?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Introduction
The evaluation of upcoming Aortic Coarctation (CoA) in new-borns with prenatal suspicion entails a close echocardiographic monitor until Arterial Duct (AD) closure, in a department with pediatric cardiological and surgical expertise. The significant number of false-positive prenatal diagnoses causes parental stress and healthcare costs.
Aim
The aim of this study was to elaborate an echocardiographic prediction model to be employed at birth when PDA is still present, in patients suspected of CoA during fetal life in order to foretell CoA requiring neonatal surgical intervention.
Methods
This retrospective monocentric study included consecutive full-term and late preterm neonates with prenatal suspicion of CoA born from 01 January 2007 to 31 December 2020. Patients were divided into two groups according to the need for aortic surgery (CoA - NoCoA). All patients underwent a comprehensive transthoracic echocardiographic exam in presence of PDA. Multivariable logistic regression was used to create a coarctation probability model (CoMOD) including isthmal (D4), transverse arch (D3) diameters, the distance between a left common carotid artery (LCA) and left subclavian artery (LSA), presence/absence of ventricular septal defect (VSD) and bicuspid aortic valve (BAV).
Results
We enrolled 87 neonates (49 male, 56%). 44 patients developed CoA in need of surgical repair. Our index CoMOD showed an AUC = 0.9382, high sensitivity (91%) and specificity (86%) in the prediction of CoA in neonates with prenatal suspicion. We classified neonates with CoMOD > 0 to be at high risk for surgical correction of CoA, with good PPV (86.9%) and NPV (90.9%).
Conclusions
CoMOD > 0 is highly suggestive of the need for CoA corrective surgery in newborns with prenatal suspicion.
Background
Coarctation of the Aorta (CoA) represents one of the most common congenital heart defects, accounting for 5% of children with Congenital Heart Disease (CHD) with an incidence of 4/10.000 live births [Citation1,Citation2]. Male seems to be more affected with a reported male-to-female ratio of 1.27–1.74 [Citation3].
CoA is defined as a discrete stenosis in the upper thoracic aorta, close to the insertion of the Arterial Duct (AD), often combined with tubular hypoplasia of the aortic arch. CoA can produce a duct-dependent systemic circulation when a critical preductal narrowing is present. It may occur as an isolated lesion or in association with almost every type of CHD, mostly as part of multiple left heart obstructive lesions [Citation4].
Critical CoA requires surgical intervention in the first weeks of life. The treatment of choice in neonates and infants with significant CoA is the surgical resection of the narrowed aortic segment. Extended CoA resection with end-to-end anastomosis through a left thoracotomy is the preferred technique in neonates with discrete CoA [Citation5]. When a long aortic segment is involved, subclavian-flap aortoplasty or patch augmentation of the hypoplastic aortic arch can be an alternative [Citation6]. If the coarctation repair is combined with the repair of an intracardiac lesion, it can be performed from an anterior approach with selective cerebral and myocardial perfusion [Citation7].
In newborns without prenatal suspicion, a pre- and post-ductal oxygen saturation is regularly assessed as neonatal screening before hospital discharge to detect major CHDs [Citation8].
The main diagnostic tool to confirm the suspicion of CoA is Transthoracic echocardiography (TTE): the typical continuous-wave Doppler finding in CoA consists of a peak systolic acceleration during systole and a continuous antegrade flow throughout diastole, creating a “saw tooth” appearance [Citation9].
In newborns, the presence of Patent Ductus Arteriosus (PDA) may disguise CoA diagnosis, as blood flows through the PDA can bypass the aortic obstruction and supply the lower body and PDA closure being itself a probable cause of CoA development [Citation10–13].
Despite technical advances, the prenatal diagnosis of CoA remains challenging with a high false-positive and false-negative rate [Citation14].
A variety of prenatal echocardiographic scoring systems have been suggested to better predict the possible neonatal CoA; nevertheless, CoA is the most frequently missed CHD diagnosis in the antenatal period, with less than one-third of the affected patients being detected at prenatal screening [Citation15,Citation16]. Thus, CoA eludes prenatal screening and becomes finally manifests in the first days of life after AD closure [Citation17].
