Abstract
Objectives
In this pilot study, the aims were to determine the feasibility of whether pain behavior in extremely and very preterm infants and perceived parental stress change when parents are involved in pain reducing measures, either actively, performing facilitated tucking or passively, observing the intervention, in comparison to the involvement of nurses only. In addition, the infant’s pain reactivity and parental stress over three time points of measurement was of interest.
Methods
Extremely and very preterm infants in need of subcutaneous erythropoietin were randomly assigned to the two intervention groups. The intervention encompassed that one parent of each infant was involved during the painful procedure: Either parents executed facilitated tucking themselves or stood by, observing the procedure. Usual care involved that nurse executed facilitated tucking. All infants received 0.5 ml of 30% oral glucose solution via cotton swab before the painful procedure. Infant pain was observed with the Bernese Pain Scale for Neonates (BPSN) and measured with the MedStorm skin conductance algesimeter (SCA) before, during, and after the procedure. Parents’ stress levels were measured before and after the painful procedure on the infant, using the Current Strain Short Questionnaire (CSSQ). Feasibility of a subsequent trial was determined by assessing recruitment, measurement and active parental involvement. Quantitative data collection methods (i.e. questionnaires, algesimeter) were employed to determine the number of participants for a larger trial and measurement adequacy. Qualitative data (interviews) was employed to determine parents’ perspectives of their involvement.
Results
A total of 13 infants (98% participation rate) were included along with their mothers. Median gestational age was 27 weeks (IQR 26-28 weeks), 62% were female. Two infants (12.5%) dropped out of the study as they were transferred to another hospital. Facilitated tucking turned out to be a good method to actively involve parents in pain reducing measures. No significant differences between the two intervention and control groups were found concerning parental stress and infant pain (p = .927). Power analysis indicated that at least N = 741 infants (power of 81%, α = .05) would be needed to obtain statistically significant results in a larger trial, as effect sizes were smaller than expected. Two of the three measurement tools – i.e. the BPSN and CSSQ) – proved easy to implement and were well accepted. owever, the SCA was challenging in this context. Measurements were also found to be time-consuming and resource-intense (i.e. health professionals as assistants).
Conclusions
Although the intervention was feasible and was readily accepted by parents, the study design was found to be challenging along with the SCA. In preparation of the larger trial, the study design needs to be revisited and adjusted. Thus, issues of time and resources may be resolved. In addition, national and international collaboration with similar neonatal intensive care units (NICU) needs to be considered. Thus, it will be possible to conduct an appropriately powered larger trial, which will yield important results to improve pain management in extremely and preterm infants in NICU.
Introduction
Every year, an estimated 15 million babies are born preterm worldwide [Citation1]. In absolute numbers, this is more than one out of ten babies [Citation2]. Many of these preterm infants would have little chance of survival without neonatal intensive care (NICU) medical treatment [Citation3]. However, NICU treatment often involves painful and stressful interventions such as venepunctures, heel pricks for blood samples, nasopharyngeal or tracheal suctioning [Citation4].
Extremely (born between the 22nd and 27th week of gestation) and very preterm (born between the 28th and 31st week of gestation) infants are most affected by painful interventions such as venepuncture [Citation5]. Repeated and persistent pain may have short and long-term consequences on the cognitive and/or motor development of these preterm infants [Citation6] and cause corresponding stress for their parents. Studies show that parental fear and stress due to the NICU stay [Citation7] may lead to negative parent-infant interactions such as a lack of bonding [Citation8]. Parental stress may impair the ability to care for infants and influence the relationship between child and parent [Citation9]. Detrimental effects on the parents are reported when the infant is experiencing multiple painful stimuli [Citation7]. Previous research shows that parental stress is significantly reduced when parents are actively involved in the pain management of their infants [Citation10,Citation11]. Thus, parents were reassured that the pain management is adequate [Citation12]. Much research on the active involvement of parents in pain-reducing measures for preterm infants focuses on skin-to-skin care (the infant lying on the parent’s chest) [Citation13,Citation14] as well as on facilitated tucking (parents holding the infant, lying in the cot, with their hands on the head and feet in a “frog position”) [Citation14,Citation15]. Both methods result in significantly lower pain in preterm infants. However, skin-to-skin care may not be applicable in any situation. Unstable infants or with chest tubes may be difficult to transfer onto the parent’s chest out of the cot. In contrast, the measure of facilitated tucking may be applied in every situation.
