Abstract
Background
Active fetal movements (AFMs) are a sign of the well-being of the baby during pregnancy and suggests the integrity of the cardiovascular, musculoskeletal, and nervous systems of the fetus. The abnormal perception of AFMs is associated with an increased risk of adverse perinatal outcomes such as stillbirth (SB) and brain damage. Several definitions of “ decreased fetal movements” have been proposed, but none of them has been universally accepted. The aim of the study is to investigate the perinatal outcomes in relation to AFMs frequency and perception in term pregnancy with an ad hoc questionnaire administered to the women before delivery.
Study design
This was a prospective case-control study on pregnant women at term referring to the Obstetric Unit of the University Hospital of Modena, Italy, between January 2020 and March 2020. A validated questionnaire was administered to women who agreed to participate in the study. Therefore, women were subdivided into the case and control groups: cases included women who experienced adverse perinatal outcomes (APO) such as perinatal mortality (SB and early neonatal mortality), operative delivery (cesarean section or vacuum) due to fetal distress, Apgar 5’ < 7, neonatal resuscitation at birth and NICU Admission, while controls were women who experienced delivery and birth without APO in the same period.
Results
Seventy-seven cases and 178 controls that compiled the questionnaire were included in the analysis. Characteristics significantly associated with APO were low education (OR 1.57, CI 95% 1.11–2.22), nulliparity (OR 1.76, CI 95% 1.20–2.58), obesity (OR 1.55, CI 95% 1.10–2.17), neonatal male gender (OR 1.92 CI95% 1.33–2.78) and centile at birth (< 10° and >90°) (OR 2.77, 95%CI 2.17, 3.55). There was no association between any answer about strengths, frequency and vigor of perceived fetal movements and APO. Even any maternal perception of fetal hiccups or uterine contractions wasn’t associated with APO. On the other hand, women who referred to frequent change positions during sleep (OR 1.55 CI95% 1.05–2.30) and women who snore (OR 1.43 CI95% 1.01–2.05) showed a statistically significant increase of APO.
Conclusions
Our data confirm the significant association between modifiable risk factors (such as obesity and low education) and APO. Thus, healthcare providers should be aware of the importance of intervention in reducing obesity, therefore snoring and related sleep apnea syndrome. Finally, changing position during sleep while not the perception of modified/reduced fetal movements significantly could induce the worst obstetric outcomes.
Background
Active fetal movements (AFMs) are a sign of the well-being of the fetus during pregnancy [Citation1]. De Vries JI et al. reported that women perceive between 33 and 88% of AFMs visualized by ultrasound [Citation2]. Normal fetal activity suggests the integrity and regular development of the cardiovascular, musculoskeletal, and nervous systems of the fetus. AFMs vary according to the gestational age [Citation3], peaking between the 28th and 34th week of gestation, and change when the pregnancy comes to term, becoming more organized, however, the frequency and intensity usually do not decrease [Citation1.Citation4].
The abnormal perception of AFMs is sometimes associated with an increased risk of adverse perinatal outcomes such as stillbirth (SB) and brain damage: if the central nervous system of the fetus is compromised, AFMs lose their variability, reduce the number, and become monotonous [Citation5].
Counting AFMs is a simple method that can be used to determine fetal well-being through the quantification and qualification of the number and type of movements that a woman perceives. The aim of this method is to reduce perinatal mortality by alerting health providers [Citation1]. On the other hand, this method could increase anxiety in pregnant women, or lead to unnecessary interventions, such as induction of labour or cesarean section, also increasing antenatal hospitalizations and prematurity [Citation1].
One of the methods to assess maternal perceptions of AFMs is quantitative and consists of the evaluation of the time to perceive 10 AFMs. This interval increases during the pregnancy, although it usually never exceeds 30 min [Citation6]. In addition to the quantitative method, the qualitative one includes an increased strength and number of movements in the semi-sitting position of women, or during the evening, after walking or in response to noise and palpation of the abdomen [Citation7].
Reduced AFMs could be explained by some conditions such as sleeping (that usually does not exceed 90 min), anterior placenta, maternal obesity, the supine position of the women, maternal distraction and anxiety, drugs, smoking, alcohol, and some pregnancy diseases such as intrauterine growth restriction, placenta insufficiency, fetomaternal hemorrhage, preeclampsia, and fetal malformation [Citation1].
