Abstract
Objectives
To evaluate the concordance of conventional autopsy (CA) and postmortem magnetic resonance (MR) after termination of pregnancy (TOP) in fetuses with prenatally detected central nervous system (CNS) anomalies. Second, to determine the most informative postmortem investigation in parental counseling.
Methods
All TOPs between 2006 and 2016 with prenatally detected CNS involvement and having a postmortem MR and CA as postmortem examinations were retrospectively analyzed and concordance levels were established.
Results
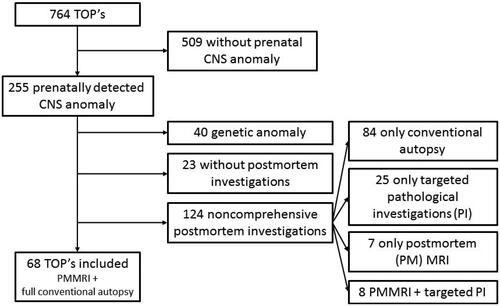
Of 764 TOPs, 255 cases had a CNS anomaly detected prenatally (33.4%). Fetal genetic anomalies (n = 40) and cases without both postmortem MR and CA were excluded, leaving 68 cases for analysis.
Disagreement between postmortem MR and CA was observed in 22 cases (32.4%). In eight cases (11.8%), more information was obtained by CA compared with MR. However, only two cases with major additional findings were found when compared with prenatal diagnosis. In 14 cases (20.6%), MR was superior to CA either because of additional cerebral anomalies undetected by CA (n = 5) and/or because of severe autolysis hindering pathology of the CNS (n = 9).
Conclusions
Our data point out that an adequate postmortem evaluation, valuable in parental counseling, can be provided by a postmortem MR in 97% of the cases.
An adequate postmortem evaluation, valuable in parental counseling, can be provided by a postmortem (PM) magnetic resonance (MR) in the majority of cases.
PM MR is an excellent postmortem imaging tool for the brain.
In cases with brain autolysis, PM MR is often the only informative PM investigation tool.
PM MR is an essential adjunct to CA in the PM evaluation of pregnancies terminated for a central nervous system (CNS) anomaly.
Key Points
Introduction
Over the past decade, conventional autopsy (CA) rates after termination of pregnancy (TOP) for congenital anomalies are declining for various reasons. These include parental concerns about disfigurement of their child and skepticism about the potential benefit of CA in both parents and clinicians [Citation1,Citation2]. Yet, the merit of postmortem (PM) investigation must not be underestimated, as it allows to determine final diagnosis and provide crucial information for counseling toward future pregnancies. The search for minimally invasive autopsy (MIA) techniques has already created promising perspectives regarding PM cross-sectional imaging, as computed tomography (CT) and magnetic resonance (MR) allow to evaluate the different organ systems in situ. The use of PM MR in this setting was first reported in 1996 by Brookes et al. [Citation3] and further investigated and validated in later years, to become a medically acceptable alternative to CA [Citation2,Citation4,Citation5], more favorably received by the parents [Citation6,Citation7]. Particularly in fetuses [Citation4] and more specifically in fetal central nervous system (CNS) anomalies [Citation2,Citation5,Citation6,Citation8–10], PM MR appears to be of greater value. Because brain tissue is highly receptive to PM changes, significantly questioning the added benefit of the pathological investigation, MR may contribute more significantly to the final diagnosis than CA, which is actually still the gold standard [Citation5,Citation6].
The purpose of our study was to evaluate the correlation of PM MR and CA in fetuses after TOP for CNS anomalies, in order to determine which PM investigation is the most informative and to determine the additional need for and contribution of CA. Furthermore, we intented to evaluate the impact of cerebral autolysis on PM MR and CA.
Material and methods
We retrospectively revised all TOP’s from a prospectively collected database to retrieve fetuses with a prenatally detected malformation involving the CNS, between 2006 and 2016. Patients are derived either from the hospital’s prenatal ultrasound screening program or referred for a second opinion.
