Abstract
Objective
The literature on the incidence of traumatic brain injury (TBI) during pregnancy is lacking. Furthermore, only studies with small sample size have analyzed the impact of TBI during pregnancy to maternal and fetal outcomes. Thus, we aim to report the incidence of TBIs during pregnancy and study the pregnancy outcomes using nationwide high-quality registers.
Methods
This nationwide retrospective register-based matched cohort study utilized two national registers. All fertile-aged (15–49 years) women with a TBI hospitalization period during pregnancy were retrieved the Care Register for Health Care. Data were then linked with the data from the National Medical Birth Register (MBR). Propensity score matching was conducted according to maternal age during pregnancy, previous cesarean section (CS), maternal smoking status, maternal body mass index, and maternal gestational diabetes. The matching was conducted using the nearest neighbor methods with a caliber width if 0.15, and with a ratio 1:3 (patients/references). Adverse maternal and fetal outcomes were compared between patient group and reference group using Chi-squared tests.
Results
A total of 392 women having a TBI during pregnancy were found. The control group consisted of 722,497 women without TBI during pregnancy. Of the TBIs occurring during pregnancy, the most common types of TBIs were concussion (S06.0) (n = 359, 91.6%), diffuse traumatic brain injury (S06.2) (n = 11, 2.8%), traumatic subdural hemorrhage (n = 7, 1.8%), and unspecified intracranial injury S06.9 (n = 6, 1.5%). The incidence rates of pregnancies with a TBI have remained similar during pregnancy in Finland, peaking at 0.8 per 1000 pregnancies in 2016. The Chi-squared test showed higher rate for CS among women with TBI than for their matched references (21.4% vs. 15.5%, p = .008). Especially, women with TBI during 3rd trimester had higher rate for CS (29.0 vs. 15.0%, p = .016).
Conclusions
The main findings of this study were that the incidence rates for TBI during pregnancy have remained similar during our study period (2004–2018). TBI during pregnancy, even a mild one, is associated with an increased rate for CS. Especially, TBI during the 3rd trimester was associated with high rate for CS, but the etiology behind this remains unknown. In addition, we found no evidence of difference in fetal outcomes, such as preterm birth, low birth weight, or need for intensive care unit. Future studies should focus on the indications for elective CS, and reasons for unplanned CS among women with TBI during pregnancy, as these could possibly provide important information on the effects of TBI on the course of childbirth.
Introduction
Traumatic brain injuries (TBIs) are common as those affect more than 10 million people worldwide annually [Citation1], with the most common causes being traffic accidents, falls, and sports related activities [Citation2]. An international study has estimated the incidence of TBI globally to be approximately 369 per 100,000 person-years [Citation3]. For fertile-aged women, TBI is reported to cause disorders in the menstrual cycle and nearly 50% of women report amenorrhea following TBI [Citation4,Citation5]. In Finland, the incidence of TBI hospitalization in fertile-aged woman has increased over twofold (150%) between 1998 and 2018 [Citation6]. However, there are little studies on the effects of TBI during pregnancy.
Currently, the effects of TBI on pregnancy and delivery are known poorly. TBIs have major complications that can affect the mother and the fetus. For instance, one of the major consequence of TBI is elevation of intracranial pressure that is known to be an independent risk factor associated with a risk of death, decrease in functional capacity, and cognition of TBI patients [Citation7,Citation8]. One of the potential risks in TBI soon before childbirth may be the elevation of cerebrospinal fluid pressure increase during delivery in a response to pain [Citation9], causing further complications in TBI patient. A recent meta-analysis published in 2023, with a total of 43 patients found that fetal death rate was highest in TBIs occurring during 2nd trimester [Citation10]. As there are no proper evidence on the subject, it is unknown whether vaginal delivery is safe for patients who have experienced TBI during pregnancy.
The aim of this nationwide register study is to report the incidence of TBI during pregnancy and to investigate the impact of TBI during pregnancy on pregnancies and deliveries.