Although scientific evidence on possible pain reduction by parental support is indicated [Citation12–14], studies on the comparison between parents’ active versus passive involvement in pain management as compared to nurse-only (usual care) are lacking. Therefore, this pilot study was conceptualized to determine the feasibility of involving parents via facilitated tucking or observing during painful procedures and to measure a change in parental stress and infant pain. An additional purpose of this pilot study was to determine the size of the cohort of preterm infants needed for a larger trial, the time, and the resources required for recruitment and data collection.
Materials and methods
Design
A three-arm pilot study using mixed methods of data collection (questionnaires, SCA, interviews) was designed to test the feasibility of the intervention encompassing parents’ active and passive involvement in procedural pain management of extreme and preterm infants hospitalized at a NICU.
Reporting of this pilot study followed the Consolidated Standards of Reporting Trials (CONSORT) guidelines [Citation16]. The authors hypothesized that the effects between the two intervention and control groups will differ as parents convey more familiarity and security either through their touch or simply by observing than nurses. As parents may be stressed during the painful procedure, it was assumed that their stress level would be elevated before and during the first measurement, but would then decrease more significantly during active involvement through facilitated tucking compared to observing. A total of time points was conceived to test the intervention and to obtain data. An overview of the entire procedure is given in supplementary Table 3.
Ethics
The pilot study protocol was approved by the ethics committees of a university hospital in the German speaking part of Switzerland and the respective cantonal ethics committee, registered with the number: 079/13, and registered on the Clinicaltrials.Gov. Protocol Registration and Results System, Identifier: NCT05656677. Informed consent was obtained from all parents who agreed to the participation of their infants and themselves in the study. The parents had 48 h to decide on participation. Parents had the right to withdraw from the study at any time without compromising the care of their infant and their relationship with the NICU team. It was highlighted that there was no disadvantage in care for parents and preterm infant, whether they participated or not, or whether they decided to withdraw.
Intervention
During a routine subcutaneous (s.c.) injection of erythropoietin to prevent anemia [Citation16,Citation17], participating parents were invited to either provide facilitated tucking to their infant or stand by in an observing mode. The facilitated tucking or the standing by were executed during the s.c. period and for three minutes after the painful procedure. The infants received 0.5% of 30% oral glucose solution on a cotton swab, 2–3 min before the s.c. injection by the nurse.
Usual care
Such s.c. injections are usually executed by two nurses, one of which applies facilitated tucking and the other injects the substance. Facilitated tucking was carried out by a nurse who was on shift that day, and the injection was carried out by the nurse who was caring for the preterm infant that day. The infants received 0.5% of 30% oral glucose solution on a cotton swab, 2–3 min before the s.c. injection by the nurse. The facilitated tucking was executed during the s.c. period, and for three minutes after the painful procedure.
Procedure
The s.c. injections generally are conducted daily. For the purpose of this pilot study, data were collected at injections of days 6, 9 and 12 by one clinical nurse specialist (CNS) and a research team that included six NICU nurses, with at least six years of professional experience. The six NICU nurses involved were blinded to the purpose of the study, whereas the CNS was not. Always three members of this research team conducted the pain assessment during the facilitated tucking or the observing by the parents. The other three members of the research team focused on the nurses’ group.
Participants
Parents of extremely and very preterm infants hospitalized in a NICU at a university hospital in the German-speaking part of Switzerland between October 2019 until November 2020 were invited to participate. A neonatologist working at the NICU identified potential participants. The CNS or the neonatologist provided information about the study to the parents, without stating its specific aim, instead referring in principle to the increased involvement of parents in pain management.