When CoA is antenatally suspected, the current practice entails birth planning in a centre with a cardiac surgical department, strict monitoring of the new-born with serial echocardiographic evaluations until AD closure in order to confirm or reject the diagnosis of CoA, in a department with pediatric cardiologic expertise. This, together with a high false-positive prenatal detection rate, produces unnecessary parental anxiety, a precocious separation of the newborn from the mother and an absence of breastfeeding in the first days of life. Moreover, it implies longer in-hospital stays, usually in tertiary cardiac center and related higher healthcare costs.
Over time, many echocardiographic parameters have been evaluated to try to predict the onset of CoA in new-borns with PDA such as: Carotid-Subclavian Artery index (CSAi) [Citation18], the ratio of the aortic isthmus diameter to descending aorta diameter (I/D) [Citation19], the distance between the Left Common Carotid Artery (LCA) and Left Subclavian Artery (LSA) [Citation20] and a combined formula developed by Soslow et al. [Citation21] to calculate the probability of CoA in new-borns with PDA and CoA suspicion. None of them has provided a definite and certain diagnostic tool for CoA prior AD closure.
Aim of the study
The aim of this study was to assess the predictive value of early neonatal echocardiographic parameters for the development of CoA in need of neonatal surgical repair in newborns suspected to have CoA during fetal life when a large AD is still present.
Materials and methods
This retrospective study was conducted at IRCCS – Sant’Orsola Hospital in Bologna, a tertiary referral center for Pediatric Cardiology and Cardiac Surgery in Italy. We identified full-term and late-preterm neonates (<30 days of age) with a prenatal suspicion of CoA born between 01 January 2007 and 31 December 2020.
Exclusion criteria were: AD closure, complex CHD, different major CHD diagnoses at birth, and major extracardiac abnormalities. Neonates with inadequate echocardiographic image quality were removed from the study.
This study protocol was reviewed and approved by the “Area Vasta Emilia Centro” Ethical Review Board according to the Helsinki Declaration (approval number 0013495). In our cohort, neonates with CoA are defined as patients with a critical aortic arch obstruction requiring surgical intervention in the first weeks of life. The decision to undergo CoA surgical repair was made by both the Pediatric cardiologic and surgical teams based on clinical findings and echocardiograms.
When the diagnosis of CoA was confirmed, patients received prostaglandin infusion to maintain the patency of the AD until surgical operation. Demographic postnatal variables and clinical data were obtained from the past medical records. Birthweight and length were expressed in centiles for gestational age using an appropriate reference range [Citation22]. The outcome of new-borns with prenatal suspicion of CoA was obtained through medical records analysis.
Echocardiographic parameters
All patients underwent a comprehensive TTE within the first hours/days of life, in presence of a large PDA, before starting the prostaglandin infusion. Recordings were saved on a digital medium and post-processed on a workstation (IntelliSpace CardioVascular 3.2).
One medical doctor (D.P.), with echocardiographic experienced and blinded to clinical outcomes, performed an off-line evaluation of the echocardiographic exams.
Dimensions, the thickness of left and right cardiac structures, and aortic diameters were measured according to the ASE/EACVI recommendations [Citation23,Citation24].
Measurements were obtained in end-diastole from the frame preceding mitral or tricuspid valve closure.
Measurements taken from the four-chamber view included: LV Volume (using the modified biplane Simpson method), RV length ad diameters. LV walls thickness and diameters (LVESD, LVEDD), aortic anulus, aortic sinuses and ascending aorta (AscAo) diameters were measured at parasternal long axis view. From the short axis view RV Outflow Tract, pulmonary artery (PA) and RV/LV diastolic diameters were obtained.
