Abstract
Introduction
A twin pregnancy involving a hydatidiform mole (HM) coexisting with a developing fetus is an extremely rare obstetric complication, which typically presents as a complete hydatidiform mole with a coexisting fetus (CHMCF) or a partial hydatidiform mole with a coexisting fetus (PHMCF).
Case presentation
A 26-year-old woman was admitted to our hospital due to a small volume of vaginal bleeding during the 31st week of pregnancy. The patient was previously healthy, and an intrauterine singleton pregnancy was detected by ultrasound on day 46 of gestation; however, bunch-of-grapes sign was observed in the uterine cavity at 24 weeks. The patient was subsequently diagnosed with CHMCF. As the patient insisted on continuing her pregnancy, she underwent hospital monitoring. Vaginal bleeding occurred in the 33rd week again and received a course of betamethasone, then continued pregnancy after bleeding stopped spontaneously. In the 37th week, a male infant weighing 3090 g was delivered by cesarean section, with an Apgar score of 10 at 1 min and a karyotype of 46XY. Placental pathology confirmed the diagnosis of a complete hydatid tumor.
Conclusion
In this report, a case of CHMCF was maintained by monitoring of blood pressure, thyroid function, human chorionic gonadotrophin, and fetal condition during pregnancy. A live newborn was delivered by cesarean section. CHMCF is a clinically rare disease with high risks; thus, it should be diagnosed carefully using several tools, including ultrasound, magnetic resonance imaging, and karyotype analysis and dynamically monitored if the patient decides to continue the pregnancy.
Introduction
A twin pregnancy with one viable fetus and a concomitant complete hydatidiform mole (CHM) is an extremely rare obstetric problem, with a frequency of 1/22,000–100,000 in all pregnancies [Citation1]. Complete hydatidiform molar pregnancy is a premalignant condition characterized by trophoblastic hyperplasia and aberrant chorionic villi [Citation2], caused by improper fertilization with diploid expression in the paternal genome [Citation2].
Owing to the high risk of severe ante- and postpartum complications, including vaginal bleeding, preeclampsia, hyperthyroidism, abortion, premature birth, intrauterine fetal death, and gestational trophoblastic neoplasia (GTN), the management of complete hydatidiform mole with a coexisting fetus (CHMCF) is difficult [Citation3]. Patients who have twin pregnancies with CHM and seemingly normal fetuses have a high risk of perinatal problems, and a correspondingly low live birth rate [Citation4,Citation5]. These significant prenatal problems associated with CHMCF decrease the likelihood of neonatal survival. GTN affects approximately one-third of such patients, who should be treated comprehensively by multiple specialists, even though there are considerable risks to consider while continuing the pregnancy. A previous report by Johnson et al. showed how expectant management can be used to maintain a normally developing fetus when the patient and family wish to continue the pregnancy [Citation2]. In the present case, the patient with CHMCF maintained the pregnancy while undergoing close monitoring in the hospital, finally giving birth to a healthy baby.
Case presentation
A 26-year-old patient (gravidity 2 and parity 0) presented to the Tianjin Central Hospital’s Emergency Department of Obstetrics and Gynecology in April 2020 with a small amount of vaginal bleeding in the 31st week of pregnancy.
The patient was previously healthy, with no family history of twin pregnancies. This patient had one spontaneous abortion in the first trimester previously, and had no history of uterine surgery. The pregnancy was a second pregnancy and a planned spontaneous conception, and an intrauterine singleton pregnancy was detected by ultrasound on day 46 of gestation at a primary hospital. The fetal size was consistent with the gestational age, with an NT value of 1.5 mm. Ultrasound at 12 weeks + 2 days of gestation still showed a singleton pregnancy, and the placenta was found to be located in the anterior wall of the uterus. A noninvasive prenatal test (NIPT) performed at 17 weeks + 3days suggested a low risk of trisomy 21, trisomy 18, and trisomy 13. Ultrasound in the primary hospital at 24 weeks + 3 days (equivalent to 24W3D) showed grid-like changes (partial mole not excluded), which suggested an existence of PHMCF. The patient continued to be monitored by the primary hospital without having adequate knowledge of her condition.