The NICU includes nine places where newborns of all weeks of pregnancy are cared for. Per annum, around 100 extreme and very preterm infants are admitted. Most admissions are internal via the delivery room, with a smaller number admitted from the outside. All intensive care ventilatory support is provided, and after surgical procedures, children are transferred to the pediatric intensive care unit. Parents can visit at any time outside of three hours per day of doctor’s rounds. Inclusion and exclusion criteria were set for preterm infants and their parents ().
Table 1. Inclusion and exclusion criteria.
Measurement of feasibility
The main objective of this pilot study was to determine the feasibility of a larger, appropriately powered study. Feasibility assessment included recruitment rate (dependent on resources and in-/exclusion criteria), and percentage of questionnaire completion (dependent on parental acceptance of active participation in the intervention and completing questionnaire three times). The pain and stress measurement over three-time points were reviewed for feasibility as well as active parental involvement. In semi-structured interviews, parents were asked about performing facilitated tucking or standing by as observers, acceptance, applicability and feasibility of the CSSQ and measurement over three time-points. The research team was also interviewed about acceptance, applicability and feasibility of the validated BPSN, the SCA over three-time points and active parental involvement. The parents were interviewed face to face after the last s.c. injection. The research team was interviewed in focus groups at the end of data collection.
Measurements of clinical outcomes
The secondary interest of the study was to test for significant differences in parental stress between the two intervention and control groups at three measurement points as well as infant pain.
To determine infant pain, two research team nurses observed the infant by using the BPSN in real-time before (2–3 min), during and (2–3 and 10 min) after the s.c. injection. The two nurses independently assessed the physiological and behavioral pain items [Citation18]. The BPSN is a multidimensional tool in German and has shown satisfactory psychometric properties among preterm and term infants (validity: r = 0.75; reliability: α = 0.8) [Citation18].
SCA was installed before the s.c. injection until the last BPSN measurement. For this purpose, a small electrode equivalent to an electrocardiogram electrode (ECG) was placed on the sole of the foot to the left and right of the ankle of the preterm infant. An initial baseline was obtained. Subsequently, SCA pain measurement was continuous. However, SCA values were recorded simultaneously with each BPSN by a research team nurse. Effectiveness of the SCA has been determined in preterm infants. The SCA demonstrated significant correlation with other assessment tools (r = 0.325, p = .027) [Citation19].
To determine parents’ stress concerning active involvement in their infant’s pain management as opposed to parents who stood by, the CSSQ was used. A pretest was carried out with two mothers prior to the start of the pilot study to examine the comprehensibility of the questions and the time required to complete the questionnaire. No problems were identified and the time required to complete the CSSQ was about five minutes. Reliability (α = 0.75) and validity (r = 0.6) of the CSSQ were deemed satisfactory [Citation20].
Randomization
Computerized block-randomization with a block length of two was used by an external statistician [Citation21]. This way, each infant had the same chance of being in one of the groups, thereby decreasing the influence of selection bias. The CNS distributed the random numbers in sealed opaque envelopes. A folder was created for each child and one envelope was assigned to each by the neonatologist involved. As soon as parents had agreed to participate, the CNS opened the respective envelope and instructed the parents on the study procedures.
Sample size
The estimation of the pilot study sample size was based on a previous study [Citation22]. The BPSN scores with and without facilitated tucking in this study (facilitated tucking: mean = 3.05, standing by/observation: mean = 4.9) and the common standard deviation (1.544) were used [Citation22]. Assuming a comparable effect, a sample size of 12 per group was assumed to have a power of 80% to detect differences at an alpha of 5% using an unpaired t-test. Therefore, a sample size of 12 preterm infants with one parent each per intervention group was calculated for the pilot study.
Quantitative analysis
Sociodemographic and questionnaire data were statistically analyzed, using IBM SPSS Statistics version 26 (IBM Corporation, Armonk, NY, USA) [Citation16].