Several definitions of “decreased fetal movements” have been proposed, but none of them have been universally accepted [Citation8] (i.e. the perception of fewer than 10 movements in 2 h [Citation4] or less than 10 movements in 12 h of the normal maternal activity or less than 4 movements in 4 h [Citation9]). The conclusion of the literature is that the quantitative method is less effective than the qualitative subjective maternal perception of the reduction/modification of AFMs in predicting the worst perinatal outcomes such as SB [Citation10]. Indeed, the Royal College of Obstetricians & Gynecologists (RCOG) guidelines declare that “there is insufficient evidence to recommend a formal cutoff of movements that can represent a warning sign. Women should develop greater awareness of the specific movement pattern of their baby” [Citation11].
The study aims to investigate the AFMs frequency and perception in late pregnancy (≥37 weeks) in relation to the perinatal outcomes with an ad hoc questionnaire administered to the women before delivery.
Methods
This was a prospective case-control study on pregnant women at term referring to the Obstetric Unit of the University Hospital of Modena, Italy, (with approximately 3.000 births annually), between January 2020 and March 2020.
All women with singleton pregnancies without a known congenital abnormality and presenting from ≥37 + 0 weeks of gestation to the Maternity Unit were included in the survey. An ad hoc questionnaire (modified by Heazell AE et al. [Citation12]) to investigate the perception and counting of AFMs was administered to women who agreed to participate in the study. The study was approved by the Local Ethical Committee. Data specific to this analysis relates to questions asked about AFMs and more specifically about changes in strength and frequency. The questionnaire is reported in the Supplementary material.
After the delivery, women were subdivided into case and control groups on the bases of the presence of adverse perinatal outcomes (APO), which include perinatal mortality (SB and early neonatal mortality), operative delivery (cesarean section or vacuum) due to fetal distress, Apgar 5′ < 7, neonatal resuscitation at birth (including both invasive ventilation such as mechanical ventilation; non-invasive ventilation such as oxygen therapy, nasal CPAP, high flow), and NICU Admission. Cases included women who experienced one or more APO; if not, women were included in the control group. We explored maternally perceived AFMs in women who experienced adverse perinatal outcomes including SB compared with a control group of women at similar gestation who had a good perinatal outcome.
Data on the course of pregnancy were collected from the hospital maternity records or from patients. Medical records were reviewed by research associates to obtain anonymized data on mothers (i.e. maternal demographics, body mass index (BMI), age, medications etc.), delivery (i.e. induction, cesarean section etc.) that were organized in a password-protected database.
Statistical analysis
Stata 16.1 (StataCorp. 2019. College Station, TX, USA) was used to analyze data. Statistical tests were designed to compare pregnancy features and perinatal outcomes in women with different perceptions of AFMs reported in the questionnaire. Comparisons between groups were made using a Welch’s t-test for continuous variables (due to the unequal group’s sample size), and a chi-square test for categorical ones. Continuous data are reported as mean standard deviation (SD). Categorical data are reported as absolute and percentage frequencies. Multivariable logistic regression was performed to evaluate the impact on APO of possible confounding variables selected according to clinical relevance criteria. Results of logistic regression are reported as the Odds Ratio (OR) with 95% confidence interval (95% CI). All probability values were 2-tailed, and a probability value of <0.05 was considered statistically significant.
Results
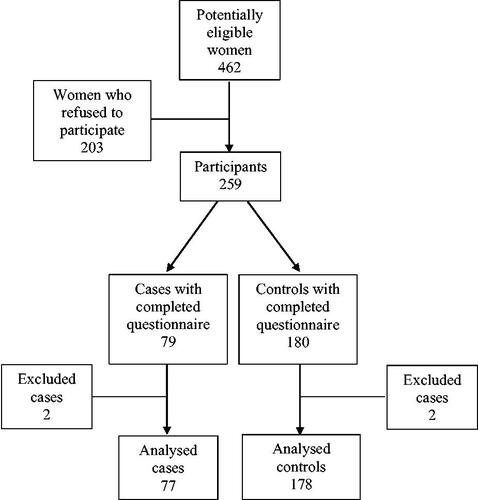
In total, 462 women were identified as potentially eligible participants. Two hundred and three did not consent to participate in the study and decline the questionnaire invitation. Four cases were excluded after data collection because of mistakes in the compilation of the questionnaire, thus 77 cases and 178 controls that compiled rightly the questionnaire were included in the analysis ().
Cases of the study included 3 SB (1.17%), 29 (4.64%) operative deliveries for fetal distress (including emergency cesarean section or vacuum application), 4 (1.56%) babies with Apgar 5′ < 7, 37 (14.5%) cases of neonatal resuscitation at birth and 5 (1.96%) infants with NICU admission.
