Abstract
Objective
COVID-19 has been reported to increase the risk of prematurity, however, due to the frequent absence of unaffected controls as well as inadequate accounting for confounders in many studies, the question requires further investigation. We sought to determine the impact of COVID-19 disease on preterm birth (PTB) overall, as well as related subcategories such as early prematurity, spontaneous, medically indicated preterm birth, and preterm labor (PTL). We assessed the impact of confounders such as COVID-19 risk factors, a-priori risk factors for PTB, symptomatology, and disease severity on rates of prematurity.
Methods
This was a retrospective cohort study of pregnant women from March 2020 till October 1st, 2020. The study included patients from 14 obstetric centers in Michigan, USA. Cases were defined as women diagnosed with COVID-19 at any point during their pregnancy. Cases were matched with uninfected women who delivered in the same unit, within 30 d of the delivery of the index case. Outcomes of interest were frequencies of prematurity overall and subcategories of preterm birth (early, spontaneous/medically indicated, preterm labor, and premature preterm rupture of membranes) in cases compared to controls. The impact of modifiers of these outcomes was documented with extensive control for potential confounders. A p value <.05 was used to infer significance.
Results
The rate of prematurity was 8.9% in controls, 9.4% in asymptomatic cases, 26.5% in symptomatic COVID-19 cases, and 58.8% among cases admitted to the ICU. Gestational age at delivery was noted to decrease with disease severity. Cases were at an increased risk of prematurity overall [adjusted relative risk (aRR) = 1.62 (1.2–2.18)] and of early prematurity (<34 weeks) [aRR = 1.8 (1.02–3.16)] when compared to controls. Medically indicated prematurity related to preeclampsia [aRR = 2.46 (1.47–4.12)] or other indications [aRR = 2.32 (1.12–4.79)], were the primary drivers of overall prematurity risk. Symptomatic cases were at an increased risk of preterm labor [aRR = 1.74 (1.04–2.8)] and spontaneous preterm birth due to premature preterm rupture of membranes [aRR = 2.2(1.05–4.55)] when compared to controls and asymptomatic cases combined. The gestational age at delivery followed a dose-response relation with disease severity, as more severe cases tended to deliver earlier (Wilcoxon p < .05).
Conclusions
COVID-19 is an independent risk factor for preterm birth. The increased preterm birth rate in COVID-19 was primarily driven by medically indicated delivery, with preeclampsia as the principal risk factor. Symptomatic status and disease severity were significant drivers of preterm birth.
Introduction
Pregnant women are at an elevated risk for severe to critical disease with the severe respiratory syndrome coronavirus (SARS-CoV-2) virus [Citation1]. Though most such infections are asymptomatic or mild, mounting data indicate an increased risk of severe complications including death compared to non-pregnant women [Citation2]. Studies indicate an increased frequency of pregnancy complications including prematurity, preeclampsia, and stillbirth [Citation2].
Prior to the pandemic, preterm birth (PTB) was recognized as the most significant perinatal challenge. PTB accounted for most adverse newborn outcomes related to maternal COVID-19 in one study [Citation3]. The evidence for increased prematurity rates with SARS-CoV-2 infection is not unambiguous, however. No increased preterm birth (PTB) rate was noted in a large single-center USA study [Citation4]. Moreover, reductions in overall PTB rates during the pandemic were reported in multiple national studies including in the US, in apparent conflict with other clinical studies [Citation5]. The observed increased prematurity rates may have been confounded by patient sociodemographic factors and the perceived need to intervene to improve maternal outcomes. A large national prospective study found that iatrogenic delivery was the main cause of prematurity. Around 60% were delivered for COVID-19 symptoms or pneumonia while a further 20% were delivered for other maternal indications combined with preeclampsia suggesting that there might not be a direct link between COVID-19 and PTB [Citation6]. Despite the growing evidence, the question of whether SARS-CoV-2 is an independent risk factor for prematurity has yet to be answered. Understanding the clinical and biological bases of COVID-19-related prematurity is a critical area for future research. Therefore, we sought to evaluate the link between SARS-CoV-2 infection and spontaneous and iatrogenic prematurity, while accounting for the possible confounding effect of disease severity, pre-existing disorders, sociodemographics and other factors. Secondly, we evaluated the relative contribution of coronavirus infection to prematurity rates when compared to traditional PTB risk factors.
