Abstract
Spontaneous preterm birth (delivery before 37 completed weeks) is the single most important cause of perinatal morbidity and mortality. The rate is increasing world-wide with a great disparity between low, middle and high income countries. It has been estimated that the cost of neonatal care for preterm babies is more than 4 times that of a term neonate admitted into the neonatal care. Furthermore, there are high costs associated with long-term morbidity in those who survive the neonatal period. Interventions to stop delivery once preterm labor starts are largely ineffective hence the best approach to reducing the rate and consequences is prevention. This is either primary (reducing or minimizing factors associated with preterm birth prior to and during pregnancy) or secondary - identification and amelioration (if possible) of factors in pregnancy that are associated with preterm labor. In the first category are optimizing maternal weight, promoting healthy nutrition, smoking cessation, birth spacing, avoidance of adolescent pregnancies and screening for and controlling various medical disorders as well as infections prior to pregnancy. Strategies in pregnancy, include early booking for prenatal care, screening and managing medical disorders and their complications, and identifying predisposing factors to preterm labor such as shortening of the cervix and timely instituting progesterone prophylaxis or cervical cerclage where appropriate. The use of biomarkers such as oncofetal fibronectin, placental alpha-macroglobulin-1 and IGFBP-1 where cervical screening is not available or to diagnosis PPROM would identify those that require close monitoring and allow the institution of antibiotics especially where infection is considered a predisposing factor. Irrespective of the approach to prevention, timing the administration of corticosteroids and where necessary tocolysis and magnesium sulfate are associated with an improved outcome. The role of genetics, infections and probiotics and how these emerging dimensions help in the diagnosis of preterm birth and consequently prevention are exciting and hopefully may identify sub-populations for targeted strategies.
Epidemiology/introduction
Preterm birth (PTB) defined as delivery before 37 completed weeks of gestation is an important cause of severe morbidity and mortality. Approximately 15 million babies are delivered preterm world-wide; 84% of these are between 32 and 36 weeks, 10% are between 28 and 32 weeks and 5% occur before 28 weeks. One million of these die before the age of 5 years accounting for 18% of all deaths in children before the age of 5 years [Citation1,Citation2]. About 35% of all neonatal deaths occur in those delivered before 28 weeks of gestation [Citation3].
About 90% of PTBs occur in low and middle-income countries (LMIC) [Citation4]. The rate varies from one Region/Country to another with an overall rate of 11%. It is highest in South East Asia and Sub-Sahara Africa. The average PTB rate is 12% in LIC, 9.4% in MIC, and 9.3% in HIC. Indeed approximately 80% of PTBs occur in Sub-Sahara Africa and South East Asian countries [Citation4]. The lowest PTB rate (4.1%) is in Belarus while the highest (19.1%) is in Bangladesh [Citation4]. Six countries including India, China, Nigeria, Pakistan, Indonesia, and the USA account for 50% of these [Citation5,Citation6]. is a map of the rates of PTB by country/region in 2014 [Citation4]. In the USA (the only HIC in this group with very high rates), social and economic discrepancies such as unequal access to maternity care and high poverty rates are major factors. For example, for the period 2014–2016, the PTB rate was 13.4% in African-Americans compared to 8.9% for Caucasians. Indeed African-Americans had a 49% higher rate of PTB than all other races and ethnicities. (March of Dimes 2021) [Citation7].
Figure 1. Estimated global rate of the rates of preterm birth in 2014. (Courtesy of Chawanpaiboon et al. Lancet 2019) [Citation4].
![Figure 1. Estimated global rate of the rates of preterm birth in 2014. (Courtesy of Chawanpaiboon et al. Lancet 2019) [Citation4].](/cms/asset/6756d852-4847-49d2-9e00-d01c0e98b33b/ijmf_a_2183756_f0001_c.jpg)
PTB has severe economic costs on individuals and society as a whole. It is greatest in countries with the highest rate. In the USA for example, in 2015 hospital charges for infants totaled $16.8 billion; premature infants accounted for half of the hospital charges for all infants and the average charge for the most severe stays was $77,000 compared to $1700 for an uncomplicated newborn. Indeed, initial hospitalization costs varied between $576 972 (range $111 152–$576 972) per infant born at 24 weeks’ gestation and $930 (range $930–$7114) per infant born at term [Citation8]. Not included in these costs are the economic consequences of the longterm functional, neurodevelopmental, behavioral and educational sequelae of preterm birth.
Approaches to prevention
Reducing the burden of preterm birth may be considered a difficult if not impossible task, but instituting appropriate and timely preventative measures rather than interventions to treat, offer the most cost-effective approach. There are two approaches to prevention – primary (pre-pregnancy and during pregnancy) and secondary, which include interventions to interrupt preterm labor (PTL). Whichever the approach, an understanding of the pathogenesis of PTL is a pre-requisite for effective prevention. The traditional classification of preterm-birth as extreme (delivery before 28 weeks), very preterm (28–32 weeks), moderate preterm (32–34 weeks) and late preterm birth (34–37 weeks) is oversimplification and preterm birth/labor must be regarded as a result of complex interaction of factors that results in variable phenotypes [Citation9,Citation10].
Preterm labor, therefore once considered a single entity is now increasingly regarded as a condition of various phenotypes which are characterized by varied biochemical and physical characteristics of the mother, fetus, and/or placenta that lead to and/or are present at the time of birth [Citation10]. Villar et al. [Citation11] and Manuck et al. [Citation12] proposed different phenotypes of preterm labor. Villar et al. identified 7 phenotypes while Manuck identified 9 phenotypes. Interestingly when each of these phenotypes was applied in clinical practice, it was easier to tailor interventions to interrupt preterm labor [Citation12]. It has therefore been concluded from these approaches that phenotyping allows for the identification of differences between very early and early spontaneous preterm birth, the role of various etiopathologies, and therefore more likely to lead to the development of effective prevention and therapies [Citation12,Citation13].
Primary prevention
The aim of this is to eliminate or significantly reduce the risk factors in the mother and her environment prior to pregnancy as well as during pregnancy. shows the impact of primary prevention on the reduction of the risk of SPTB [Citation5,Citation14–16]
Table 1. Effectiveness of interventions in primary prevention (data are presented as the OR changes with each factor).
Pre-pregnancy: This must include optimizing the health of the woman prior to pregnancy, reducing pregnancy in adolescents and unintended pregnancies, and promoting birth spacing. In a recent study, from high-income countries, Tessema et al. [Citation17] showed that inter-pregnancy intervals of < 6 months were associated with a significantly increased risk of PTB. Other pre-pregnancy measures include better planning of pregnancies (pregnancy by choice rather than by accident), optimizing maternal weight, promoting healthy nutrition including fortification/supplementation of essential foods with micronutrients, and promoting vaccination of children and adolescence [Citation5]. Additionally, risk factors for preterm birth should be identified and eliminated if possible prior to conception. These include screening for, diagnosing, and managing mental health disorders and preventing intimate partner violence, screening for and treatment of STIs and HIV/AIDS; promoting cessation of smoking and recreational drug use including exposure to secondary smoke and screening for and managing chronic disease such as diabetes mellitus, hypertension, etc [Citation5].
Pregnancy: Encouraging early booking for antenatal care, screening for risk factors and eliminating them, and managing complications when they arise such as anemia (as shown in the systematic review and meta-analysis by Rahmati et al. [Citation18]
Secondary prevention
This focuses primarily on those at risk of spontaneous preterm birth (SPTB) although there is an increasing realization that since PTB occurs in a significant number of women without risk factors, screening should be considered universal (i.e. can be extended to the general population). Those at risk for interventions include previous pregnancy loss >16 weeks or preterm birth, short cervix, history of 3 or more previous mid-trimester miscarriages, cervical trauma (such as trachelectomy and cone biopsy), incidental finding of a short cervix or presenting with a dilated cervix and multiple pregnancies.
(a) Previous loss >16 weeks (previous miscarriage or spontaneous delivery >16 weeks)
While most preterm births occur in previously uncomplicated or first pregnancies, one of the most predictive risk factors are previous preterm birth. Following a previous spontaneous PTB (SPTB) the risk of recurrent is approximately 14%; this rises exponentially to 30% after two previous PTBs and to 40% after 3 [Citation19]. There is a tendency for these to recur with the rates higher for earlier gestations [Citation20] and also related to causes such as cervical weakness/insufficiency [Citation19]. If this is indeed the case, can the identification of those at risk of having cervical weakness/insufficiency be followed by timely interventions to reduce the risk of SPTB?