Only patients having consented to both PM MR and full CA were included. There were no particular exclusion criteria, except for cases with an established genetic diagnosis after prenatal investigation. Therefore, cases with a genetic anomaly detected by molecular karyotyping were further excluded from the analysis. CNS anomalies were stratified into seven major subclasses and cases were classified according to their most prominent lesion. We classified the cases into (1) Isolated severe ventriculomegaly (ISV), (2) neural tube defects (NTD), (3) midline anomalies, (4) destructive disorders (congenital infections (mainly congenital cytomegalovirus infections), intracranial hemorrhage and congenital porencephaly, hydrancephaly and periventricular leukomalacia) (5) profileration disorders, (6) tumor/cyst and (7) hemorrhage.
Extensive prenatal ultrasound (transabdominal and transvaginal whenever possible) was performed by an expert in fetal medicine (GE Voluson 730 E, GE Voluson E8 and E10, 4-8MhZ abdominal probe and a 5-9MhZ vaginal probe (GE Healthcare Belgium, Kouterveldstraat 20, 1831 Diegem, Belgium) according to the ISUOG guidelines [Citation11]. Prenatal MR on a Siemens Aera 1.5 Tesla MR using 2 body coils included a T2 half-Fourier acquisition single-shot turbo spin-echo-(T2 HASTE) and fast imaging with steady-state free precession-sequences, respectively, coronal and sagittal plane with reference to the mother; a T2 HASTE of three fetal brain planes; a T2 echo planar imaging (epi), T1 turbo spin echo (TSE) and diffusion-weighted imaging (DWI) of fetal brain in axial plane; T2 HASTE of three fetal body planes and a T1 volumetric interpolated breath-hold examination with fat saturation of the coronal fetal body plane.
Parental request for TOP was accepted by the multidisciplinary feto-maternal board whenever the fetal condition implied a major morbidity or mortality, in concordance with local legislation. A legally defined reflection period of six days minimal was implemented between the parental request and the performance of TOP.
The TOP protocol implied administration of Mifepristone 48 h prior to induction with Misoprostol. A feticide with Fentanyl 0.1mg/mL 2 cc and Lidocaine 2% 10 cc proceeded induction of labor in gestations beyond 22 weeks. Post-TOP investigations were performed within 4 h after delivery whenever possible. However, in cases of involuntary delay, the fetal body was cooled to 4 °C within 4 h after delivery to allow for further analysis as soon as possible. PM investigations combined MR and CA including histology of fetus and placenta. PM MR was performed using a Philips Healthcare Ingenia 3 Tesla MR, using the smallest possible coil, with scanning times of 1.5 up to 2 h, depending on the fetal size and gestational age. Used sequences include a T2 three-dimensional (3D) volume isotropic turbo spin echo acquisition, T1 3D magnetization prepared rapid acquisition gradient recalled echo (MPRAGE) and susceptibility weighted imaging in the axial plane of the fetal brain, as well as T1 MPRAGE and 3D T2 TSE of the fetal body. The prenatal and PM MR images were analyzed by a fetal MR specialist and autopsy was performed by a certified perinatal pathologist. Suspicion of skeletal abnormalities led to an additional X-ray or CT examination.
All data were retrieved from a computerized database including medical, sonographic, pathological and genetic data as well as digitized ultrasound-, X-ray- and MR images.
A favorable ethical opinion was granted by the Ethical Committee of the University Hospital Leuven (ML8404).
Statistical analysis was performed with SISA statistics [Citation12]. To compare the rate of agreement between the different groups, the Fisher’s exact test or a Chi2 analysis was used. Mean values of categories were compared with a t-test. A p-value at <.05 was considered to be statistically significant.
Results
Of 255 TOP’s with CNS anomalies in our tertiary center, 68 cases were included for analysis. The flowchart of excluded cases is depicted in .
Figure 1. Flowchart included and excluded cases. TOP: termination of pregnancy; CNS: central nervous system; PMMR: postmortem MR; PI: Pathological investigations. Genetic anomaly: chromosomal anomaly + anomaly detectable by array comparative genomic hybridization (CGH).
The distribution of detected CNS anomalies is represented in . Mean gestational age (GA) at TOP was 24.4 weeks (SD ± 5.5). A prenatal MR was performed in 60.3% (41/68) of the cases.
Table 1. Conventional autopsy provides more information than postmortem.
