Abstract
Objective
To evaluate the efficacy of preoperative low-residue diet on postoperative ileus in women undergoing elective cesarean section (CS).
Methods
This is a surgeon-blind, randomized controlled trial enrolling pregnant women at ≥39 weeks of gestation undergoing elective CS. Patients were preoperatively randomized to receive either low-residue diet (arm A) or free diet (arm B) starting from three days before surgery. The primary outcome was the postoperative ileus. The secondary outcomes were the postoperative pain (assessed through VAS scale), the quality of the surgical field (scored using a 5-point scale, from poor to excellent), postoperative complications, and the length of hospital stay. Perioperative data were collected and compared between groups.
Results
A total of 166 patients were enrolled and randomized in arm A (n = 83) and arm B (n = 83). Postoperative ileus over 24 h was significantly shorter in arm A, compared to arm B (19.3% vs 36.2%). The surgical evaluation of small intestine was scored ≥3 in 96.4% of arm A patients versus 80.7% in arm B, while evaluation of large intestine, respectively, in 97.7% and 81.9%. Postoperative pain after 12 h from CS was significantly lower in arm A (VAS, 3.4 ± 1.7) compared to arm B (VAS, 4.1 ± 1.8). There were no significant differences as regards postoperative pain at 24 and 48 h, nausea/vomit, surgical complications, and hospital stay.
Conclusions
Implementation of a preoperative low-residue diet for women scheduled for elective CS would reduce postoperative ileus and pain. Further large-scale studies are required before translating these research findings into routine obstetrical practice.
1. Introduction
Cesarean section (CS) represents one of the most commonly performed surgeries worldwide, with nearly 19 million procedures recorded yearly [Citation1]. Considering this high surgical burden, increasing interest has been raised in investigating the role of enhanced recovery after surgery (ERAS) protocol in the obstetric surgical care [Citation2].
Enhanced recovery after surgery is a multidisciplinary, evidence-based, standardized program to improve the perioperative care and recovery of surgical patients with both clinical (reductions in length of stay, complications, and readmissions, earlier return to daily activities) and health system benefits (reduction in costs) [Citation2,Citation3]. As the first ERAS pathway was developed in colorectal surgery nearly two decades ago, these protocols have been successfully applied in several surgical specialties, including orthopedic, urologic, and gynecologic surgery [Citation4–12]. However, its application in the field of obstetrics for women undergoing CS has attracted less critical attention, and the literature is still limited [Citation3,Citation13–15].
Among the key elements of ERAS protocols, bowel preparation and preoperative nutrition care have been, and continues to be, a long-standing matter of debate [Citation16]. This acquires even more importance for obstetric patients where at least two patients are impacted and a special focus on fetal health is required. Currently, the ERAS Society guidelines do not recommend preoperative routine mechanical bowel preparation (oral and/or rectally administered solutions) because the putative beneficial role on reducing the risk of postoperative infections and improving the quality of the operative field is counterbalanced by the patient discomfort, dehydration, and electrolyte imbalance [Citation17–19]. On the other hand, the impact of preoperative nutrition care is rarely described in recent literature for most surgical disciplines [Citation9,Citation20]. The implementation of preoperative nutrition interventions into the surgical pathway may be both effective and safe in improving intra- and postoperative outcomes [Citation21]. In addition to pre‑operative fasting, patients encounter additional postoperative hunger due to delay in return of bowel function (ileus), causing decreased wound healing, prolonged hospital stay, and patient discomfort [Citation22]. Reducing the duration of postoperative ileus may not only improve maternal recovery, but also facilitate maternal–neonatal bonding [Citation13]. Several measures have been tried to reduce the duration of postoperative ileus after surgery, including the nasogastric tube, adequate hydration, early ambulation, and chewing gum, all showing only limited benefits [Citation17–19]. Only few ERAS Society guidelines recommend the routine inclusion of specific preoperative diets before surgery and there are no specific indications for obstetric surgical patients [Citation23–25].
The aim of the present study, therefore, is to evaluate the impact of preoperative low-residue diet on postoperative ileus in women undergoing elective CS.