Those that are likely to have a weak/insufficient cervix can be identified from (a) history - including previous mid-trimester miscarriages, previous preterm birth or cervical trauma from surgery such as trachelectomy/cone biopsy (b) asymptomatic/symptomatic (dilated/short cervix) identified as part of routine anomaly ultrasound evaluation or in those presenting with preterm labor and (c) those presenting as emergency with a dilated cervix, contracting or passing a show or mucus [Citation21,Citation22].
The first evidence of cervical weakness/insufficiency as a cause of preterm birth was reported in 1678, however, it was almost 200 years later that GT Gream associated cervical trauma with cervical weakness in a publication titled “Dilatation or division of the cervix uteri” [Citation23]. It then took nearly one century before Shirodkar VN published his seminal experience with cerclage that the possibility of intervention to reduce preterm birth in those with cervical weakness/insufficiency was considered possible [Citation24]. Two years later McDonald reported on the modified cerclage technique [Citation25]. In the period that followed, the use of cervical cerclage gained widespread adoption without robust evidence including in women who had had one mid-trimester miscarriage.
The key question then was whether a history of a mid-trimester miscarriage or preterm labor was diagnostic of cervical weakness/insufficiency and thus an indication for cervical cerclage? The RCOG/MRC randomized controlled trial was the first multicenter study to address this. It concluded that “the operation had an important beneficial effect in 1:25 (95% CI 1:12–1:300) cases [Citation26]. Its use is associated with increased medical intervention and puerperal pyrexia. Nevertheless, this trial suggests that on balance, cervical cerclage should be offered to women at high risk, such as those with a history of three or more pregnancies ending before 37 weeks.” This trial was criticized for several reasons including heterogeneity of the population studied, the selection criteria, and the type of cerclage used. Despite this, it remained the evidence-basis for recommending interventions in women with a history suggesting cervical weakness/insufficiency. Subsequent sub-analysis did confirm the benefit of cerclage in those with three of more mid-trimester losses - the so-called history indicated cerclage [Citation22,Citation27].
The advent of cervical assessment with ultrasound provided an opportunity to evaluate the validity of the RCOG/MRC trial findings. Althiusius et al. [Citation28] from Amsterdam, in the cervical incompetence prevention randomized cerclage trial (CIPRACT) showed that “Transvaginal ultrasonographic serial follow-up examinations of the cervix in women at risk for cervical incompetence, with secondary intervention as indicated, was a safe alternative to the traditional cerclage.” They further stated that “transvaginal ultrasonographic follow-up examination of the cervix could save the majority of women from un-necessary intervention and that placement of a cerclage could reduce the incidence of preterm delivery <34 weeks.” These seminal observations were followed by several studies including those by Berghella et al. [Citation29] and Berghella and Mackeen [Citation30], which showed that up to 60% of women with a prior history of pregnancy loss >16 weeks did not shorten and therefore in this group, only 40% would benefit from cerclage. This conclusion was further confirmed in a meta-analysis in 2011 by Berghella et al. [Citation31]. Consequently, current recommendations are for serial cervical assessment from 14 weeks of gestation in women with a history of pregnancy loss >16 weeks. For these women, a cervical cerclage is indicated if the cervical length is ≤25 mm before 28 weeks of gestation [Citation22,Citation31]. For those with a cervix >25mm, there is no evidence of benefit from any intervention [Citation32].
Is cervical cerclage the only option for those with ultrasound-diagnosed cervical shortening? In the 2000s several studies reported on the potential efficacy of progesterone in reducing the risk of SPTB in women with a short cervix. The seminal paper by Conde-Agudelo et al. [Citation33] showed that vaginal progesterone was as effective as cervical cerclage in those with an ultrasound-proven short cervix. Currently, therefore this is an alternative to cervical cerclage. There were no differences in the efficacy of vaginal progesterone versus cerclage in women with a cervix ≤ 25 mm in all the outcomes measured [Citation33]. In an updated systematic review and meta-analysis, comparing vaginal progesterone, intramuscular 19-OHPC, cerclage, and pessary, Jarde et al. [Citation34] concluded that “vaginal progesterone was the only intervention with consistent effectiveness for preventing preterm birth in singleton at-risk pregnancies overall and in those with a previous preterm birth.” In a more recent network systematic review and meta-analysis, Care et al. concluded that for singleton pregnancies identified to be at high risk of spontaneous preterm birth either because of a history or SPTB or a short cervical length, vaginal progesterone should be considered the preventative treatment of choice [Citation27].
Progesterone can be administered either parenterally or intravaginally. Sacconne et al. [Citation35] in a systematic review of studies comparing the efficacy of vaginal versus intramuscular progesterone in the prevention of SPTB in those with an ultrasound shortened cervix concluded that daily vaginal progesterone (either as a suppository or gel) started from 16 weeks’ gestation is a reasonable, if not a better alternative to weekly 17-OHPC injection for the prevention of SPTB in women with a singleton pregnancy and prior SPTB. Romero et al. [Citation36] in their updated meta-analysis that included data from the OPIMUM trial concluded that vaginal progesterone decreases the risk of preterm birth before 34 weeks in women with singleton pregnancy and a short cervix. The RCOG [Citation21], NICECitation23, [Citation23] and the SMFM [Citation37] recommend offering prophylactic vaginal progesterone or prophylactic cervical cerclage to women who have both (a) history of SPTB (≤34+0 weeks or) or mid- trimester loss (from 16+0 weeks onwards) and (b) cervical length of ≤25mm on TVS at 16+0–24+0 weeks. In these women, this should be started between 16+0 and 24+0 weeks and continued until at least 34+0 weeks [Citation21,Citation22,Citation37,Citation38]. For those with a cervical length of 25 mm or less between 16+0 and 26+0 and a history of PPROM or cervical trauma, prophylactic cerclage should be considered [Citation22]. These women should have serial follow-up with USS monitoring of cervical length, best started at 13–14 weeks and then 1–2 weekly until 28 weeks rather than cerclage electively. In those requiring cervical cerclage, either a Shirodkar or McDonald suture is equally effective. However, where the woman has had a failed cerclage, then the best option would be transabdominal rather that a high (Shirodkar/double McDonald) cerclage. In the recently concluded MAVRIC trial, Shennan et al. [Citation39] showed that “transabdominal cerclage - inserted laparoscopically or by laparotomy and either as an interval procedure or during pregnancy is the treatment of choice for women with failed vaginal cerclage.” They showed that this was superior to high vaginal cerclage in the reduction of early preterm birth and fetal loss in women with a previous failed vaginal cerclage. High vaginal cerclage does not confer this benefit.
An alternative to progesterone and cervical cerclage in these high risk women is the cervical pessary (Arabin pessary). Although early studies had suggested that this may indeed reduce the risk of SPTB, recent studies [Citation40] and systematic reviews and meta-analysis have concluded that the use of the cervical pessary (Arabin pessary) does not prevent SPTB or improve outcome in high risk pregnancies [Citation31,Citation41]. Despite these conclusions, the recently published network systematic review and meta-analysis by Care et al. [Citation27] showed that the pessary reduced the risk of SPTB with only moderate certainty with an aOR of 0.65 (95%CI 0.39–1.08). On balance, therefore this should not be considered in this high-risk group.
(b) Asymptomatic
Since a significant proportion of women who deliver preterm have no history, it has been suggested that all women should have a cervical assessment at the time of their anomaly ultrasound scan [Citation42]. In those with a short cervix (defined as ≤25mm) the question is whether to insert a prophylactic cerclage or not. A systematic review by Berghella et al. [Citation43] concluded that “in singleton gestations without a prior spontaneous PTB, but with a cervical length of ≤25mm in the second trimester, cerclage does not seem to prevent preterm delivery or improve neonatal outcome. However, in these pregnancies, cerclage seems to be efficacious at lower cervical lengths, such as <10mm and when tocolysis or antibiotics are used as additional therapy.” A more recent study by Gulersen et al. [Citation44] confirmed these meta-analysis findings and concluded that cervical cerclage should be considered in asymptomatic women with an extremely short cervical length (≤10 mm). In a previous study in 2020 the same group [Citation45] had followed-up asymptomatic women with a short cervix (≤25mm) at 23–28 weeks’ gestation in an effort to determine the interval from identifying a short cervix to delivery and showed that the risk of SPTB in asymptomatic women with a sonographic short cervix increased as cervical length decreased. The risk was substantially higher in women with a cervical length of ≤10mm. Women with a cervical length of ≤10mm also had the shortest interval to delivery. Furthermore, they found that delivery within 1 or 2 weeks was highly unlikely regardless of the cervical length at the time of enrollment. They suggested from their findings that management decisions such as timing of the administration of antenatal corticosteroids in asymptomatic women with a cervical length of ≤25mm at 23–28 weeks’ gestation may be delayed until additional indications were present. Over 80% of those with a cervix ≤10mm went on to have preterm delivery. On the basis of these findings, they recommended that for asymptomatic women (i.e. those with no previous history) and a cervix that is >10mm and ≤25mm at <28 weeks’ gestation, vaginal progesterone should be the primary treatment as there is no benefit from cervical cerclage but if the cervix is ≤10mm then cerclage is recommended as 80% of these will delivery <34 weeks.