Materials and methods
In this nationwide retrospective register-based matched cohort study, data from the Care Register for Health Care were linked with the data from the National Medical Birth Register (MBR) to evaluate the effects of TBIs during pregnancy on maternal and fetal outcomes. Both registers are maintained by the Finnish Institute for Health and Welfare. The study period was from 1 January 2004 to 31 December 2018.
All fertile-aged (15–49 years) women with a hospitalization period with a TBI diagnosis during our study period were retrieved from the Care Register for Health Care. The Care Register for Health Care contains data on the patients treated in hospitals as inpatients, surgeries, and outpatient care in specialized healthcare (including emergency department visits) in secondary and tertiary level units. Finland has universal healthcare with minimal costs per visit for patients and thus, all Finnish inhabitants are eligible to this social healthcare in public healthcare [Citation11]. The quality of the Care Register for Health Care has shown to be good [Citation12]. TBI was defined as a hospitalization period following TBI based on ICD-10 (International Classification of Diseases 10th revision) codes. The following ICD-10 codes from the Care Register for Health Care were included: S06.0 (Concussion), S06.1 (Traumatic cerebral edema), S06.2 (Diffuse traumatic brain injury), S06.3 (Focal traumatic brain injury), S06.4 (Epidural hemorrhage), S06.5 (Traumatic subdural hemorrhage), S06.6 (Traumatic subdural hemorrhage), S06.8 (Other specified intracranial injury), and S06.9 (Unspecified intracranial injury). To identify only new TBIs and not the control appointments, only TBIs with over one-year period from the previous appointment with the TBI diagnosis were included in this study.
Data retrieved from the Care Register for Health Care were combined with data from the National MBR using the pseudonymized identification number of the mother. The MBR contains information on all pregnancies, delivery statistics, and the perinatal outcomes of births with a birthweight of ≥500 g or a gestational age ≥22 + 0. The MBR has a coverage of 100% [Citation13,Citation14]. The dates of the TBI diagnosis and the dates of the pregnancies were used to identify the pregnancies with a TBI occurring during pregnancy. Multiple pregnancies and pregnancies with missing maternal pre-pregnancy body mass index (BMI) were excluded from the analysis. Multiple pregnancies were excluded, as these are more commonly more complicating events for both, fetuses and mothers and therefore they are not comparable to singleton pregnancies [Citation15].
Statistics
The annual incidences of TBIs during pregnancy were calculated using the annual total number of deliveries obtained from the MBR. During our study period, the annual total number of deliveries decreased from 57,527 pregnancies in 2004 to 46,964 pregnancies in 2018 in Finland. The incidences are presented as TBIs per 1000 pregnancies with 95% confidence intervals (CIs). The CIs for the incidences were calculated using Poisson’s regression.
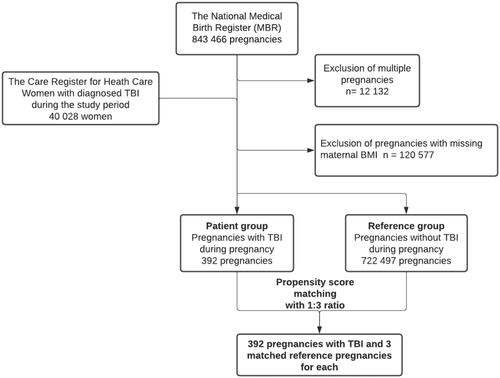
A flowchart of patient selection is presented in . A total of 392 patients with TBI during pregnancy were identified, and the control group consisted of 722,497 pregnancies without TBI. Propensity score matching resulted in 392 patients with three reference pregnancies for each patient, comprising a total of 1176 matched pregnancies in the reference group. Propensity score matching was conducted using the nearest neighbor methods with a caliber width if 0.15, and with a ratio 1:3 (patients/references), as the size of our data was large, and the covariate balance remained acceptable. Covariate balance was measured by calculating the z-difference between the patient group and the reference groups [Citation16]. A z-difference of less than ±1 was considered acceptable. Propensity score matching was conducted according to maternal age during pregnancy, previous CS, maternal smoking status, maternal BMI, and maternal gestational diabetes (GDM). The matching variables were selected according to previous knowledge on clinical risk factors for adverse maternal or fetal outcomes [Citation17–23]. Details of maternal smoking status during pregnancy are collected during visits to maternity clinics and can be either nonsmoker, smoking during first semester, smoker or unknown. According to a reliability study on the MBR in 1991, the reliability of the smoking status variable was found to be good [Citation24]. GDM was diagnosed using the 75 g 2-h oral glucose tolerance test. A flowchart on forming the study groups and how the propensity score was conducted is shown in .