2. Materials and methods
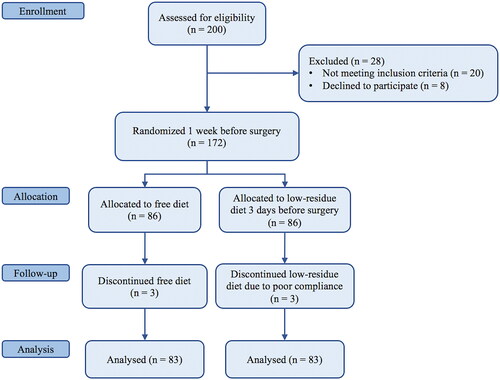
The present study is a surgeon-blind prospective randomized controlled trial. The study was reported following the Consolidated Standards of Reporting Trials (CONSORT) guideline () [Citation26]. From October 2018 to November 2020, pregnant women at ≥39 weeks of gestation scheduled for elective CS at the Department of Gynecology of Policlinico Umberto I (Sapienza University of Rome) were enrolled in the study. Written informed consent was obtained from all subjects. The study was approved by the local Institutional Review Board and retrospectively registered (protocol number: 0724/2018).
Exclusion criteria included: (a) urgent/emergent surgery; (b) patients with gastrointestinal disorders; (c) complicated pregnancy (e.g. active infection, placental adhesive disorders, hypertensive disorders), which would potentially prolong their hospitalization; (d) incomplete medical records. One week before the surgery, patients were randomized to receive preoperative low-residue diet starting three days before surgery (Arm A) or free diet (Arm B). A low-fiber diet simply reduces fiber intake by eliminating or limiting high-fiber foods such as raw fruits and vegetables. Patients received clear explanations, practical schemes, and a list of allowed foods, tailored to fit individual needs (see and Citation2). They were also encouraged to maintain daily records of foods they consumed, which were then checked by unblind investigators. Randomization assignment was performed using the block randomization method (block size of 4) to ensure a balance in sample size across groups over time.
Table 1. List of allowed and not allowed foods.
Table 2. Preoperative low-residue diet (starting three days before CS).
Preoperatively, all patients were submitted to a general and obstetric history, complete physical and obstetric examination, obstetric ultrasound, blood exams, and EKG. All patients received antibiotic prophylaxis with cefazolin 2 g 30 min before incision and antithrombotic prophylaxis with low-molecular-weight heparins 12 h before surgery. Liquid and solid fasting was maintained starting from 8 h before the intervention. Perioperative data were recorded. All patients followed a liquid diet for 12 h after surgery. All CSs were performed by the same surgeon using a Pfannenstiel incision. The surgeon was not aware of which diet the patients had followed before surgery.
The primary outcome was the postoperative ileus. The primary endpoint was the passage of flatus within the first 24 h after CS. The secondary outcomes were the postoperative pain, the quality of the surgical field, postoperative complications, and the length of hospital stay. The Clavien–Dindo classification system was used to describe grade I–IV postoperative complications [Citation27]. During surgery, the surgeon was asked to evaluate the degree of small and large bowel preparation and the overall appropriateness of the surgical field using a 5-point scale (poor, sufficient, medium, good, excellent) [Citation28]. Blood loss was evaluated indirectly by pre and postoperative hemoglobin levels after standardization of postoperative fluid intake. All patients were asked to indicate their degree of nausea, pain, and abdominal swelling at 12, 24, and 48 h postoperatively, through a VAS scale. Postoperative ileus (time to first passage of flatus) and postoperative hospital stay were recorded as well as the request and administration of analgesics (IV or IM) and any short-term postoperative complication. The standard analgesic used was intravenous 1000 mg paracetamol every 6–8 h as needed.
The sample size was calculated by considering a 70% rate of passage of flatus within the first 24 h after CS and a 90% rate in patients receiving low-residue diet before surgery. Group sample sizes of 78 in group one and 78 in group two achieve 86% power to detect a difference between the group proportions of −0.2000. The proportion in group one (the low-residue diet group) is assumed to be 0.9000 under the null hypothesis and 0.7000 under the alternative hypothesis. The test statistic used is the two-sided Fisher’s Exact test. The significance level of the test was targeted at .05. We considered a dropout percentage of about 10%, and then we enrolled 172 patients; after the screening procedures we assigned 83 patients in the free-diet group and 83 patients in the low-residue group.