(c) Cervical length in symptomatic (presenting with contraction)
In women presenting with threatened preterm labor (i.e. with uterine contractions), a high proportion will not progress to deliver. Identifying those who will progress to deliver in the absence of interventions is an important step in management. Would assessing the cervix identify those who will progress to deliver and therefore enable institution of preventative measures?
Several prospective studies have investigated the value of cervical length measurement in these women. When the data from these studies were put together in a meta-analysis, Berghella et al. [Citation43] concluded that there is a significant association between knowledge of TVS CL and lower incidence of PTB and later gestational age at delivery. They further found a significant 36% reduction in the primary outcome (associated with interventions). Ho et al. [Citation46] in a prospective study titled “Prediction of time of delivery using cervical length measurements in women with threatened preterm labor” concluded that “cervical length measurement at the time of presentation was significantly associated with the risk of preterm delivery in women presenting with threatened preterm labor and a short cervix”. Cervical length measurement was also helpful in predicting time of delivery within 14 days from presentation. The negative predictive value and predictive accuracy of CL as a single measure were significant. Wong et al. [Citation47] in a similar follow-up study found that using a cut off of 27.5 mm at presentation predicted deliveries within 1 week in 78% of their cohort. Furthermore, a repeat measurement of ≤27.5 mm a day after admission predicted delivery within a week in 100% of cases. While the numbers from these studies are small, they provide a compelling approach to assessing the cervix in those presenting with preterm labor.
Where transvaginal assessment of the cervix is not available, consideration must be given to the use of biochemical point-of-care tests to diagnosis labor. shows the various biomarkers for diagnosis of PTL and their sensitivity, specificity, PPV and NPV. In clinical practice ofFN and IGFBP-1 are the two most commonly used. Oncofetal fibronectin (ofFN) should be offered to women presenting at ≤30+0 weeks and in whom cervical length measurement is indicated but is not available to determine the likelihood of delivery within 48 h. ofFN test is negative if it is ≤50 ng/m implying that delivery is unlikely but if positive (>50ng/ml) the woman should be managed as in labor and delivery anticipated [Citation22].
Table 2. Accuracy of various biomarkers for the diagnosis of PTL.
(d) Multiple pregnancies
Multiple pregnancy is an important risk factor for spontaneous preterm birth (SPTB) and the gestational age at delivery falls with increasing number of fetuses. The rate of SPTB before 37 weeks of gestation is 7–12% in singletons and increases exponentially to 50–60% in twins, 80–95% in triplet pregnancies and to 100% in quadruplet or more pregnancies [Citation48,Citation49]. Prevention of SPTB must therefore include avoidance of iatrogenic multiple pregnancies especially those of higher order (3 or more). Several authorities/societies/bodies have, in a drive to influence the rate of SPTB, recommended and in some cases legislated for single embryo transfer in assisted reproduction techniques (ART). Where there are 3 or more fetuses, there is general support for selective reduction. Despite this, ART remains unregulated in several parts of the world where unfortunately there are inadequate facilities for neonatal care/support, meaning morbidity and mortality are higher with preterm delivery.
While the drive to reduce multiple pregnancies has affected SPTB in some societies, emphasis has also been on how to reduce this in spontaneous multiple pregnancies especially twins. The options that have been investigated especially in twin pregnancies include routine cervical length monitoring with or without cervical cerclage, use of the mechanical devices (the Arabin vaginal pessary) and the administration of progesterone.
The evidence for use of cervical length as a screening tool comes from two groups of patients - those who are either asymptomatic or present with threatened labor (symptomatic). In the asymptomatic group, previous studies reported conflicting conclusions on the value of cervical length measurement, however, in a meta-analysis in 2010, Conde-Agudelo et al. [Citation50] concluded that “in asymptomatic women with twins, a cervical length <25mm at 20–24 weeks was associated with a 25% risk of delivery <28 weeks of gestation.” These conclusions were supported by another systematic review by Lim et al. [Citation51] who also concluded that in asymptomatic women with a twin pregnancy, a cervical length measurement at 20–24 weeks of gestation was a good predictor of spontaneous preterm birth.” In a sub-analysis, Conde-Agudelo et al. [Citation52] showed that where the cervical length was <20mm at 20–24 weeks, the risk of spontaneous delivery was 42% before 32 weeks and 62% before 34 weeks. However, when repeated measurements were made to assess changes in cervical length, it was concluded that shortening of the cervix over time had low predictive accuracy for preterm birth at <34 weeks. In an individual patient data (IPD) meta-analysis published in 2015, Kindinger et al. [Citation52] in an analysis of 12 twin cohorts showed that a cervical length <30mm at 18 weeks of gestation was most predictive of spontaneous preterm birth ≤28 weeks of gestation. When the cervical length measurements were made > =22 weeks, the prediction of later SPTB (28–34 weeks) was much better. From this analysis, they recommended cervical length measurements in twin pregnancies from 18 weeks of gestation but only for single measurements. In a more recent study Meller et al. [Citation53] in a single center study concluded than serial measurements showed a better performance than a single one in mid-gestation for the prediction of SPTB. A single CL measurement in mid-gestation was the worst predictor of SPTB but measurement at 28 weeks predicted SPTB in 50% of cases (<25mm).
The Society for Maternal-Fetal Medicine (SMFM) recommends that “routine cervical length screening in multiple pregnancies is not indicated,” [Citation38] while the International Society for Ultrasound in Obstetrics and Gynecology (ISUOG) states that “for twin pregnancies, cervical length measurement is the preferred method of screening for preterm birth in twins; 25 mm is the cut off most commonly used in the second trimester.” [Citation54] It would seem that there is no consensus on the use of cervical length monitoring in twin pregnancies primarily because the data available do not provide convincing evidence of benefits.
Where cervical length monitoring has been instituted the crucial question has been how to manage those women with a short cervix. Reports of the benefits of various options have remained contradictory. In a meta-analysis of the value of vaginal progesterone in those with a short cervix, Romero et al. [Citation55] concluded that ‘administration of vaginal progesterone to asymptomatic women with a twin gestation and a sonographic short cervix in the mid-trimester reduces the risk of preterm birth occurring at <30–<35 gestational weeks, neonatal mortality and some measures of neonatal morbidity, without any demonstrable deleterious effects on childhood development. More recently in a randomized double-blind trial in 14 centers, Caritis et al. [Citation56] and a systemic review and meta-analysis [Citation57] concluded that “treatment with intramuscular 17-alpha-hydroxyprogesterone caproate versus placebo did not reduce the rate of preterm birth in women with triplet pregnancy.” Rehal et al. [Citation58] in a randomized trial of early vaginal progesterone versus placebo in women with pregnancies showed that universal treatment with vaginal progesterone did not reduce the incidence of spontaneous preterm birth between 24+0 and 33+6 weeks’ gestation. However, in a post hoc time-to-event analysis, they showed that progesterone may reduce the risk of SPTB before 32 weeks’ gestation in women with a cervical length of <30mm.”
With regards to comparing vaginal progesterone with other preventative options, Roeckner et al. [Citation59] performed a systematic review and quantitatively compared efficacy and perinatal outcomes of cervical pessary, cervical cerclage, vaginal progesterone and injectable progesterone for the prevention of preterm birth <35 weeks in women with twin pregnancy and a sonographic short cervix. They showed that when compared with the other agents, vaginal progesterone appears to be the most effective in lowering the risk of preterm birth in twin gestations with a sonographic short cervix while placement of a cerclage appears to elevate the risk. Indeed, Roechner et al. [Citation60] recommended that in twin pregnancy, vaginal progesterone should be the agent of choice in those with a short cervix.