Methods
Study design
The State of Michigan was the epicentre of repeated COVID-19 outbreaks prompting a state-wide collaboration among 14 obstetric centers [Citation7]. Institutional Review Board (IRB) approval was obtained to gather prospective and retrospective maternal and newborn data from the electronic medical records. This multicentre case-control study was standardized across each site by the application of the same inclusion and exclusion criteria prior to enrolment. This report covers the period from March to December 1st, 2020. SARS-CoV-2-positive women (cases) were primarily defined as those with positive reverse transcription-polymerase chain reaction (RT-PCR) test from nasopharyngeal swabs. RT-PCR were performed at the time of the presentation to the Labor and Delivery unit for delivery. Controls were the next 2–3 subjects delivered in the same obstetric unit within one month of the index study case who were RT-PCR negative and without clinical suspicion of COVID-19 disease. During the initial stages of the outbreak and the shortage of testing kits, only a minority of Labor and Delivery units implemented universal screening of all obstetric patients, regardless of symptomatology. Therefore, controls recruited for the early cases included asymptomatic pregnant women admitted with no prior history or clinical suspicion or subsequent diagnosis of COVID-19 prior to delivery hospitalization and discharge. Disease severity was defined as follows: an asymptomatic, mild disease with no pneumonia, moderate: with pneumonia, severe: respiratory/cardiovascular failure, and critical: shock/organ failure based on a careful review of the medical records. The guidelines by the Society for Maternal-Fetal Medicine for the management of pregnant patients with COVID-19 was used to confirm consistency in determining disease severity, and the diagnosis of pneumonia and multi-organ dysfunction [Citation8]. All pregnant individuals with COVID-19 were included regardless of delivery on index hospitalization. To ensure that data was consistent in each study site, the same variables were collected and were deidentified before it was combined for the analysis. The data collection was overseen by the Office of Women’s Health at Wayne State University, Detroit, Michigan.
Statistical analysis
Power Analysis: At the time of the initiation of the study the review of Schwartz et al [Citation9] reported a prematurity rate, primarily from Wuhan City, China of 28.2%. Data from Hubei Province, in which Wuhan is located, reported a prematurity rate pre-Covid-19 of 9.4% an approximately 3-fold increase in prematurity associated with COVID-19 infection in pregnancy [Citation10]. Based on a prematurity rate in the State of Michigan of 10% (March of Dimes. 2019 Report Card. https://www.marchofdimes.org/mission/reportcard.aspx reviewed March 2020) the sample size needed to detect a 3-fold increase in the prematurity rate in the SARS-CoV-2 infected group at a power of 0.9 and significance level of p-value <.01 was calculated to be 121 cases and 121 controls [Citation7,Citation11].
Demographic data analysis: Demographic and clinical characteristics were compared between groups using two-tailed Fisher’s exact test for categorical variables and t-tests for continuous variables. All data analysis were performed using the R statistical langue and environment (www.r-project.org) version 3.6.
Crude relative risk determinations: The effect of SARS-CoV-2 infection defined as a two- (control vs case), three- (control, asymptomatic case, symptomatic case), and four- (control, asymptomatic case, cases with mild/moderate symptoms, case with severe symptoms) group exposure variable was assessed on a given pregnancy outcome (e.g. preterm birth). Relative risk estimates in two- and three-group- analyses were performed using robust Poisson regression models [Citation12] using the geepack package [Citation13] in R using the control group as reference. In addition, for assessing the effect of disease symptomatology (three group-) and severity (four group- comparisons), respectively, chi-squared tests for trend in proportions were also performed.
Adjusted relative risk determinations: Missing entries for demographic and obstetrical history variables considered relevant as possible confounders were imputed using the R package Multivariate Imputation by Chained Equations (MICE) [Citation14], using default parameters. The variables included in the imputation procedure were Body Mass Index (BMI), maternal age, number of previous term births, number of previous preterm births, race, ethnicity, county of residence, insurance status, history of smoking, substance use disorder, gravidity, number of fetuses, and fetal sex. The details of the adjusted relative risk analysis are described in the supplementary section.
Effect modification: To determine whether the effect of SARS-CoV-2 infection, treated as a binary variable, on the risk of prematurity was modified by specific factors (e.g. race, ethnicity) interaction terms were allowed in the Poisson regression model between exposure and potential effect modifiers. The significance of the interaction was assessed via a χ2 test implemented in the anova function in the geepack package.