The maternal and fetal characteristics of the study population are presented in detail in . The majority of participants were Italian women. Participants ages were distributed across the reproductive lifespan, with the largest group under 35 years of age in both groups (67.5% cases, 64.0% controls). There was no difference in smoking habits, gestational weight gain, and gestational age at delivery. Characteristics significantly associated with APO were low education (OR 1.57, 95% CI: 1.11–2.22), nulliparity (OR 1.76, 95% CI: 1.20–2.58), obesity (OR 1.55, 95% CI: 1.10–2.17), neonatal male gender (OR 1.92, 95% CI: 1.33–2.78) and centile at birth (<10° and >90°) (OR 2.77, 95% CI: 2.17–3.55).
Table 1. Maternal and fetal characteristics.
The prevalence of each variable relating to AFMs and their univariable ORs associated with APO are presented in . There was no association between any answer about strengths, frequency, and vigor of perceived AFMs and APO. Even any maternal perception of fetal hiccups or uterine contractions in the last weeks wasn’t associated with APO. On the other hand, women who referred to frequent change positions during sleep (OR 1.55 CI95% 1.05–2.30) and women who snore (OR 1.43 CI95% 1.01–2.05) showed a statistically significant increase of APO.
Table 2. A univariable analysis evaluating the perception of fetal movements and adverse perinatal outcomes risk.
Also, no difference in APO was found in relation to the number of admissions to Obstetrics Emergency for reduced AFMs or with the quality of provider counselling regarding the perception of AFMs.
The multivariate logistic regression, adjusted for confounding variables for the APO risk such as smoking and inadequate weight gain, confirmed the impact of low education, nulliparity and BMI ≥30 kg/m2 on APO ().
Table 3. Multivariable logistic regression evaluating the confounding factors for the risk of adverse perinatal outcomes.
Discussion
This study, through questionnaire interpretation, showed that the majority of women with the worst perinatal outcome referred to changing position during sleep and snoring. The perception of different patterns of AFMs was not associated with a substantial reduction in the risk of APO including SB. Our finding differs from other data showing that a decrease in the strength or frequency of AFMs is associated with an increased risk of late SB, particularly if this is a recurrent phenomenon (OR 2.36 rising to 5.11) [Citation15]. Nevertheless, our data confirm the relationship between snoring and APO as reported in another study where snore appears to play a role in various obstetric diseases such as diabetes, hypertension, preterm birth, and intrauterine growth restriction [Citation16]. This phenomenon is also more frequent in overweight/obese women and is associated with sleep apnea syndrome. This condition involves less oxygenation of the maternal blood and therefore of the fetus [Citation17], leading to vascular malperfusion of the placenta [Citation18] that leads to worst perinatal outcomes. Indeed, in our population, obese women are significantly more represented in the cases group.
On the other hand, our data showed that women who change position during sleep, without side preference, present a significantly increased risk for APO. This finding does not confirm the relationship between the backside position and SB described by another study [Citation19]. Indeed, the recent NICE review investigated the association between “going to sleep position” (considered a proxy for sleeping position) and outcomes and concluded that because of the observational nature of most studies relying on women’s recall, should not be possible to establish an association between the woman’s sleeping position and outcomes [Citation20]. Moreover, this review did not assess the effectiveness of any interventions to modify sleeping positions while the committee agreed to recommend advising women to consider using for example pillows so that they can maintain their position when sleeping [Citation20].
Our study confirms the well-known risk factors for SB described in the literature [Citation21,Citation22] and in the Emilia Romagna Regional report of perinatal mortality [Citation23] such as low education, nulliparity, obesity, neonatal male gender, and neonate small or large for gestational age at birth. These results pointed out the attention on the modifiable factors (low education and obesity) and on the matter of the interventions that should be encouraged to prevent this awful outcome.
One of the strengths of our study is the prospective case-control design. Moreover, this study reported detailed information about the maternal perception of AFMs with a specific survey performed before delivery. Indeed, the questionnaire was administered between 37 weeks and the delivery by a hospital midwife during the fetal monitoring (i.e. during cardiotocography). This way attempted to avoid the recall bias present in several studies that performed the questionnaire by phone after delivery. The limitation of the study is the small sample size and lack of late neonatal outcomes. Moreover, the study was based on maternal perception of AFM that might be influenced by placental localization, maternal BMI, fetal sleep-wake cycle, state of anxiety, level of attention, smoking and consumption of alcohol.
In conclusion, our data confirm the significant association between modifiable risk factors (such as obesity and low education) and adverse perinatal outcomes. Considering this finding, healthcare providers should be aware of the importance of intervention in reducing obesity and therefore snore and related sleep apnea syndrome. Finally, changing position during sleep while not the perception of modified/reduced AFMs significantly could induce the worst obstetric outcomes. Further studies are required to determine whether interventions can decrease the frequency of changing position and snoring during sleeping and the incidence of adverse perinatal outcomes.