As for the use of mechanical devices, the recently concluded randomized trial by Norman et al. [Citation61] concluded that mechanical devices such as the Arabin pessary do not decrease preterm birth in women with twin pregnancies and therefore should not be offered/used.
Norman et al. [Citation62] undertook a randomized, double-blind, placebo-controlled study and meta-analysis of Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT). 500 women with twin pregnancies recruited from nine UK National Health Service hospitals were randomized either to daily vaginal progesterone gel 90 mg (n = 250) or to placebo gel (n = 250) for 10 weeks from 24 weeks’ gestation. The combined proportion of intrauterine death or delivery before 34 weeks of pregnancy was 24.7% (61/247) in the progesterone group and 19.4% (48/247) in the placebo group (odds ratio [OR] 1.36, 95% CI 0.89–2.09; p = 0.16). The rate of adverse events did not differ between the two groups. A meta-analysis including these data generated a pooled OR of 1.16, 95% CI 0.89–1.51). These data suggest that progesterone, routinely administered vaginally, does not prevent preterm birth in women with twin pregnancies (who do not have a short cervix). Indeed NICE in its guideline in 2019 did not recommend cervical length screening in twin pregnancies not only because of some inconsistencies between studies with evidence suggesting that cervical length was a moderate predictor of spontaneous preterm birth in twin pregnancy but also because establishing that a woman is at risk of preterm birth ought to allow an intervention to be offered, and the evidence that such an intervention like vaginal progesterone may reduce this risk in subgroups of women with a short cervix was inconsistent [Citation22].
Conflicting data from using vaginal progesterone may be linked to the varied doses of progesterone in the studies. The “Vaginal Progesterone for the Prevention of Preterm Birth in Twins (POPPET)” trial will hopefully address this as it randomizes 200 mg versus 400 mg [ClinicalTrials.gov Identifier: NCT03540225. Completion date Dec 2022] [Citation63].
Interestingly in a recent retrospective study, Zhang et al. [Citation64] developed a dynamic model to predict the risk of spontaneous preterm birth at < 32 weeks in twin pregnancy using maternal demographic characteristics, transvaginal cervical length and funneling at 20–24 weeks. When this model was applied to a cohort of 252 women with twin pregnancy, it predicted SPB <32 weeks with a sensitivity of 80.0%, specificity of 88.17%, positive predictive value of 50.33% and negative predictive value of 96.71% - much better than single variables. They concluded that using such a validated dynamic nomogram model to predict the individual probability of early preterm birth better represented the complex etiology of preterm labor and should hopefully improve its prediction and indication of interventions. More studies are needed to affirm these interesting observations.
Notwithstanding the limitations in the current evidence on the prevention of SPTB in multiple pregnancies (twins), we recommend a pragmatic approach that includes a transvaginal cervical assessment at 16–24 weeks and then offering either vaginal progesterone or cerclage to those with a cervical length of < =25mm. For those with twins and a previous history of SPTB, vaginal progesterone should be offered from 16 weeks of gestation. In those with triplet pregnancies, since the risk of SPTB is over 80%, we would recommend routine use of vaginal progesterone from 14–16 weeks irrespective of the cervical length. Vaginal progesterone should be continued until at least 32 weeks of gestation.
Infections and Preterm labor
Infections are an important cause of preterm labor [Citation22]. Just how common these are in those presenting with a short cervix and how they can be identified remains a major challenge. Romero et al. [Citation65] who advocate amniocentesis for these women showed that intra-amniotic infections were present in 52% of cases. In some of these cases there was no evidence of inflammation [Citation66]. The most common organisms were Ureaplasma urealyticum, Gardinella vaginalis, Candida albicans and Fusibacterium spp [Citation67]. Among the women with a negative amniotic fluid culture, 55% delivered after 34 weeks of gestation. Various other studies have also shown high infections rates, though not as high as 52%. Where organisms were not identified, inflammation was found to be present in a high proportion of case.
Based on these observations, it would seem logical to exclude infections in women presenting with preterm labor and a short cervix or those with an asymptomatic short cervix especially if cerclage is considered since such a procedure may be associated with severe morbidity and mortality if there is infection/inflammation (e.g. higher chance of membrane rupture, preterm labor and possible severe maternal infectious morbidity). The only way to make the diagnosis of intrauterine infection or inflammation is by amniocentesis followed by gram stain, WBC, test for glucose, cytokines and/or culture [Citation67]. Increasingly there are many new rapid point-of-care tests that can be performed such as those for interleukin 6 and MMP-8 generating immediate results that could guide management. The problem is that not many patients will accept amniocentesis and not many obstetricians are willing to offer it. With the identification of infections, do antibiotics reduce the risk of preterm birth? Joon et al. [Citation67] undertook a prospective study of 22 women who had (1) singleton pregnancy; (2) painless cervical dilatation of >10 mm between 16.0 and 27.9 weeks of gestation; (3) intact membranes and absence of uterine contractions, (4) transabdominal amniocentesis for the evaluation of the microbiologic and inflammatory status of the amniotic cavity and antibiotic treatment (ceftriaxone, clarithromycin, and metronidazole) administered parenterally. Follow-up amniocentesis was routinely offered to monitor the microbiologic and inflammatory status of the amniotic cavity. Treatment success was defined as the resolution of intra-amniotic infection/inflammation or delivery ≥34 weeks of gestation. It was concluded that in patients with cervical insufficiency and intra-amniotic infection/inflammation, administration of antibiotics (such as a combination of ceftriaxone, clarithromycin, and metronidazole) was followed by resolution of the intra-amniotic inflammatory process or intra-amniotic infection in 75% of patients and was associated with treatment success in about 60% of cases. It would therefore seem reasonable to consider empirical antibiotics in those with a short/dilating cervix especially where an emergency cerclage is being considered (where amniocentesis has not been performed).
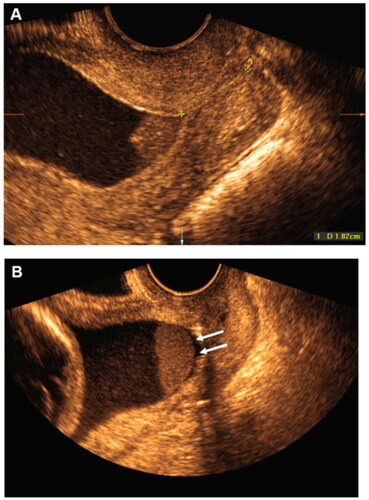
While amniocentesis is the gold standard for identifying intra-amniotic infection/inflammation, ultrasound has increasingly been able to identify a sub-population of women with possible intra-amniotic infections based on the presence of a 'sludge” defined by Romero et al. [Citation68] as sonographic appearance of echogenic, dense free-floating aggregates of debris within the amniotic cavity (). Kusovanovic et al. [Citation69] published a series on the significance of the sludge in SPTB and showed that the shorter the cervix the more likely this was present and furthermore the presence of a sludge was significantly associated with SPTB <32 weeks. These observations have been confirmed by others [Citation70–72]. In a more recent case report by Yeo et al. [Citation73], antibiotics were administered to a woman with a sludge and dilated short cervix and not only did the sludge disappear but the cervix returned to normal and the pregnancy progressed to term. However, Ryan et al. [Citation74] 2020 in a large series failed to demonstrate benefits from antibiotics in these women. Thus, while the presence of a sludge may be considered an indication for antibiotics especially where the cervix has started coning or shortening, there is a need for more data for robust conclusions to be made.
Interventions in those presenting with dilated cervix
A proportion of women, with or without a prior history will present with a dilated cervix and often bulging membranes. Previously, these women were offered bed rest which proved ineffective but the emergency/rescue/physical examination indicated cerclage has been shown to prolong some of the pregnancies and indeed allow for the administration of corticosteroids [Citation21,Citation22]. Althuisius et al. [Citation75] in a randomized trial showed that when compared to bed rest in women presenting before 27 weeks, with loss of cervical mucus, vaginal pressure, mild/no contractions, and visible membranes on speculum examination) cervical cerclage was effective in reducing the rate of PTB. Several other studies have confirmed these seminal observations [Citation76,Citation77]. The problem is often technical – how to reduce the membranes prior to inserting the cerclage without rupturing them.