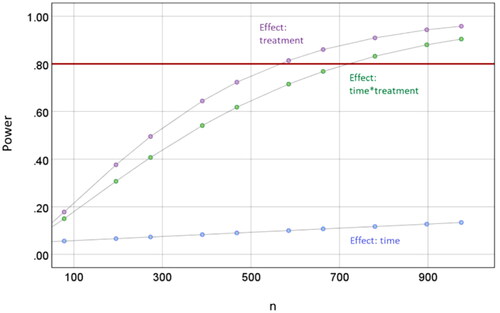
Drawing on the results of this pilot study (mean scores, standard deviation, correlation of values between time-points), sample size calculation was performed to determine the total number of infants needed to detect a significant effect over time (time*group) with a power of 80% for the larger study. All calculations were carried out by an external statistician.
Qualitative Analysis
For parents and the research team respectively an interview guide was developed (see supplementary Table 4). Both interview guides comprised an introduction, open-ended questions, supplemented with in-depth questions and a conclusion. The open-ended questions for the parents addressed the facilitated tucking and the application of the CSSQ. The research team was questioned on the use of the BPSN, the SCA and active parental involvement. All interviews were audiotaped, transcribed, and analyzed by using thematic analysis according to Braun and Clarke [Citation23]. Two analysts (AE and LS) independently coded the transcripts for themes. The following six analytic steps were performed: Familiarization with the data, generating initial codes, searching for themes, reviewing themes, defining and naming themes, and producing the report. The analysts discussed differences and agreed on major themes such as the involvement of the parents, handling of the SCA device, and the timing of the measurement.
Results
Participants’ characteristics, participation rate, drop-out percentage
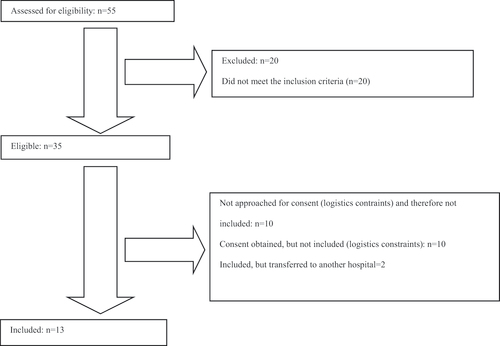
A total of 15 preterm infants who met the inclusion criteria were enrolled. Of the 15 preterm babies included, two were transferred to another hospital and therefore could not be included in the study. Eventually, five boys and eight girls were included. Most participants were very preterm infants, with one pair of twin girls. Only mothers were involved in the intervention. Demographic and clinical data are provided in , mean, median, IQR numbers and range in supplementary Table 5. As this was a pilot study no further statistical testing was performed.
Table 2. Demographic and clinical data.
Feasibility of Recruitment
Data were collected over two years from October 2019 until November 2020. On average, every month a preterm infant was included. Instead of the intended 12 infants per group, only 13 in total were recruited. Based on the inclusion/exclusion criteria, 25 parents were identified for participation in the study. 15 declared their willingness to participate in the study, 10 refrained. The majority cited stress from the unexpected situation with a premature baby as a reason for not participating as well as having to commit to three s.c. injection at three different times, which could potentially collide with work obligations ().
Estimation of sample size for larger study
Median pain scores ranged from 2.5−5.0 (nurses) vs. 2.0−5.0 (parents) 30 min before injection, 2.5−4.5 (nurses) vs. 3.0−4.0 (parents) 2–3 min before injection, 6.5–10 (nurses) vs. 6.0−8.0 (parents) during injection, 3.0−5.0 (nurses) vs. 2.0−4.0 (parents) 2–3 min after injection and 2.0−.5 (nurses) vs. 2.0−3.0 (parents) 30 min after injection (supplementary Table 5). The greatest effect (before vs. after injection) was observed 2–3 min before vs. 2–3 min after injection. Therefore, these two time-points were used for sample size calculation for the larger trial. In addition, all three time-points were pooled to get more stable estimations of the potential effect. Mean pooled pain scores (over all injection) of 3.83 (nurses) 3.62 (parents) were observed 2–3 min before injection and 4.33 (nurses) vs. 3.32 (parents) 2–3 min after injection. Generalized linear models determined a sample size of 741 infants overall to have a power of 81% to detect an effect (time*group) ().
