Abstract
Background
Hypertensive disorders of pregnancy and fetal growth restriction share common etiopathological origins and could be caused by maternal hemodynamic maladaptation to pregnancy.
Objective
The aim of our study is to evaluate if there is a correlation between maternal hemodynamic detected by UltraSonic Cardiac Output Monitor (USCOM®) during the first trimester and the pregnancy outcome.
Study design
We recruited a nonconsecutive series of women in the first trimester of pregnancy with no previous history of hypertensive disorders. We measured the pulsatility index uterine arteries and performed a hemodynamic evaluation by USCOM® device. After delivery, we reported the development of hypertensive disorders or intrauterine fetal growth restriction later during gestation.
Results
A total of 187 women were enrolled during the first trimester; 17 (9%) developed gestational hypertension or preeclampsia while 11 (6%) delivered a restricted growth fetus. Mean uterine artery pulsatility index above the 95th percentile was significantly more frequent in both women who developed hypertension and those with fetal growth restriction compared to controls. Hemodynamic parameters (reduced cardiac output and increased total vascular resistance) were significantly different in the group that developed hypertensive disorders, compared to uncomplicated pregnancy. ROC curves demonstrated the usefulness of uterine artery pulsatility index in the prediction of fetal growth restriction, while hemodynamic parameters were significantly associated to the development of hypertensive disorders.
Conclusions
Hemodynamic maladaptation to pregnancy may predispose to the development of hypertension, while we demonstrated a significative relationship between growth restriction and mean uterine pulsatility index. Further studies are needed to assess the value of hemodynamics evaluation in screening protocols of preeclampsia.
Introduction
Hypertensive disorders of pregnancy and fetal growth restriction are two of the major concerns in Obstetrics, often sharing a common etiopathogenesis [Citation1–3]. Much effort has focused on the early identification of women at greater risk of developing these complications, in order to implement effective preventive strategies, such as the administration of acetylsalicylic acid [Citation4,Citation5]. In recent times, the hypothesis that these hypertensive disorders may originate, rather than being the cause, from a maladaptation of the maternal organism to pregnancy has emerged [Citation6–8]. During uncomplicated gestations, we observe a reduction in mean arterial pressure and total peripheral resistance (TVR), accompanied by a specular increase in cardiac output (CO) [Citation9]. In pregnancies complicated by hypertensive disorders and/or fetal growth restriction, it has now been widely demonstrated that these changes in maternal hemodynamics are lacking, with a persisting low cardiac output and high peripheral resistances [Citation10–13]. To carry out these assessments in a noninvasive, simple and reproducible way, even in the hands of operators not dedicated to maternal echocardiography, the USCOM® (Ultrasonic Cardiac Output Monitor) system was introduced [Citation14–16]. In the present study, we therefore evaluated if the hemodynamic evaluation, assessed by this technology, could correlate with the development of hypertensive disorders and/or fetal growth restriction later in pregnancy in an unselected group of women referred to our Obstetric Unit for the screening of first trimester aneuploidies.
Materials and methods
This was a prospective study conducted in the unit of Obstetrics and Prenatal Medicine at Sant’Orsola-Malpighi University Hospital in Bologna, Italy. We recruited a nonconsecutive series of women referred for the first trimester screening of chromosomal abnormalities with a gestational age between 11 and 13 + 6 weeks and who subsequently delivered in our Obstetric Unit. We excluded smoking patients, twin pregnancies, women with previous hypertensive disorder of pregnancies or previous growth restricted fetus, with chronic hypertension, kidney disease or pre-gestational diabetes mellitus. We also excluded from the analysis those fetuses with fetal anatomical abnormalities such as cystic hygroma, holoprosencephaly and other malformations visible in the first trimester. Both primigravid and patients with previous uncomplicated pregnancy were included, such as both spontaneous pregnancies and pregnancies achieved with homologous assisted fertilization techniques. Demographic, ultrasound and biochemical data were collected. Patients with high-risk test and a chromosomal abnormality confirmed at the chorionic villous sampling were excluded from the analysis. During the ultrasound, we measured the pulsatility index of right and left uterine artery (UTPI) by transabdominal method, thus calculating the mean pulsatility [Citation17]. The cutoff used to define an increased mean pulsatility in the first trimester was 2.35, as reported by the recent guidelines of ISUOG [Citation18]. We then proceeded with the hemodynamic evaluation, performed by the same expert operator, carried out using USCOM®, after 15 min of rest in the supine position. We evaluated blood pressure, the stroke volume (SV, volume of blood pumped by the heart per cardiac cycle, cm3), cardiac output (CO, l/min), total peripheral vascular resistances (TVR, dyne s/cm5) and inotropic index (INO, Watt/m2). CO, SV and TVR were then indexed and their percentile were calculated, by means of the Excel normograms calculator developed by the London group [Citation19]. After delivery, we collected the outcomes of those pregnancies by consulting clinical charts and records. We reported the onset of hypertensive disorders or intrauterine fetal growth restriction (IUGR) during pregnancies, the mode of delivery and its gestational age, neonatal weigh, Apgar score, the admission to Neonatal Intensive Care Unit, pH and base excess at delivery and postpartum hemorrhages defined as a blood loss of more than one liter.
