Abstract
Objective
The aim of this study was to investigate the association and predictive value between intertwin discordance in first trimester biometries crown-rump length (CRL) and nuchal translucency (NT), and the first trimester biochemical markers PAPP-A and free β-hCG in relation to birth weight discordance (BWD) ≥25% in monochorionic diamniotic (MCDA) twin pregnancies.
Methods
First trimester screening information and pregnancy outcome data on MCDA twin pregnancies with delivery from July 2008 to July 2017 were retrieved from the Danish Fetal Medicine Database. CRL discordance was divided into: <10% (reference group) and ≥10%. NT discordance was divided into: <20% (reference group) and ≥20%. The twin pregnancies were classified according to BWD into the following groups: <10% (reference group), 10–24.9%, and ≥25% including cases undergoing umbilical cord occlusion due to selective fetal growth restriction (sFGR). The twin pregnancies with the most severe BWD (BWD ≥25%) were subdivided into three groups including cases with only one growth-restricted (<10th centile) infant defined as sFGR, and cases where both twins were <10th centile. Median multiples of the median (MoM) values of PAPP-A and free ß-hCG were compared with the group with BWD <10% using the Wilcoxon two-sample test. The ability of CRL discordance and NT discordance to predict BWD ≥25% was examined by the area under the receiver operator characteristic (ROC) curve.
Results
A total of 762 MCDA pregnancies were included. The proportion of pregnancies with CRL discordance ≥10% and NT discordance ≥20% was significantly higher in the group with severe BWD discordance (27.0% vs. 4.7% (p < 0.001) and 40.9% vs. 23.9% (p = 0.001), respectively). When examining the three subgroups of severe BWD, we found a significantly higher percentage of pregnancies with CRL discordance ≥10% in the group where umbilical cord occlusion was performed (52.6% vs. 4.7% in the group with BWD <10% (p < 0.001)) and in the group of BWD ≥25% with sFGR (21.7% vs. 4.7% (p < 0.001)). Additionally, a significantly higher percentage of pregnancies with NT discordance ≥20% was found in the group where umbilical cord occlusion was performed (52.6% vs. 23.9% (p = 0.005)) and in the group with both twins <10th centile (66.7% vs. 23.9% (p = 0.003)). No statistically significant differences were found when comparing levels of PAPP-A and free β-hCG MoMs with the group with BWD <10%. In ROC curves, CRL discordance yielded an AUC for prediction of BWD ≥25% of 0.70 (95% CI 0.63–0.76), and for NT discordance AUC was 0.59 (95% CI 0.52–0.66)). OR for any BWD ≥ 25% was 6.7 (95% CI 3.8–12.0) for pregnancies with a CRL discordance ≥10% compared to pregnancies with a CRL discordance <10%.
Conclusions
This study shows that a discordance in CRL and NT in MCDA twins are both significantly associated with development of BWD. The most important predictor remains CRL discordance ≥10%, thereby suggesting the unequal growth pattern in many cases with BWD is evident already in the first trimester of the pregnancy. No association was found between first trimester biochemical markers and severe BWD.
Introduction
Monochorionic diamniotic twin pregnancies (MCDA) account for about 20% of all twin gestations [Citation1]. Monochorionic twin pregnancies are known to have a significantly higher risk of adverse outcome including a higher risk of low birth weight and increased morbidity and mortality [Citation2]. Selective fetal growth restriction (sFGR), where one fetus is significantly smaller than the other, affects about 10% of MCDA pregnancies and is thought to be caused by a discrepancy in the placental territory [Citation3,Citation4]. Severe sFGR may be treated by induced preterm birth or with intervention, such as selective feticide (cord occlusion) of the smallest fetus [Citation3,Citation5]. Early identification of pregnancies at increased risk of developing sFGR may be relevant in order to counsel the parents, to plan intensified follow-up and, if indicated, early intervention.
It has previously been shown that both crown-rump length (CRL) and nuchal translucency thickness (NT) discordance is associated with sFGR in MCDA twins [Citation6,Citation7]. Pregnancy-associated plasma protein-A (PAPP-A) in the first trimester has been extensively investigated regarding adverse pregnancy outcome in singleton pregnancies. Pregnant women delivering small-for-gestational-age (SGA) neonates have lower PAPP-A levels in the first trimester [Citation8–10], but the association is less well described in twins and has primarily been assessed in selected small cohorts.