Feasibility of assessment procedures and the active parental involvement
All six mothers who performed facilitated tucking reported a high level of acceptance and considered the CSSQ easy to complete. They welcomed active involvement, whereas passive observation was described as exhausting. Sometimes, the mothers found it difficult to attend all three times of the s.c. injections. The completion rate of included participants was 100%, with no withdrawal. For the research team, data collection was very time-consuming in addition to daily work with scarce staff resources. When parents were involved, data collection had to take place during visiting hours outside of daily doctor rounds, which constituted another difficulty.
The research team nurses generally found the SCA installation to be easy. However, applying and reading the values of the SCA at the same time as the BPSN was sometimes difficult. The BPSN was simple and part of daily routine. Having mothers executing facilitated tucking was met with high acceptance.
Clinical outcomes
Conducting data collection at each time-point was generally considered feasible. However, executing several measurements all at the same time was very challenging and complex. In addition, the SCA manipulation necessitated a trained person. Also, the SCA had never been used at this NICU before and was new for everyone. All mothers and nurses found adhering to the three measurement points per s.c. injection difficult. The mothers’ stress was found to be lower (range 1.50–3.50, median 2.50, IQR 1.67–3.00) and decreased over time when they executed facilitated tucking themselves (range 0.83–3.33, median 1.50, IQR 1.17–1.83). Pain intensity of the preterm infants under facilitated tucking performed by mothers decreased over time compared to the control group. An overview of the results for interventions and control group is given in supplementary Table 5.
Discussion
This pilot study examined the feasibility of a larger study to investigate active parental involvement using facilitated tucking or observation, which are easy to implement, compared with nurses’ facilitated tucking. Recruitment procedure, sample size, study design, data collection, and acceptability of active parental involvement were also considered.
The recruitment process was found to have significant hurdles. With the requirement that participating parents needed to be fluently speaking and writing German, the group of potential candidates for the study was small. In addition, almost half the parents who approached about the study (i.e. 10 parents) declined participation due to the stressful situation, the time commitment demanded by the intervention, and potential collisions with the parents’ work. Therefore, only mothers participated.
To carry out all necessary measures, a large research team (i.e. 6 NICU nurses and one CNS) was needed. Currently, there are severe shortages of qualified health professionals. Therefore, a study with such high demands on resources needs to be well thought through and prepared ahead [Citation24].
Additionally, coordination and organization of data collection need to be considered. The measurements in this intervention took place over three days and at time-points. Thus, not only did parents need to adjust their schedules but also the research team needed to organize themselves and coordinate. Flexibility in such neonatal studies is basically required, time of birth is unplanned, painful interventions cannot always be planned, and health status in very premature infants can change rapidly. Therefore, not only the study design but also the data collection methods need to be reviewed. A quasi-experimental design [Citation25,Citation26] or a crossover design [Citation25] might be more applicable to the larger study.
Facilitated tucking, whether performed by mothers or nurses, was easily implemented and accepted. Holding the infant’s head and feet at the same time is easy for one person and has an immediate calming effect on both the preterm infant and the person doing the facilitated tucking [Citation15]. In this pilot study, nurses already used facilitated tucking when conducting painful interventions such as s.c. injections. Therefore, the usual care in this NICU is already improved in comparison to the usual care described in a previous study where nurses only stroked the child [Citation22].
The BPSN was well known to the nurses and, thus, easily used. However, for subsequent studies, an instrument adapted to the target sample needs to be included such as the BPSN-R [Citation26] or the Premature Infant Pain-revised (PIPP-R) [Citation27].