Moreover, we evaluated whether there was a significant difference in a-priori risk for PTB [Citation15] and medically indicated or iatrogenic preterm birth [Citation16] in COVID-19 pregnancies versus controls. Finally using Wilcoxon non-parametric testing, the median (interquartile range) of a-priori PTB calculated risk was compared between the controls and COVID-19 groups. A p-value <.05 was considered a significant result.
Results
Of the N = 1459 patients in the analysis set, 25.3% (N = 369) were SARS-CoV-2 (positive) cases, and 74.7% (N = 1090) were controls. Table S1 summarizes the demographic and clinical characteristics of the groups. Data was missing for <1.7% of women for each variables considered as potential confounders, except for race (4.3%) and county of residence (7.7%). These were imputed to allow the calculation of adjusted relative risks (aRR). When compared to controls, COVID-19 cases had a higher frequency of clinical and demographic risks factors of preterm delivery, i.e. race, chronic hypertension, diabetes. There were no significant differences in gestational age at the time of delivery, delivery method, prior preterm birth, and parity. The relative risk for all major prematurity-related outcomes is shown in and . Additionally, 29.3% of the controls did not have an RT-PCR test while 70.7% had a negative RT-PCR test. Clinical assessment (asymptomatic and negative history and exam) was used to include the patients as controls in the absence of RT-PCR test result.
Figure 1. Effect of COVID-19 on prematurity rates. (A)Medically indicated and spontaneous prematurity rates by COVID-19 status. ***p < .001; *p < .05, by Fisher’s exact test. (B) Adjusted relative risk with COVID-19 for prematurity related outcomes by Poisson regression models. PTB: preterm birth; iPTB: medically indicated PTB; PPROM: preterm prelabor rupture of membranes; sPTB: spontaneous PTB; sPTB_Other: spontaneous PTB with intact membranes; Early PTB: PTB <34 weeks. iPTB_PE: medically indicated prematurity involving preeclampsia; iPTB_Other: medically indicated prematurity for causes other than preeclampsia
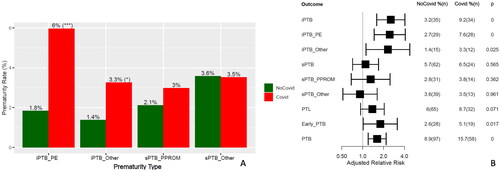
Table 1. Crude and adjusted relative risk carried by Covid19 for prematurity related outcomes
Preterm birth (<37 weeks)
The rate of preterm birth (PTB) was 10.6% (154/1459) in the full cohort, and 8.8% (96/1090) and 15.7% (58/369) in controls and cases, respectively. There was therefore a significant increase in the relative risk of PTB with SARS-CoV-2 infection, RR = 1.77(1.3–2.39) and remained true after accounting for relevant confounding factors [aRR = 1.62(1.2–2.18)] (). Of note, the relative risk associated with coronavirus infection on the rate of PTB while significant was lower than for established risk factors such as a history of a prior preterm delivery (aRR = 3.8) and twin gestations (aRR = 4.8) while comparable to that of history of chronic hypertension (aRR = 2.4), substance use disorder (aRR = 2.3), and insurance status (aRR = 1.6) (Figure S1).
The effect of infection on the overall prematurity rate was modified by a history of previous preterm delivery. Although the rate of preterm birth was highest among cases who had a history of preterm delivery (43.5%), the magnitude of the effect of coronavirus in women with a history of preterm delivery (aRR = 1.1) was lower (p interaction = 0.04) than that for those without such history (aRR = 2.0). This is likely due to the higher baseline risk in women with a history of prior PTB (32.8%) (Table S2).