Full correlation between PM MR and CA, irrespective of prenatal diagnosis, was observed in 67.6% (46/68) of the cases. In five cases, TOP was performed because of congenital cytomegalovirus (CMV) fetopathy; pathological investigation of the brain anomalies was inhibited by autolysis but histology revealed virocytes in all fetal organs. In 5/46 cases, both PM MR and CA revealed major additional findings, when compared with prenatal diagnosis.
Discrepancy between PM MR and CA, irrespective of prenatal diagnosis, was observed in 22 cases (32.4%).
In eight cases (11.8%), CA provided more information than MR. In 4/8 cases, cerebral histology added additional value over MR: (1) in a case with a partial agenesis of the corpus callosum and polymicrogyria (GA: 26 weeks), histology showed a pericallosal lipoma and CMV inclusions; (2) in a second case with arthrogryposis (GA:18 weeks) and abnormal midline structures, histological analysis demonstrated migration disorders such as schizencephalic cysts, heterotopia, abnormal perisylvian cortex and hypoplastic pyramidal tracts; (3) another case with microcephaly and delayed gyration (GA:23 weeks) showed on histological analysis additional calcifications and leptomeningeal heterotopia, diagnosing an early form of Aicardi-Goutières syndrome; (4) the tumor type was determined by cerebral histology in one case. In the four remaining cases, gross pathological PM investigation in addition to the PM MR findings revealed a facial dysmorphism and talipes equinovarus, in one case each, and a more detailed characterization of a cardiac defects in two cases. The detection of the cerebral findings was equal by autopsy and MR ().
Table 2. Cases in which PM MR detects major additional findings, undetected prenatally and undetected by CA, influencing final diagnosis and parental counseling.
Hence, parental counseling changed only in two cases with major additional findings undetected prenatally and by PM MR, but revealed by CA (, case 1 and 2), determining the recurrence risk.
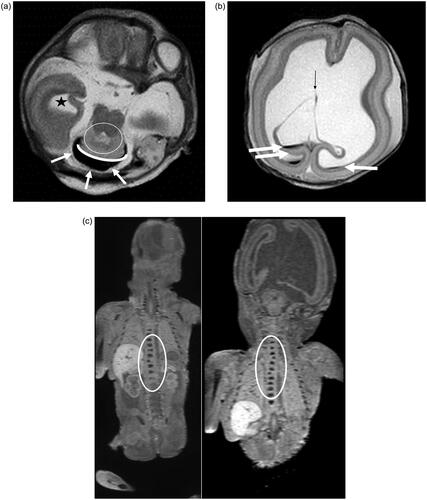
In 14 cases (20.6%), PM MR was superior to CA, irrespective of prenatal findings. In nine cases, cerebral pathological examination was partially (n = 2) or completely (n = 7) uninformative because of severe autolysis, while on PM MR a detailed analysis of cerebral anatomy was feasible. One case with a polymalformative syndrome, arthrogryposis and extreme cerebellar hypoplasia, presented on MR with an additional aneurysmal dilation of venous sinuses and cerebral clefts and cysts, not depicted by CA. Pathological investigation showed a cleft palate, neither detected prenatally nor on PM MR, but considered of minor impact. In another case, additional cerebral findings – periventricular and subcortical cysts and clefts – not diagnosed prenatally, were detected by PM MR after TOP at 26 weeks for hydrocephalus, callosal dysgenesis and cerebellar hypoplasia, fetal growth restriction and fetal akinesia sequence (). Furthermore, in three cases, extracerebral findings, undetected prenatally, were revealed by PM MR: a cleft palate in one and vertebral anomalies in two cases ().
Figure 2. Distribution of detected CNS anomalies. X-axis = number of cases. ISV = isolated severe ventriculomegaly; NTD = neural tube defect.