Data should also be collected directly after the completion of the painful procedure. At this moment, the biggest differences have been found concerning the measurement of pain between intervention and control groups [Citation13]. For the SCA, a trained person was needed to obtain valid data [Citation28]. To facilitate data collection, video recordings could be considered. An additional person would be necessary, available at all times for data collection. However, the data could have captured the infant exclusively, and would allow for blinding persons to the data analysis [Citation29,Citation30]. In the pilot study, the CNS could have conducted the video recordings. However, more than one person would have been necessary to adjust for all the various time-points of data collection.
Additionally, the daily routine at the NICU constituted a barrier to data collection. In a future study, the interventions and data collection need to be scheduled at times more convenient to the NICU’s daily routine, or to negotiate parent involvement during doctors’ rounds. However, by the end of the study, parent visiting hours had been adjusted. Parents were finally allowed to be present during doctors’ rounds, which constitutes a fundamental improvement for future parental involvement in pain-relieving interventions.
Despite such improvements, obtaining a larger sample for a larger study constitutes a very high barrier. Low case numbers of preterm infants in NICU are well-known internationally [Citation14]. Considering that for the larger study, a sample of more than 700 preterm infants, each with one parent, is simply not feasible at one NICU. Therefore, the study design needs to be revisited, and a more suitable design needs to be identified to account for the low case numbers. Additionally, collaboration with other NICU nationally and internationally is a MUST.
One of the strengths of the study was that it was very carefully conducted, providing important data for future studies.
Conclusion
Overall, the pilot study was a good way to determine the feasibility of a larger study. However, it was found that the employed design may need to be revisited in order to account for the time-intense commitment of the parents and the resource-intense intervention. Besides the measurements employed, integrating video recording may contribute to simplifying data collection. In addition, integrating national and international NICU to access a larger sample of potential study participants is essential for a successful larger study.
Supplemental Material
Download MS Word (19.1 KB)Acknowledgments
The authors thank Prof. Dr. Maya Zumstein-Shaha for her support as scientific advisor and all participating parents, preterm infants and NICU staff for their cooperation.
Disclosure statement
The authors have no conflicts of interest to declare.
Additional information
Funding
References
- Lincetto O, Banerjee A. World prematurity day: improving survival and quality of life for millions of babies born preterm around the world. Am J Physiol Lung Cell Mol Physiol. 2020;319(5):L871–L874.
- Purisch SE, Gyamfi-Bannerman C. Epidemiology of preterm birth. Semin Perinatol. 2017;41(7):387–391.
- Helenius K, Sjörs G, Shah PS, et al. Survival in very preterm infants: an international comparison of 10 national neonatal networks. Pediatrics. 2017;140(6):e20171264.
- Bucsea O, Riddell RP. Non-pharmacological pain management in the neonatal intensive care unit: managing neonatal pain without drugs. Semin Fetal Neonatal Med. 2019;24(4):101017.
- Myrhaug HT, Brurberg KG, Hov L, et al. Survival and impairment of extremely premature infants: a meta-analysis. Pediatrics. 2019;143(2):e20180933.
- Williams MD, Lascelles BDX. Early neonatal pain – a review of clinical and experimental implications on painful conditions later in life. Front Pediatr. 2020;8:30.
- McNair C, Chinian N, Shah V, et al. Metasynthesis of factors that influence parents’ participation in pain management for their infants in the NICU. J Obstet Gynecol Neonatal Nurs. 2020;49(3):263–271.
- Medina IMF, Granero-Molina J, Fernández-Sola C, et al. Bonding in neonatal intensive care units: experiences of extremely preterm infants’ mothers. Women Birth. 2018;31(4):325–330.
- Brady M, Stevens E, Coles L, et al. You can spend time… but not necessarily be bonding with them’: Australian fathers’ constructions and enactments of infant bonding. J Soc Pol. 2017;46(1):69–90.