Continuous data were summarized by mean, median, and standard deviation. Categorical data were summarized by counts and percentage. Parametric tests were used after evaluation of the normal distribution of the data to be analyzed. Student’s t-test was used for continuous parametric variables, and the χ2test was used for categorical variables. The Mann–Whitney test was used for nonparametric data. Statistical significance was set at a p value of <.05.
3. Results
A total of 166 patients scheduled for cesarean delivery were enrolled: 83 in arm A and 83 in arm B. Patients’ characteristics are detailed in . No statistically significant differences in terms of demographic characteristics and comorbidities were reported between the two groups. Briefly, the mean ages (± SD) were 34.4 ± 6.2 years and 34.42 ± 7.5 years, respectively, in arm A and B. Previous CS was the most frequent indication for elective CS, being 51.8% and 56.6% respectively, in arm A and B, followed by maternal indications (25.3% vs 21.7%, respectively), maternal request (14.5% vs 15.7%, respectively), fetal indications (10.8% vs 7.2%, respectively), and malpresentation (2.4% vs 3.6%, respectively).
Table 3. Clinical characteristics of OB group patients.
Intraoperative variables and complications as well as the evaluation of the quality of surgical field are listed in . There are no differences regarding pre- and post-operative hemoglobin level and operating time. Hysterectomy for uterine atony was reported in four cases: one case (1.2%) in arm A and three cases (3.6%) in arm B.
Table 4. Intraoperative variables, complications, and quality of the surgical field.
A statistically significant difference was reported about postoperative ileus after cesarean delivery. Postoperative ileus was significantly shorter in arm A patients, being <24 h in 80.7% of patients versus 63.8% in arm B. The evaluation of small and large intestine preparation was significantly different between the two groups: the final score was graded as ≥3 in 96.4% of patients in arm A and 80.7% in arm B for small intestine, and, respectively, 97.6% and 81.9% for large intestine. Postoperative surgical data and complications are detailed in . Postoperative pain after 12 h from CS was significantly lower in arm A (VAS 3.4 ± 1.7) compared to arm B (VAS 4.1 ± 1.8). There were no significant differences as regards pain at 24 and 48 h, nausea and vomit at 24 h, postoperative complications, and postoperative hospital stay.
Table 5. Postoperative variables and complications.
4. Discussion
In light of the elevated burden of CS worldwide, the adoption of ERAS programs also within the field of obstetric surgery could significantly improve the quality and cost of care. Pregnant women undergoing elective CS must cross many of the same perioperative steps as patients submitted to other elective surgeries; however, implementation of ERAS for CS seems to be unusual as compared to other surgical specialties.
During 2018–2019, the ERAS Society published the guidelines for perioperative care in CS divided into three parts: antenatal and preoperative [Citation17], intraoperative [Citation18], and postoperative [Citation19]care. In 2019, the Society for Obstetric Anesthesia and Perinatology (SOAP) provided a consensus document regarding Enhanced Recovery after Cesarean (ERAC) [Citation29]. All these recommendations agree that oral or mechanical bowel preparation should not be used before CS, as the only two trials available in the obstetrics field failed to demonstrate any benefit. Nonetheless, there are neither data nor specific indications on the alternative use of preoperative diets aiming to reduce the risk of infection and adverse postoperative outcomes, although it is a simple, safe, and noninvasive intervention. Compared to mechanical bowel preparation, normocaloric low-fiber diet has demonstrated to be equally effective and better tolerated by patients in several settings, including colorectal and gynecologic surgery [Citation9,Citation10]. A large number of nutrition care interventions aimed at improving postoperative morbidity are a result of practice-based knowledge handed down by experienced mentors rather than evidence-based evidence.