The aim of this study was to investigate the association and predictive value of discordant first trimester ultrasound markers and the biochemical markers PAPP-A and free beta-human chorionic gonadotrophin (free β-hCG) and birth weight discordance (BWD) in a large non-selected national cohort of MCDA twin pregnancies.
Materials and methods
This was a register-based national cohort study. From the national Danish Fetal Medicine Database [Citation11] information was retrieved on first trimester markers including CRL, NT, maternal serum PAPP-A, and free β-hCG and data on birth weight from all women with MCDA twin pregnancies delivering between July 2008 and July 2017 in Denmark. The twin pregnancies were classified according to BWD into the following groups: BWD <10%, BWD between 10% and 24.9%, and BWD ≥25%. BWD was calculated using the formula: BWD = (larger weight – smaller weight)/larger weight*100 [Citation12]. The twin pregnancies with the most severe BWD (BWD ≥25%) consisted of three subgroups: Cases undergoing umbilical cord occlusion due to sFGR (included in this group although BWD could not be calculated due to only one liveborn infant), cases where only one infant was growth restricted (< 10th centile) defined as sFGR, and cases where both twins were <10th centile. All MCDA twins undergoing cord occlusion in the same period were reviewed to assess whether the indication for the procedure was sFGR and if so, the pregnancy was included in the group with a BWD ≥25%. Pregnancies treated with umbilical cord occlusion for other reasons than sFGR were excluded from the cohort. Pregnancies were excluded where outcome data was unclear or missing. So were pregnancies with missing values of PAPP-A and free β-hCG and pregnancies with chromosomal or morphological abnormalities.
The CRL discordance was calculated as the difference in the twin CRL measurements divided by the CRL of the larger twin and was expressed as a proportion. The CRL discordance was divided into two groups, CRL discordance <10% (reference group) and CRL discordance ≥10%.
The NT discordance was calculated as the difference in the twin NT measurements divided by the NT of the larger twin and was expressed as a proportion. The NT discordance was divided into two groups, NT discordance <20% (reference group) and NT discordance ≥20%. Both CRL discordance ≥10% and NT discordance ≥20% are considered significant discordances according to International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) and is set to enable comparisons with similar studies [Citation13,Citation14].
The levels of PAPP-A and free β-hCG were expressed as multiples of the median (MoM), which were adjusted for method of conception, ethnicity, gestational age, maternal weight, parity, smoking, and number of fetuses. Baseline maternal characteristics recorded at the time of the NT scan included maternal age, body mass index (BMI), smoking status, parity, ethnicity, and method of conception.
For women in BWD groups 10–24.9% and ≥25%, the proportion with a CRL discordance ≥10% and a NT discordance ≥20% was examined and compared to the group with BWD <10% using the Chi-squared test or the Fisher’s exact test. Median MoM values of PAPP-A and free ß-hCG were compared with the group with BWD <10% using the Wilcoxon two-sample test. Odds ratios for any BWD ≥25% as well as for umbilical cord occlusion, BWD ≥25% with sFGR and BWD ≥25% and two twins <10th centile were calculated using logistic regression analysis. The ability of CRL discordance and NT discordance to predict BWD ≥25% was examined by the area under the receiver operator characteristic (ROC) curve.
The study was approved by the Danish Data Protection Agency (2012-58-0004) and the Danish Fetal Medicine Database (RH-2018-30).
Results
We retrieved data from 762 MCDA twin pregnancies where information on first trimester screening markers and birth outcome was available. shows maternal characteristics according to BWD in the study population. A BWD <10% was found in 404 (53.0%) pregnancies. Pregnancies with a BWD 10–24.9% were recorded in 268 (35.2%) pregnancies, while BWD ≥25% was found in 90 (11.8%). We found no major differences in any of the maternal characteristics between the BWD groups.
Table 1. Maternal characteristics according to birth weight discordance in the study population (n = 762 monochorionic diamniotic twin pregnancies).