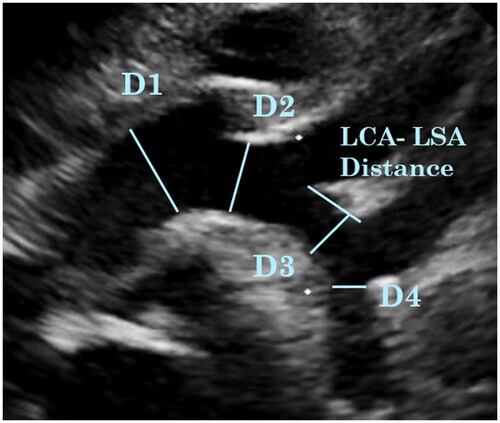
The aortic arch measurements were taken from the suprasternal notch view: D1 = arch diameter prior to Innominate artery (IA), D2 = arch diameter between IA and LCA, D3 = arch diameter between LCA and LSA, LCA-LSA Distance = length of the distal transverse arch between the LCA and LSA, D4 = aortic isthmic diameter ().
The three epiaortic vessels (IA, LCA and LSA) diameters were measured and the aortic arch branching pattern was noted. All vessel diameters were measured in end-systole from the inner edge to inner edge.
Morphological features of the aortic valve and aortic arch as well as the presence of associated minor CHDs were noted. All measurements were performed as described by Lang [Citation23].
For every patient, the Body Surface Area (BSA) was calculated using Haycock formula [Citation25] and corresponding children z-scores for the evaluation of cardiac chamber dimensions and arterial dimensions were assessed according to the most appropriate published guidelines [Citation26,Citation27].
The following measures were calculated: diastolic left-to-right ventricular diameter (LV/RV), D2/D1, D3/D1, D4/D1, LCA/D3, D1/Sinuses, D3/Sinuses, D4/Sinuses, D4/Abdominal Aorta, D3/weight, D4/weight, D3/BSA, D4/BSA. CSAi index was calculated as ratio of the aortic arch diameter (D3) at the LSA, to the distance between LCA and LSA.
Statistical analysis
Categorical variables were expressed as frequency, continuous variables were presented as mean ± standard deviation if normally distributed. Differences among categorical variables were analyzed using Chi-square test and normally distributed continuous variables with independent samples t test.
Univariable regression analysis was conducted to identify predictors associated with development of CoA.
Parameters significant on univariable analysis were then tested in a pairwise fashion, and variables were excluded when they were no longer significant on multivariate analysis. Moreover, variables were excluded when not contributing to the overall area under the curve (AUC).
We fit a logistic regression model, or coarctation probability model (CoMOD), to estimate the probability of CoA in need of surgical repair in the first weeks of life, based on significant predictors at univariable regression analysis. A p Value < .05 was considered statistically significant. All analyses were performed using STATA/IC version 15.
Results
Out of the 143 neonates with prenatal suspicion of CoA, 24 patients were excluded because of major extracardiac malformation, complex CHD or different CHD diagnosis at birth and 32 neonates were removed due to inadequate echocardiographic image quality.
We enrolled 87 neonates (49 male, 56%), with a median gestational age of 272 ± 11.4 days and birth weight 3136 ± 524 grams. Our population was then divided in two groups No CoA (43 patients, 49%) and CoA requiring surgical intervention (44 patients, 51%). There were no statistical significative differences in prenatal and birth characteristics (). All patients presented large PDA with bidirectional shunt during the echocardiographic exam. Due to PDA presence and to the increased pulmonary vascular resistance at birth, we can observe a lower SpO2 in the inferior limbs in both populations (Table-1a Supplementary Material), but differential SpO2 and differential Systolic Arterial Pressure (between the superior and inferior limbs) were similarly distributed between CoA and No Coa patients and almost all neonates in CoA group presented femoral pulses at birth (Table-1a, Supplementary Material).
Table 1. Prenatal and birth characteristics.
Table 2(a) (Supplementary Material) shows the LV main echocardiographic measures. The only significant difference between the two populations was the presence of Ventricular Septal Defect (VSD), which resulted more frequent in CoA group.
Of the 27 patients with VSD and CoA, 13 had a hemodynamically significant VSD, which underwent pulmonary artery banding together with CoA corrective surgery.
The main RV echocardiographic measures are reported in Table 3(a) (Supplementary Material). None of these parameters resulted noticeably different between coarctated and not coarctated neonates, neither the relation between RV/LV diameters (Table 2(a), Supplementary Material).
