated in both early and late-onset patients relative to healthy controls. The correlation study showed that DCN levels and TNF-α levels in the peripheral blood of patients with preeclampsia were positively correlated. This suggests that both DCN and TNF-α are involved in the pathogenesis of preeclampsia and the immune inflammatory response in the body, and they may be complementary to each other.
MAPK signaling pathway is one of the important signaling pathways in living organisms. MAPK1 is a major member of the MAPK system, and the MAPK signaling pathway undergoes a three-stage kinase cascade signaling approach that leads to biological effects. The process is the phosphorylation of mitogen-activated protein kinase kinase (MAPKK) by mitogen stimulation, which activates MAPKK and activates MAPK, which then acts directly on its corresponding substrate in the nucleus to phosphorylate the protein in question, leading to protein synthesis, gene transcription, and the regulation of apoptosis and differentiation. Zhang et al. [Citation25] examined factors related to mitochondrial DNA damage in rat models of lung and liver and showed that stimulus signals such as tissue damage can cause increased infiltration of neutrophils at damaged sites and activation of p38 MAPK signaling pathway proteins, resulting in neutrophil activation and increased levels of TNF-α and interleukin-8 (IL-8), triggering an inflammatory response in the body. Sun et al. [Citation26] reported that intra-serum p38MAPK, interleukin-6 (IL-6) and TNF-α levels were significantly increased in a rat model of acute myocardial infarction. Vascular endothelial cell injury is a major pathophysiological alteration in PE, and activation of neutrophils and adhesion to vascular endothelial cells to stimulate an inflammatory response is thought to be an important cause of endothelial injury in PE patients, exacerbating systemic small vessel spasm in PE [Citation27]. Exogenous and endogenous stimuli activate MAPK signaling pathways, inducing actin remodeling in endothelial cells and altered endothelial cell permeability, resulting in disruption of endothelial cell integrity in response to oxidative stress [Citation28]. It has also been shown that in a hypoxic/reoxygenated model of oxidative stress in human chorionic villous trophoblast cells (HRT8/SVneo cells), the MAPK signaling pathway is activated, affecting trophoblast invasion and thus participating in the pathogenesis of PE [Citation29]. In this study, we showed that peripheral neutrophil MAPK1 mRNA levels were elevated in both early and late-onset patients relative to healthy controls, and correlation studies showed that peripheral blood neutrophil MAPK1 mRNA expression levels and TNF-α levels were positively correlated in pre-eclamptic patients. It is inferred that activation of the MAPK signaling pathway in pre-eclamptic patients may lead to excessive activation of neutrophils through regulation of physiological processes such as inflammatory responses, growth, differentiation, proliferation and survival, resulting in disruption of endothelial cell integrity in response to oxidative stress, leading to placental “shallow bedfellows” or MAPK1 mRNA is involved in the pathogenesis of pre-eclampsia through the upregulation of cytokines such as TNF-α.
In summary, the group retrospectively measured the expression levels of TNF-α, DCN and neutrophils MAPK1 mRNA in blood samples stored 2–4 weeks prior to enrollment in the group. The results showed statistically significant differences in the expression levels of TNF-α, DCN and neutrophil MAPK1 mRNA in the early-onset PE group compared to the control group, and no statistically significant differences in the late-onset PE group compared to the control group, implying that all three have a better predictive value for the development of early-onset PE. In addition, the present study found that peripheral blood TNF-α, DCN, and neutrophil MAPK1 mRNA expression levels were increased in both early and late-onset preeclampsia patients, with the effect being stronger in early-onset preeclampsia patients. Additionally, both DCN and MAPK1 mRNA expression levels were positively correlated with TNF-α levels. Therefore, TNF-α, DCN and neutrophils MAPK1 mRNA expression levels in peripheral blood circulation are expected to be biomarkers for predicting the onset of preeclampsia. However, due to the limited number of cases in this study, the non-matching of gestational weeks tested, and the lack of classification of disease severity by gestational age and PE, the results may be biased to some extent and need to be confirmed by further studies.
Ethical approval
This study was approved by the Ethics Committee of Jinshan Branch of Shanghai Sixth People’s Hospital. Written informed consent was obtained from the parents for publication of clinical details, clinical images, and videos.
Authors’ contributions
YNZ finished the conception, design, and implementation of the project, and prepared the manuscript. GPG assisted in the conception and design of the project, provided technical assistance. All authors approved the final manuscript and agreed to be accountable for all aspects of the work.
Institutional review board statement
Ethical review and approval were waived for this study (approval number: jszxyy202233), due to the study not meeting criteria for research involving human subjects.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statment
The datasets supporting the conclusions of this article are included within the manuscript. The demanders may contact the corresponding author.
Additional information
Funding
References
- Liong S, Barker G, Lappas M. Bromodomain protein BRD4 is increased in human placentas from women with early-onset preeclampsia. Reproduction. 2018;155(6):573–582.
- Yang H, He W, Eriksson M, et al. Inherited factors contribute to an inverse association between preeclampsia and breast cancer. Breast Cancer Res. 2018;20(1):6–14.
- Wu L, Chen Y, Guan X, et al. Relationship between pre-pregnancy BMI and the occurrence and clinical characteristics of pre-eclampsia. Chin J Obstet Gynecol. 2021;56(2):96–101.
- Xu J, Gu Y, Sun J, et al. Reduced CD200 expression is associated with altered Th1/Th2 cytokine production in placental trophoblasts from preeclampsia. Am J Reprod Immunol. 2018;17(2):e12763.