- Filippa M, Poisbeau P, Mairesse J, et al. Pain, parental involvement, and oxytocin in the neonatal intensive care unit. Front Psychol. 2019;10:715.
- Palomaa A-K, Korhonen A, Pölkki T. Factors influencing parental participation in neonatal pain alleviation. J Pediatr Nurs. 2016;31(5):519–527.
- Balice-Bourgois C, Zumstein-Shaha M, Simonetti GD, et al. Interprofessional collaboration and involvement of parents in the management of painful procedures in newborns. Front Pediatr. 2020;8:394.
- Johnston C, Campbell‐Yeo M, Disher T, et al. Skin‐to‐skin care for procedural pain in neonates. Cochrane Database Syst Rev. 2017;2(2):CD008435.
- Eissler A, Zwakhalen S, Stoffel L, et al. Systematic review of the effectiveness of involving parents during painful interventions for their preterm infants. J Obstet Gynecol Neonatal Nurs. 2022;51(1):6–15.
- Francisco ASPG, Montemezzo D, Ribeiro SNDS, et al. Positioning effects for procedural pain relief in NICU: systematic review. Pain Manag Nurs. 2021;22(2):121–132.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11(1):1–8.
- Kling PJ. Iron nutrition, erythrocytes, and erythropoietin in the NICU: erythropoietic and neuroprotective effects. Neoreviews. 2020;21(2):e80–e8.
- Schenk K, Cignacco E, Stevens B, et al. Individuelle kontextfaktoren in der validierung des berner schmerzscores für neugeborene: ergebnisse der validierungsstudie. Zeitschrift Für Geburtshilfe Und Neonatologie. 2017;221(S 01):P03–11.
- Passariello A, Montaldo P, Palma M, et al. Neonatal painful stimuli: skin conductance algesimeter index to measure efficacy 24% of sucrose oral solution. The Journal of Maternal-Fetal & Neonatal Medicine. 2020;33(21):3596–3601.
- Muller B, Basler H. Kurzfragebogen zur aktuellen beanspruchung. Manual Beltz Test Weinheim: Germany; 1993.
- Burger B, Vaudel M, Barsnes H. Importance of block randomization when designing proteomics experiments. J Proteome Res. 2021;20(1):122–128.
- Axelin A, Salanterä S, Lehtonen L. Facilitated tucking by parents’ in pain management of preterm infants—a randomized crossover trial. Early Hum Dev. 2006;82(4):241–247.
- Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Res Psychol. 2006;3(2):77–101.
- Ranney ML, Griffeth V, Jha AK. Critical supply shortages—the need for ventilators and personal protective equipment during the covid-19 pandemic. N Engl J Med. 2020;382(18):e41.
- Fatollahzade M, Parvizi S, Kashaki M, et al. The effect of gentle human touch during endotracheal suctioning on procedural pain response in preterm infant admitted to neonatal intensive care units: a randomized controlled crossover study. J Matern Fetal Neonatal Med.. 2022;35(7):1370–1376.
- Schenk K, Stoffel L, Bürgin R, et al. Acute pain measured with the modified bernese pain scale for neonates is influenced by individual contextual factors. Eur J Pain. 2020;24(6):1107–1118.
- Gibbins S, Stevens BJ, Yamada J, et al. Validation of the premature infant pain profile-revised (PIPP-R). Early Hum Dev. 2014;90(4):189–193.
- Walas W, Halaba ZP, Szczapa T, et al. Procedural pain assessment in infants without analgosedation: comparison of newborn infant parasympathetic evaluation and skin conductance activity-a pilot study. Front Pediatr. 2021;9:746504.
- Xie W, Wang X, Huang R, et al. Assessment of four pain scales for evaluating procedural pain in premature infants undergoing heel blood collection. Pediatr Res. 2021;89(7):1724–1731.
- Brahnam S, Nanni L, McMurtrey S, et al. Neonatal pain detection in videos using the iCOPEvid dataset and an ensemble of descriptors extracted from Gaussian of local descriptors. ACI. 2020;19:122–143.