In the echocardiographic parameters of the Aorta are reported. Bicuspid Aortic Valve (BAV) was more prevalent in CoA population, while Bovine Arch was more frequently present in No CoA group. The dimensions D4, D3 and relative z-scores [Citation26] resulted significantly reduced in CoA group. Also, the D1, D2 and Abdominal Aorta appeared markedly smaller in CoA group, but this difference was not evident with z-scores. LCA- LSA distance resulted noticeably longer in CoA cohort.
Table 2. Echographic aortic parameters.
We also analyzed the relation between different parameters of the aortic arch, body weight and BSA (). We observed a different distribution of D3, D4 diameters indexed on weight, BSA and related to aortic sinuses.
Table 3. Derived parameters for aortic arch evaluation.
Univariable regression analysis was conducted to identify variables associated with development of CoA. summarizes the results of univariable regression analysis.
Table 4. Univariate regression Analysis for CoA.
Parameters significant on univariable analysis were then tested in a multivariable analysis, fitting a logistic regression model, named Coarctation Model (CoMOD) to estimate an index and a probability of CoA. We selected D3, D4, the presence/absence of BAV and VSD, LCA-LSA distance to be included in the model based on clinical relevance. The predictors met statistical significance at an α level of 0,05.
Lower D3 and D4 diameters, higher LCA-LSA distance and presence of BAV and/or VSD were associated with increased risk of CoA.
The equation for the model is:
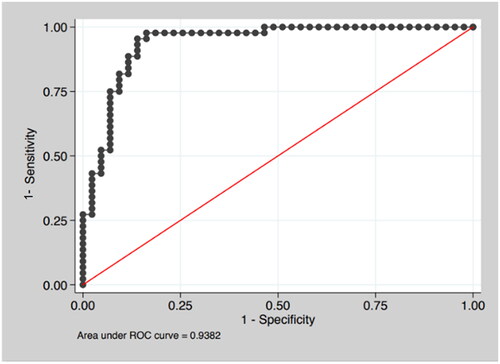
In the ROC Curve obtained with CoMOD applied to our population is reported. The model showed an AUC = 0.9382, high sensitivity (90.9%) and specificity (86.05%) ().
Table 5. Multivariable logistic model estimating the probability to develop CoA.
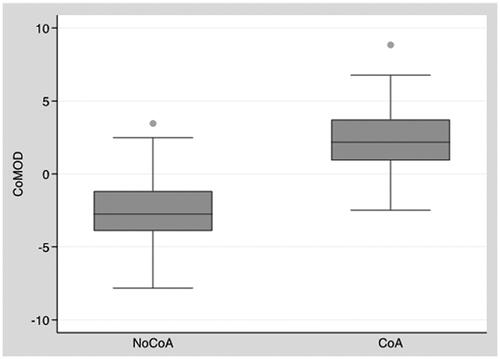
Inserting each individual measurement into the CoMOD equation provides an index allowing us to estimate the risk of CoA: an index >0 () demonstrated a good sensibility and specificity in predicting surgical CoA ().
Table 6. Diagnostic accuracy of CoA with CoMOD >0.
Discussion
Even if prenatal diagnostic tools have improved, antenatal detection of aortic coarctation remains challenging [Citation28]. Detecting CoA in fetal screening programs is associated with a high number of false positive diagnoses because it relies on indirect and nonspecific signs, especially cardiac asymmetry with right ventricle dominance. This implies that in many cases (>50–60%) CoA is postnatally excluded [Citation29]. Our results confirm this tendency and outline the need to have a reliable system to exclude or confirm CoA as soon as possible in the neonatal period.
Over the years, the method of diagnosis for CoA has changed from using clinical data, with or without preoperative catheter confirmation, to relying almost exclusively on echocardiography. This noninvasive assessment of aortic narrowing and flow measurement, with determination of instant gradient over the coarctation has become the gold standard but it still exhibits some limits, especially in neonatal period, when ductus arteriosus is patent. In fact, the presence of PDA may obscure the diagnosis of CoA until it fully constricts, as aortic flow through the PDA may bypass the juxtaductal obstruction and supply the lower body [Citation30].
Numerous previous studies have examined echocardiographic findings of patients with a definitive diagnosis of CoA and compared them with those subjects with no evidence of CoA and normal heart, but still, no definitive parameters allow a certain diagnosis of CoA prior AD closure.