The proportion of pregnancies with CRL discordance ≥10% was significantly higher in the group with a BWD discordance ≥25% compared to the group with a BWD discordance <10% (27.0% vs. 4.7% [p < 0.001], Supplementary material 1). Likewise, the proportion of pregnancies with NT discordance ≥20% was significantly higher in the group with a BWD ≥25% compared to the women with a BWD discordance <10% (40.9% vs. 23.9% (p = 0.001)). No statistically significant differences were found when comparing levels of PAPP-A and free β-hCG MoMs in groups with BWD 10-24.9% or BWD ≥25% with the group with BWD <10%.
When dividing the group with BWD ≥25% into the three subgroups, we found a significantly higher percentage of pregnancies with CRL discordance ≥10% in the group where umbilical cord occlusion was performed (52.6% vs. 4.7% in the group with BWD <10% (p < 0.001)) (Supplementary material 1). This was also the case in the group of BWD ≥25% with sFGR (21.7% vs. 4.7% [p < 0.001]). Additionally, a significantly higher percentage of pregnancies with NT discordance ≥20% was found in the group where umbilical cord occlusion was performed (52.6% vs. 23.9% (p = 0.005)) and in the group with both twins <10th centile (66.7% vs. 23.9% (p = 0.003)) when compared to the group with BWD <10%.
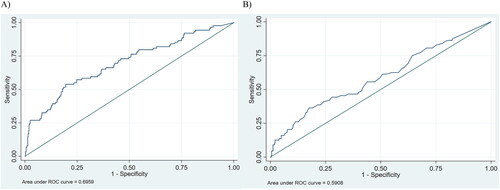
Among the twin pregnancies with a CRL discordance ≥10%, 17% belonged to the group with cord occlusion, 22% to the group with sFGR and 1.3% to the group with both twins <10th centile. The corresponding proportions were 1.3%, 6.7%, and 1.3%, respectively, for the twin pregnancies with CRL discordance <10% (Supplementary material 2). OR for any BWD ≥25% was 6.7 (95% CI 3.8–12.0) for pregnancies with a CRL discordance ≥10% compared to pregnancies with a CRL discordance <10%. The OR for umbilical cord occlusion was 15.7 (95% CI 6.1–40.4) and OR was 3.9 (95% CI 2.0–7.8) for BWD ≥25% with sFGR if CRL discordance was ≥10% compared to CRL discordance <10%. In ROC curves, CRL discordance yielded an AUC for prediction of BWD ≥25% of 0.70 (95% CI 0.63–0.76), and for NT discordance AUC was 0.59 (95% CI 0.52–0.66)). For a 25% false-positive rate, the sensitivity for prediction of BWD ≥25% was 57% for CRL discordance and 39% for NT discordance. Adding NT discordance to CRL discordance did not increase the AUC (0.70 [95% CI 0.64–0.77]). Correspondingly, the sensitivity was unchanged, 57% for a 25% false-positive rate ().
Discussion
In this national cohort of MCDA twins, we found a significant association between BWD ≥25% and both CRL and NT discordance in the first trimester. There was no association between a large BWD and the first trimester biochemical markers PAPP-A and free β-hCG.
With a large sample size of 762 MCDA pregnancies, our study validates the previous findings that CRL discordance is strongly associated with the development of sFGR in MCDA pregnancies. In another study from Denmark including 260 MCDA pregnancies, the relationship between CRL discordance and BWD was investigated [Citation14]. The study found that in MCDA pregnancies with CRL discordance ≥10%, the odds of BWD ≥20% were three-fold compared to concordant MCDA pregnancies, which is in line with an OR of 3.9 in our study for sFGR. Pandya et al. found a high predictive value evaluating 261 MCDA pregnancies of the combination of CRL discordance and abnormal cord insertion for sFGR according to the conventional criteria (estimated fetal weight [EFW] less than 10th centile for one fetus and a fetal weight discordance of at least 25% or more between both fetuses) [Citation6]. Finally, Memmo et al. stated that a significant CRL discrepancy at 11–14 weeks was found to be a useful predictor of sFGR, but not of twin-twin transfusion syndrome [Citation15].