Clinical findings
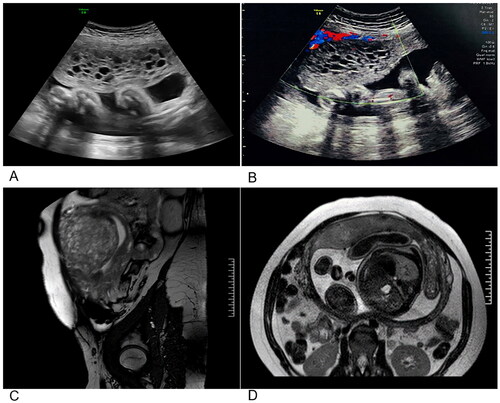
Upon admission to our hospital in the 31st week of pregnancy, an ultrasound showed an intrauterine singleton pregnancy (equivalent to 31 weeks + 6 days), with the placenta located in the anterior wall of the uterus. In addition, part of the placental tissue showed a ‘bunch-of-grapes’ appearance (134 × 52 mm) in the uterine cavity with multiple anechoic spaces, which was identified as a hydatidiform mole (). Magnetic resonance imaging (MRI) image revealed a singleton, cephalic pregnancy. Multiple honeycomb-like vesicles with slightly shorter T1 and longer T2 signals were observed in the left anterior wall (near the fundus), measuring 62 mm × 70 mm × 102 mm, and were clearly demarcated from the adjacent placenta, consistent with CHMCF (). The patient’s HCG (Human chorionic gonadotrophin) level was 87,704.0 mIU/ml, urea level was 3.54 mmol/L, creatinine level was 50.2 μmol/L, and TSH level was 1.764 uIU/ml, FT4 level was 0.81 ng/dl. During the physical examination, enlarged thyroid or thyroid nodules were not identified.
Figure 1. Results of imaging studies performed at 31 weeks. (A,B) Ultrasound appearance of the pregnancy, and the molar component. (C) Sagittal MRI image. (D) Transverse MRI image.
Diagnostic assessment and intervention
Based on the imaging and laboratory findings, a diagnosis of a CHCMF was made. The risks involved were communicated, and karyotyping was recommended to the patient and her family. The patient refused invasive chromosomal testing and wished to continue the pregnancy. Accordingly, the patient underwent close monitoring at a prenatal diagnostic center. After discharge from our hospital, the patient had regular prenatal examinations until delivery. Prenatal examinations were scheduled in advance, and the patient visited the hospital at the agreed appointments.
The patient experienced a recurrence of vaginal bleeding at 33 weeks with a volume comparable to the typical menstrual volume. She received a course of betamethasone, and the bleeding stopped spontaneously., The HCG level was 75129.00 mIU/ml, TSH level was 2.545 uIU/ml, and FT4 level was 0.80 ng/dl in this stage. The results of renal function and routine urine tests were normal; however, no kidney ultrasound was performed. She had no symptoms of preeclampsia and continued to receive regular checkups without hospitalization.
Outcomes
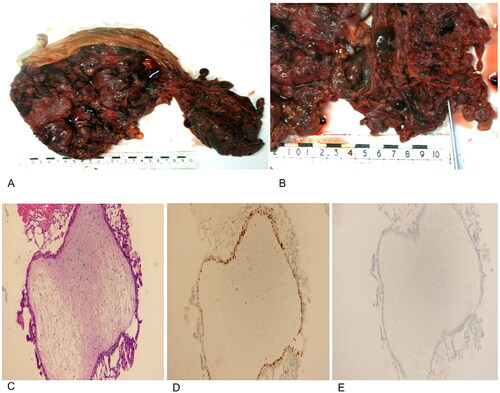
In the patient’s subsequent routine examination before delivery at 36 weeks, no serious complications were identified, and the timing of delivery was difficult to decide. Considering the high risk of CHMCF, the patient was advised to give birth at 37 weeks upon reaching full-term. At this time, the patient’s HCG level was 66133.00 mIU/ml, TSH level was 1.605 uIU/ml, FT4 level was 0.85 ng/dl, urea was 3.69 mmol/L, and creatinine was 52.2 μmol/L. The ultrasonography at 36 weeks showed an intrauterine, singleton pregnancy with moles and the location of the placenta in the anterior wall. The chest radiograph results were normal. The patient was then admitted to the hospital for the purpose of terminating the pregnancy. A 3090 g live male infant was delivered by cesarean section under epidural anesthesia. The infant had an Apgar score of 10 at 1 min, and a karyotype of 46XY. Pathological findings suggested diffuse hyperplasia of placental trophoblasts, vesicular, connected by fibrin. Pathological examination of the placenta revealed a 100 × 50 mm section of placenta-like tissue consisting of a large number of blisters of different sizes attached to a single 250 × 190 × 30 mm placenta (). Immunohistochemistry showed the cytosolic embryonic cells to be P57 (-) and Ki-67 90% (+), which supported the diagnosis of complete hydatid tumor (). Lung and brain metastases were not found. A thyroid function test was performed during the postpartum follow-up, and the results were normal.