Several approaches have been used with varying degrees of success to reduce bulging membranes prior to emergency cerclage insertion [Citation78]. These include amniocentesis [Citation79] either transvaginally (through bulging membranes) [Citation80] or transabdominal; using a metreurynter with the patient in the knee-chest position [Citation81]; amniocentesis after filling of the bladder [Citation82]; Foley catheter (or wet sponge and ovum forceps [Citation83–85]; sterile cellular porcine implant [Citation86] and pulling margins of the dilated cervix over the bulging membranes without touching them [Citation78]. More recently Lv et al. [Citation87] reported on the use of a purposely designed uni-concave balloon for reduction of bulging membranes with good results. The advantage of this device is mainly with in its approach to membrane reduction with minimal risk of rupture, however, it is likely to be more expensive than other conventional approaches. illustrates the application of this device. Irrespective of the approach, it is now generally accepted that with cervical dilatation and bulging membranes, the outcome with an emergency cerclage is better than with conservative management [Citation15]. The timing for this intervention is in general between 16+0 and 27+6 weeks [Citation22] but may be modified depending on the facilities for neonatal care (i.e. could be extended upwards). Infections are a common cause of the cervical changes (shortening and dilatation) in these patients, hence this procedure (reduction of bulging membrnaes and insertion of cerclage) should be covered with a course of antibiotics – preferably a combination against gram negative gram positive and anaerobes. A typical combination will include ceftriaxone, clarithromycin and metronidazole (see infections above).
Figure 3. Placement of balloon to reduce membranes prior to cerclage insertion with Uniconcave catheter (Courtesy of Lv et al. 2000) [Citation77].
![Figure 3. Placement of balloon to reduce membranes prior to cerclage insertion with Uniconcave catheter (Courtesy of Lv et al. 2000) [Citation77].](/cms/asset/636a8e40-ff3e-459e-ad45-38e83eb0b4a8/ijmf_a_2183756_f0003_c.jpg)
Preterm premature rupture of fetal membranes (PPROM)
PPROM precedes spontaneous preterm birth in about a third of cases [Citation88]. While this cannot be prevented, the consequences can be minimized through appropriate, reliable and timely diagnosis followed by monitoring and delivery when appropriate. The diagnosis of ruptured membranes is clinical with confirmation of fluid coming through an exposed cervix with a speculum or a pool of clear fluid in the posterior fornix [Citation22]. Where no pooling is seen in the posterior fornix or coming through the cervix (i.e. the diagnosis is uncertain from clinical examination), biochemical point-of-care (bed-side) test with placental alpha microglobulin-1 (PAMG-1) or insulin-like growth factor binding protein-1 (IGFBP-1) test of vagina fluid should be used [Citation22,Citation89]. Vital in those with confirmed PPROM is the exclusion of co-infections especially with group β hemolytic streptococcus. Screening for infections must therefore be undertaken especially in those presenting <34 weeks of gestation. The presence of GBS has been shown to increase the rate of preterm delivery 3-fold in those presenting before 32 weeks of gestation [Citation90]. Identification of specific infectious organisms will determine the type of antimicrobial therapy to be offered but without results, the women should be placed on a combination such as ampicillin and erythromycin (or 1 g azithromycin as a single dose if erythromycin is unavailable) for about least 5–7 days [Citation22,Citation91,Citation92].
Once the diagnosis has been confirmed, management will depend on the gestational age and associated complications (fetal or maternal). In general, for PPROM after 37+0 weeks of gestation, management is often conservative for 24 h and if spontaneous labor does not start, it should be induced. For pregnancies <37+0 weeks, where there are no complications, expectant management is recommended [Citation89,Citation91]. For those below 34+0 weeks, a course of corticosteroids should be given and managed expectantly. Consideration should be given to administering corticosteroids to those between 34+0 and 35+6 weeks. Management of PPROM is outside the scope of this review.
Tocolysis
The role of tocolysis in prolonging pregnancy has been reviewed in several meta-analysis and there is no evidence of significant prolongation of pregnancy, however their use allows time for the administration of corticosteroids to improve morbidity in the neonate and when appropriate in-utero trnasfer [Citation93]. Where tocolysis is to be used it should be offered to those between 26+0 and 33+6 [Citation22]. The tocolytics of choice are nifedipine and the oxytocin antagonist – atosiban. Magnesium sulfate should be given to those between 24+0 an 29+6 and considered for those between 23+0 and 23+6 and 30+0 and 33+6 weeks primarily for neuroprotection [Citation22].
Free fetal DNA and increased risk of preterm labor
Cell-free deoxyribonucleic acid (DNA) is widely used to screen for fetal aneuploidy. Most of fetal cell-free DNA (cfFDNA) in the maternal blood is released from the syncytiotrophoblast as a result of cellular apoptosis and necrosis. Elevated levels may be indicative of underlying placental dysfunction, which has been associated with preterm birth [Citation94]. While studies have demonstrated that cfFDNA is increased in pregnancies complicated by spontaneous preterm birth [Citation95,Citation96], there are limited data on the association between cfFDNA levels and preterm birth in asymptomatic women in the first and second trimesters. Studies have failed to find an association between first-trimester cfFDNA levels and preterm birth [Citation97,Citation98]; but there is conflicting evidence associating elevated second-trimester cfFDNA levels with subsequent spontaneous preterm birth. In a retrospective study of 1349 women with singleton pregnancies at increased risk for aneuploidy who had cell-free DNA testing at 10–20 weeks’ gestation, [Citation99] 119 (8.8%) delivered preterm birth [prior to 37 weeks] with 49 cases (3.6%) delivering prior to 34 weeks. There was no significant association between fetal fraction and preterm birth for cfFDNA levels measured at 10–14 weeks’ gestation, however, there were significant associations for measurements at 14.1–20.0 weeks’ gestation. cfFDNA levels greater than or equal to the 95th percentile at 14.1–20.0 weeks’ gestation were significantly associated with an increased risk for preterm birth less than 37 and 34 weeks’ gestation (aOR 4.59; 95% CI, 1.39–15.2; aOR 22.0; 95% CI 5.02–96.9, respectively). This maybe more predictive for measurements made after 20 weeks [Citation94]. While these findings are encouraging more data are needed to confirm the possible use of elevated cfDNA in the second trimester as a biomarker for SPTB.
Genetics and preterm labor
There is evidence that the duration of gestation and the risk of preterm birth is influence by genetics [Citation100,Citation101]. Twin and family studies for example, suggest that 30 to 40% of the variation in birth timing and in the risk of preterm birth arises from genetic factors that largely but not exclusively reside in the maternal genome [Citation102–105]. Women who give birth post-term are at increased risk for subsequent post-term deliveries [Citation106] and similarly, a maternal history of preterm birth [Citation107] is a strong risk factor for preterm birth. Taken together, these findings suggest that individual influences that are stable over time contribute to variations in the length of gestation. These influences could be genetic—either a woman’s contingent of nuclear and mitochondrial DNA or the meta-genome that she shares with her microbiome constituency—or they could reflect unchanging environmental exposures [Citation108]. Indeed, several studies have shown that genetic factors are likely to be important not only in isolation but also in combination with other genetic or environmental factors. For example, variation in the progesterone receptor has been implicated as a maternal risk factor for SPTB though not consistently [Citation109–111]. Similarly, polymorphisms in genes that encode inflammatory cytokines initially identified as a possible risk factor have not been consistently associated with preterm birth [Citation106]. More recently genomewide association studies investigating the genetic associations with preterm birth have identified variants at the EBF1, EEFSEC, AGTR2, WNT4, ADCY5, and RAP2C loci associated with gestational duration and variants at the EBF1, EEFSEC, and AGTR2 loci associated with preterm birth [Citation108]. If these are confirmed in further studies, then potentially women at genetic risk of SPTB could be identified prior to pregnancy or in early pregnancy and appropriate steps taken to prevent SPTB. There are currently no trials on how such genetically identified at risk women should be managed to reduce the risk of SPTB. This may partly be because the evidence is still emerging and yet to be robust. It is only when the evidence is strong enough that clinical trials can be undertaken in this regard.
Cervical elastogrpahy
Advances in technology are enabling the trial of new potential tools for identifying high-risk women for SPTB. One such technology is elastography. The E-Cervix™ (WS80A; Samsung, Seoul, Korea) elastography is a quantification tool that allows for the measurement of the stiffness ratio (HR) of the cervix using strain elastography. In a study of 95 singleton pregnancies with threatened preterm labor and no prior preterm birth, Nazzaro et al. [Citation112] showed that women who delivered preterm had significantly lower HR compared to those who did not. They concluded that cervical elastography with the E-cervix may be useful for the assessment of women presenting to obstetrics triage with threatened preterm labor. These are preliminary and exciting data that need to be replicated for use in clinical practice.