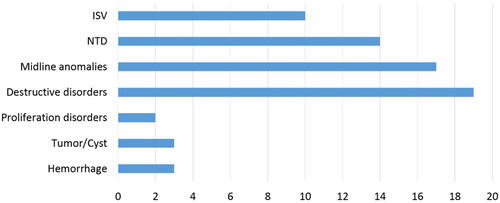
Figure 3. Case: Prenatal diagnosis of hydrocephalus, cerebellar hypoplasia and aqueductal stenosis. TOP at GA 27 weeks. PM MR shows hydrocephalus, rhombencephalosynapsis, CC dysgenesis, vertebral anomalies. CA shows hydrocephalus, rhombencephalosynapsis, CC dysgenesis. Vertebral anomalies are only depicted on PM MR, demonstrating superiority of PM MR over CA in this case. (a) T2-weighted image of the brain in the axial plane. Dilated right temporal horn of the lateral ventricle (black star). Black fluid surrounding the cerebellum (white arrows). The dentate nuclei appear fused (White circle) and there is no vermian structure visible (curved white line). (b) T2-weighted image of the brain in the axial plane. Severe ventriculomegaly with blood layering as a normal post mortem finding and destruction of the leaflets of the cavum septi pellucidi (thin black arrow). Furthermore, the normal layering of the cerebral mantle is seen with the germinative matrix, subventricular zone, subplate and cortical plate. (c) T1-weighted image of the body in the coronal plane. Abnormal ossification of a thoracic vertebral body (T11) in the lower half of the thoracic spine. (d) T1-weighted image of the body in the coronal plane. Abnormal ossification of two thoracic vertebral bodies (T1 and T4) in the upper half of the thoracic spine.
Subsequently, in these 14 cases, correlation with prenatal diagnosis revealed that after performance of PM MR, parental counseling remained unchanged in seven out of nine cases with autolysis. In the two other cases, PM MR showed an aqueductal stenosis, undetected prenatally and considered to be a major additional finding. In the remaining five cases, the major additional findings determined the counseling significantly by influencing recurrence risk.
Table 3. The time interval between feticide, delivery and PM MR in different groups with and without feticide or autolysis.
Feticide might enhance brain autolysis either by direct impact on the fetal brain by the drugs used to cause asystole, or related to the expanded delay between fetal demise and the moment of autopsy [Citation8]. In total, 32.3% (22/68) of the cases presented cerebral autolysis, partially or completely hindering pathological investigation in 14 cases [20.6% (14/68)]. Feticide was performed in 67.6% of the cases (46/68), and was associated with brain autolysis in 39.1% (18/46). In the absence of a feticide, only 18.1% (4/22) of the brains were autolytic (p = .05). When autolysis was present, the mean time interval between feticide and delivery was 30.6 h (SD ± 15.5) compared with 21.2 h (SD ± 16.0) without brain autolysis (p =.05) ().
Discussion
We aimed to evaluate the correlation of PM MR and CA in fetuses after TOP for CNS anomalies and to determine the most valuable PM examination to direct parental counseling.
Our data favor PM MR over CA in 20.6% (14/68) of the cases. Indeed in 13.2% (9/68), severe autolysis hindered cerebral pathological examination partially (n = 2) or completely (n = 7), while MR disclosed a detailed analysis of cerebral anatomy. Altogether, MR was equivalent or superior to CA in 97.1% of the cases (66/68) in establishing the final diagnosis.
Only the additional histological information on the brain malformation overclassed MR in parental counseling. CA is also superior in case of associated cardiac anomalies, especially in fetuses < 24 weeks of GA [Citation13].
Brain autolysis occurred more frequently after feticide and after a prolonged delay between feticide and delivery. However, this observation did not influence our results, probably because the time interval between feticide and delivery was used as a proxy for exact time delay between feticide and the actual pathological examination, which is actually shorter.
In order to confirm prenatal diagnosis or to detect additional findings with possible effect on final diagnosis and parental counseling, PM examinations remain useful. The declining autopsy rates, and the subsequent search for minimally invasive postmortem investigation tools enhanced comparative studies on the added value of PM MR over CA [Citation3,Citation4,Citation8]. Brookes et al. [Citation3] studied whole body MR in 20 stillborn or aborted fetuses, to reveal that PM MR yielded major findings additional to CA in 20% (4/20), and was of equal value in 40% (12/20). They concluded that MR was equivalent or superior to CA in 60% (12/20). Griffiths et al. [Citation8] specifically studied PM MR of the CNS in fetuses and stillborn neonates, and found PM MR was superior over CA in 9.4% (3/32) and equal in 88% (28/32) of the cases. So PM MR was at least equivalent or superior to CA in 96.9% (31/32).