Our study is the first attempt to investigate, in a randomized controlled design, the use of a preoperative low-fiber diet in pregnant women undergoing elective CS. The idea of a study comparing preoperative free and low-residue diets arose from the belief that several perioperative outcomes, such as pain, nausea/vomiting, postoperative ileus, and quality of the surgical field might be improved with a very minimal intervention. Our findings are encouraging, since either the quality of the surgical field and postoperative ileus and pain were significantly better in women subjected to preoperative low-fiber diet. In particular, post-CS pain poses a burden on women in the postpartum period as it may interfere with maternal–fetal bonding and impair maternal recovery [Citation13]. As mothers may be concerned for the underlying side effects of using analgesic agents during breastfeeding, an alternative approach to reduce pain after CS would be preferable [Citation30]. In this scenario, a simple and safe intervention such as low-residue diet has the potential to enhance the quality of CS and improve both maternal and neonatal outcomes.
The main strength of our study was the prospective, randomized study design. The structure of our intervention, however, was not without limitations and the generalizability of our study may be limited as it was performed at a single center. Our findings are consistent with ERAS implementation in nonobstetric populations and represent an opportunity to improve the preoperative nutrition care within ERAS programs for CS.
5. Conclusions
This prospective randomized clinical trial adds to the paucity of literature on ERAS for obstetric patients and suggests the potential benefits of preoperative low-residue diet on improving postoperative ileus after CS and maternal outcomes. Low-residue diet started three days before elective CS was associated with reduced postoperative ileus and pain when compared with free diet. Reducing the duration of postoperative ileus may not only improve patient comfort and recovery, but also facilitate maternal–neonatal bonding and reduce the cost of patient care. These results are encouraging but further large-scale, multicenter studies are required before being able to translate them into routine obstetrical practice.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki Declaration (as revised in Tokyo 2004) and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board of Sapienza University of Rome (Umberto I Hospital).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Gibbons L, Belizán J, Lauer J, et al. The global numbers and costs of addition- ally needed and unnecessary caesarean sections performed per year: overuse as a barrier to universal coverage. (Background paper no. 30). Geneva: World Health Organization; 2010.
- Suharwardy S, Carvalho B. Enhanced recovery after surgery for cesarean delivery. Curr Opin Obstet Gynecol. 2020;32(2):113–120.
- Fay EE, Hitti JE, Delgado CM, et al. An enhanced recovery after surgery pathway for cesarean delivery decreases hospital stay and cost. Am J Obstet Gynecol. 2019;221(4):349.e1–349–e9.
- Bisch SP, Jago CA, Kalogera E, et al. Outcomes of enhanced recovery after surgery (ERAS) in gynecologic oncology–a systematic review and meta-analysis. Gynecol Oncol. 2021;161(1):46–55.
- Bogani G, Sarpietro G, Ferrandina G, et al. Enhanced recovery after surgery (ERAS) in gynecology oncology. Eur J Surg Oncol. 2021;47(5):952–959.
- Scheib SA, Thomassee M, Kenner JL. Enhanced recovery after surgery in gynecology: a review of the literature. J Minim Invasive Gynecol. 2019;26(2):327–343.
- Wijk L, Udumyan R, Pache B, et al. International validation of enhanced recovery After surgery society guidelines on enhanced recovery for gynecologic surgery. Am J Obstet Gynecol. 2019;221(3):237.e1–237.e11. e1
- Nelson G, Bakkum-Gamez J, Kalogera E, et al. Guidelines for perioperative care in gynecologic/oncology: enhanced recovery after surgery (ERAS) society recommendations-2019 update. Int J Gynecol Cancer. 2019;29(4):651–668.
- Alvarez-Gonzalez MA, Pantaleon MA, Flores-Le Roux JA, et al. Randomized clinical trial: a normocaloric low-fiber diet the day Before colonoscopy is the most effective approach to bowel preparation in colorectal cancer screening colonoscopy. Dis Colon Rectum. 2019;62(4):491–497.
- Slim K, Vicaut E, Launay-Savary M-V, et al. Updated systematic review and meta-analysis of randomized clinical trials on the role of mechanical bowel preparation before colorectal surgery. Ann Surg. 2009;249(2):203–209.