Figure 2. Pathology of the patient’s placenta. (A,B) Gross pathological assessment following delivery. A placenta from a normal pregnancy is presented adjacent to the complete molar tissues. (C) Hematoxylin-eosin staining results of the complete hydatidiform mole. (D) Ki-67 expression in the complete hydatidiform mole (+). (E) p57 expression in the complete hydatidiform mole (-).
After delivery, the patient was instructed to undergo monitoring of HCG levels every 2 weeks until 3 months postpartum and every 1–2 months thereafter until the levels were normalized. HCG level became normal in 150 days after delivery. Chest radiography was performed one month post-partum, yielding normal results. The patient was suggested to have sex at least 42 days after delivery. The patient adopted the method of instrumental contraception since the first sex post-partum. The patient still carried the possibility of trophoblastic disease if re-impregnated, and was informed of this risk at the end of her pregnancy. The patient was instructed to visit a hospital with a prenatal diagnosis center and gynaecological oncology center for evaluation after 12 weeks if she was to become pregnant again.
Discussion and conclusions
CHMCF and partial hydatidiform mole with coexisting fetus (PHMCF) can be classified into three subtypes according to cytogenetics:
CHMCF: 46 chromosomes (23 maternal and 23 paternal) and a complete hydatidiform mole (46 chromosomes, all paternal).
PHMCF: 46 chromosomes (23 maternal and 23 paternal) and a partial hydatidiform mole (69 chromosomes: 23 maternal and 46 paternal).
Triploid fetus: partial mole (69 chromosomes: 23 maternal and 46 paternal) [Citation2].
Newborns may still survive in cases of CHMCF and PHMCF; however, the above mentioned third type commonly ends in a miscarriage or intrauterine stillbirth [Citation6]. The NIPT reported in this paper showed a low risk of trisomy 21, trisomy 18, and trisomy 13, as well as a newborn karyotype of 46XY after delivery. In addition, the placental pathology confirmed the presence of a normal placenta with an intact hydatidiform mole. As a result, the patient was defined as a rare case of a full hydatidiform mole and a viable fetus in twins (CHMCF). Because of the low number of pregnancies with CHMCF that have continued into the third trimester, it is vital to recognize that conditions may emerge in which it is not safe to continue the pregnancy due to concerns for the patient or the low possibility of a viable fetus [Citation2]. Prenatal care and follow-ups related to the increased risk of GTN have been the focus of the majority of the CHMCF literature. Currently, few studies have focused on diagnostic methods and differential diagnosis, particularly in light of recent molecular improvements in GTN risk stratification [Citation7].
The diagnostic methods for CHMCF and PHMCF during pregnancy include ultrasonography, MRI, and karyotype analysis. Ultrasound is used as the diagnostic tool of choice in all cases, with the ‘bunch-of-grapes’ sign appearing in the first trimester and the ‘snowstorm’ indications appearing in the uterine cavity in the second trimester. However, it is difficult to diagnose this disease in the first trimester. Diagnosis is usually made between 12 and 14 weeks [Citation8–10]. The detection rate for CHMCF and PHMCF during pregnancy is 68% and the accuracy rate is only 43–68% [Citation11]. In recent years, the accuracy of CHMCF diagnosis has greatly improved with the application of nuclear magnetic resonance technology and cytogenetics. In addition, MRI has several advantages in diagnosing CHMCF owing to its wide imaging range and strong tissue contrast. MRI can thus be used to reveal the relationship between a hydatidiform mole, fetus, and normal placenta, as well as aid in the differential diagnosis of CHMCF and PHMCF, and determine the invasion of the myometrium. The optimal time for diagnosis is 14–18 weeks [Citation12,Citation13]. However, there have been no reports on the accuracy of MRI in diagnosing CHMCF [Citation14]. Therefore, we used MRI to confirm the CHMCF in the patient.