Nevertheless, the real weight of these retrospective studies remains uncertain as the cohorts are small, and the ultrasound findings are not blinded to radiologists and pathologists [Citation14]. In a prospective validation study, Thayyil et al. [Citation4] were the first to show that an extensive minimally invasive investigation (consisting of MR and other PM imaging techniques, antemortem or PM blood sampling, external examination of the fetal body, genetic tests and placental analysis but without histological tissue sampling) performed equally good as CA. Furthermore, PM MR reveals significant information in over 50% of fetuses with a non-diagnostic CA [Citation5]. Yet, compelling evidence for PM MR and MIA to replace CA is lacking.
Nevertheless, PM MR should be encouraged as an adjuvant to conventional brain autopsy in fetuses with suspected brain anomalies [Citation2,Citation5]. Major advantages include the in situ analysis of the fetal brain, the reduced impact of autolysis and the avoidance of disfiguration of normal anatomy by prelevation. Fantasia et al. [Citation15] recently reported a prospective study with PM MRI concordancy rate of 99% in CNS anomalies comparable to our study.
Furthermore, the obtained images can be stored and reevaluated at any time. Additionally, the more frequent use increases normal PM MR skills, further augmenting its accuracy [Citation16,Citation17].
Our study showed several limitations. First, in this retrospective study, radiological and pathological analysis was not performed blinded to the prenatal findings but after multidisciplinary discussion of prenatal imaging studies. Therefore, it remains impossible to estimate the true added value of PM MR over CA. However, in those cases complicated by autolysis of the brain tissue, the additional value of PM MR seems clear.
Second, in this paper, we did not address the feasibility of directed fetal biopsy and the histological diagnostic accuracy of small tissue samples. Some of the pathological findings may be limited in size, multifocal or in area’s difficult to reach. Targeted biopsies may be challenging and need the joined efforts of the radiologist, the fetal medicine specialist and the pathologist. Dedicated sampling aids have to ensure sufficient amount of tissue for histological investigation and can be retrieved either by laparoscopy or by an ultrasound- or CT-guided needle sampling [Citation14].
A third limitation which needs further assessment consists of defining the conditions which might benefit from additional histological investigation. So far, we understand that not all congenital malformations need histological confirmation in order to correctly counsel the parents. Therefore, it would be of utmost importance to define the MR characteristics that definitely call for targeted biopsy and histological investigation. The reasons of a prenatal MRI performance rate in only 60% of cases are multiple. Some patients did not consent. In other cases, the prognosis was felt to be so poor that the decision to opt for TOP was taken before considering the possible clinical relevance of MRI. Furthermore, we relied mainly on the Paladini paper [Citation18] stating that in a tertiary referral center with good neurosonographic expertise in the assessment of fetal malformations, MRI is likely to be of help in a limited proportion of cases. In our center, the mean GA at fetal MRI is 27 weeks (SD3.9).
Finally, as prenatal diagnosis moves toward the first trimester, TOP occurs at a much earlier GA, at which the diagnostic value of PM MR still has to be established. PM and post-TOP imaging in smaller fetuses therefore may be enhanced by smaller coils and 9-Tesla output machines, or micro-CT [Citation5,Citation19–22]
In conclusion, our data point out that a PM MR is an excellent PM imaging method for the brain, particularly in cases with brain autolysis. CA including histological analysis remains of particular value in a minority of the cases. Therefore, we advise PM MR as an essential adjunct to CA in the PM evaluation of pregnancies terminated for a CNS anomaly. The implementation of PM MR is widely accepted as an adjunct to CA and as alternative whenever CA is refused by the parents.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Rüegger CM, Bartsch C, Martinez RM, et al. Minimally invasive, imaging guided virtual autopsy comparted to conventional autopsy in foetal, newborn and infant cases: study protocol for the paediatric virtual autopsy trial. BMC Pediatr. 2014;14:15.
- Thayyil S. Less invasive autopsy: an evidenced based approach. Arch Dis Child. 2011;96(7):681–687.