Figure 1. Flowchart of the study population. Data from the MBR were combined with data on the diagnosed TBI in the Care Register for Health Care. Multiple pregnancies and pregnancies with missing body mass index (BMI) were excluded from the analysis.
Continuous variables were interpreted as mean with standard deviation or as median with interquartile range based on variable distribution. Categorized variables were presented as absolute numbers and percentages with 95% CIs. The 95% CIs for rates were calculated using Poisson’s regression. Finnish research legislation regarding register studies and the secondary use of routinely collected healthcare data prevents reporting of events with absolute numbers below 5 due to possible identification of an individual. Therefore, all numbers below 5 are presented as <5 in tables. After propensity score matching, the adverse maternal and fetal outcomes were compared between patient group and reference group using Chi-squared tests. Adverse maternal outcomes were overall cesarean section (CS), induction of labor, uterine curettage, and manual placenta removal. Due to low number of pregnancies, CS classified as elective or unplanned (included emergency and urgent CS). The adverse neonatal outcomes were needed for intensive care unit (NICU), preterm birth (<37 + 0 weeks), low birth weight (LBW, <2500 grams), and perinatal mortality.
In the subgroup analysis, women with TBI were divided into three groups, based on which trimester the TBI occurred. These patients were then compared to their matched pairs. The date of giving birth and registered length of the pregnancy were used to calculate the starting date of the pregnancy. Information about the time of the TBI in relation to the progression of the pregnancy was gained by dividing the pregnancy into trimesters using the length of the pregnancy. Statistical analysis was performed using R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). The results of this study are reported according to the STROBE guidelines [Citation25].
Ethics
All methods were carried in accordance with Finnish regulations. The Ethical Committee of Tampere University hospital waived the ethical committee evaluation of all retrospective studies utilizing routinely collected healthcare data and this decision is based on the law of medical research 488/1999 and the law of patient rights 785/1992. In accordance with the Finnish regulations (the law of secondary use of routinely collected healthcare data 552/2019), no informed written consent was required because of the retrospective register-based study design and the patients were not contacted. Both the National MBR and the Care Register for Health Care have the same unique pseudonymized identification number for each patient. The pseudonymization was made by the Finnish data authority Findata. The authors did not have access to the pseudonymization key, as it is maintained by Findata. Permission for use of these data was granted by Findata after evaluation of the study protocol (permission number: THL/1756/14.02.00/2020).
Results
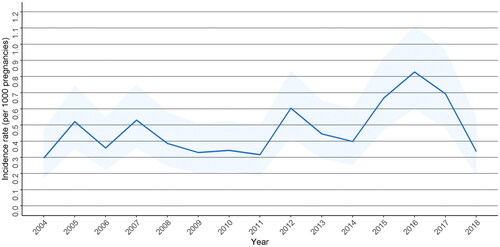
In total, 40,028 suffered a TBI during our study period. A total of 392 women had a TBI during pregnancy. The matched reference group consisted of 1176 women without TBI during pregnancy. Of the TBIs occurring during pregnancy, the most common type of TBIs were concussion (S06.0) (n = 359, 91.6%), diffuse traumatic brain injury (S06.2) (n = 11, 2.8%), traumatic subdural hemorrhage (n = 7, 1.8%), and unspecified intracranial injury S06.9 (n = 6, 1.5%). No other TBI diagnosis occurred more than five times. The incidence rates of pregnancies with a TBI have remained similar during pregnancy in Finland, peaking at 0.8 per 1000 pregnancies in 2016 ().