- Valero L, Alhareth K, Gil S, et al. Nanomedicine as a potential approach to empower the new strategies for the treatment of preeclampsia. Drug Discov Today. 2018;23(5):1099–1107.
- LaMarca B, , Cornelius DC, , Lamarca B. The role of inflammation in the pathology of preeclampsia. Clin Sci. 2016;130(6):409–419.
- Dundar B, Dincgez Cakmak B, Aydin Boyama B, et al. Maternal serum glycodelin levels in preeclampsia and its relationship with the severity of the disease. J Matern-Fetal Neo Med. 2018;31(21):2884–2892.
- Kang YE, Seong KY, Yim SG, et al. Nanochannel-driven rapid capture of Sub-nanogram level biomarkers for painless preeclampsia diagnosis. Biosens Bioelectron. 2020;163:112281.
- Founds SA, Powers RW, Patrick TE, , et al. A comparison of circulating TNF-α lpha in obese and lean women with and without pre- eclampsia. Hypertens Pregnancy. 2008;27(1):39–45.
- Jarvelinen H, Sainio A, Wight TN. Pivotal role for decorin in angiogenesis. Matrix Biol J Int Soc Matrix Bio. 2015;43:15–26.
- Khan Gas Girish GV, Lala N, et al. Decorin is a novel VEGFR-2 binding antagonist for the human extravillous trophbiast. Mol Endocrinol. 2011;25(8):1431–1443.
- Lim W, Yang C, Jeong M, et al. Coumestrol induces mitochondrial dysfunction by stimulating ROS production and calcium ion influx into mitochondria in human placental choriocarcinoma cells. Mol Hum Reprod. 2017;23(11):786–802.
- Song GY, Na Q, Wang D, et al. Microarray expression profile of lncRNAs and mRNAs in the placenta of non-diabetic macrosomia. J Dev Orig Hlth Dis. 2018;9(2):191–197.
- Staff AC. Long-term cardiovascular health after stopping pre-eclampsia. Lancet. 2019;394(10204):1120–1121.
- Cornelius DC, Hogg JP, Scott J, et al. Administration of interleukin-17 soluble receptor C suppresses TH17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancy. Hypertension. 2013;62(6):1068–1073.
- Ou M, Zhang Q, Zhao H, et al. Polyunsaturated fatty acid diet and upregulation of lipoxin A4 reduce the inflammatory response of preeclampsia. J Proteome Res. 2020;20(1):132–140.
- Mg A, Dj B, Me C, et al. The role of TNF-α and TLR4 polymorphisms in the placenta of pregnant women complicated by preeclampsia and in silico analysis. Int J Biol Macromol. 2019;134:1205–1215.
- Hung T-H, Charnock-Jones DS, Skepper JN, et al. Secretion of tumor necrosis factor-alpha from human placental tissues induced by hypoxia-reoxygenation causes endothelial cell activation in vitro: a potential mediator of the inflammatory response in preeclampsia. Am J Pathol. 2004;164(3):1049–1061.
- Iacob D, Cai J, Tsonis M, et al. Decorin-mediated inhibition of proliferation and migration of the human trophoblast via different ryrosine kinase receptors. Endocrinology. 2008;149(12):6187–6197.
- Nandi P, Siddiqui MF, Lala PK. Restraint of trophoblast invasion of the uterus by decorin: role in pre‐eclampsia. Ame J Reproduct Immunol. 2016;75(3):351–360.
- Lai J, Chen F, Chen J, et al. Overexpression of decorin promoted angiogenesis in diabetic cardiomyopathy via IGF1R-AKT-VEGF signaling. Sci Rep. 2017;7:44473.
- Buraschi S, Neill T, Goyal A, et al. Decorin causes autophagy in endothelial cells via Peg3. Proc NatI Acad Sci USA. 2013;110(28):2582–2591.
- Zen A, Lafont A, Durand E, et al. Effect of adenovirus-mediated overexpression of decorin on metalloproteinases, tissue inhibitors of metalloproteinases and cytokines secretion by human gingival fibroblasts. Matrix Biol. 2003;22(3):251–258.
- Lala N, Girish GV, Cloutierbosworth A, et al. Mechanisms in decorin regulation of vascular endothelial growth actor-Induced human trophoblast migration and acquisition of endothelial phenotype. Biol Reprod. 2012;87(3):1166–1170.
- Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–112.
- Sun SJ, Wu XP, Song HL, et al. Baicalin ameliorates isoproterenol-induced acute myocardial infarction through iNOS, inflammation, oxidative stress and P38MAPK pathway in rat. Int J Clin Exp Med. 2015;8(12):22063–22072.
- Possomato-Vieira JS, Khalil RA. Mechanisms of endothelial dysfunction in hypertensive pregnancy and preeclampsia. Adv Pharmacol Pharm. 2016;77:361–431.
- Corre I, Paris F, Huot J. The p38 pathway, a major pleiotropic Cascade that transduces stress and metastatic signals in endothelial cells. Oncotarget. 2017;8(33):55684–55714.
- Liu X, Deng Q, Luo X, et al. Oxidative stress-induced Gadd45α inhibits trophoblast invasion and increases sFlt1/sEng secretions via p38 MAPK involving in the pathology of pre-eclampsia. J Matern-Fetal Neo Med. 2016;29(23):3776–3785.