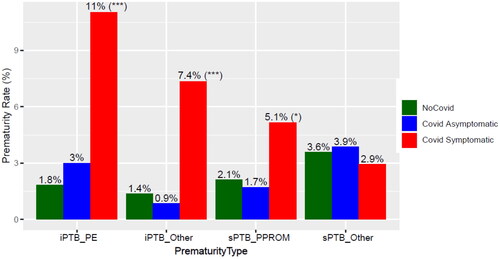
We then investigated the impact of symptomatology on the PTB rate in coronavirus infection. The increased PTB rate with infection was driven by a higher rate among symptomatic cases (26.5%) (Table S3). The increase in symptomatic cases was observed not only for medically indicated or iatrogenic preterm birth (iPTB) but also for spontaneous PTB (sPTB) involving PPROM (). In contrast, the rate of PTB in asymptomatic cases (9.4%) was similar to that of controls (8.9%), and their gestational age at delivery were similar (Figure S2). Analysis based on COVID-19 severity found that the rate of PTB was 23.5% in the Mild/Moderate COVID-19 group versus 47.1% in the severe disease group. A test for an increasing trend in the rate of PTB with COVID-19 severity resulted in significant findings for both three- and four-group analyses (Table S3 and Table S4). Furthermore, the gestational age at delivery followed a dose-response relation with disease severity, as more severe cases tended to deliver earlier (Wilcoxon p < .05 for all pairwise comparisons, except for asymptomatic COVID-19 vs. controls) (Figure S4). A more objective indicator of severe disease is the requirement for maternal ICU admission. There were 19 cases of women admitted to the ICU, of which 17 were in the COVID-19 group. The rate of preterm birth was 58.8% among such ICU cases versus 13.7% among non-ICU admission [RR = 4.3(2.05–8.14)] (Table S4). Socio-demographic variables such as insurance type, race, and ethnicity, county of residence did not emerge as being significant effect modifiers for PTB.
Figure 2. Effect of COVID-19 symptomatology on prematurity rates. Medically indicated and spontaneous prematurity rates are shown by COVID-19 status and symptoms. ***p < .001; *p < .05, by Fisher’s exact test comparing the frequency of PTB in Covid symptomatic vs all other patients. sPTB: spontaneous PTB; sPTB_Other: spontaneous PTB with intact membranes; Early PTB: PTB <34 weeks. iPTB_PE: medically indicated prematurity involving preeclampsia; iPTB_Other: medically indicated prematurity for causes other than preeclampsia.
Early preterm birth (<34 weeks)
The overall rate of early preterm birth (< 34 weeks) was 3.2% (46/1459) in the full cohort, 2.6% (28/1090) among controls, and 5.1% (19/369) in cases. The relative risk for early preterm birth with coronavirus infection was significant after adjustment for relevant covariates listed in [aRR = 1.8(1.02–3.16)]. The rate of early PTB increased with the presence of COVID-19 symptoms, disease severity (Table S3), and maternal ICU admission [RR = 7.4(2.4–19.3)] (Table S4).
Although the rate of early PTB was similar for cases with a male (4.9%) or female (5.4%) fetus, the relative risk of early PTB with COVID-19 was higher in pregnancies with a female versus a male fetus, due to the lower baseline risk of early PTB female (1.3%) vs male (3.8%) fetus in controls (Figure S3).
Medically indicated preterm birth (<37 weeks)
The overall rate of medically indicated/iatrogenic preterm birth (iPTB) was 4.7% (69/1459) in the full cohort, 3.2% (35/1090) among controls, and 9.2% (34/369) among cases (p:0.000). The main risk factors for iPTB in decreasing order of the magnitude of the effect were: preeclampsia, COVID-19 status, followed by other standard indications for iPTB [Citation13] (Figure S5). The adjusted relative risk of iPTB with associated with COVID-19 infection was aRR = 2.55 (1.6–4.08) ().
Similar to the overall rate of PTB, the rate of iPTB was increased in cases, especially in those who were symptomatic (18.4%) and those with severe disease (35.3%) (Table S3, ). Of note, iPTB involving preeclampsia aRR = 2.46(1.47–4.12) and also for other indications aRR = 2.32(1.12–4.79) was increased with COVID-19 (). A test for an increasing trend in the rate of iPTB with COVID-19 symptomatology and severity was highly significant (all p <.05). Among cases, the risk of iPTB increased with maternal ICU admission [RR = 6.4(2.7–13.4)] (Table S4).
Spontaneous preterm birth (<37 weeks)
The overall rate of spontaneous PTB (sPTB) was 5.7% in controls and 6.5% in cases (). There was a trend towards an increased risk of sPTB due to PPROM in symptomatic women versus controls [RR = 2.1(0.97–4.4)]. Also, there was a significant risk of PPROM in symptomatic COVID-19 cases compared to the combined group of asymptomatic cases plus controls [RR = 2.2(1.05–4.55), aRR = 1.92(0.87–4.3)]. We were likely underpowered for an adjusted sub-analysis given the overall low rate of PPROM (3%) and the fact that aRR and crude RR were comparable.