Figure 2. Incidence rates as per 1000 pregnancies with 95% confidence intervals of pregnancies with TBI during years 2004–2018 in Finland.
Propensity score matching led to 392 pregnancies in patient group, and 1176 matched references. The differences in the matching variables between the two groups were low, as the z-differences remained between ±1. Women in the TBI group were younger than the reference group including all pregnancies without TBI (28.5 years, SD 6.5 vs. 29.8 years, SD 5.4). High rate of smokers after 1st trimester was found in the TBI group (18.4%, CI 14.4–23.1) (). The women in TBI group had lower rate of spontaneous vaginal birth (66.1% vs. 75.6%) and higher rates of assisted vaginal (12.5% vs. 8.7%), unplanned CS (11.7% vs. 9.0%), and elective CS (9.7% vs. 6.5%) compared to the matched reference group adjusted for confounding factors (). A total of 6 (1.5%, CI 0.6–3.3) emergency CS were found among women with TBI during pregnancy, and 11 (0.9%, CI 0.5–1.7) emergency CS among matched references.
Table 1. Patient characteristics before and after propensity score matching.
Table 2. Maternal and fetal outcomes in pregnancies with traumatic brain injury (TBI) during pregnancy, when compared to reference group consisting of pregnancies without.
In a subgroup analysis for TBIs during different stages of pregnancy, a high rate of labor induction was seen in pregnancies with a TBI during first trimester compared to the reference group (24.2%, 95% vs. 19.2%, 95%). In addition, the rate of CS was higher in all TBI groups compared to the reference groups (). The Chi-squared test showed higher rate for CS among women with TBI than for their matched references (21.4% vs. 15.5%, p = .008). Especially, women with TBI during 3rd trimester had higher rate for CS. (29.0 vs. 15.0%, p = .016). No evidence of difference was found in the fetal outcomes ().
Table 3. Subgroup analyses maternal and fetal outcomes in pregnancies with traumatic brain injury during pregnancy, when compared to reference group consisting of matched pregnancies without TBI based on which trimester the TBI occurred.
Table 4. Statistical significance measured for adverse maternal and fetal outcomes between patient group and matched references using Pearson’s Chi-squared test.
When only 33 cases of severe TBIs included (concussion excluded), no evidence of difference was found in the rate for CS (24.2% vs. 16.2%, p = .434), or neonatal outcomes, when compared to their matched references.
Discussion
The main findings of this study were that the incidence rates for TBI have remained similar during our study period. Also, TBI during pregnancy was associated with increased rate for CS during pregnancy. Interestingly, especially, TBI during 3rd trimester was associated with high rate for CS. In addition, we found no evidence of difference in fetal outcomes, such as preterm birth, LBW, or NICU between women with TBI during pregnancy and reference group.
The previous studies on the effects of TBIs on maternal and fetal outcomes during pregnancy are lacking, and therefore, the results of this study should provide basic information on rare events such as TBIs during pregnancy. A recent study in Finland using a large nationwide study-sample found that TBIs occurring before pregnancy increased the odds for CS, and instrumental vaginal deliveries among women with previous TBI [Citation6]. This study concluded that history of TBI should be acknowledged as a possible factor affecting the delivery and health of the neonate [Citation6]. In our study, the odds for CS was also higher, which is in line with these results. TBI in the 3rd trimester, despite the lowest absolute number of deliveries, showed important difference in the rates for CS. However, due to crude nature of our data, the exact reason for this remains unknown. Also, over 50% of CS deliveries in this group were elective CS, but the rate for unplanned CS was also high. A TBI in the 3rd trimester might be a reason to convert a trial of labor into elective CS, but as the indications for elective CS is not collected in the MBR, the reason for the elective CS remains unknown. One possible explanation might be the short time difference between TBI and delivery, which could lower the threshold for elective CS by the clinician and mother, as the capability to give birth in a short period after TBI remains unknown due to lack of knowledge on this topic. In addition, post TBI psychological symptoms might also have effect on the decision about the mode of delivery [Citation26]. Especially, TBIs in 3rd trimester should be acknowledged as a possible factor affecting the course of delivery and more research on this topic is warranted. Future studies should focus on the indications for elective CS, and reasons for unplanned CS among women with TBI during pregnancy, as these could possibly provide important information on the effects of TBI on the course of childbirth.