A study from Arora et al. including 58 MCDA pregnancies described that first trimester CRL discordance ≥10% and NT discordance ≥20% were not useful predictors of adverse outcomes (twin–twin transfusion syndrome, sFGR or intrauterine fetal demise) [Citation7]. Another study of 65 MCDA pregnancies claimed that NT was effective in predicting twin-twin transfusion syndrome in MCDA pregnancies but with a rather poor positive predictive value of 36%. Intertwin discordances such as CRL discordance did not show sufficient efficacy for predicting adverse outcomes and the study did not find any combinations of predictive tools to improve screening performance [Citation16]. Both studies have a markedly lower sample size and include other adverse outcomes in their assessment which may explain why the results differ from our study.
PAPP-A and free β-hCG are well-described, not only as first trimester markers for chromosomal abnormalities but also as markers of placental function. A low PAPP-A MoM is related to low birth weight and FGR in singleton pregnancies [Citation17]. Studies on first trimester biomarkers in MCDA twin pregnancies are limited. Two other studies have assessed PAPP-A levels in twins with discordant growth. Saletra-Bielińska et al. (n = 304) reported that both high and low concentrations of PAPP-A seem to be statistically significantly related to pregnancy outcome [Citation18]. Iskender et al. (n = 104) did not find any significant correlation between low concentrations of PAPP-A and adverse pregnancy outcomes although they describe a statistically non-significant tendency toward fetal growth disorders in patients with low PAPP-A concentrations [Citation19]. Our data exclusively assess MCDA twins and do not confirm the association found by Saletra-Bielińska et al. but although not statistically significant, our results showed a possible tendency toward lower PAPP-A MoM in pregnancies with a large BWD where both twins are FGR (BW < 10th percentile).
Although discordance in first trimester ultrasound markers is associated with fetal growth discordance according to the presented data and other reports [Citation8,Citation17], the predictive value is currently modest (AUC 0.70) and improved performance probably requires inclusion of more markers in a multi model algorithm. Placental growth factor is now increasingly used in screening for preeclampsia and could potentially also be useful in screening algorithms for adverse outcome in twins [Citation20]. Nevertheless, we believe that the reported data may be useful in counseling Danish parents with MCDA twins. Some reassurance regarding the risk of severe BWD can be given in pregnancies where no or a small discordance in ultrasound markers is detected. No severe BWD was observed in 90% of pregnancies with a CRL discordance <10% or an NT discordance <20% in this study. However, severe BWD is only one of several possible complications in MCDA pregnancies, thus studies including additional markers and models assessing the overall risk of adverse outcome for MCDA twins are warranted.
Strength and limitations
The strengths of this study include the large study cohort and the availability of outcome data. We found no significant differences in maternal characteristics between the BWD groups. Moreover, the proportion of cases with BWD >25% of 11.8% in our study is in accordance with the literature. Therefore, we believe our findings are representative of the Danish MCDA population. A limitation of our study is that it is register-based and, therefore, an implicit risk of incorrect data transferal exists regarding type of classification and registration. Furthermore, there was no access to information regarding ultrasound EFW. Additionally, we only had 10 cases with two twins under the 10th percentile. A larger number of pregnancies in this group could possibly make the association with PAPP-A MoM significant. The Danish Fetal Medicine Database does not contain data on ultrasound Doppler measurements and, therefore, we were not able to divide the pregnancies with severe BWD into sFGR subtypes according to the Gratacos classification [Citation21].
Conclusion and perspectives
In conclusion, this study shows that a discordance in CRL and NT in MCDA twins are both significantly associated with development of BWD. The most important predictor remains CRL discordance ≥10%, thereby suggesting the unequal growth pattern in many cases with BWD is evident already in the first trimester of the pregnancy. No association was found between first trimester biochemical markers and severe BWD.
Supplemental Material
Download MS Word (36 KB)Acknowledgments
We would like to thank sonographers and medical doctors working in Fetal Medicine departments at hospitals around Denmark, who have been collecting data for the study and for the Danish Fetal Medicine Database.
Disclosure statement
No conflict of interest was reported by the authors.
Additional information
Funding
References
- Fichera A, Prefumo F, Stagnati V, et al. Outcome of monochorionic diamniotic twin pregnancies followed at a single center. Prenat Diagn. 2015;35(11):1057–1064.
- Kristiansen MK, Joensen BS, Ekelund CK, et al. Perinatal outcome after first-trimester risk assessment in monochorionic and dichorionic twin pregnancies: a population-based register study. BJOG. 2015;122(10):1362–1369.