The rate of preterm labor (PTL), was significantly increased in symptomatic cases (12.5%) versus controls (6%) after adjusting for confounders listed in [aRR = 1.74(1.04–2.8)] while the rate of PTL was also associated with the severity of disease in unadjusted analyses (Tables S3 and Table S4).
Relative contribution of coronavirus infection to prematurity rates compared to traditional PTB risk-factors
To put in perspective the contribution of coronavirus infection to preterm birth, we determined for each prematurity outcome the partial R2 for predictors retained in Poisson regression models. For medically indicated PTB, COVID-19 was second only to chronic hypertension in terms of % of variance explained (Table S2), while for PTB overall, the factors explaining most of the variability in this outcome were a history of preterm delivery, chronic hypertension, twin gestation and insurance type. The severity of prematurity as judged by the average gestational age at delivery did not differ between the COVID-19 and non-COVID-19 PTB groups (Figure S2).
A-priori risk factors for PTB and likelihood of COVID-19 disease
The a-priori risk for PTB was higher in cases that delivered prematurely compared to controls, whether the patient was in the infected or non-infected group. The calculated a-priori PTB risk was higher for COVID-19 cases overall compared to controls: 0.053 (0.05–0.10) versus 0.05 (0.03–0.07), (Wilcoxon test p <.01). While 25% of patients in the control group had a prior risk score of 7% or more, 31% of patients in the COVID-19 had a prior risk of 7% or more (Fisher’s exact test, p =.02). The 7% cut-off was chosen as the 3rd quartile of prior risk in the control group. Finally, between the PTB groups, there was no difference in the a-priori PTB risk for those with COVID-19 vs those in the control group (Figure S6). Overall, these findings suggest that cases at higher a-priori risk for PTB may be more susceptible to SARS-CoV-2 infection. This is made plausible by the extensive overlap between PTB risk factors and COVID-19 risk/susceptibility factors e.g. race and socioeconomic factors.
Discussion
Principal findings
After controlling for confounding factors, we found an increased risk of PTB and early PTB in COVID-19 pregnancies. There was also a dose-response relation between gestational age at delivery and COVID-19 severity. The increase in the rate of PTB was driven largely by an increase in medically indicated or iatrogenic PTB. Indicated PTB had a strong correlation with the diagnosis of preeclampsia. Preterm labor (PTL) was significantly increased in symptomatic COVID-19 pregnancies and those with greater disease severity. Further, there was an increase in spontaneous PTB due to PPROM in symptomatic cases compared to others. Overall, however, the effect of traditional risk factors such as a prior history of PTB and multiple gestation on the rate of PTB were greater than, and that of hypertensive disorders and substance abuse comparable to, that of COVID-19.
National cohort studies using an uninfected control group [Citation3,Citation6] and a multi-national study [Citation17] report increased prematurity rates with COVID-19 in contrast to the study of Adhikarj et al. [Citation4]. In an attempt to further understand the possible biologic mechanisms driving PTB outcomes in COVID-19, we examined subcategories of PTB including iatrogenic and spontaneous PTB and preterm labor. We observed a significant increase in indicated PTB after controlling for confounders. Further, indicated PTB was driven primarily by the association with preeclampsia which exceeded all other indications combined. Preeclampsia rates are known to be elevated in symptomatic COVID-19 compared to asymptomatic cases [Citation17,Citation18]. Moreover, the more severe the disease, the earlier the gestational age at delivery was reported by the same authors. Prematurity has been shown to be strongly linked to preeclampsia [Citation19]. Increased rates of medically indicated PTB have been confirmed in a case-controlled national cohort [Citation20]. A detailed investigation of the basis of medically indicated deliveries is absent from most studies. One exception was the study of Cruz Melguizo et al [Citation6] which found that 47.7% of PTB in COVID-19 cases were iatrogenic compared to 21.3% (p <.001) in controls. Among the COVID-19 group close to 80% of such deliveries were performed to improve maternal COVID-19 condition.
Given the established role of inflammation and infection in PTB [Citation21,Citation22] and the systemic inflammation induced by the SARS-COV-2 virus, both in the maternal and fetal circulations [Citation23], we evaluated the risk of spontaneous prematurity. While the risk of spontaneous PTB was not increased overall, we found a statistically significant trend in the risk of PTL with COVID-19 severity and with symptomatology. PPROM as a cause of sPTB was increased in symptomatic cases. A metanalysis reported an increased risk for preterm labor [Citation24] and increased risk of PPROM [Citation6,Citation17] as well as PROM [Citation24] in affected cases. In addition, a large national cohort study found an increased rate of amniotic fluid infection in COVID-19 cases [Citation25]. We did not find an increased frequency of cervical incompetence/shortening/premature dilation among cases.