Interestingly, the fetal outcomes among women with TBI during pregnancy were not impaired. This is an important finding, as there are no previous studies using sufficient number of patients with TBI during pregnancy. A recent meta-analysis about TBIs during pregnancy found that the fetal death rate was highest in TBIs occurring during 2nd trimester [Citation10]. However, no fetal death was observed in mild TBI group [Citation10]. However, the total number of patients in this meta-analysis was only 43 [Citation10]. The latest study examining the effects of TBIs before pregnancy found that the odds for preterm deliveries, and NICU was higher among women with TBI before pregnancy [Citation6]. However, our results suggest that TBI at any trimester of pregnancy is not crucial for the health of neonate. However, it is good to note that most of the TBIs in this study are presumably mild TBIs, and the conclusions about the effects of moderate or severe TBIs cannot be taken. In an expert’s viewpoint on the management of moderate and severe TBIs during pregnancy, the status of the mother and fetus is critical and the management is balancing between maternal health and neonatal health [Citation27], meaning that the results of these studies are most likely not comparable to these.
The strength of our study is the large nationwide study population with a long study period, making it possible to compare largest patient groups so far on this topic. On such rare events as TBIs during pregnancy, no previous studies have been able to investigate these events using proper data. Therefore, most of the previous studies have been case reports or studies using small population. The register data used in our study are routinely collected with structured forms with national instructions, which ensures good coverage and reduces possible reporting and selection bias [Citation14]. Furthermore, the quality and coverage of both registers included in this study is high [Citation14,Citation12]. The advantage of this study compared to previous ones is the large national research material in a country with uniform delivery-related guidelines and attitudes.
The main limitation of our study is the missing clinical information on TBIs (e.g. radiological findings and TBI severity indices such as Glasgow Coma Scale). Also, our study includes only women who ended up giving birth, as those possible patients suffering a truly severe TBI leading to miscarriage or even death are not available in our data. Also, majority of the patients in this study suffered a concussion, meaning that patient group is probably mostly consisting of the patients with mild TBI.
Conclusions
The main findings of this study were that the incidence rates for TBI during pregnancy have remained similar during our study period (2004–2018). TBI during pregnancy, even a mild one, is associated with an increased rate for CS. Especially, TBI during the 3rd trimester was associated with high rate for CS, but the etiology behind this remains unknown. In addition, we found no evidence of difference in fetal outcomes, such as preterm birth, low birth weight, or NICU, and therefore, it appears that mild TBIs during pregnancy have less impact on fetal health. Future studies should focus on the indications for elective CS, and reasons for unplanned CS among women with TBI during pregnancy, as these could possibly provide important information on the effects of TBI on the course of childbirth.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hyder AA, Wunderlich CA, Puvanachandra P, et al. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. 2007;22(5):341–353.
- Ng SY, Lee AYW. Traumatic brain injuries: pathophysiology and potential therapeutic targets. Front Cell Neurosci. 2019;13:528.
- GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(1):56–87.
- Koskinen S, Alaranta H. Traumatic brain injury in Finland 1991–2005: a nationwide register study of hospitalized and fatal TBI. Brain Inj. 2008;22(3):205–214.
- Ripley DL, Harrison-Felix C, Sendroy-Terrill M, et al. The impact of female reproductive function on outcomes after traumatic brain injury. Arch Phys Med Rehabil. 2008;89(6):1090–1096.