- Gratacos E, Ortiz JU, Martinez JM. A systematic approach to the differential diagnosis and management of the complications of monochorionic twin pregnancies. Fetal Diagn Ther. 2012;32(3):145–155.
- Kalafat E, Thilaganathan B, Papageorghiou A, et al. Significance of placental cord insertion site in twin pregnancy. Ultrasound Obstet Gyne. 2018;52(3):378–384.
- Townsend R, Khalil A. Twin pregnancy complicated by selective growth restriction. Curr Opin Obstet Gynecol. 2016;28(6):485–491.
- Pandya VM, Colmant C, Stirnemann J, et al. Comparison of crown-rump length discordance and abnormal cord insertions as first-trimester predictors of poor outcome in monochorionic diamniotic twin pregnancies. J Matern Fetal Neonatal Med. 2020;15:1–5.
- Arora S, Prasad S, Sharma A, et al. First-trimester Crown-Rump length (CRL) and nuchal translucency (NT) discordance in monochorionic twins: an ominous sign or a benign feature? J Obstet Gynaecol India. 2020;70(5):349–354.
- Hansen YB, Myrhoj V, Jorgensen FS, et al. First trimester PAPP-A2, PAPP-A and hCGbeta in small-for-gestational-age pregnancies. Clin Chem Lab Med. 2016;54(1):117–123.
- Khalil A, Sodre D, Syngelaki A, et al. Maternal hemodynamics at 11–13 weeks of gestation in pregnancies delivering small for gestational age neonates. Fetal Diagn Ther. 2012;32(4):231–238.
- Gaccioli F, Aye I, Sovio U, et al. Screening for fetal growth restriction using fetal biometry combined with maternal biomarkers. Am J Obstet Gynecol. 2018;218(2):S725–S737.
- Danish Fetal Medicine Database (DFMD) [Internet]. Copenhagen: DFMS; 2023. [cited 2023 March 2]. Available from: https://www.dfms.dk/
- Khalil A, Beune I, Hecher K, et al. Consensus definition and essential reporting parameters of selective fetal growth restriction in twin pregnancy: a Delphi procedure. Ultrasound Obstet Gynecol. 2019;53(1):47–54.
- Khalil A, Rodgers M, Baschat A, et al. ISUOG practice guidelines: role of ultrasound in twin pregnancy. Ultrasound Obstet Gynecol. 2016;47(2):247–263.
- Johansen ML, Oldenburg A, Rosthoj S, et al. Crown-rump length discordance in the first trimester: a predictor of adverse outcome in twin pregnancies? Ultrasound Obstet Gynecol. 2014;43(3):277–283.
- Memmo A, Dias T, Mahsud-Dornan S, et al. Prediction of selective fetal growth restriction and twin-to-twin transfusion syndrome in monochorionic twins. BJOG. 2012;119(4):417–421.
- Mogra R, Saaid R, Tooher J, et al. Prospective validation of first-trimester ultrasound characteristics as predictive tools for twin-twin transfusion syndrome and selective intrauterine growth restriction in monochorionic diamniotic twin pregnancies. Fetal Diagn Ther. 2020;47(4):321–327.
- Dugoff L, Hobbins JC, Malone FD, et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (the FASTER trial). Am J Obstet Gynecol. 2004;191(4):1446–1451.
- Saletra-Bielińska A, Kosińska-Kaczyńska K, Szymusik I, et al. Both low and high PAPP-A concentrations in the first trimester of pregnancy are associated with increased risk of delivery before 32 weeks in twin gestation. J Clin Med. 2020;9(7):2099.
- Iskender C, Tarim E, Cok T, et al. Obstetrical complications associated with first-trimester screening markers in twin pregnancies. J Obstet Gynaecol Res. 2013;39(11):1495–1499.
- Boutin A, Demers S, Gasse C, et al. First-trimester placental growth factor for the prediction of preeclampsia in nulliparous women: the great obstetrical syndromes cohort study. Fetal Diagn Ther. 2019;45(2):69–75.
- Gratacós E, Lewi L, Muñoz B, et al. A classification system for selective intrauterine growth restriction in monochorionic pregnancies according to umbilical artery doppler flow in the smaller twin. Ultrasound Obstet Gynecol. 2007;30(1):28–34.