A major challenge of assessing the risk of prematurity attributable to COVID-19 is the extensive overlap of pre-existing risk factors for prematurity and iatrogenic prematurity with those for moderate/severe COVID-19 infection. To clarify this issue, we performed Poisson regression using PTB risk factors (e.g. substance use, insurance status and twin gestation, and COVID-19 status) for PTB prediction. COVID-19 emerged as a significant independent risk factor for PTB, early PTB, and indicated PTB. Consistent with some other publications, we found that the frequency of PTB in COVID-19 was increased with symptomatology and disease severity [Citation26–29]. Finally, variables such as insurance type, race, and ethnicity did not emerge as being significant effect modifiers.
Strengths and limitations
Our study had several strengths. A well-curated database was used which contained extensive clinical information allowing us to address detailed questions The control group allowed significance testing of the observed changes. We restricted the study to a large but well-defined geographic area with fairly uniform medical and obstetric standards for the management of COVID-19 pregnancies, minimizing variability. Further, one of the strengths of our study was the large cohort included. The sample size of the analysis set (369 cases and 1089 controls) exceeded by a factor of >3 the minimum sample size we initially anticipated based on power analysis [Citation7,Citation11]. This large cohort was included to limit potential confounders and also increase the power for subgroup analysis.
Our study does have limitations including the primarily retrospective approach. While it is possible that patients with COVID-during the first trimester or early pregnancy COVID-19 might present less frequently to the hospital for evaluation compared to infection later in gestation, whether or not a patient was admitted at the time of diagnosis she would still be included as a study case.
Designations such as iatrogenic/medically indicated prematurity are partly subjective increasing the risk of misclassification. Additionally, a fraction of controls did not undergo RT-PCR testing due to test unavailability during the early phase of the epidemic, increasing the risk of misclassification. Finally, the case series was powered to address the impact of COVID-19 on prematurity overall and not for all sub-categories of outcomes analysed. Another limitation of this study was that multiple aspects of the COVID-19 disease has continued to markedly change over time: virus mutations and thus clinical disease, type and availability of RT-PCR tests, availability and uptake of home testing which were not as accurate, type and availability of therapeutic agents. All of the above factors introduce variability in diagnosis, presentation and clinical severity and potentially obstetric impact of the disease. We thus wanted to focus on the “classical” COVID-19 disease. We made the determination that it was most important to limit the analysis to a given time period and use the available standards for disease diagnosis in that particular period.
Our findings confirm an increased risk of PTB in the following subgroups of COVID-19 pregnancies: those that are symptomatic, with moderate or severe clinical status, and infected women diagnosed with preeclampsia. This is important for patient counselling and prognostication.
Conclusions
In conclusion, we found that COVID-19 was an independent risk factor for PTB. Yet, as a risk factor for PTB, COVID-19 was not as strong as a history of PTB or twin gestation. COVID-19 severity and symptomatology increased prematurity risk. The increase in PTB and early PTB in pregnancies with COVID-19 was driven primarily by medically indicated prematurity of which preeclampsia was the primary driver.
Supplemental Material
Download MS Word (381.6 KB)Acknowledgments
We would like to acknowledge the contributions of the Office of Women’s Health at Wayne State University who served as the coordinating center for and those of the many participating institutions in the State of Michigan Collaborative.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References:
- Lokken EM, Taylor GG, Huebner EM, et al. Higher severe acute respiratory syndrome coronavirus 2 infection rate in pregnant patients. Am J Obstet Gynecol. 2021;225(1):75.e1–75.e16.
- Jamieson DJ, Rasmussen SA. An update on COVID-19 and pregnancy. Am J Obstet Gynecol. 2022;226(2):177–186.
- Gurol-Urganci I, Jardine JE, Carroll F, et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study. Am J Obstet Gynecol. 2021;225(5):522.e1–522.e11.
- Adhikari EH, Moreno W, Zofkie AC, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open. 2020;3(11):e2029256.
- Shah PS, Ye XY, Yang J, et al. Preterm birth and stillbirth rates during the COVID-19 pandemic: a population-based cohort study. CMAJ. 2021;193(30):E1164–E1172.