- Vaajala M, Kuitunen I, Nyrhi L, et al. Pregnancy and delivery after traumatic brain injury: a nationwide population-based cohort study in Finland. J Matern Fetal Neonatal Med. 2022;35(25):9709–9716.
- Steyerberg EW, Wiegers E, Sewalt C, et al. Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: a European prospective, multicentre, longitudinal, cohort study. Lancet Neurol. 2019;18(10):923–934.
- Badri S, Chen J, Barber J, et al. Mortality and long-term functional outcome associated with intracranial pressure after traumatic brain injury. Intensive Care Med. 2012;38(11):1800–1809.
- Marx GF, Zemaitis MT, Orkin LR. Cerebrospinal fluid pressures during labor and obstetrical anesthesia. Anesthesiology. 1961;22(3):348–354.
- Al Fauzi A, Apriawan T, Ranuh I, et al. Traumatic brain injury in pregnancy: a systematic review of epidemiology, management, and outcome. J Clin Neurosci. 2023;107:106–117.
- Keskimaki I, Tynkkynen LK, Reissell E, et al. Finland: health system review. Health Syst Transit. 2019;21(2):1–166.
- Sund R. Quality of the Finnish hospital discharge register: a systematic review. Scand J Public Health. 2012;40(6):505–515.
- Gissler M, Shelley J. Quality of data on subsequent events in a routine Medical Birth Register. Med Inform Internet Med. 2002;27(1):33–38.
- Gissler M, Teperi J, Hemminki E, et al. Data quality after restructuring a national medical registry. Scand J Soc Med. 1995;23(1):75–80.
- Norwitz ER, Edusa V, Park JS. Maternal physiology and complications of multiple pregnancy. Semin Perinatol. 2005;29(5):338–348.
- Kuss O. The z-difference can be used to measure covariate balance in matched propensity score analyses. J Clin Epidemiol. 2013;66(11):1302–1307.
- Bergholt T, Skjeldestad FE, Pyykönen A, et al. Maternal age and risk of cesarean section in women with induced labor at term—a Nordic register-based study. Acta Obstet Gynecol Scand. 2020;99(2):283–289.
- Zhuang W, Lv J, Liang Q, et al. Adverse effects of gestational diabetes-related risk factors on pregnancy outcomes and intervention measures. Exp Ther Med. 2020;20(4):3361–3367.
- Poston L, Harthoorn LF, van der Beek EM, et al. Obesity in pregnancy: implications for the mother and lifelong health of the child. A consensus statement. Pediatr Res. 2011;69(2):175–180.
- Yang Z, Phung H, Freebairn L, et al. Contribution of maternal overweight and obesity to the occurrence of adverse pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2019;59(3):367–374.
- Lurie S, Ribenzaft S, Boaz M, et al. The effect of cigarette smoking during pregnancy on mode of delivery in uncomplicated term singleton pregnancies. J Matern Fetal Neonatal Med. 2014;27(8):812–815.
- Knopik VS. Maternal smoking during pregnancy and child outcomes: real or spurious effect? Dev Neuropsychol. 2009;34(1):1–36.
- Kuitunen I, Huttunen TT, Ponkilainen VT, et al. Incidence of obese parturients and the outcomes of their pregnancies: a nationwide register study in Finland. Eur J Obstet Gynecol Reprod Biol. 2022;274:62–67.
- Gissler M, Teperi J, Forssas E, et al. Syntymärekisterin Luotettavuustutkimus 1991. Stakes, aiheita; No. 11; 1993.
- von EE, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349.
- Ryan LM, Warden DL. Post concussion syndrome. Int Rev Psychiatry. 2003;15(4):310–316.
- Di Filippo S, Godoy DA, Manca M, et al. Ten rules for the management of moderate and severe traumatic brain injury during pregnancy: an expert viewpoint. Front Neurol. 2022;13:911460.