- Brookes JA, Hall-Craggs MA, Sams VR, et al. Non-Invasive perinatal necropsy by magnetic resonance imaging. Lancet. 1996;348(9035):1139–1141.
- Thayyil S, Sebire NJ, Chitty LS, et al. Post-mortem MRI versus conventional autopsy in fetuses and children: a prospective validation study. Lancet. 2013;382(9888):223–233.
- Arthurs OJ, Thayyil S, Pauliah SS, et al. Diagnostic accuracy and limitations of post-mortem MRI for neurological abnormalities in fetuses and children. Clin Radiol. 2015;70(8):872–880.
- Cannie M, Votino C, Moerman P, et al. Acceptance, reliability and confidence of diagnosis of fetal and neonatal virtuopsy compared with conventional autopsy: a prospective study. Ultrasound Obstet Gynecol. 2012;39(6):659–665.
- Kang X, Cos T, Guizani M, et al. Parental acceptance of minimally invasive feta land neonatal autopsy compared with conventional autopsy. Prenat Diagn. 2014;34(11):1106–1110.
- Griffiths PD, Variend D, Evans M, et al. Postmortem MR imaging of the fetal and stillborn central nervous system. AJNR Am J Neuroradiol. 2002;24:22–27.
- Woodward PJ, Sohaey R, Harris DP, et al. Postmortem fetal MR imaging: comparison with findings at autopsy. AJR Am J Roentgenol. 1997;168(1):41–46.
- Breeze ACG, Jessop FA, Set PAK, et al. Minimally-invasive fetal autopsy using magnetic resonance imaging and percutaneous organ biopsies: clinical value and comparison to conventional autopsy. Ultrasound Obstet Gynecol. 2011;37(3):317–323.
- Salomon LJ, Alfirevic Z, Berghella V, et al. Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol. 2011;37(1):116–126.
- Fisher exact test and T-test on SISA statistics (online). Available from http://www.quantitativeskills.com/sisa/.
- Taylor AM, Sebire NJ, Ashworth MT, et al. Post-Mortem cardiovascular magnetic resonance imaging in fetuses and children: a masked comparison study with conventional autopsy. Circulation. 2014;129(19):1937–1944.
- Thayyil S, Chitty LS, Robertson NJ, et al. Minimally invasive fetal postmortem examination using magnetic resonance imaging and computerized tomography: current evidence and practical issues. Prenat Diagn. 2010;30(8):713–718.
- Fantasia I, Murru F, Bussani R, et al. Accuracy and clinical utility of standard postmortem radiological imaging after early second trimester termination of pregancy. Eur J Obstet Gynecol Reprod Biol. 2022;273:75–80.
- Arthurs OH, Barber JL, Taylor AM, et al. Normal perinatal and paediatric postmortem magnetic resonance imaging appearances. Pediatr Radiol. 2015;45(4):527–535.
- Sebire NJ, Miller S, Jacques TS, et al. Post-mortem apparent resolution of fetal ventriculomegaly: evidence from magnetic resonance imaging. Prenat Diagn. 2013;33(4):360–364.
- Paladini D, Quarantelli M, Sglavo G, et al. Accuracy of neurosonography and MRI in clinical management of fetuses referred with Central nervous system abnormalities. Ultrasound Obstet Gynecol. 2014;44(2):188–196.
- Sebire NJ, Wade A, Taylor AM, et al. Body weight lower limits of fetal postmortem MRI at 1.5 T. Ultrasound Obstet Gynecol. 2016;48(1):92–97.
- Kang X, Cannie MM, Arthurs OJ, et al. Postmortem whole-body magnetic resonance imaging of human fetuses: a comparison of 3-T vs 1.5-T MR imaging with classical autopsy. Eur Radiol. 2017;8:3542–3553.
- Hutchinson JC, Kang X, Shelmerdine SC, et al. Postmortem microfocus computed tomography for early gestation fetuses: a validation study against conventional autopsy. Am J Obstet Gynecol. 2018;218(4):445.e1-12–445.e12.
- Lombardi CM, Zambelli V, Botta G, et al. Postmortem microcomputed tomography (micro-CT) of small fetuses and hearts. Ultrasound Obstet Gynecol. 2014;44(5):600–609.