Statistical analysis
Numerical variables were summarized as mean ± standard deviation and as median [interquartile range]; categorical variables were summarized as frequencies and percentages. To investigate the presence of systematic differences according to hypertension, IUGR or either conditions, we performed the Mann–Whitney test or Fisher’s exact test, where appropriate. Accuracy and predictive ability of all numerical independent variables was further evaluated with nonparametric receiver operating characteristic (ROC) analysis. More specifically, the optimal cut-point value was determined using the Youden method, which maximizes the sum of the sensitivity and specificity. All analyses were carried out using Stata software, version 15 (StataCorp, 2017, Stata Statistical Software: Release 15, College Station, Texas, USA: StataCorp LP). The significance level was set at 5%.
Ethics
The study protocol was approved by our local ethics committee (147/2019/Oss/AOUBo) and coheres the ethical guidelines of the “World Medical Association Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects” adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964 and amended by the 59th WMA General Assembly, Seoul, South Korea, October 2008.
Results
For the purpose of the study, 187 women were enrolled during the first trimester of pregnancy. The summary of the demographic, ultrasound and hemodynamic data detected by USCOM® are shown in . Of these, 26 women (14%) developed subsequently hypertensive disorders of pregnancy and/or fetal growth restriction; in particular, 17 (9%) developed gestational hypertension or preeclampsia while 11 (6%) delivered a restricted growth fetus (two of them had both complications). The data regarding pregnancy outcomes are reported in .
Table 1. Characteristics of women enrolled.
Table 2. Pregnancy outcomes of the population enrolled.
We then compared the demographic, biochemical, ultrasound and hemodynamic characteristics of the women who developed hypertensive disorders and/or IUGR, as reported in . Patients who developed hypertension in pregnancy are significantly older than the others, which is not confirmed for the IUGR, as well as in this group the use of assisted fertilization is more frequent. As for gestational age at delivery and neonatal weight, they are obviously lower than in patients with uncomplicated pregnancy, while we have not reported statistically significant differences regarding the mode of delivery and the onset of postpartum hemorrhage. The onset of hypertension is significantly more frequent in those pregnancy conceived with assisted reproductive technologies.
Table 3. Association of hypertension and intrauterine growth restriction (IUGR) with demographic, biochemical, ultrasound, and hemodinamics parameter.
Mean-UTPI above the 95th percentile was significantly more frequent in both women who developed hypertension and those with fetal growth restriction compared to patients with uncomplicated pregnancy. The hemodynamic parameters (CO, TVR, SV), instead, were significantly different in the group that developed hypertensive disorders, compared to uncomplicated pregnancy, showing a reduced CO and an increase in TVR since the first trimester of gestation. We also aimed to evaluate how these parameters can predict hypertension/preeclampsia (), IUGR () or at least one of the two (). Age, BMI, and biochemical markers were not significantly related with the development of these pregnancy complications.
Table 4. Accuracy and predictive ability of potential risk factors for hypertension; point estimates are presented along with 95% confidence intervals.
Table 5. Accuracy and predictive ability of potential risk factors for intrauterine growth restriction (IUGR).
Table 6. Accuracy and predictive ability of potential risk factors for hypertension or intrauterine growth restriction (IUGR).