To our knowledge, this study is the first to use a logistic regression model to analyze the prognostic ability of echocardiographic parameters to predict the development of CoA requiring surgical correction, in neonates with PDA and prenatal suspicion of this condition.
Our cohort consisted of term and late pre-term neonates with an average weight adequate for gestational age and without adaptation problems to extrauterine life. We based our study on previously published indices and known risk factors associated with CoA and reached to find out an interesting score able to help clinicians to be oriented in the diagnosis and predict the development of the disease.
As attended, BAV and VSD were more frequent in CoA group, confirming the major prevalence of these anomalies associated with CoA when compared to the healthy general population [Citation9,Citation31].
Unexpectedly, bovine arch morphology was significantly more present in No CoA group strengthening the hypotheses of Vigneswaran et al. [Citation32] of the bovine arch being a confounding factor for CoA suspicion in fetal life. Their hypothesis consisted of the presence of an underdeveloped distal arch in this population due to reduced blood flow in utero.
As expected, the dimension of the posterior arch (D3) and isthmus (D4) and relative z-scores resulted in significant narrowed in CoA Group, and the LCA-LSA distance was confirmed to be longer in CoA patients as previously described [Citation18,Citation21,Citation33]. Nevertheless, the mean z-score of D3 and D4 resulted superior to −2 SD, bringing into question this threshold as a pathologic criterion in CoA and its use in the diagnostic field.
With regards to aortic arch indexed values, we noticed some interesting strong differences between groups. In particular, D4/Weight index and its cutoff >1 showed a good relation to unobstructed aortic arches: the higher the value, the less probable the development of CoA (AUC 0.8009 and 0.7119 for D4/Weight and D4/Weight >1 respectively). To our knowledge, it is the first time that D4 (aortic isthmus) has been related to body weight. This rapid tool could be employed as the first approximate evaluation of the aortic arch in newborns. Moreover, based on our experience, using this ratio could be more helpful in the arch evaluation than z-score, which we have proven not to be reliable as an absolute value.
Peng et al. [Citation34] previously showed CSAi to be a simple and reproducible measure that can identify neonates at risk for CoA even in the presence of AD. Despite the high sensitivity and specificity of the CSAi, they underlined that it should not be applied alone, but rather in combination with other supportive clinical data before deciding on the need for surgical intervention.
Soslow et al. fit a regression model comprehensive of CSAi and LCA/D3 to help identify CoA in neonates with PDA [Citation22]. When investigated in our population, and at univariate logistic regression analysis, these two relations appeared significantly different between the two groups, with AUC respectively of 0.7944 and 0.6773. In our population, a lower cutoff of CSAi of 0,8 [33] detected 82% of patients with CoA, but still, 28% of patients in No CoA group were included.
We evaluated the most significant derived parameters (CSAi, LCA/D3, D4/Weight, D4/D1, D4/Sinuses) at multivariate regression analysis, but we excluded them from the final regression model because of their loss of statistical power when compared to integer parameters.
According to this thought, we decided to include in the model only linear dimensions and simple anomalies typically associated with the disease object of our study: D3, D4, LCA-LSA distance, BAV and VSD presence.
The resulting CoMOD showed good sensitivity (86.05%) and sensibility (90.9%) in our cohort with a positive predictive value of 86,9% and a negative predictive value of 90.2%, when applying a cutoff value of 0. Therefore, CoMOD allows good risk stratification in new-borns with prenatal suspicion of CoA in presence of PDA using five simple echocardiographic parameters.
Given the dynamic remodeling of aortic isthmus during ductal closure, close clinical monitoring of impending coarctation is usually recommended in neonates with prenatal suspicion. Our score, if prospectively validated, may allow better patient streaming, reduce inappropriate neonatal transfer to intensive pediatric cardiology units, enhance costs containment, reduce family stress and improve mother-infant bonding and breastfeeding.
Study limitations
CoMOD was created from a retrospective cohort and it needs to be validated prospectively. Both cohorts were relatively small and further validations on a larger patient population are necessary. Our cohort consisted of new-borns with prenatal suspicion of CoA and didn’t include pair-matched normal controls.