Supplemental Material
Download MS Word (83.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Mangesi L, Hofmeyr GJ. Fetal movement counting for assessment of fetal wellbeing. Cochrane Database Syst Rev. 2007;(1):CD004909. DOI:10.1002/14651858.CD004909.pub2. Update in: Cochrane Database Syst Rev. 2015;10:CD004909.
- de Vries JI, Fong BF. Normal fetal motility: an overview. Ultrasound Obstet Gynecol. 2006;27(6):701–711.
- Bradford BF, Cronin RS, McKinlay CJD, et al. A diurnal fetal movement pattern: findings from a cross-sectional study of maternally perceived fetal movements in the third trimester of pregnancy. PLoS One. 2019;14(6):e0217583.
- Linde A, Georgsson S, Pettersson K, et al. Fetal movement in late pregnancy - a content analysis of women’s experiences of how their unborn baby moved less or differently. BMC Pregnancy Childbirth. 2016;16(1):127.
- Hantoushzadeh S, Sheikh M, Shariat M, et al. Maternal perception of fetal movement type: the effect of gestational age and maternal factors. J Matern Fetal Neonatal Med. 2015;28(6):713–717.
- Koshida S, Ono T, Tsuji S, et al. Fetal movement frequency and the effect of associated perinatal factors: multicenter study. Women Birth. 2019;32(2):127–130.
- Bradford B, Maude R. Maternal perception of fetal movements in the third trimester: a qualitative description. Women Birth. 2018;31(5):e287–e293.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality: a systematic review and meta-analysis. Obstet Gynecol. 2020;135(2):453–462.
- Winje BA, Saastad E, Gunnes N, et al. Analysis of 'count-to-ten’ fetal movement charts: a prospective cohort study. BJOG. 2011;118(10):1229–1238.
- Norman JE, Heazell AEP, Rodriguez A, et al. AFFIRM investigators. Awareness of fetal movements and care package to reduce fetal mortality (AFFIRM): a stepped wedge, cluster-randomised trial. Lancet. 2018;392(10158):1629–1638.
- Royal College of Obstetricians & Gynaecologists. Reduced Fetal Movements, Green-top Guideline No. 57, February 2011.
- Heazell AEP, Warland J, Stacey T, et al. Stillbirth is associated with perceived alterations in fetal activity - findings from an international case control study. BMC Pregnancy Childbirth. 2017;17(1):369.
- Rasmussen KM, Yaktine AL. IOM pregnancy weight guidelines. Weight gain During pregnancy: reexamining the guidelines. Institute of medicine (US) and national research council (US). Washington (DC): National Academies Press (US); 2009.
- Bertino E, Spada E, Occhi L, et al. Neonatal anthropometric charts: the italian neonatal study compared with other european studies. J Pediatric Gastroenterol Nutr. 2010;51(3):353–361.
- Heazell AE, Frøen JF. Methods of fetal movement counting and the detection of fetal compromise. J Obstet Gynaecol. 2008;28(2):147–154.
- Ge X, Tao F, Huang K, et al. Maternal snoring may predict adverse pregnancy outcomes: a cohort study in China. PLoS One. 2016;11(2):e0148732.
- Kalkhoff SM, Lutgendorf MA, Morrison TC, et al. A randomized controlled trial of sleep study surveillance with targeted autoregulated positive airway pressure therapy for obstructive sleep apnea in pregnancy. Am J Obstet Gynecol MFM. 2022;4(3):100571.
- Avagliano L, Monari F, Po’ G, et al. The burden of placental histopathology in stillbirths associated With maternal obesity. Am J Clin Pathol. 2020;154(2):225–235.
- Heazell A, Li M, Budd J, et al. Association between maternal sleep practices and late stillbirth - findings from a stillbirth case-control study. BJOG. 2018;125(2):254–262.
- NICE National Guideline Alliance (UK). Maternal sleep position during pregnancy: antenatal care: evidence review W. London: National Institute for Health and Care Excellence (NICE); 2021.
- Escañuela Sánchez T, Meaney S, O'Donoghue K. Modifiable risk factors for stillbirth: a literature review. Midwifery. 2019;79:102539.
- Po’ G, Salerno C, Monari F, et al. Stillbirth Emilia-Romagna audit group. Potentially preventable antepartum stillbirths in a high-resource setting: a prospective audit-based study. Eur J Obstet Gynecol Reprod Biol. 2021;258:228–234.
- Po’ G, Monari F, Zanni F, et al. Stillbirth Emilia-Romagna audit group. A regional audit system for stillbirth: a way to better understand the phenomenon. BMC Pregnancy Childbirth. 2019;19(1):276.