Corticosteroids
The evidence for the benefits of the use of antenatal corticosteroids to accelerate fetal lung maturation in women at risk of preterm birth is overwhelmingly in support especially of a single course [Citation113]. It is associated with a reduction in the risk of perinatal and neonatal death and RDS and intraventricular hemorrhage. This evidence is robust, regardless of resource setting (high, middle or low). While the immediate benefits (i.e. especially in the neonatal period) are obvious, follow-up studies of these children into child and adulthood to investigate any longer-term effects of antenatal corticosteroids are less robust. In a recent systematic review and meta-analysis of studies that have investigated the long-term outcomes associated with antenatal corticosteroid exposure, Nina et al. concluded that while the use of corticosteroids is associated with improvements in neonatal morbidity, greater emphasis must be placed on prevention especially as increasingly, evidence is emerging on the potential harm (such as increased risk of neurocognitive disorders and behavioral disturbances) of corticosteroids, especially in babies whose mothers were given these but were delivered at term [Citation114]. This conclusions underlie the importance of long-term follow-up studies especially those involving the pituitary-adrenal axis.
Conclusion
Prevention of spontaneous preterm birth remains the key tool for mitigating this important cause of perinatal morbidity and mortality and long-term health problems. Primary prevention aims to improve maternal health prior and during pregnancy while secondary prevention includes cervical length measurements in those with a prior pregnancy loss after 16 weeks, cervical trauma (trachelectomy or cone biopsy), identifying those with a short cervix at routine ultrasound scan or when they present with threatened preterm labor and those presenting with a dilated cervix. For all these women with cervical changes, a cerclage is an option that has been shown to significantly delay delivery even when the cervix is dilated and membranes are bulging. The use of vaginal progesterone has been shown to be as effective as cerclage in those with a history of previous pregnancy loss >16 weeks who are asymptomatic but have a short cervix. In twin pregnancies it would seem that cervical length measurement at around 18–20 weeks may identify those at risk while for those presenting in threatened preterm labor, cervical length measurement is a useful tool to identify those who will progress to deliver and therefore require more intensive care, corticosteroids and tocolysis. The advent of modeling is generating potential tools for use in stratifying women into high-risk categories that would require closer monitoring. Such models as those reported by Stocks et al. [Citation115] will allow for better planning and individualized care. This would be supplemented by genetics and the use of biomarkers such as free fetal DNA and possibly newer biomarkers [Citation116].
Acknowledgements
Open Access funding provided by the Qatar National Library.
Additional information
Funding
References
- Kinney V, Rhoda NR. Understanding the causes of preterm birth: solutions depend on context. Lancet Glob Health. 2019;7:e1000–e1001.
- Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37–46–e46.
- Blencowe H, Cousens S, Chou D, et al. LawnJ on behalf of the born too soon preterm birth action group born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(S1):S2.
- Chawanpaiboon S, Vogel JP, Moller A-B, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37–e46.
- Born Too Soon: The Global Action Report on Preterm Birth. https://www.efcni.org/wp-content/uploads/2018/03/BornTooSoonExecSummary_v05.pdf.
- Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–2172.
- National Center for Health Statistics. Final Mortality data and Marchof Dimes 2021 Premature Birth Report Card. https://www.marchofdimes.org/peristats/tools/reportcard.aspx. Accessed 12 August 2022
- Petrou S, Yiu HH, Kwon J. Economic consequences of preterm birth: a systematic review of the recent literature (2009–2017). Arch Dis Childh. 2018;0:1–10.
- Barros FC, Papageorghiou AT, Victora CG, et al. The distribution of clinical phenotypes of preterm birth syndrome: implications for prevention. JAMA Pediatr. 2015;169(3):220. Published on line January 5th 2015
- Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;362(6):529–535.
- Villar J, Papageorghiou AT, Knight HE, et al. The preterm birth syndrome: a prototype phenotypic classification. Am J Obstet Gynecol. 2012;206(2):119–123.
- Manuck TA, Esplin MS, Biggio J, et al. The phenotype of spontaneous preterm birth: application of a clinical phenotyping tool. Am J Obstet Gynecol. 2015;212(4):487.e1–487.e11.
- ACOG Bulletin Summary Number 234. Prediction and prevention of spontaneous preterm birth. Obstetrics and Gynecology. 2021;138:320–233.
- Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. The Lancet. 2008;371(9606):75–84.
- Bhutta Z, Yakoob M, Salam R, et al. 2011. Global review of interventions related to maternal, newborn and child health (MNCH): what works and can be scaled up?. Pakistan: Aga Khan University.
- Barros AJ, Ronsmans C, Axelson H, et al. Equity in maternal, newborn, and child health interventions in countdown to 2015: a retrospective review of survey data from 54 countries. Lancet. 2012;379(9822):1225–1233.
- Tessema GA, Marinovich ML, Håberg SE, et al. Interpregnancy intervals and adverse birth outcomes in high-income countries: an international cohort study. PLOS One. 2021;16(7):e0255000.
- Rahmati S, Azami M, Badfar G, et al. The relationship between maternal anemia during pregnancy with preterm birth: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2020;33(15):2679–2689.
- Mazaki-Tovi S, Kusanovic JP, Erez O, et al. Recurrent preterm birth. Semin Perinatol. 2007;31(3):142–158.
- Yang J, Baer RJ, Berghella V, et al. Recurrence of preterm birth and early term birth. Obstet Gynecol. 2016;128(2):364–372.
- Shennan AH, Story L. on behalf of the royal college of obstetricians and gynaecologists. Cervical Cerclage. RCOG Green-Top Guideline. 2022;129:1178–1210.
- Preterm labour and birth NICE guideline [NG25] Published: 20 November 2015 Last updated: 02 August 2019. National Institute for Health and Care Excellence. Preterm Labour and Birth. NICE Guideline 25. London: NICE; 2015.
- Gream GT. Dilatation or division of the cervix uteri. The Lancet. 1865;85(2171):381.
- Shirodkar VN. A new method of operative treatment for habitual abortions in the second trimester of pregnancy. Antiseptic. 1955;52:299–300.
- McDonald IA. Suture of the cervix for inevitable miscarriage. BJOG: Int J Obstet Gynaecol. 1957;64(3):346–350.
- Final report of the Medical Research Council/Royal College of Obstetricians and Gynaecologists multicentre randomised trial of cervical cerclage. MRC/RCOG Working Party on CervicalCerclage. BJOG 1993;100:516–23.
- Care A, Nevitt S, Medley N, et al. Interventions to prevent spontaneous preterm birth in women with singleton pregnancy who are at high risk: systematic review and network meta-analysis. BMJ. 2022;376:e064547.
- Althuisius SM, Dekker GA, van Geijn HP, et al. Cervical incompetence prevention randomized cerclage trial(CIPRACT): study design and preliminary results. Am J ObstetGynecol. 2000;183(4):823–829.
- Berghella V, Haas S, Chervoneva I, et al. Patients with prior second-trimester loss: prophylactic cerclage or serial transvaginal sonograms? Am J Obstet Gynecol. 2002;187(3):747–751.
- Berghella V, Mackeen AD. Cervical length screening with ultrasound-indicated cerclage compared with history-indicated cerclage for prevention of preterm birth: a meta-analysis. Obstet Gynecol. 2011;118(1):148–155.
- Berghella V, Timothy R, Jeff S, et al. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth. Obstet Gynecol. 2011;117(3):663–671.
- Phung J, Williams KP, McAullife L, et al. Vaginal progesterone for prevention of preterm birth in asymptomatic high-risk women with a normal cervical length: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2022;35:7093–7101.
- Conde-Agudelo A, Romero R, Da Fonseca E, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated in direct comparison meta-analysis. Am J Obstet Gynecol. 2018;219(1):10–25.
- Jarde A, Lutsiv O, Beyene J, et al. Vaginal progesterone, 17-OHPC, cerclage and pessary for preventing preterm birth in at risk singleton pregnancies: an updated systematic review and network meta-analysis. BJOG. 2019;126(5):556–567.
- Saccone G, Khalifeh A, Elimian A, et al. Vaginal progesterone vs intramuscular 17α-hydroxyprogesterone caproate for prevention of recurrent spontaneous preterm birth in singleton gestations: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2017;49(3):315–321.