Dynamic evaluations such as isthmal and ductal flow patterns were not studied as they were not recoverable for all exams.
Conclusions
CoMOD is a new model derived from a retrospective observation of new-borns with prenatal suspicion of CoA and PDA. A CoMOD > 0 predicts the onset of CoA needing neonatal surgery with good sensitivity and specificity employing 5 simple echocardiographic items (D3, D4, BAV, VSD, LCA-LSA distance). If the good reliability of CoMOD will be confirmed, it could help identify neonates with a high risk of CoA, who need to be observed in an intensive way versus patients at low risk of CoA, who can be early discharged or hospitalized in non-intensive settings, reducing family stress, healthcare-related costs and improving mother-infant bonding and breastfeeding in this group of patients.
More rapid tools for a gross evaluation of the adequacy of D4 in presence of PDA are: D4/Weight >1, D4/D1 > 50%, CSAi >0.8. This is the first time to our knowledge that the aortic isthmus diameter is directly related to body weight.
BAV and VSD are more frequent in patients with CoA, while the common origin of IA and LCA could be a confounding factor in fetal life, being more common in patients not developing CoA.
To conclude, Aortic Coarctation remains an insidious entity to be detected, both in the antenatal and post-natal period. Further studies are needed, but our index CoMOD could be a useful tool in association with clinical assessment to guide the management of these patients in the neonatal period.
Author contributors’
Dr Bartolacelli, Dr Palleri, Dr Balducci, Dr Ragni, conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. Dr Bartolacelli, Dr Palleri, Dr Bonetti, Dr Hasan, Dr Fabi, designed the data collection instruments, collected data, carried out the initial analyses. Dr Bartolacelli, Dr Palleri, Dr Egidy Assenza, Dr Mariucci, Dr Angeli reviewed and revised the manuscript. Professor Gargiulo and Dr Donti conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (24.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- van der Linde D, Konings EEM, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58(21):2241–2247.
- Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002; 39(12):1890–1900.
- Campbell M, Polani PE. The aetiology of coarctation of the aorta. Lancet. 1961; 1(7175):463–468.
- Shone JD, Sellers RD, Anderson RC, et al. The developmental complex of ‘parachute mitral valve,’ supravalvular ring of left atrium, subaortic stenosis, and coarctation of aorta. Am J Cardiol. 1963;11:714–725.
- Brown ML, Burkhart HM, Connolly HM, et al. Coarctation of the aorta: lifelong surveillance is mandatory following surgical repair. J Am Coll Cardiol. 2013;62(11):1020–1025.
- Nguyen L, Cook SC. Coarctation of the aorta: strategies for improving outcomes. Cardiol Clin. 2015; 33(4):521–530, vii.
- Luciani GB, Hoxha S, Angeli E, et al. Selective versus standard cerebro-myocardial perfusion in neonates undergoing aortic arch repair: a multi-center study. Artif Organs. 2019;43(8):728–735.
- Ewer AK, Furmston AT, Middleton LJ, et al. Pulse oximetry as a screening test for congenital heart defects in newborn infants: a test accuracy study with evaluation of acceptability and cost-effectiveness. Health Technol Assess. 2012;16(2): v-xiii, 1–184.
- Roos-Hesselink JW, Schölzel BE, Heijdra RJ, et al. Aortic valve and aortic arch pathology after coarctation repair. Heart. 2003;89(9):1074–1077.
- Rudolph AM, Heymann MA, Spitznas U. Hemodynamic considerations in the development of narrowing of the aorta. Am J Cardiol. 1972; Oct30(5):514–525.
- Sanders SP, MacPherson D, Yeager SB. Temporal flow velocity profile in the descending aorta in coarctation. J Am Coll Cardiol. 1986; Mar 17(3):603–609.
- Ho SY, Anderson RH. Coarctation, tubular hypoplasia, and the ductus arteriosus. Histological study of 35 specimens. Br Heart J. 1979; Mar41(3):268–274.
- Russell GA, Berry PJ, Watterson K, et al. Patterns of ductal tissue in coarctation of the aorta in the first three months of life. J Thorac Cardiovasc Surg. 1991;102(4):596–601.