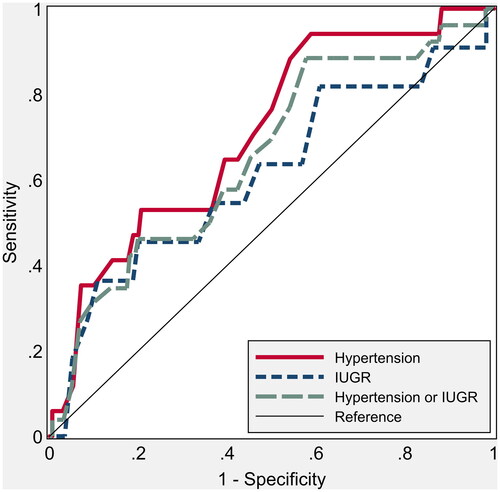
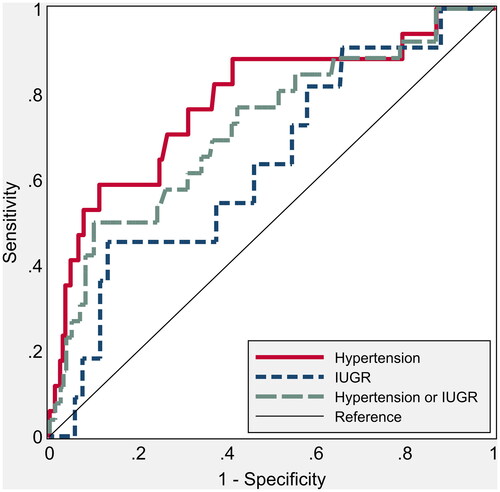
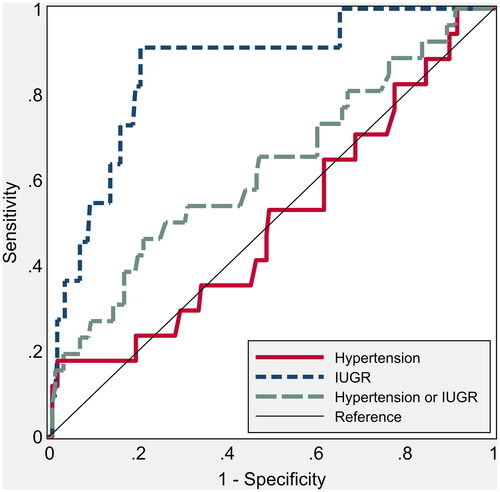
The ROC curve of mean UTPI for the prediction of either hypertension/IUGR shows an AUC (area under the curve) of 62.4 (48.9 to 74.8, p = .04) and an AUC of 85.6 (71.0 to 94.1, p = .001) for IUGR alone. It was not related, instead, in the first trimester with the onset of hypertensive disorders alone (), AUC of 50.3 (34.8 to 66.2, p = .95). CO instead showed an AUC of 69.6 (56.9 to 82.0, p = .005) for the prediction of hypertension, while it was not significantly predictive for IUGR (AUC of 60.0; 36.2 to 78.0, p = .21, ). Finally, the TVR were significantly predictive of hypertension (AUC of 78.2; 61.9–89.8, p < .001), while similarly to CO they were not significantly related to the development of IUGR (AUC of 63.7; 46.7 to 80.8, p = .126, ).
Figure 1. Nonparametric receiver operating characteristic (ROC) curves for umbilical artery pulsatility index (UTPI) to predict hypertension and intrauterine growth restriction. The areas under the ROC curve are shown in .
Discussion
Our study demonstrates that since the first trimester there is a clear correlation between maternal hemodynamics, uterine artery Doppler and the development of hypertensive disorders or fetal growth restriction [Citation13,Citation20].
In many studies these complications have often been considered together, as they have a common etiology [Citation2,Citation3,Citation21]. We demonstrated a significative relationship between those combined outcomes with UTPI, hemodynamic parameters (such as CO, TVR, and SV) and the use of techniques of assisted reproduction.
In this paper, we also analyzed IUGR and hypertensive disorders separately. As regards the prediction of IUGR, we reported that the only significantly predictive factor is the mean UTPI, as shown by the ROC curve, while maternal hemodynamics plays a significant role in the prediction of hypertensive disorders alone, with higher peripheral resistance and lower cardiac output. Obviously the sample is small and needs further investigation, but we can speculate that the alteration of the mean UTPI plays a more “localized” role, leading to the development of fetal IUGR. On the contrary, an hemodynamic maladaptation to pregnancy may predispose to the development of hypertension, supporting the hypothesis of a “systemic” and cardiovascular origin of preeclampsia [Citation22,Citation23].
The strength of this study is the prospective enrollment of an unselected population of pregnant women in the first trimester of pregnancy. However, the incidence of mean UTPI and TVR above the 95th percentile or of CO below the 5th percentile is higher than what can be expected from a low-risk population. This is partly explained by the fact that the women referred to our center are older and with a higher prevalence of ART, thus causing a selection bias. These can be also the reasons for the quite high prevalence of hypertensive disorders of pregnancy in our population. A limitation is the small sample and worthy of enlargement.