In this case, ultrasonography at 24 + 3 weeks revealed that CHMCF could not be ruled out. To confirm the diagnosis of CHMCF, chorionic villus biopsy, amniocentesis, and umbilical cord blood biopsy can reliably aid in karyotype analyses, while excluding triploidy and other genetic illnesses. SNP sequencing for karyotyping has also been used to clarify CHMCF [Citation15]. In addition, MRI is helpful when making an accurate diagnosis of CHMCF, PHMCF, partial hydatidiform mole with a viable fetus, and molar degeneration [Citation14]. Although this patient did not undergo invasive fetal karyotyping, the risk of triploidy was low in the NIPT. Diagnosis was made using a combination of ultrasound, MRI, and prenatal diagnostic techniques. Unfortunately, the patient did not undergo karyotype analysis in the first and second trimesters in the primary hospital due to the lack of equipment. After referral to our hospital the patient rejected to undergo karyotype analysis due to the worrying the safety of the fetus. After delivery, a karyotype of 46XY was confirmed.
During pregnancy, the levels of endocrine biomarkers change frequently in pregnant women. Prior reports have indicated that blood HCG levels show a certain relationship with a patient’s condition and prognosis [Citation16]. HCG levels should be regularly monitored after delivery. Chemotherapy should be administered when HCG levels do not decline consistently, or when metastases to the lungs or other sites occur. In addition, the pathological results of P57 (-) and Ki-67 90% (+) in cytosolic embryonic cells support the diagnosis of complete hydatidiform mole [Citation17,Citation18]. Thyroid levels also have great effects in pregnancy women. Maternal hypothyroxinemia and hyperthyroidism associated preterm labor, pregnancy loss, and complicate fetal health problems [Citation19,Citation20]. The cutoff value of TSH 2.5 mIU/L, and > 2.5 mIU/L is identified with higher risks of adverse pregnancy outcomes [Citation19, Citation21,Citation22]. Lee et al. finds that a TSH level of > 4 mIU/L is associated with approximately 2-fold increased risks of prematurity and neonatal respiratory distress syndrome in offspring [Citation21]. Although statistically non-significant, elevated TSH is associated with increased risks of preeclampsia/eclampsia, fetal loss, and low birth weight [Citation21]. As reported, a baby with biochemical euthyroid status following CHMCF can be delivered successfully under careful monitoring of the thyroid levels while the mother had gestational trophoblastic hyperthyroidism [Citation23]. During the monitoring of the present case of our study, the patient’s TSH was 1.764 uIU/ml in 31 weeks. Then, TSH increased to 2.545 uIU/ml when she had vaginal bleeding in 33 weeks. TSH was 1.605 uIU/ml in 37 weeks, and she gave birth to a healthy fetus.
The possibility of continuing the pregnancy remains controversial. In Japanese studies, the incidence of GTN is significantly higher in CHMCF than in complete hydatidiform nevus, and serious complications can occur; therefore, pregnancy termination is generally recommended [Citation24]. However, it has also been reported that 60% of expectantly managed CHMCF can result in surviving neonates and that the risk of GTN was not influcenced by elective termination of pregnancy [Citation25]; therefore, continuing the pregnancy in patients with CHMCF can be considered an acceptable option [Citation25]. The 2020 RCOG guidelines state that patients with confirmed or suspected CHMCF should be referred to a fetal medicine center and a gestational trophoblastic disease (GTD) center. In addition, patients with CHMCF should be advised regarding the perinatal risks and GTN outcomes. Invasive perinatal diagnostic tests are required when the type of coexistence between the mole and fetus cannot be determined [Citation26]. Studies have shown that 80.3% of CHMCF cases have perinatal complications, including vaginal bleeding (67%), preeclampsia (14.3%), hyperthyroidism (25%), intrauterine stillbirth (29%), and GTN (34%) [Citation27]. Thus, the probability of a surviving neonate in CHMCF varies from 30% to 50% [Citation28,Citation29].
Our patient with CHMCF was transferred to a prenatal diagnostic center upon admission, where they were closely monitored. There were no serious complications, and the pregnancy was terminated at 37 weeks. Although there is no recommended mode of delivery for CHMCF, the American Institute for Cancer Research recommends curettage at the time of cesarean delivery to help with the continued decline in HCG levels after delivery. A meta-analysis found C-section is more common than vaginal delivery among patients with CHMCF (52 patients received C-section [72.2%] vs. 20 patients received vaginal delivery [27.8%]) [Citation30]. However, the incidence of GTN between the two modes of delivery has no significant difference [Citation30]. C-section is recommended in the second trimester among PHMCF patients as reported by Zeng et al. [Citation31]. Rare cases of CHMCF patients continue into the third trimester, it is highly alerted that it may be unsafe in some situations for the mother and/or surviving fetus to continue the pregnancy [Citation2]. To avoid a possible postpartum hemorrhage, we suggested that the patient undergo a cesarean section, which allowed the delivery of a healthy fetus and a reduction of other complications at the same time.