- Familiari A, Morlando M, Khalil A, et al. Risk factors for coarctation of the aorta on prenatal ultrasound: a systematic review and meta-analysis. Circulation. 2017;135(8):772–785.
- Wren C, Reinhardt Z, Khawaja K. Twenty-year trends in diagnosis of life-threatening neonatal cardiovascular malformations. Arch Dis Child Fetal Neonatal Ed. 2008; Jan93(1):F33–35.
- Tegnander E, Williams W, Johansen OJ, et al. Prenatal detection of heart defects in a non-selected population of 30,149 fetuses–detection rates and outcome. Ultrasound Obstet Gynecol. 2006;27(3):252–265.
- Hornberger LK, Sahn DJ, Kleinman CS, et al. Antenatal diagnosis of coarctation of the aorta: a multicenter experience. J Am Coll Cardiol. 1994;23(2):417–423.
- Dodge-Khatami A, Ott S, Bernardo SD, et al. Carotid-subclavian artery index: new echocardiographic index to detect coarctation in neonates and infants. Ann Thorac Surg. 2005;80(5):1652–1657.
- Lu C-W, Wang J-K, Chang C-I, et al. Noninvasive diagnosis of aortic coarctation in neonates with patent ductus arteriosus. J Pediatr. 2006;148(2):217–221.
- Morrow R, Huhta W, Murphy JC, et al. Quantitative morphology of the aortic arch in neonatal coarctation. J Am Coll Cardiol. 1986;8(3):616–620.
- Soslow JH, Kavanaugh-McHugh A, Wang L, et al. A clinical prediction model to estimate the risk for coarctation of the aorta in the presence of a patent ductus arteriosus. J Am Soc Echocardiogr. 2013;26(12):1379–1387.
- Bertino E, Spada E, Occhi L, et al. Neonatal anthropometric charts: the Italian neonatal study compared with other European studies. J Pediatr Gastroenterol Nutr. 2010;51(3):353–361.
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the european association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14.
- Wasserman MA, Shea E, Cassidy C, et al. Recommendations for the adult cardiac sonographer performing echocardiography to screen for critical congenital heart disease in the newborn: from the American society of echocardiography. J Am Soc Echocardiogr. 2021;34(3):207–222.
- Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978; Jul93(1):62–66.
- Cantinotti M, Giordano R, Scalese M, et al. Nomograms for two-dimensional echocardiography derived valvular and arterial dimensions in Caucasian children. J Cardiol. 2017;69(1):208–215.
- Cantinotti M, Scalese M, Murzi B, et al. Echocardiographic nomograms for chamber diameters and areas in Caucasian children. J Am Soc Echocardiogr. 2014;27(12):1279–1292.e2.
- Franklin O, Burch M, Manning N, et al. Prenatal diagnosis of coarctation of the aorta improves survival and reduces morbidity. Heart. 2002;87(1):67–69.
- Gómez-Montes E, Herraiz I, Gómez-Arriaga PI, et al. Gestational age-specific scoring systems for the prediction of coarctation of the aorta. Prenat Diagn. 2014;34(12):1198–1206.
- Vergales JE, Gangemi JJ, Rhueban KS, et al. Coarctation of the aorta - the current state of surgical and transcatheter therapies. Curr Cardiol Rev. 2013;9(3):211–219.
- Darabian S, Zeb I, Rezaeian P, et al. Use of noninvasive imaging in the evaluation of coarctation of aorta. J Comput Assist Tomogr. 2013;37(1):75–78.
- Vigneswaran TV, Bellsham-Revell HR, Chubb H, et al. Echocardiography in neonates with a prenatal suspicion of coarctation of the aorta. Pediatr Cardiol. 2020;41(4):772–780.
- Mivelaz Y, Di Bernardo S, Meijboom EJ, et al. Validation of two echocardiographic indexes to improve the diagnosis of complex coarctations. Eur J Cardiothorac Surg. 2008;34(5):1051–1056.
- Peng DM, Punn R, Maeda K, et al. Diagnosing neonatal aortic coarctation in the setting of patent ductus arteriosus. Ann Thorac Surg. 2016;101(3):1005–1010.