- Cruz Melguizo S, de la Cruz Conty ML, Carmona Payan P, et al. Pregnancy outcomes and SARS-CoV-2 infection: the panish obstetric emergency group study. Viruses. 2021;13(5):853.
- Bahado-Singh R, Hassan SS, Szymanska M, et al. Starting a regional collaborative research group for COVID-19 in pregnancy: the Southern Michigan experience. J Perinat Med. 2020;48(9):883–891.
- Society of Maternal Fetal Medicine. Management considerations for pregnant patients with COVID-19. 2022. Available from: https://s3.amazonaws.com/cdn.smfm.org/media/2734/SMFM_COVID_Management_of_COVID_pos_preg_patients_2-2-21_(final).pdf
- Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12(2):194.
- Xu H, Dai Q, Xu Y, et al. Time trends and risk factor associated with premature birth and infants deaths due to prematurity in Hubei Province, China from 2001 to 2012. BMC Pregnancy Childbirth. 2015;15:329.
- Pettirosso E, Giles M, Cole S, et al. COVID-19 and pregnancy: a review of clinical characteristics, obstetric outcomes and vertical transmission. Aust N Z J Obstet Gynaecol. 2020;60(5):640–659.
- Chen W, Qian L, Shi J, et al. Comparing performance between log-binomial and robust Poisson regression models for estimating risk ratios under model misspecification. BMC Med Res Methodol. 2018;18(1):63.
- Yan J, Fine J. Estimating equations for association structures. Stat Med. 2004;23(6):859–874; discussion 875–877, 879–880.
- van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18(6):681–694.
- American College of Obstetricians and Gynecologists Gynecologists’ Committee on Practice Bulletins-Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG Practice Bulletin, Number 234. Obstet Gynecol. 2021;138(2):e65–e90.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice Society for Maternal-Fetal Medicine. Medically indicated late-preterm and early-term deliveries: ACOG Committee Opinion, Number 831. Obstet Gynecol. 2021;138(1):e35–e39.
- Papageorghiou AT, Deruelle P, Gunier RB, et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am J Obstet Gynecol. 2021;225(3):289.e1–289.e17.
- Lai J, Romero R, Tarca AL, et al. SARS-CoV-2 and the subsequent development of preeclampsia and preterm birth: evidence of a dose-response relationship supporting causality. Am J Obstet Gynecol. 2021;225(6):689–693.e1.
- Ferrero DM, Larson J, Jacobsson B, et al. Cross-country individual participant analysis of 4.1 million singleton births in 5 countries with very high human development index confirms known associations but provides no biologic explanation for 2/3 of all preterm births. PLOS One. 2016;11(9):e0162506.
- Martinez-Perez O, Prats Rodriguez P, Muner Hernandez M, et al. The association between SARS-CoV-2 infection and preterm delivery: a prospective study with a multivariable analysis. BMC Pregnancy Childbirth. 2021;21(1):273.
- Romero R, Espinoza J, Goncalves LF, et al. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25(1):21–39.
- Romero R, Espinoza J, Goncalves LF, et al. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11(5):317–326.
- Garcia-Flores V, Romero R, Xu Y, et al. Maternal-fetal immune responses in pregnant women infected with SARS-CoV-2. Nat Commun. 2022;13(1):320.
- Jafari M, Pormohammad A, Sheikh Neshin SA, et al. Clinical characteristics and outcomes of pregnant women with COVID-19 and comparison with control patients: a systematic review and meta-analysis. Rev Med Virol. 2021;31(5):1–16.
- Epelboin S, Labrosse J, De Mouzon J, et al. Obstetrical outcomes and maternal morbidities associated with COVID-19 in pregnant women in France: a national retrospective cohort study. PLOS Med. 2021;18(11):e1003857.
- DeBolt CA, Bianco A, Limaye MA, et al. Pregnant women with severe or critical coronavirus disease 2019 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol. 2021;224(5):510.e1–510.e12.
- Delahoy MJ, Whitaker M, O’Halloran A, et al. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confirmed COVID-19 – COVID-NET, 13 states, March 1-August 22, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(38):1347–1354.
- Khoury R, Bernstein PS, Debolt C, et al. Characteristics and outcomes of 241 births to women with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection at five New York city medical centers. Obstet Gynecol. 2020;136(2):273–282.
- Metz TD, Clifton RG, Hughes BL, et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19). Obstet Gynecol. 2021;137(4):571–580.