In conclusion, the patient with CHMCF in our report continued her pregnancy under close monitoring of blood pressure, thyroid function, HCG, and fetal condition and delivered a live male infant in the 37th week. Nevertheless, CHMCF is an uncommon obstetric illness with great risks, especially when the patient wishes to continue the pregnancy. It is important to distinguish CHMCF from PHMCF, for which ultrasonography, MRI, and karyotype analysis are important diagnostic tools. Blood pressure, thyroid function, HCG, and fetal condition should be monitored during pregnancy, and chest radiography or lung CT should be performed if necessary. The timing of delivery should be after 34 weeks, as this greatly increases the probability of fetal survival. There is no medical evidence for choosing cesarean section or vaginal delivery, thus the benefits and downsides of each delivery method can be weighed in each case. After delivery, HCG levels should be monitored continuously, and GTN should be treated aggressively.
Consent to publish identifiable information
Written consent to publish identifiable information related with this case study has been obtained from the patient.
Author Contributions
GW: Guarantor of integrity of the entire study and contributor to the study concepts, study design, definition of intellectual content, data analysis, statistical analysis, manuscript editing, and manuscript review. HYC: Literature review, clinical studies, experimental studies, data acquisition, and manuscript preparation. XC: literature research, experimental studies, data acquisition, and manuscript preparation. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability Statement
All data analyzed during this study are included in the published article.
Additional information
Funding
References
- Vaisbuch E, Ben-Arie A, Dgani R, et al. Twin pregnancy consisting of a complete hydatidiform mole and co-existent fetus: report of two cases and review of literature. Gynecol Oncol. 2005;98(1):19–23.
- Johnson C, Davitt C, Harrison R, et al. Expectant management of a twin pregnancy with complete hydatidiform mole and coexistent normal fetus. Case Rep Obstet Gynecol. 2019;2019:8737080.
- Lipi LB, Philp L, Goodman AK. A challenging case of twin pregnancy with complete hydatidiform mole and co-existing normal live fetus - A case report and review of the literature. Gynecol Oncol Rep. 2020;31:100519.
- Sun SY, Melamed A, Joseph NT, et al. Clinical presentation of complete hydatidiform mole and partial hydatidiform mole at a regional trophoblastic disease center in the United States over the past 2 decades. Int J Gynecol Cancer. 2016;26:367–370.
- Atuk FA, Binti J, Basuni M. Molar pregnancy with normal viable fetus presenting with severe pre-eclampsia: a case report. J Med Case Rep. 2018;12:140.
- Ghassemzadeh S, Farci F, Kang M. Hydatidiform mole. Treasure Island (FL): statPearls Publishing; 2022.
- McHenry A, Magriples U, Hui P, et al. Complete hydatidiform mole and coexisting fetus with gastroschisis: a case report highlighting the importance of diagnostic genotyping. Pediatr Dev Pathol. 2021;24(6):575–580.
- Sheik S, Al-Riyami N, Mathew NR, et al. Twin pregnancy with a complete hydatidiform mole and a coexisting live fetus: rare entity. Sultan Qaboos Univ Med J. 2015;15:550–553.
- Himoto Y, Kido A, Minamiguchi S, et al. Prenatal differential diagnosis of complete hydatidiform mole with a twin live fetus and placental mesenchymal dysplasia by magnetic resonance imaging. J Obstet Gynaecol Res. 2014;40(7):1894–1900.
- Marcorelles P, Audrezet MP, Le Bris MJ, et al. Diagnosis and outcome of complete hydatidiform mole coexisting with a live twin fetus. Eur J Obstet Gynecol Reprod Biol. 2005;118(1):21–27.
- Gabra MG, Gonzalez MG, Bullock HN, et al. Cell-Free DNA as an addition to ultrasound for screening of a complete hydatidiform mole and coexisting normal fetus pregnancy: a case report. AJP Rep. 2020;10(2):e176-8–e178.
- Braga A, Obeica B, Werner H, et al. A twin pregnancy with a hydatidiform mole and a coexisting live fetus: prenatal diagnosis, treatment, and follow-up. J Ultrason. 2017;17(71):299–305.