- Romero R, Nicolaides KH, Conde-Agudelo A, et al. Vaginal progesterone decrerases preterm birth < =34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPTIMUM study. Ultrasound Obstet Gynecol. 2016;48:308–317.
- Cervical cerclage for the woman with prior adverse pregnancy. https://www.smfm.org/publications/98-cervical-cerclage-for-the-woman-with-prior-adverse-pregnancy-outcome. Accessed 13th April 2022.
- McIntosh J, Feltovich H, Berghella V, et al. The role of routine cervical length screening in selected high- and low-risk women for preterm birth prevention. Am J Obstet Gynecol. 2016;215(3):B2–B7.
- Shennan AH, Chandiramani M, Bennett P, et al. MAVRIC: a multicenter randomized controlled trial of transabdominal vs transvaginal cervical cerclage. Am J Obstet Gynecol. 2020; 222(3):261.e1–261.e9.
- Mastantuoni E, Saccone G, Gragnano E, et al. Cervical pessary in singleton gestations with arrested preterm labor: a randomized clinical trial. Am J Obstet Gynecol Maternal Fetal Medicine. 2021;3(2):100307.
- Jin XH, Li D, Huang LL. Cervical pessary for prevention of preterm birth: a meta-analysis. Sci Rep. 2017;7(1):42560.
- RANZCOG Best Practice Statement: measurement of cervical length for prediction of preterm birth. First endorsed by RANZCOG: November 2008. Current: November 2021.
- Berghella V, Ciardulli A, Rust OA, et al. Cerclage for sonographic short cervix in singleton gestations without prior spontaneous preterm birth: systematic review and meta-analysis of randomized controlled trials using individual patient-level data. Ultrasound Obstet Gynecol. 2017; Nov50(5):569–577.
- Gulersen M, Bornstein E, Domney A, et al. Cerclage in singleton gestations with an extremely short cervix (≤10 mm) and no history of spontaneous preterm birth. Am J Obstet Gynecol MFM. 2021;3(5):100430.
- Gulersen M, Divon MY, Krantz D, et al. The risk of spontaneous preterm birth in symptomatic women with a short cervix (< =25mmm) at 23-28 weeks’ gestation. Am J Obstet Gynecol. 2020;2:100059.
- Ho N, Liu C, Nguyen A, et al. Prediction of time of delivery using cervical length measurement in women with threatened preterm labour. J Matern Fetal Neonatal Med. 2019;34(16):2649–2654.
- Wong TTC, Yong X, Tung JSZ, et al. Prediction of labour onset in women who present with symptoms of preterm labour using cervical length. BMC Pregnancy Childbirth. 2021;21(1):359.
- Kalikkot Thekkeveedu R, Dankhara N, Desai J, et al. Outcomes of multiple gestation births compared to singleton: analysis of multicenter KID database. Matern Health, Neonatol Perinatol. 2021;7(1):15.
- Thekkeveedu K, et al. Outcomes of multiple gestation births compared to singleton: analysis of multicentre KID database. Maternal Health, Neonatal Perinatol. 2021;7:15.
- Conde-Agudelo A, Romero R, Hassan SS, et al. Transvaginal sonographic cervical length for the prediction of spontaneous preterm birth in twin pregnancies: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010;203(2):128.e1–128.e12.
- Lim AC, Hegeman MA, Huis I, et al. Cervical length measurement for the prediction of preterm birth in multiple pregnancies: a systematic review and bivariate meta-analysis. Ultrasound Obstet Gynecol. 2011;38(1):10–17.
- Conde-Agudelo A, Romero R. Predictive accuracy of changes in transvaginal sonographic cervical length over time for preterm birth: a systematic review and meta-analysis. Am J Obstet Gynecol. 2015;213(6):789–801.
- Meller C, Izbizky G, Aiello H, et al. Cervical length as a screening for spontantous preterm birth in uncomplicated twins: one vs serial measurements. J Maternal Fetal Neonatal Med. 2022;35(21):4097–4103.
- Khalil A, Rodgers M, Baschat A, et al. ISUOG Practice Guidelines: role of ultrasound in twin pregnancy. Ultrasound Obstet Gynecol. 2016;47:247–263.
- Romero R, Conde-Agudelo A, El-Refaie W, et al. Vaginal progesterone decreases preterm birth and neonatal morbidity and mortality in women with a twin gestation and a short cervix: an updated meta-analysis of individual patient data. Ultrasound Obstet Gynecol. 2017;49(3):303–314.
- Caritis SN, Rouse DJ, Peaceman AM, et al. Prevention of preterm birth in triplets using 17 alpha-hydroxyprogesterone caproate: a randomized controlled trial. Obstet Gynecol. 2009;113(2 Pt 1):285–292.
- Almutairi A, Aljohani HI, Al-Fadel NS. 17-Alpha-Hydroxyprogesterone vs. Placebo for Preventing of Recurrent Preterm Birth: a systematic review and meta-analysis of randomized trials. Front Med. 2021. published: 01 December
- Rehal A, Benkő Z, De Paco Matallana C, et al. Early vaginal progesterone versus placebo in twin pregnancies for the prevention of spontaneous preterm birth: a randomized, double-blind trial. Am J Obstet Gynecol. 2021; 224(1):86.e1–86.e19.
- Roeckner JT, Sanchez-Ramos L. The comparative efficacy of cervical pessary, cerclage, vaginal and parenteral progesterone for the prevention of preterm birth in women with a sonographic short cervix and a singleton gestation: a systematic review and network meta-analysis. Am J Obstet Gynecol. 2017;216(1):S382.
- Roeckner JT, Mitta M, Sanchez-Ramos L, et al. Twin pregnancies with short cervix: vaginal progesterone is agent of choice for preterm prevention. Am J Obstet Gynecol. 2019;220(1):S368–S369.
- Norman JE, Norrie J, MacLennan G, et al. The Arabin pessary to prevent preterm birth in women with a twin pregnancy and a short cervix (STOPPIT-2): an open-label randomised trial and updated meta-analysis. PLOS Med. 2021; 18(3):e1003506.
- Norman JE, Mackenzie F, Owen P, et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, double-blind, placebo-controlled study and meta-analysis. Lancet. 2009;373(9680):2034–2040.
- ClinicalTrials.gov Identifier: NCT03540225. Completion date Dec 2022. Chiu lee Liona Poon - Chinese University Hongkong; 2018.
- Zhang J, Zhan W, Lin Y, et al. Development and external validation of a normogram for predicting preterm birth at <32 weeks in twin pregnancy. Sci Reproduction. 2021;11:12430.
- Romero R, Gonzalez R, Sepulveda W, et al. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol. 1992; Oct167(4 Pt 1):1086–1091.
- Jung E, Romero R, Yoon BH, et al. Bacteria in the amniotic fluid without inflammation: early colonization vs. contamination. J Perinat Med. 2021;49(9):1103–1121.
- Oh KJ, Romero R, Park JY, et al. Evidence that antibiotic administration is effective in the treatment of a subset of patients with intra-amniotic infection/inflammation presenting with cervical insufficiency. Am J Obstet Gynecol. 2019;221(2):140.e1–140.e18.
- Romero R, Kusanovic JP, Espinoza J, et al. What is amniotic fluid sludge? Ultrasound Obstet Gynecol. 2007;30(5):793–798.
- Kusanovic JP, Espinoza J, Romero R, et al. Clinical significance of the presence of amniotic fluid sludge in asymptomatic patients at high risk of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2007;30(5):706–714.
- Hatanaka AL, Fraca MA, Nishimoto TE, et al. Antibiotic treatment for patients with amniotic fluid “sludge. To prevent spontaneous preterm birth: a historically controlled observational study. Acta Obstet Gynecol Scand. 2019;98:1157–1163.
- Himaya E, Rhalmi N, Girard M, et al. Midtrimester intra-amniotic sludge and the risk of spontaneous preterm birth. Amer J Perinatol. 2011;28(10):815–820.
- Pérez-Pedregosa J, Ruiz MC, Medina TB, et al. Amniotic sludge and short cervix as inflammation and intraamniotic infection markers. OGIJ. 2017;7(2):00239.
- Yeo L, Romero R, Chaiworapongsa T, et al. Resolution of acute cervical insufficiency after antibiotics in a case with amniotic fluid sludge. J Matern-Fetal Neonatal Med. 2022;35(25):5416–5426.
- Cuff RD, Carter E, Taam R, et al. Effect of antibiotic treatment of amniotic fluid sludge. Am J Obstet Gynecol MFM. 2020;2(1):100073.