Considering those data, we could hypothesize the inclusion of maternal hemodynamic assessment by USCOM® device in the screening protocols of preeclampsia [Citation15]. In particular, it would be interesting to evaluate whether the introduction of prophylaxis with acetylsalicylic acid to those women with low CO and high TVR can reduce the onset of hypertensive disorders later in pregnancy.
The hemodynamic changes that occur since the first trimester of pregnancy can predict the onset of some obstetrics complications, providing important information for the future management of those pregnancies.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chaiworapongsa T, Chaemsaithong P, Yeo L, et al. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10(8):466–480.
- Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol. 2006;195(1):40–49.
- Mecacci F, Avagliano L, Lisi F, et al. Fetal growth restriction: does an integrated maternal hemodynamic-placental model fit better? Reprod Sci. 2021;28(9):2422–2435.
- Roberge S, Nicolaides K, Demers S, et al. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216(2):110.e6–120.e6.
- Rolnik DL, Wright D, Poon LCY, et al. ASPRE trial: performance of screening for preterm pre-eclampsia. Ultrasound Obstet Gynecol. 2017;50(4):492–495.
- Melchiorre K, Giorgione V, Thilaganathan B. The placenta and preeclampsia: villain or victim? Am J Obstet Gynecol. 2022;226(2):S954–S962.
- Thilaganathan B. Pre-eclampsia is primarily a placental disorder: against: pre-eclampsia: the heart matters. BJOG. 2017;124(11):1763.
- Thilaganathan B. Placental syndromes: getting to the heart of the matter. Ultrasound Obstet Gynecol. 2017;49(1):7–9.
- Melchiorre K, Sharma R, Khalil A, et al. Maternal cardiovascular function in normal pregnancy: evidence of maladaptation to chronic volume overload. Hypertension. 2016;67(4):754–762.
- Melchiorre K, Sharma R, Thilaganathan B. Cardiac structure and function in normal pregnancy. Curr Opin Obstet Gynecol. 2012;24(6):413–421.
- Melchiorre K, Sharma R, Thilaganathan B. Cardiovascular implications in preeclampsia: an overview. Circulation. 2014;130(8):703–714.
- Melchiorre K, Sutherland GR, Baltabaeva A, et al. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension. 2011;57(1):85–93.
- Melchiorre K, Thilaganathan B. Maternal cardiac function in preeclampsia. Curr Opin Obstet Gynecol. 2011;23(6):440–447.
- Montaguti E, Di Donna G, Pilu G. Usefulness of USCOM® evaluation in women with chronic hypertension who developed severe preeclampsia with low platelets and elevated liver enzymes. J Matern Fetal Neonatal Med. 2022;35(25):4942–4945.
- Montaguti E, Youssef A, Cavalera M, et al. Maternal hemodynamic assessment by USCOM® device in the first trimester of pregnancy. J Matern Fetal Neonatal Med. 2022;35(25):5580–5586.
- Mulder E, Basit S, Oben J, et al. Accuracy and precision of USCOM versus transthoracic echocardiography before and during pregnancy. Pregnancy Hypertens. 2019;17:138–143.
- Khalil A, Nicolaides KH. How to record uterine artery doppler in the first trimester. Ultrasound Obstet Gynecol. 2013;42(4):478–479.
- Sotiriadis A, Hernandez-Andrade E, da Silva Costa F, et al. ISUOG practice guidelines: role of ultrasound in screening for and follow-up of pre-eclampsia. Ultrasound Obstet Gynecol. 2019;53(1):7–22.
- Vinayagam D, Thilaganathan B, Stirrup O, et al. Maternal hemodynamics in normal pregnancy: reference ranges and role of maternal characteristics. Ultrasound Obstet Gynecol. 2018;51(5):665–671.
- Melchiorre K, Sutherland GR, Liberati M, et al. Maternal cardiovascular impairment in pregnancies complicated by severe fetal growth restriction. Hypertension. 2012;60(2):437–443.
- Foo FL, Mahendru AA, Masini G, et al. Association between prepregnancy cardiovascular function and subsequent preeclampsia or fetal growth restriction. Hypertension. 2018;72(2):442–450.
- Gagliardi G, Tiralongo GM, LoPresti D, et al. Screening for pre-eclampsia in the first trimester: role of maternal hemodynamics and bioimpedance in non-obese patients. Ultrasound Obstet Gynecol. 2017;50(5):584–588.
- Kalafat E, Thilaganathan B. Cardiovascular origins of preeclampsia. Curr Opin Obstet Gynecol. 2017;29(6):383–389.