- Aguilera M, Rauk P, Ghebre R, et al. Complete hydatidiform mole presenting as a placenta accreta in a twin pregnancy with a coexisting normal fetus: case report. Case Rep Obstet Gynecol. 2012;2012:405085. 2012:
- Imafuku H, Miyahara Y, Ebina Y, et al. Ultrasound and MRI findings of twin pregnancies with complete hydatidiform mole and coexisting normal fetus: two case reports. Kobe J Med Sci. 2018;64(1):E1–5.
- Hamard A, Heitzmann A, Ceccaldi C, et al. Association of placental mesenchymal dysplasia with a live female fetus and complete hydatidiform mole: report of a challenging case confirmed by molecular genotyping analysis. Int J Gynecol Pathol. 2021;41(3):251–257.
- Nobuhara I, Harada N, Haruta N, et al. Multiple metastatic gestational trophoblastic disease after a twin pregnancy with complete hydatidiform mole and coexisting fetus, following assisted reproductive technology: case report and literature review. Taiwan J Obstet Gynecol. 2018;57(4):588–593.
- Chen Y, Shen D, Gu Y, et al. The diagnostic value of Ki-67, P53 and P63 in distinguishing partial hydatidiform mole from hydropic abortion. Wien Klin Wochenschr. 2012;124(5-6):184–187.
- Missaoui N, Landolsi H, Mestiri S, et al. Immunohistochemical analysis of c-erbB-2, bcl-2, p53, p21WAF1/Cip1, p63 and Ki-67 expression in hydatidiform moles. Pathol Res Pract. 2019;215(3):446–452.
- Lee SY, Pearce EN. Testing, monitoring, and treatment of thyroid dysfunction in pregnancy. J Clin Endocrinol Metab. 2021;106(3):883–892.
- Dumitrascu MC, Nenciu AE, Florica S, et al. Hyperthyroidism management during pregnancy and lactation (review). Exp Ther Med. 2021;22(3):960.
- Lee SY, Cabral HJ, Aschengrau A, et al. Associations between maternal thyroid function in pregnancy and obstetric and perinatal outcomes. J Clin Endocrinol Metab. 2020;105(5):e2015–23–e2023.
- Hernández M, López C, Soldevila B, et al. Impact of TSH during the first trimester of pregnancy on obstetric and foetal complications: usefulness of 2.5 mIU/L cut-off value. Clin Endocrinol. 2018;88(5):728–734.
- Raj R, Uy EM, Hager M, et al. Delivery of euthyroid baby following hyperthyroidism in twin gestation with coexisting complete hydatidiform mole. Case Rep Endocrinol. 2019;2019:2941501.
- Matsui H, Sekiya S, Hando T, et al. Hydatidiform mole coexistent with a twin live fetus: a national collaborative study in Japan. Hum Reprod. 2000;15(3):608–611.
- Lin LH, Maestá I, Braga A, et al. Multiple pregnancies with complete mole and coexisting normal fetus in North and South america: a retrospective multicenter cohort and literature review. Gynecol Oncol. 2017;145(1):88–95.
- Tidy J, Secki M, Hancock BW. Management of gestational trophoblastic disease: green-top guideline no. 38 - June 2020. BJOG. 2021;128(3):e1-27.
- Zilberman Sharon N, Maymon R, Melcer Y, et al. Obstetric outcomes of twin pregnancies presenting with a complete hydatidiform mole and coexistent normal fetus: a systematic review and meta-analysis. BJOG. 2020;127(12):1450–1457.
- Sebire NJ, Foskett M, Paradinas FJ, et al. Outcome of twin pregnancies with complete hydatidiform mole and healthy co-twin. Lancet. 2002;359(9324):2165–2166.
- Giorgione V, Cavoretto P, Cormio G, et al. Prenatal diagnosis of twin pregnancies with complete hydatidiform mole and coexistent normal fetus: a series of 13 cases. Gynecol Obstet Invest. 2017;82(4):404–409.
- Wang G, Cao J, Xu X, et al. Delivery management of a complete hydatidiform mole and co-existing viable fetus: a meta-analysis and systematic review. J Gynecol Obstet Hum Reprod. 2022;51(1):102269.
- Zeng C, Chen Y, Zhao L, et al. Partial hydatidiform mole and coexistent live fetus: a case report and review of the literature. Open Med. 2019;14:843–846.