- Althuisius SM, Dekker GA, Hummel P, et al. Cervical incompetence prevention randomized cerclage trial: emergency cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2003;189(4):907–910.
- Ehsanipoor RM, Seligman NS, Saccone G, et al. Physical examination indicated cerclage. A systematic review and meta-analysis. Obstet Gynecol. 2015;126(1):125–135.
- Chatzakis C, Efthymiou A, Sotiriadis A, et al. Emergency cerclage in singleton pregnancies with painless cervical dilatation: a meta-analysis. Acta Obstet Gynecol Scand. 2020;99(11):1444–1457.
- Kilani Z, Hamarsheh M, Kilani S, et al. A Novel Technique of Emergency Cerclage for Mid Trimester Cervical Dilatation Annals of Infertility & Reproductive Endocrinology Published: 10 May2018.
- Locatelli A, Vergani P, Bellini P, et al. Amnioreduction in emergency cerclage with prolapsed membranes: comparison of two methods for reducing the membranes. Am J Perinatol. 1999;16(2):73–77.
- Makino Y, Makino I, Tsujioka H, et al. Amnioreduction in patients with bulging prolapsed membranes out of the cervix and vaginal orifice in cervical cerclage. J Perinat Med. 2004;32(2):140–148.
- Ogawa M, Sanada H, Tsuda A, et al. Modified cervical cerclage in pregnant women with advanced bulging membranes: knee- chest positioning. Acta Obstet Gynecol Scand. 1999;78(9):779–782.
- Ochi M, Ishikawa K, Itoh H, et al. Aggressive management of prolapsed fetal membranes earlier than 26 weeks’ gestation by emergent McDonald cerclage combined with amniocentesis and bladder overfilling][article in Japanese]. Nihon Sanka Fujinka Gakkai Zasshi. 1994; 46(4):301–307.
- Wong GP, Farquharson DF, Dansereau J. Emergency cervical cerclage: a retrospective review of 51 cases. Amer J Perinatol. 1993;10(05):341–347.
- Debby A, Sadan O, Glezerman M, et al. Favorable outcome following emergency second trimester cerclage. Int J Gynaecol Obstet. 2007;96(1):16–19.
- Levin I, Salzer L, Maslovitz S, et al. Outcomes of mid-trimester emergency cerclage in twin pregnancies. Fetal Diagn Ther. 2012;32(4):246–250.
- Tsapanos VS, Decavalas GO, Adonakis GL, et al. Late or emergency (salvage) cerclage of a dilated cervix after tissue support with pelvicol implant: a case. J Biomed Mater Res B Appl Biomater. 2005;72(2):368–372.
- Lv M, Zhao B, Chen Y, et al. Balloon tamponade for successful emergency cervical cerclage. J Obstet Gynaecol Res. 2020;46(3):418–424.
- Linehan LA, Walsh J, Morris A, et al. Neonatal and maternal outcomes following midtrimester preterm premature rupture of the membranes: a retrospective cohort study. BMC Pregnancy Childbirth. 2016;16(1):25.
- Bond DM, Middleton P, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2017;3(3):CD004735.
- Regan JA, Chao S, James LS. Premature rupture of membranes, preterm delviery and group β streptococcal colonization of mothers. Am J Obstet Gynecol. 1981;141(2):184–186.
- Thomson AJ,. Care of women presenting with suspected preterm prelabour rupture of membranes from 24 + 0Weeks of gestation. BJOG. 2019;126:e152–166.
- ACOG. Practice bulletin no 217. Prelabor Rupture of Membranes. Obstet Gynecol. 2020;135:739–743.
- Miyazaki C, Moreno Garcia R, Ota E, et al. Tocolysis for inhibiting preterm birth in extremely preterm birth, multiple gestations and in growth-restricted fetuses: a systematic review and meta-analysis. Reprod Health. 2016;13:4.
- van Boeckel SR, Davidson DJ, Norman JE, et al. Stock SJ cell-free fetal DNA and spontaneous preterm birth. Reproduction. 2018;155(3):R137–R145.
- Leung TN, Zhang J, Lau TK, et al. Maternal plasma fetal DNA as a marker for preterm labour. The Lancet. 1998;352(9144):1904–1905.
- Darghahi R, Mobaraki‐Asl N, Ghavami Z, et al. Jalilvand FEffect of cell‐free fetal DNA on spontaneous preterm labor. J Adv Pharm Technol Res. 2019;10(3):117–120.
- Poon LCY, Musci T, Song K, et al. Maternal plasma cell-free fetal and maternal DNA at 11-13 weeks’ gestation: relation to fetal and maternal characteristics and pregnancy outcomes. Fetal Diagn Ther. 2013;33(4):215–223.
- Quezada MS, Francisco C, Dumitrascu-Biris D, et al. Fetal fraction of cell-free DNA in maternal plasma in the prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2015;45(1):101–105.
- Dugoff L, Barberio A, Whittaker PG, et al. l Cell-free DNA fetal fraction and preterm birth. Am J Obstet Gynecol. 2016;215(2):231.e1–231.e7.
- Bezold KY, Karjalainen MK, Hallman M, et al. The genomics of preterm birth: from animal models to hu- man studies. Genome Med. 2013;5(4):34.
- Plunkett J, Feitosa MF, Trusgnich M, et al. Mother’s genome or maternally-in- herited genes acting in the fetus influence gestational age in familial preterm birth. Hum Hered. 2009;68(3):209–219.
- Kistka ZA, DeFranco EA, Ligthart L, et al. Heritability of parturition timing: an extended twin design analysis. Am J Obstet Gynecol. 2008;199(1):43.e1–5.
- Boyd HA, Poulsen G, Wohlfahrt J, et al. Maternal contributions to preterm delivery. Am J Epidemiol. 2009;170(11):1358–1364.
- Clausson B, Lichtenstein P, Cnattin- Gius S. Genetic influence on birthweight and gestational length determined by studies in offspring of twins. BJOG: Int J Obstet Gynaecol. 2000;107(3):375–381.
- York TP, Eaves LJ, Lichtenstein P, et al. Fetal and maternal genes’ influence on gestational age in a quantitative genetic analysis of 244,000 swedish births. Am J Epidemiol. 2013;178(4):543–550.
- Kistka ZA-F, Palomar L, Boslaugh SE, et al. Risk for post-term delivery after previous post-term delivery. Am J Obstet Gynecol. 2007;196(3):241.e1–241.e6.
- Plunkett J, Muglia LJ. Genetic contributions to preterm birth: implications from epidemiological and genetic association studies. Ann Med. 2008;40(3):167–195.
- Zhang G, Feenstra B, Bacelis J, et al. Genetic associations with gestational duration and spontaneous preterm birth. N Engl J Med. 2017;377(12):1156–1167.
- Ehn NL, Cooper ME, Orr K, et al. Evaluation of fetal and maternal genetic variation in the progesterone receptor gene for contributions to preterm birth. Pediatr Res. 2007;62(5):630–635.
- Diaz-Cueto L, Dominguez-Lopez P, Cantillo-Cabarcas J, et al. Progesterone receptor gene polymorphisms are not associated with preterm birth in a hispanic population. Int J Gynaecol Obstet. 2008;103(2):153–157.
- Guoyang L, Morgan T, Bahtiyar MO, et al. Single nucleotide polymorphisms in the human progesterone receptor gene and spontaneous preterm birth. Reprod Sci. 2008;15:147–155.
- Roberts D, Brown J, Medley N, et al. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3(3):CD004454.
- Nazzaro G, Saccone G, Miranda M, et al. Cervical elastography using E-cervix for prediction of preterm birth in singleton pregnancies with threatened preterm labor. J Matern-Fetal Neonatal Med. 2022;35(2):330–335.
- Ninan K, Liyanage SK, Murphy KE, et al. Evaluation of long-term outcomes associated with preterm exposure to antenatal CorticosteroidsA systematic review and meta-analysis. JAMA Pediatr. 2022;176(6):e220483.
- Stock SJ, Horne M, Bruijn M, et al. Development and validation of a risk prediction model for preterm birth for women with preterm labour symptoms (the QUIDS study): a prospective cohort study and individual participant data meta-analysis. PLOS Med. 2021; 18(7):e1003686.
- Bachkangi P, Taylor AH, Bari M, et al. Prediction of preterm labour from a single blood test: the role of the endocannabinoid system in predicting preterm birth in high-risk women. Eur J Obstet Gynecol Reprod Biol. 2019;243:1–6.