- Slim K, Vicaut E, Panis Y, et al. Meta-analysis of randomized clinical trials of colorectal surgery with or without mechanical bowel preparation. Br J Surg. 2004;91(9):1125–1130.
- Oliveira L, Wexner SD, Daniel N, et al. Mechanical bowel preparation for elective colorectal surgery. A prospective, randomized, surgeon-blinded trial comparing sodium phosphate and polyethylene glycol-based oral lavage solutions. Dis Colon Rectum. 1997;40(5):585–591.
- Laronche A, Popescu L, Benhamou D. An enhanced recovery programme after caesarean delivery increases maternal satisfaction and improves maternal-neonatal bonding: a case control study. Eur J Obstet Gynecol Reprod Biol. 2017;210:212–216.
- Kleiman AM, Chisholm CA, Dixon AJ, et al. Evaluation of the impact of enhanced recovery after surgery protocol implementation on maternal outcomes following elective cesarean delivery. Int J Obstet Anesth. 2020;43:39–46.
- Teigen NC, Sahasrabudhe N, Doulaveris G, et al. Enhanced recovery after surgery at cesarean delivery to reduce postoperative length of stay: a randomized controlled trial. Am J Obstet Gynecol. 2020;222(4):372.e1–372.e10.
- Ljungqvist O, Thanh NX, Nelson G. ERAS-Value based surgery. J Surg Oncol. 2017;116(5):608–612.
- Wilson RD, Caughey AB, Wood SL, et al. Guidelines for antenatal and preoperative care in cesarean delivery: enhanced recovery After surgery society recommendations (part 1). Am J Obstet Gynecol. 2018;219(6):523.e1–523.e15.
- Caughey AB, Wood SL, Macones GA, et al. Guidelines for intraoperative care in cesarean delivery: enhanced recovery after surgery society recommendations (part 2). Am J Obstet Gynecol. 2018;219(6):533–544.
- Macones GA, Caughey AB, Wood SL, et al. Guidelines for postoperative care in cesarean delivery: enhanced recovery After surgery (ERAS) society recommendations (part 3). Am J Obstet Gynecol. 2019;221(3):247.e1-247–e9.
- Lijoi D, Ferrero S, Mistrangelo E, et al. Bowel preparation before laparoscopic gynaecological surgery in benign conditions using a 1-week low fibre diet: a surgeon blind, randomized and controlled trial. Arch Gynecol Obstet. 2009;280(5):713–718.
- Smeets BJJ, Luyer MDP. Nutritional interventions to improve recovery from postoperative ileus. Curr Opin Clin Nutr Metab Care. 2018;21(5):394–398.
- Wrench IJ, Allison A, Galimberti A, et al. Introduction of enhanced recovery for elective caesarean section enabling next day discharge: a tertiary centre experience. Int J Obstet Anesth. 2015;24(2):124–130.
- Bisch S, Nelson G, Altman A. Impact of nutrition on enhanced recovery after surgery (ERAS) in gynecologic oncology. Nutrients. 2019;11(5):1088.
- Sandrucci S, Beets G, Braga M, et al. Perioperative nutrition and enhanced recovery after surgery in gastrointestinal cancer patients. A position paper by the ESSO task force in collaboration with the ERAS society (ERAS coalition). Eur J Surg Oncol. 2018;44(4):509–514.
- Weimann A, Braga M, Carli F, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. 2017;36(3):623–650.
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332–c332.
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196.
- Muzii L, Bellati F, Zullo MA, et al. Mechanical bowel preparation before gynecologic laparoscopy: a randomized, single-blind, controlled trial. Fertil Steril. 2006;85(3):689–693.
- Bollag L, Lim G, Sultan P, et al. Society for obstetric anesthesia and perinatology: consensus statement and recommendations for enhanced recovery After cesarean. Anesth Analg. 2021;132(5):1362–1377.
- Di Mascio D, Caruso G, Prata G, et al. The efficacy of abdominal binders in reducing postoperative pain and distress after cesarean delivery: a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2021;262:73–79.